Abstract
The Bateman domain (CBS subdomain) of IMP dehydrogenase (IMPDH), a rate-limiting enzyme of the de novo GMP biosynthesis, is evolutionarily conserved but has no established function. Deletion of the Bateman domain has no effect on the in vitro IMPDH activity. We report that in vivo deletion of the Bateman domain of IMPDH in Escherichia coli (guaBΔCBS) sensitizes the bacterium to growth arrest by adenosine and inosine. These nucleosides exert their growth inhibitory effect via a dramatic increase in the intracellular adenylate nucleotide pool, which results in the enhanced allosteric inhibition of PRPP synthetase and consequently a PRPP deficit. The ensuing starvation for pyrimidine nucleotides culminates in growth arrest. Thus, deletion of the Bateman domain of IMPDH derepresses the synthesis of AMP from IMP. The growth inhibitory effect of inosine can be rescued by second-site suppressor mutations in the genes responsible for the conversion of inosine to AMP (gsk, purA, and purB) as well as by the prsA1 allele, which encodes a PRPP synthetase that is insensitive to allosteric inhibition by adenylate nucleotides. Importantly, the guaBΔCBS phenotype can be complemented in trans by a mutant guaB allele, which encodes a catalytically disabled IMPDHC305A protein containing an intact Bateman domain. We conclude that the Bateman domain of IMPDH is a negative trans-regulator of adenylate nucleotide synthesis, and that this role is independent of the catalytic function of IMPDH in the de novo GMP biosynthesis.
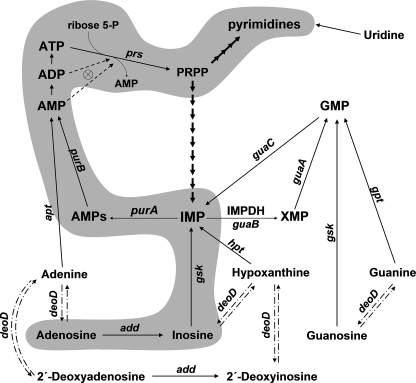
Inosine 5′-monophosphate dehydrogenase (IMPDH)3 catalyzes the first committed reaction in the de novo synthetic pathway of GMP, the NAD-dependent oxidation of IMP to XMP (1). IMP is the last common precursor of both guanylate and adenylate nucleotides and also serves as a substrate for adenylosuccinate (AMPs) synthetase, which commits IMP to adenylate nucleotide biosynthesis (Fig. 1). Sources of IMP include de novo synthesis starting from 5-phosphoribosyl 1-pyrophosphate (PRPP), regeneration from AMP and GMP, as well as salvage of hypoxanthine and inosine by phosphoribosylation and phosphorylation, respectively (1). Inhibition of IMPDH depletes the cellular guanylate pool and is antiproliferative, which has led to the establishment of IMPDH as a target for anti-tumor, immunosuppressive, and antimicrobial therapies (2, 3).
FIGURE 1.
The de novo and salvage pathways for purine nucleotide biosynthesis. The pathway of adenosine/inosine toxicity is highlighted. Dashdot lines indicate the reversible reactions catalyzed by purine nucleoside phosphorylase (deoD); most strains employed in this study are null for the enzyme. Dashed lines indicate feedback regulation of PRPP synthetase by ADP and AMP. The gene designations are: purA, adenylosuccinate synthetase; purB, adenylosuccinate lyase; guaB, IMP dehydrogenase; guaA, GMP synthetase; deoD, purine nucleoside phosphorylase; hpt, guanine-hypoxanthine phosphoribosyltransferase; gpt, guanine phosphoribosyltransferase; gsk, guanosine-inosine kinase; apt, adenine phosphoribosyltransferase; add, adenosine deaminase; guaC, GMP reductase; prs, PRPP synthetase.
A multitude of crystal structures of IMPDH from various sources have been reported, and these have collectively created a detailed picture of the catalytic domain structure-function relationships (for reviews see Refs. 4 and 5). IMPDH is a tetramer with each subunit consisting of two structurally discrete domains (6). The larger catalytic domain is an α-β barrel of about 400 amino acids. The approximately 120-residue subdomain is inserted within the center of the dehydrogenase sequence and is composed of two tandem repeats of an amino acid sequence motif with homology to the enzyme cystathionine β-synthase (CBS). Pairs of CBS sequences are common in many proteins of unrelated functions and are also known as Bateman domains (7, 8). Despite the nearly absolute conservation of a Bateman domain in the several hundred known IMPDH sequences, the physiological function of this structure remains a mystery. Amino acid substitutions in the Bateman domain of human IMPDH type 1 are associated with the RP10 form of autosomal dominant retinitis pigmentosa, a hereditary degenerative disease of the retina (9-11). However, extensive evidence indicates that amino acid substitutions in the Bateman domain of IMPDH, as well as a complete deletion of the structure, do not impair the in vitro catalytic activity of the enzyme (12-15). The subdomain therefore appears irrelevant to the catalytic function of the core domain and has been speculated to have an as yet to be discovered moonlighting role. This is in sharp contrast to several other enzymes, such as AMP-dependent protein kinase and cystathionine β-synthase, which are allosterically regulated by binding of adenosine-containing compounds to their Bateman domains (16, 17).
In our previous study we created a bacterial model that allowed us to gain initial insights into the possible in vivo functions of the Bateman domain of IMPDH (18). A guanine prototrophic Escherichia coli strain (MP101, guaBΔCBS) was constructed in which the Bateman domain coding sequence was deleted from the chromosomal guaB gene for IMPDH while preserving the catalytic function of the core enzyme. The metabolic effects of this mutation allowed us to conclude that the Bateman domain of IMPDH plays an important role in maintaining the physiological adenylate nucleotide pool and in the regulation of the purine nucleotide turnover.
In the present study we employed metabolic phenotype screening to identify additional phenotypic manifestations of the guaBΔCBS mutation. We found that growth of the guaBΔCBS mutant on minimal media is arrested by adenosine or inosine, and that these exert their growth inhibitory effect via a dramatic increase in the adenylate nucleotide pool size. The increase in the intracellular concentrations of the adenylate nucleotides leads to enhanced allosteric inhibition of PRPP synthetase and, as a result, starvation for PRPP. An insufficient PRPP supply causes depletion of the pyrimidine nucleotide pool and culminates in growth arrest. We provide evidence that the Bateman domain of IMPDH negatively regulates the biosynthesis of AMP, possibly via inhibition of AMPs synthetase, and that this regulation is independent of the IMPDH catalytic function.
EXPERIMENTAL PROCEDURES
Strains and Plasmids—The antibiotic concentrations used throughout this study were as follows: tetracycline, 20 μg/ml; kanamycin, 35 μg/ml; chloramphenicol, 12.5 μg/ml. All cell growth procedures were carried out at 37 °C. MOPS medium (19) supplemented with 0.4% glucose as carbon source was used as the standard minimal salts growth medium. Some growth phenotypes were also verified in M9 minimal salts medium with 0.2% glucose (data not shown). MOPS medium containing 0.0125% glucose was used for glucose-limited growth. All minimal media were routinely supplemented with 1 μg/ml thiamine.
For the strain sources and cloning strategies please refer to Table 1. For oligonucleotide sequences see Table 2. The P1vir transductions and molecular cloning procedures were carried out as described elsewhere (20, 21). The genetic identities of the recombinant DNA constructs and strains were verified by PCR and sequencing.
TABLE 1.
Strains and plasmids used in this study
| Strain/plasmid | Parent/genotype | Ref./source/construction |
|---|---|---|
| BW25113 | lacIqrrnBT14 ΔlacZWJ16hsdR514 ΔaraBADAH33 ΔrhaBADLD78 | 22 |
| MP101 | [BW25113] guaBΔCBS | 18 |
| JW1615 | [BW25113] add::kan | Keio collection (44) |
| JW5401 | [BW25113] guaB::kan | Keio collection (44) |
| JW0466 | [BW25113] gsk::kan | Keio collection (44) |
| JW4135 | [BW25113] purA::kan | Keio collection (44) |
| JW0101 | [BW25113] guaC::kan | Keio collection (44) |
| TT25401 | tet+ | TetR; J. Roth laboratorya |
| MP310 | [BW25113] guaBΔCBSadd::kan | MP101 × P1(JW1615), Kanr |
| MP330 | [BW25113] guaBΔCBSgsk::kan | MP101 × P1(JW0466), Kanr |
| MP255 | [BW25113] deoD::tet | Recombineering, BW25113 deoD → tet, Tetr |
| MP350 | [BW25113] guaBΔCBSdeoD::tet | Recombineering, MP101 deoD → tet, Tetr |
| MP2501 | [BW25113] deoD::tet purA::kan | MP255 × P1(JW4135), Kanr Tetr |
| MP3501 | [BW25113] guaBΔCBSdeoD::tet purA::kan | MP350 × P1(JW4135), Kanr Tetr |
| MP402 | [BW25113] purB::kan | Recombineering, BW25113 purB → kan, Kanr |
| MP4021 | [BW25113] deoD::tet purB::kan | MP255 × P1(MP402), Kanr Tetr |
| MP4022 | [BW25113] guaBΔCBSdeoD::tet purB::kan | MP350 × P1(MP402), Kanr Tetr |
| pGUAB | pCC1 | guaBwt gene for IMPDH, native promoter (18) |
| pGUA6 | pGUAB | guaBTGT(914-916)→GCG gene for IMPDHC305A, native promoter |
| pGSK | pCC1 | gsk gene for guanosine-inosine kinase under control of constitutive PblaTEM promoter |
| pPRS | pCC1 | Wild-type prs allele for PRPP synthetase, native promoter |
| pPRS-A1 | pPRS | prsA1 allele for ADP/AMP-insensitive PRPP synthetase (prsA386C), native promoter (23) |
J. Roth, unpublished data.
TABLE 2.
Sequences of the oligonucleotides used in this study
| Primer | Sequence |
|---|---|
| DM481 | 5′-GCCAAAAAGGTCAATAGAAACGTTG |
| DM482 | 5′-GCATAGGTCATACAAATGGATATTACAGAC |
| DM483a | 5′-CGGTGTTGACCGTGTGCTGACAGTGGCTCTGCACGCTGAACAGATTCAGGGTTTC |
| DM484 | 5′-GAAACCCTGAATCTGTTCAGCGTGCAGAGCCACTGTCAGCACACGGTCAACACCG |
| DM479b | 5′-AAATACATTCAAATATGTATCCGCTCATGAGACAATAACCCTGTTTCAATAATTTCACACAGGAAAC AGCTATGAAATTTCCCGGTAAACGTA |
| DM480 | 5′-TTAACGATCCCAGTAAGACTCTTCC |
| DM429a | 5′-CGGCATTGGCCCTGGCTCTATCGCGACAACTCGTATCGTGACTGGCG |
| DM430 | 5′-CGCCAGTCACGATACGAGTTGTCGCGATAGAGCCAGGGCCAATGCCG |
| DM465c | 5′-GAGCGTTGACTCCGCCTTTGTTATGTCACAAAAAGGATAAAACACACCAAACACCCCCCAAAA CC |
| DM466c | 5′-CTCCCGCTCCGGCTTCACAAGGCAATCGCCTTGCAGCGAAACACAACACACAACCACACCACA CCAC |
| DM475c | 5′-CTCAGGCAAAACAAATTCTTGCTCATTTAACCCCGGAGTTGTGATCTGGAGCTGCTTCGAAGT TCC |
| DM476c | 5′-CTTAATAAGCAGGCCGGACAGCATCGCCATCCGGCACTGATACGAGGTATGAATATCCTCCTT AGTTCC |
The mutagenic sequence is underlined.
The 5′ extension containing the PblaTEM promoter sequence is underlined.
The 5′ extension for recombination with the target gene is underlined.
The E. coli strains MP255 and MP350 were constructed from BW25113 and MP101, respectively, by replacing the deoD gene for purine nucleoside phosphorylase with a tet cassette that confers resistance to tetracycline. The sequences of the DM465 and DM466 oligonucleotides used for tet cassette amplification from the TT25401 genomic DNA are presented in Table 2. Similarly, the DM402 strain was created by replacing the purB gene of BW25113 with a kanamycin resistance cassette. The DM475 and DM476 PCR primers, carrying purB-complementary 5′ adaptors, were used to amplify the kan cassette from the pKD4 DNA. A standard recombineering protocol (22) was employed for the deoD::tet and purB::kan gene replacements.
The pGSK single-copy plasmid was created using the Copy Control PCR Cloning kit (Epicenter). The oligonucleotides DM479 and DM480 were used to amplify the gsk gene for guanosine-inosine kinase and place it under control of the PblaTEM promoter. Purified E. coli BW25113 genomic DNA was used as the template. The resulting PCR product was cloned in the pCC1 single-copy number vector and sequenced to verify the construct.
The single-copy pGUA6 plasmid, which encodes a C305A active site mutant of IMPDH, was constructed using a standard QuikChange site-directed mutagenesis procedure (Stratagene) with the DM429/DM430 oligonucleotide pair. Our previously constructed pGUAB plasmid, a single-copy pCC1 derivative containing the guaBwt gene preceded by the PguaBA native promoter, was employed as a template (18).
The wild-type prs gene for PRPP synthetase was amplified from the E. coli BW25113 genomic DNA using the DM481/DM482 primer pair and cloned in pCC1, yielding the pPRS plasmid. A standard QuikChange site-directed mutagenesis procedure (Stratagene) was subsequently employed to create the pPRS-A1 plasmid harboring the previously described prsA1 allele encoding a A386C single amino acid substitution variant of PRPP synthetase (23). The DM483 and DM484 oligonucleotides were employed for the mutagenesis.
Measurements of Nucleotide Pools—The intracellular nucleotide pools were measured by 33Pi labeling of exponentially growing cells as described (24). Briefly, about 104 freshly grown cells were inoculated into 1 ml of glucose-limited MOPS minimal medium, containing 0.0125% glucose as carbon source, up to 10 μCi/ml H333PO4 (40-158 Ci/mg), and the appropriate antibiotics. Purine auxotrophic strains were supplemented with 0.1 mm guanine or adenine, as appropriate. After overnight growth at 37 °C the culture typically attained a terminal A600 of about 0.2. To allow the cells to resume growth, 20% glucose was added to a final concentration of 0.4%. Other additives, when indicated, were also added at this point. Cell growth was monitored by measurements of A600 on a calibrated spectrophotometer. After incubation at 37 °C with shaking for an additional 2 h the cultures typically had an A600 of 0.2-0.6 depending on the strain background and additives used. For nucleotide extraction, a 150-μl portion of the culture was mixed with ice-cold 11 n formic acid to a final concentration of 0.5 n. The inorganic phosphate was precipitated along with any acid-insoluble cell debris using a precipitation reagent that was prepared on the day of use by mixing 400 mm sodium tungstate, 500 mm tetraethylamine·Cl, and 500 mm procaine in the ratio 5:4:1 (24). Of this mixture, 14.3 μl was added to each 157-μl formic acid extract. The sample was vortexed and centrifuged at >10,000 × g at 4 °C for 10 min. The supernatant was chromatographed two-dimensionally on polyethyleneimine-cellulose plates as described (24). The radiolabeled nucleotide spots were visualized by phosphorimaging followed by quantification using the MultiGauge software (Fujifilm). The ATP concentration in the BW25113 strain was previously estimated to be 3.5 mm (18). Concentrations of the rest of the labeled nucleotides were calculated by comparison of their intensities to the intensity of the ATP spot in BW25113. All reported nucleotide concentrations are the average of at least three independent measurements.
Assays of Enzyme Activities in Cell Extracts—The cells were grown overnight in 20-ml batches in glucose-limited MOPS medium. The terminal A600 of the overnight cultures was typically 0.2. Cell growth was restored by re-addition of glucose to the medium to a final concentration of 0.4%. Where indicated, 4 mm inosine was added simultaneously with glucose. The cultures were incubated in a shaking water bath at 37 °C for 2 h. The A600 was measured and the cells were harvested at 4 °C by centrifugation at 5,000 × g for 20 min. The cells were washed by resuspending in 600 μl of cold 100 mm Tris·HCl, pH 7.46, 2 mm EDTA, 0.1 mm dithiothreitol followed by spinning at 10,000 × g. The BugBuster Master Mix reagent (Novagen), containing 30 μg/ml phenylmethanesulfonyl fluoride was used for protein extraction. One ml of BugBuster reagent was added per 100 A600 of bacteria. The extracts were spun at 15,000 × g at 4 °C for 10 min and the supernatant was diluted 20-fold with cold 100 mm Tris·HCl, pH 7.46, 2 mm EDTA, 0.1 mm dithiothreitol. The protein concentration in the extracts was measured using the Bio-Rad Bradford protein assay. Enzyme activities were normalized to the total protein concentrations in the 20-fold diluted extracts.
IMPDH activity was assayed essentially as described (18), with minor modifications. The 20-μl reaction mixture contained 0.2 mm [8-14C]IMP, 0.2 mm NAD, 50 mm Tris·HCl, pH 7.6, 150 mm KCl, 0.1 mm dithiothreitol, and 90-150 ng/μl of protein. The reaction was incubated at 37 °C for 30 min and stopped by addition of 5.5 n formic acid to a final concentration of 0.6 n. The production of [8-14C]XMP was monitored by TLC as previously described (24).
Measurements of the AMPs synthetase activity were carried out using a modification of a previously described procedure (25). The reaction was started by combining 2.5 μl of cell extract (1-1.75 μg of protein) with 20.5 μl of assay mixture containing 0.4 mm [8-14C]IMP, 2 mm GTP, 8 mm Mg(CH3COO)2, 50 mm HEPES, pH 7.0, and 8 mm aspartic acid. After incubation at 37 °C for 30 min, the reaction was quenched by addition of 2.5 μl of cold 5.5 n formic acid. The production of [8-14C]AMPs was monitored by thin-layer chromatography. Two microliters of the reaction mixture were spotted on a polyethyleneimine-cellulose plate and airdried. The plate was then immersed in methanol for 10 min, dried, and developed in the Ta buffer (24).
The activity assays for guanosine-inosine kinase were performed essentially as described (26). Ten μl of cell extract (4-7 μg of protein) was mixed with 10 μl of assay mixture, giving final concentrations in the assay of 80 mm Tris·HCl, pH 7.6, 35 mm MgCl2, 35 mm KCl, 2 mm ATP, and 0.25 mm [8-14C]guanosine. The reaction mixture was incubated at 37 °C for 30 min and quenched by addition of 2.5 μl of 5.5 n formic acid. Two μl of the reaction was spotted on a polyethyleneimine-cellulose thin layer plate that was developed in methanol up to the application line and then further developed in deionized water.
Anti-IMPDH Antibody and Western Blot Analysis—Polyclonal anti-IMPDH antibodies to the E. coli enzyme were custom-made by 21st Century Biochemicals (USA) using rabbit immunizations with the following two synthetic peptides: CLPNTADLSTQLTKTIRL and CVHDVTITKESPNYRLGS. These amino acid sequences are not part of the Bateman domain sequence. The antiserum was affinity purified and used for a standard immunoblot analysis of the crude BugBuster E. coli extracts. The protein load in each lane was standardized using the Bradford Bio-Rad protein assay.
RESULTS
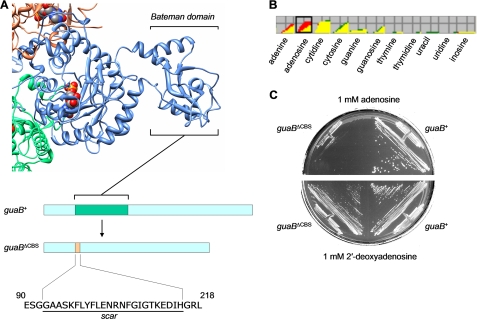
The guaBΔCBS Allele Sensitizes a Wild-type E. coli Strain to Adenosine and Inosine—In our previous study, we created and characterized an E. coli strain carrying a chromosomal mutation in the guaB gene for IMPDH (18). Specifically, the nucleotide sequence encoding the Bateman domain of the dehydrogenase was excised and replaced with an in-frame 24-amino acid “scar” sequence, as illustrated in Fig. 2A. The resulting strain, MP101 (guaBΔCBS), was prototrophic for guanine and grew with a wild-type rate on minimal salts media, indicating that the IMPDH enzymatic function is sustained. However, the mutation resulted in a 1.7-fold increase in the intracellular ATP pool as well as alteration of the purine nucleotide turnover rates of the mutant, indicating for the first time that the Bateman domain of IMPDH may play a regulatory role in the purine nucleotide homeostasis (18).
FIGURE 2.
The guaBΔCBS mutation sensitizes E. coli to growth inhibition by adenosine. A, the structure of the homologous Streptococcus pyogenes IMPDH tetramer (Protein Data Bank code 1ZFJ). A single subunit of the tetramer is shown along with the contacting parts of the two adjacent subunits. The individual subunits are shown in blue, orange, and green. The substrate (IMP) is shown in a sphere representation. The positioning of the Bateman domain inside the dehydrogenase sequence and its replacement with a scar sequence are illustrated in a schematic bar representation. UCSF Chimera was used for structure visualization (43). B, results of the Biolog phenotype microarray screening: an overlay diagram of the respiration rates of the guaB+ wild-type strain (BW25113, red) and guaBΔCBS mutant (MP101, green) using nucleosides and bases as nitrogen source. C, growth of the guaB+ wild-type strain and guaBΔCBS mutant on MOPS minimal media supplemented with 0.4% glucose as carbon source and 1 mm adenosine or 2′-deoxyadenosine.
In search for unexpected manifestations of the guaBΔCBS mutation we employed Biolog phenotype microarray technology, which couples cell respiration on various nitrogen and carbon sources to reduction of a tetrazolium dye and production of purple color (27). Microarrays PM1-PM8 were used, which allowed testing of 768 growth conditions in a single experiment. Compared with the guaB+ isogenic strain, the guaBΔCBS mutant respired significantly more slowly with adenosine as the nitrogen source (Fig. 2B). To confirm the results obtained on Biolog plates, we grew the wild-type (BW25113) and guaBΔCBS strain (MP101) on a minimal salts medium supplemented with 0.4% glucose as carbon source and 1 mm adenosine as nitrogen source (data not shown) as well as on standard MOPS minimal medium (which contained ammonia as nitrogen source and 0.4% glucose as carbon source) supplemented with 1 mm adenosine. The guaBΔCBS mutant was unable to grow on minimal media in the presence of adenosine, irrespective of whether ammonia was present as a source of nitrogen, suggesting that the lack of growth results from adenosine toxicity rather than a defect in utilization of adenosine as the sole nitrogen source. Surprisingly, the guaBΔCBS strain was not affected by the presence of equivalent concentrations of 2′-deoxyadenosine (Fig. 2C). Similar results were obtained with liquid MOPS cultures (data not shown).
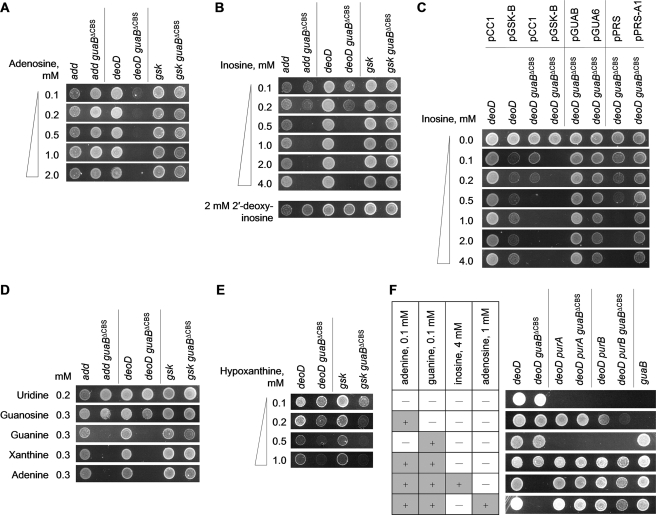
In E. coli, adenosine and 2′-deoxyadenosine are metabolized in the same way and can be either deaminated to produce ammonia and either inosine or 2′-deoxyinosine, respectively, or reversibly phosphorylyzed into adenine and the respective sugar phosphate (1). The selective toxicity of adenosine but not 2′-deoxyadenosine indicates that adenosine does not cause growth inhibition via adenine. In support of such an interpretation, a guaBΔCBS deoD strain, which lacks purine nucleoside phosphorylase and is therefore unable to cleave adenosine to form adenine, retained susceptibility to adenosine (Fig. 3A). In contrast, an adenosine deaminase knock-out mutation rendered the subdomain deletion mutant (guaBΔCBS add) resistant to adenosine (Fig. 3A), which indicated that adenosine has to be converted to inosine to cause growth arrest. In agreement with this result, the adenosine-resistant guaBΔCBS add strain was susceptible to growth arrest by 0.5 mm inosine, but not 2 mm 2′-deoxyinosine (Fig. 3B).
FIGURE 3.
Agar dilution spot assay on MOPS glucose minimal media. Appropriate antibiotics were used as described under “Experimental Procedures.” Freshly grown cells were spotted on the agar surface (104 colony forming units per spot) and the plates were incubated for 36-48 h at 37 °C. Most phenotypes were verified in MOPS liquid batch cultures with similar results. A, sensitivity testing of various concentrations of adenosine and second-site suppressors of adenosine toxicity. B, sensitivity testing of various concentrations of inosine. C, effects of gsk overexpression and the prsA1 and guaBC305A alleles on inosine toxicity. The strain genotypes are shown in combination with the plasmids used for gene expression and complementation. D, the effect of nucleosides and bases on growth in the presence of 4 mm inosine. E, growth on minimal media supplemented with hypoxanthine. F, growth phenotypes of purine-auxotrophic derivatives of the deoD guaB+ and deoD guaBΔCBS strains. The presence of purine additives in each lane are indicated by plus signs.
As shown in Fig. 1, metabolism of inosine can proceed in two directions: phosphorylation to IMP by inosine-guanosine kinase (gsk) or phosphorolysis to hypoxanthine and ribose 1-phosphate by the action of purine nucleoside phosphorylase (deoD), the same enzyme that metabolizes adenosine and guanosine. As shown in Fig. 3B, the toxic effect of inosine was preserved in the guaBΔCBS deoD strain. In the absence of deoD, the only route of inosine metabolism is phosphorylation to IMP by guanosine-inosine kinase (Fig. 1). Indeed, as demonstrated in Fig. 3, A and B, transduction of a gsk mutation into the guaBΔCBS mutant rendered the resulting strain (guaBΔCBS gsk) completely adenosine- and inosine-insensitive. Collectively, these results indicate that to impose growth arrest adenosine must be converted to IMP via a consecutive action of adenosine deaminase and guanosine-inosine kinase (Fig. 1).
Notably, the guaBΔCBS mutant could also be rescued from the toxic effects of inosine by addition of guanosine to the growth media, but not guanine (Fig. 3D). The gsk gene product is a bifunctional guanosine-inosine kinase with guanosine being a much better substrate than inosine (28), and guanosine appears to prevent the conversion of inosine to IMP by substrate competition. The lack of growth inhibition by 2′-deoxyinosine is probably due to a poor, if any, utilization of this substrate by guanosine-inosine kinase (29, 30).
The guaBΔCBS Allele Relaxes Control over Adenylate Nucleotide Synthesis—Metabolic effects of a nucleoside are most readily studied in a system where it cannot be interconverted with the nucleobase. Consequently, all further experiments were carried out using derivatives of the wild-type and guaBΔCBS strains carrying a deletion of the deoD gene for purine nucleoside phosphorylase, termed MP255 (deoD) and MP350 (deoD guaBΔCBS), respectively (Table 1).
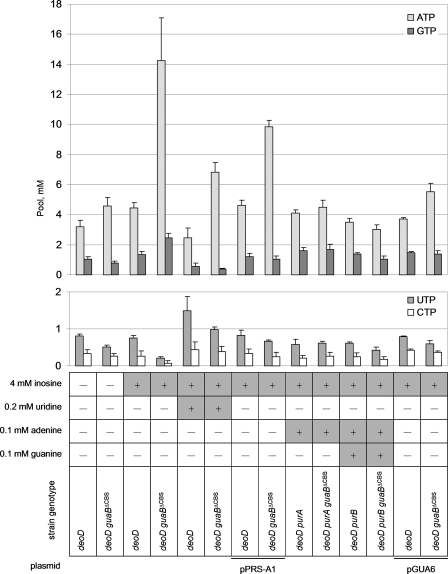
To trace the mechanism of growth inhibition by the IMP precursors, we measured how the nucleotide pools of the wild-type and subdomain deletion strains responded to inosine addition. As shown in Fig. 4, the “wild-type” guaB+ deoD stain responded to added inosine by only a slight increase in the ATP and GTP levels. In contrast, in the deoD guaBΔCBS mutant the ATP pool increased up to 4-fold and the GTP pool increased about 3-fold after a 2-h incubation with 4 mm inosine, compared with a culture where no inosine was used. A modest accumulation of IMP over the wild-type levels was detected in inosine-treated deoD guaBΔCBS cells ([IMP]MP255 = 0.13 ± 0.06 mm; [IMP]MP350 = 0.24 ± 0.05 mm); the statistical significance of this observation was marginal (p = 0.08). In contrast, the pyrimidine nucleotide pools of the inosine-challenged deoD guaBΔCBS mutant were almost undetectable (at least 3.5-fold lower than in the guaB+ strain), suggesting that pyrimidine starvation may be the underlying cause of the growth arrest. Indeed, addition of 0.2 mm uridine rescued the inosine-mediated growth inhibition of the deoD guaBΔCBS strain (Fig. 3D) and reversed the pyrimidine nucleotide pool change (Fig. 4). Uridine also lessened the inosine-induced swelling of the ATP pool although it was unable to bring it back to the wild-type value (Fig. 4). This suggests that pyrimidine starvation is secondary to the adenylate nucleotide pool increase in the mechanism of inosine toxicity.
FIGURE 4.
Nucleotide pools in strains grown on minimal media supplemented with the compounds indicated. The average of 3 independent measurements is given. Error bars are standard deviations.
It has been reported that an increase in the adenylate nucleotide pool leads to an enhanced allosteric inhibition of PRPP synthetase (prs) by ADP and AMP, which results in a decreased availability of PRPP for pyrimidine nucleotide biosynthesis (26). If such an interpretation were correct, the guaBΔCBS mutant would be insensitive to inosine if it carried the previously described prsA1 allele encoding a PRPP synthetase mutant that is insensitive to allosteric inhibition by adenylates (23). As expected, introduction of a single-copy pPRS-A1 plasmid containing the prsA1 allele rendered the recipient deoD guaBΔCBS mutant inosine-resistant (Fig. 3C), although it did not prevent the inosine-induced swelling of the ATP pool (Fig. 4). In contrast, pPRS, the parent plasmid harboring the wild-type allele for PRPP synthetase, did not confer inosine resistance upon the subdomain deletion mutant (Fig. 3C). Although we did not conduct direct measurements of the PRPP pools due to a low stability of PRPP in acid extracts, suppression of inosine toxicity by prsA1 strongly suggests that the PRPP pools are depleted in the guaBΔCBS mutant following inosine addition. Collectively, these results indicate that the guaBΔCBS mutation relaxes control over the adenylate nucleotide biosynthesis in the presence of IMP precursors (adenosine and inosine), which leads to increased intracellular concentrations of adenylate nucleotides, PRPP deficit, and thus starvation for pyrimidine nucleotides.
Notably, PRPP is also a precursor of the amino acids tryptophan and histidine. Depletion of PRPP has been shown to elicit starvation for these amino acids and result in stringent response with marked accumulation of ppGpp (26). However, we did not observe accumulation of ppGpp in any of our strains following addition of inosine, and supplementation of the growth media with amino acids did not rescue the inosine-induced growth arrest of the guaBΔCBS mutant (data not shown). We did not investigate the reason behind this observation, but other reports have suggested that amino acid synthesis may be relatively resistant to a partial depletion of the PRPP pool (31).
Mutations in the Adenylate Biosynthetic Genes Are Second-site Suppressors of Inosine Toxicity—To obtain additional evidence of the relaxed control over the ATP synthesis in the guaBΔCBS mutant and to provide insights into a possible mechanism of this dysregulation, we introduced into the deoD guaBΔCBS strain additional mutations that inactivated the genes responsible for the de novo synthesis of AMP from IMP. As shown in Fig. 1, in E. coli, as well as in all other organisms capable of de novo AMP synthesis, IMP is converted to adenylate nucleotides in a two-step process, catalyzed by the consecutive action of AMPs synthetase (purA) and AMPs lyase (purB). As expected, deletion of either of the two genes in the deoD guaBΔCBS background rendered the resulting strains (deoD purA guaBΔCBS and deoD purB guaBΔCBS, respectively) auxotrophic for adenine as well as completely insensitive to growth inhibition by inosine (Fig. 3F). This result confirms that inosine-dependent swelling of the adenylate nucleotide pool in the guaBΔCBS strain proceeds through direct conversion of IMP to AMP.
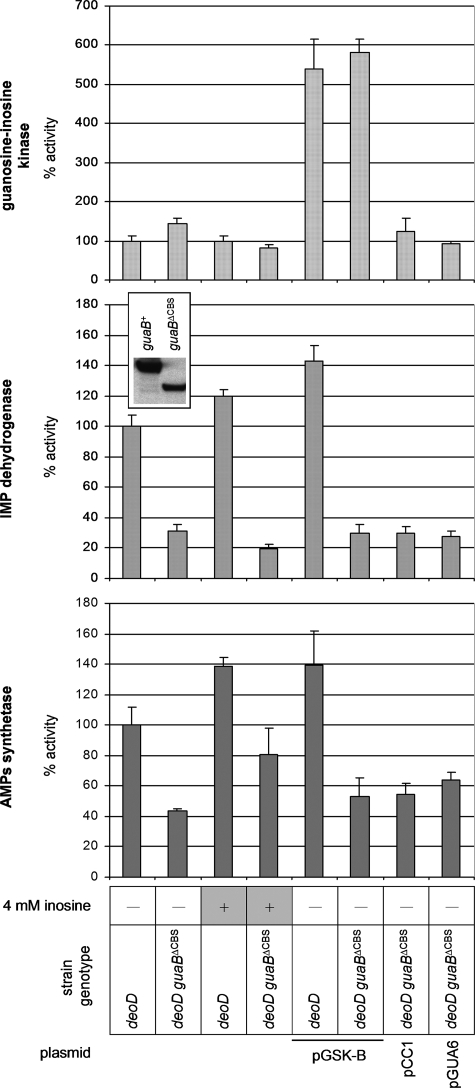
The Bateman Domain of IMPDH as a Possible Trans-regulator of AMPs Synthetase—In light of the above data, a central question remains: which enzyme in the chain of reactions leading from inosine to AMP is responsible for the uncontrolled increase of the pool of adenylate nucleotides? First, we measured the activities of AMPs synthetase, guanosine-inosine kinase, and IMPDH in crude extracts of the deoD guaB+ and deoD guaBΔCBS strains in the presence or absence of inosine (Fig. 5). The measured activity of guanosine-inosine kinase in the deoD guaBΔCBS strain was 1.5-fold higher than in the deoD guaB+ strain grown on the minimal medium, although the two activities were virtually indistinguishable when measured during growth in the presence of inosine. In agreement with the results of our previous study (18), the activity of AMPs synthetase in the deoD guaBΔCBS mutant was about 40% of the deoD guaB+ wild-type strain value, and we now find that it increased about 2-fold following inosine addition. Finally, the activity of IMPDH in the deoD guaBΔCBS strain extract was one-third of the wild-type strain value and decreased even further when inosine was added to the minimal culture medium. The 1.5-fold increase in the guanosine-inosine kinase activity, albeit modest, raises suspicion that it may be the principal factor behind the enhanced conversion of inosine to AMP and the resulting growth inhibition. However, even a 5-fold overexpression of guanosine-inosine kinase from a constitutive PblaTEM promoter did not significantly sensitize the wild-type strain to inosine (Figs. 3C and 5). In addition, compared with the deoD guaB+ wild-type strain, the deoD guaBΔCBS mutant displayed an increased sensitivity to hypoxanthine, which is converted to IMP in a gsk-independent fashion (although the difference in growth rates was less pronounced in this case due to a significant inhibition of the wild-type strain). This result suggests that the biosynthetic route to IMP is less important than an upstream event in the mechanism of the ATP pool expansion (Fig. 3E).
FIGURE 5.
Enzyme activities in crude cell extracts. The measured activities of IMPDH, AMPs synthetase, and guanosine-inosine kinase were normalized with respect to the protein load in each reaction and compared with the wild-type values. The average of 4 measurements is given. Error bars are standard deviations. The inset shows an immunoblot analysis of the wild-type and guaBΔCBS cell extracts with a polyclonal anti-IMPDH antibody demonstrating a change in the quantity and electrophoretic mobility of IMPDH following subdomain deletion. Thirty μg of protein was loaded onto each lane. A representative of three independent experiments is shown.
We previously observed a decrease in the in vivo activity of IMPDH following the subdomain deletion (18), and this decrease is sustained in the deoD background (Fig. 4). As demonstrated by an immunoblot analysis with a polyclonal anti-IMPDH antibody (Fig. 4, inset), the change in the in vivo enzyme activity is mirrored by a decreased total IMPDH concentration in the cell extract. Additionally, MP101 extracts probed with the anti-IMPDH antibody routinely demonstrated an accumulation of lower molecular weight proteins that were not observed in the wild-type E. coli lysates and likely represent IMPDH proteolytic fragments (data not shown). This observation supports the interpretation that the reduction of IMPDH activity accompanying our replacement of the subdomain with the “scar” sequence resulted from an enhanced in vivo degradation of the mutant IMPDH protein rather than a catalytic defect. In any case, a decreased IMPDH enzymatic activity per se does not confer inosine sensitivity, as clearly demonstrated by the fact that the JW5401 (guaB::kan) strain, a guanine auxotroph carrying a complete deletion of the guaB gene, is insensitive to inosine (Fig. 3F). Additionally, we observed that the guanylate nucleotide precursors guanine and xanthine are incapable of reversing the toxic effects of inosine (Fig. 3D), which further suggests that starvation for guanylate nucleotides does not play a role in the mechanism of the inosine-induced increase in the adenylate nucleotide pool.
As mentioned above, deletions of the purA gene encoding AMPs synthetase and purB gene for AMPs lyase rendered the guaBΔCBS mutant unable to convert IMP to AMP and, consequently, inosine-resistant. Indeed, as shown in Fig. 4, no significant increase in the ATP pool or decrease in the pyrimidine nucleotide pools was detected in the deoD purA guaBΔCBS and deoD purB guaBΔCBS strains growing in the presence of inosine. However, a significant difference in the AMPs accumulation patterns was observed between MP4022 (deoD purB guaBΔCBS) and its wild-type counterpart MP4021 (deoD purB guaB+). These strains are deficient in the last reaction of the de novo AMP synthesis, the conversion of AMPs to AMP by AMPs lyase, but retain a functional AMPs synthetase and are therefore proficient in the synthesis of AMPs from IMP. Following inosine addition, a very large, millimolar scale, accumulation of AMPs was observed in MP4022 ([AMPs]MP4022 = 8.1 ± 1.8 mm), up to 3-fold higher compared with the MP4021 wild-type strain ([AMPs]MP4021 = 2.8 ± 1.1 mm). In contrast, no inosine-induced accumulation of IMP was noted in the deoD purA guaBΔCBS strain that lacks the enzyme that is one step earlier in the AMP biosynthetic pathway (data not shown). It should be noted that measurements of IMP are complicated and generally somewhat less reliable than the rest of nucleotides. The use of a Pi precipitation reagent improves the visibility of nucleoside monophosphates by removing the phosphate front and results in a more reliable IMP quantification (24), allowing us to conclude with reasonable confidence that no significant accumulation of IMP takes place in any of our strains following addition of inosine. The much greater inosine-induced accumulation of AMPs, rather than IMP, suggests that the swelling of the adenylate nucleotide pool in the guaBΔCBS mutant may result from an increased in vivo activity of AMPs synthetase, despite the fact that the in vitro activity of this enzyme in crude extract is lowered by the guaBΔCBS mutation. The lack of correlation between in vitro enzyme activities and nucleotide pools has been previously reported (32) and is usually ascribed to in vivo regulation of enzyme activity by the components of a crowded cellular milieu.
Trans-complementation of Subdomain Deletion with a Catalytically Deficient Full-length IMPDH—We speculated that if the Bateman domain of IMPDH played a regulatory role that was not directly associated with the core domain catalysis, the phenotypic traits of the guaBΔCBS mutation might be rescued by a catalytically deficient IMPDH containing an intact Bateman domain and supplied in trans. The chemical mechanism of the IMPDH reaction proceeds through a covalent adduct between the 2-position of the IMP purine ring and the sulfur of an active site cysteine (Cys305 in the E. coli enzyme). In the subsequent steps, hydride transfer from the covalent enzyme-IMP species to NAD yields a thioimidate intermediate that is then hydrolyzed (5). A C305A mutant of IMPDH would therefore be defective in the first chemical step of the reaction but would likely keep its native structure. We cloned the wild-type E. coli guaB in the pCC1 single-copy vector under control of the native PguaB promoter and used the resulting plasmid, pGUAB, as the template for a site-directed mutagenesis procedure. The new vector carried a guaBTGT(914-916)→GCG gene for IMPDHC305A and was termed pGUA6. As expected, transformation of MP350 (deoD guaBΔCBS) with pGUA6 failed to increase the IMPDH activity of the strain, measured in a crude extract (Fig. 5). This confirms that the C305A amino acid substitution renders IMPDH catalytically ineffective. However, in contrast to an MP350/pCC1 “empty” vector control, the MP350/pGUA6 strain was resistant to growth inhibition by inosine (Fig. 3C) and demonstrated only very modest changes of the purine and pyrimidine nucleotide pools following inosine addition (Fig. 4). Thus, the presence of an intact Bateman domain is both necessary and sufficient for the negative regulation of adenylate nucleotide biosynthesis, irrespective of whether it is attached to an enzymatically intact or a catalytically disabled dehydrogenase core domain.
DISCUSSION
The realization that the Bateman domain of IMP dehydrogenase is dispensable for the in vitro catalytic activity of the enzyme has generated the now predominant view that the physiological importance of IMPDH may extend beyond its primary role in the de novo biosynthesis of GMP (4, 11). The recent discovery that point mutations in the Bateman domain of IMPDH type 1 cause human retinitis pigmentosa yet confer no detectable catalytic defect reinforced this view, renewed the interest in IMPDH biology, and suggested that further in vivo studies were necessary (14, 33-38). Having only one gene for IMPDH and being the most easily genetically manipulated system, E. coli seems to be an ideal organism for in vivo studies of IMPDH. We have previously demonstrated that replacement of the subdomain of E. coli IMPDH with a short scar sequence results in dysregulation of the purine nucleotide pool sizes and their turnover rates (18). The present study identified additional phenotypes associated with the guaBΔCBS mutation and has provided evidence that the Bateman domain of IMPDH is a negative regulator of the adenylate nucleotide synthesis.
We demonstrate that deletion of the Bateman domain of IMPDH sensitizes E. coli to growth arrest by adenosine and inosine via their enhanced conversion to ATP, as illustrated by the highlighted area in Fig. 1. The accumulation of adenylate nucleotides results in an increased allosteric inhibition of PRPP synthetase by AMP and ADP, which depletes cellular stores of PRPP, a common precursor of both purine and pyrimidine nucleotides. Starvation for pyrimidine nucleotides ensues which culminates in growth arrest. The toxic effect of adenosine and inosine can be rescued by uridine (which replenishes the pyrimidine nucleotide pool), guanosine (which competes with inosine for guanosine-inosine kinase, reducing the conversion of inosine to IMP), or by second-site suppressor mutations that inactivate the enzymes responsible for the conversion of inosine to AMP. Additionally, the guaBΔCBS mutation can be complemented with either the prsA1 allele for a PRPP synthetase mutant that is insensitive to allosteric inhibition by adenylate nucleotides, or by the full-length, catalytically disabled IMPDHC305A.
Several observations suggest that an increased utilization of IMP by AMPs synthetase, rather an enhanced IMP production by guanosine-inosine kinase, is the main driving force behind the inosine-induced swelling of the guaBΔCBS adenylate nucleotide pool. First, overexpression of guanosine-inosine kinase does not sensitize the wild-type guaB+ strain to inosine. Second, the guaBΔCBS mutant is also sensitive to growth inhibition by hypoxanthine, which is converted to IMP by a different enzyme. Finally, only modest accumulation of IMP over the wild-type levels is detected in the deoD guaBΔCBS strain following inosine addition (probably attributable to the slightly higher activity of guanosine-inosine kinase in this strain), whereas high levels of AMPs accumulate in the inosine-treated deoD purB guaBΔCBS mutant, indicative of an inappropriately high in vivo activity of AMPs synthetase.
Although both ATP and GTP pools increase in the guaBΔCBS mutant following inosine treatment, only ATP plays a role in growth inhibition. This result is consistent with the reported observation that an excess of guanylate nucleotides is growth inhibitory only so long as the pathway of conversion of GMP to AMP is intact, permitting accumulation of both purines (26). It should be noted that inhibition of de novo AMP synthesis by purA and purB mutations in the guaBΔCBS background restricts the inosine-induced accumulation of both ATP and GTP. The mechanism underlying this observation is unclear, but it indicates that the increase in the GTP pool is secondary to the ATP accumulation. Moreover, it provides additional evidence that IMP accumulation is not a central factor in the inosine-induced swelling of the ATP pool. Indeed, if that were the case, a mutation in the purA gene would increase (not decrease) the GTP pool by further increasing IMP availability. In contrast, the IMP pool was barely detectable in the purA guaBΔCBS mutant following inosine addition, suggesting that salvage of inosine is tightly coupled with IMP utilization. This is in agreement with other reports that suggest that purine precursor assimilation is coupled to the synthesis of pathway end products, and that phosphorylated intermediates, such as IMP, do not accumulate under most circumstances (18, 39, 40). We note that the ATP concentration in the guaBΔCBS mutant has already increased during growth on minimal media with no purine supplementation (18). Apparently, supplementation with adenosine or inosine provides an additional source of IMP that is converted to adenylate nucleotides by a derepressed AMPs synthetase.
The location of both IMPDH and AMPs synthetase at a major branch point in the purine nucleotide synthesis has generated the so far unsubstantiated speculation that the two enzymes are allosterically regulated by the intracellular levels of nucleotides or other stimuli. Several cellular metabolites have been shown to act as competitive inhibitors of AMPs synthetase, but the in vivo significance of these observations is typically unclear (41). Likewise, an in vivo importance of the classical competitive feedback inhibition of IMPDH by GMP is doubtful because of the large GMP concentrations needed for such inhibition to occur (18, 42). No allosteric regulation of either enzyme has been reported. Our observation that a catalytically disabled full-length IMPDH can complement the guaBΔCBS mutation in trans indicates that the catalytic function of IMPDH in de novo GMP biosynthesis and its regulatory role in ATP homeostasis may indeed be independent. Whether this regulation involves direct interactions of IMPDH with AMPs synthetase remains to be elucidated. The possibility of influencing growth by inhibiting the function of the Bateman domain of IMPDH suggests that it may represent a novel pharmacological target for drug development.
Acknowledgments
We thank Drs. W. Kruger, M. Andrake, and S. Karakashev for critical reading of this manuscript. We are grateful to Dr. A. T. Yeung, M. Hussein, and Dr. M. Andrake for helpful technical discussions. We thank Drs. S. Moore and R. T. Sauer for a gift of P1vir and the J. Roth laboratory for a gift of E. coli TT25401.
This work was supported, in whole or in part, by National Institutes of Health Grants GM072425 and CA06927. This work was also supported by an appropriation from the Commonwealth of Pennsylvania. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, or any other sponsoring organization. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: IMPDH, inosine 5′-monophosphate dehydrogenase; AMPs, adenylosuccinate; XMP, xanthosine 5′-monophosphate; PRPP, 5-phosphoribosyl 1-pyrophosphate; ppGpp, guanosine 3′-diphosphate 5′-diphosphate; CBS, cystathionine β-synthase; MOPS, 4-morpholinepropanesulfonic acid.
References
- 1.Zalkin, H., and Nygaard, P. (1996) in Escherichia coli and Salmonella: Cellular and Molecular Biology (Neidhardt, F. C., ed) pp. 561-579, ASM Press, Washington, D. C.
- 2.Pankiewicz, K. W., and Goldstein, B. M. (2003) Inosine Monophosphate Dehydrogenase: A Major Therapeutic Target, Oxford University Press, Washington, D. C.
- 3.Pankiewicz, K. W., Patterson, S. E., Black, P. L., Jayaram, H. N., Risal, D., Goldstein, B. M., Stuyver, L. J., and Schinazi, R. F. (2004) Curr. Med. Chem. 11 887-900 [DOI] [PubMed] [Google Scholar]
- 4.Hedstrom, L., and Gan, L. (2006) Curr. Opin. Chem. Biol. 10 520-525 [DOI] [PubMed] [Google Scholar]
- 5.Pimkin, M., and Markham, G. D. (2009) Adv. Enzymol. Relat. Areas Mol. Biol. 76 1-53 [PubMed] [Google Scholar]
- 6.Zhang, R., Evans, G., Rotella, F. J., Westbrook, E. M., Beno, D., Huberman, E., Joachimiak, A., and Collart, F. R. (1999) Biochemistry 38 4691-4700 [DOI] [PubMed] [Google Scholar]
- 7.Ignoul, S., and Eggermont, J. (2005) Am. J. Physiol. 289 C1369-C1378 [DOI] [PubMed] [Google Scholar]
- 8.Bateman, A. (1997) Trends Biochem. Sci. 22 12-13 [DOI] [PubMed] [Google Scholar]
- 9.Bowne, S. J., Sullivan, L. S., Blanton, S. H., Cepko, C. L., Blackshaw, S., Birch, D. G., Hughbanks-Wheaton, D., Heckenlively, J. R., and Daiger, S. P. (2002) Hum. Mol. Genet. 11 559-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennan, A., Aherne, A., Palfi, A., Humphries, M., McKee, A., Stitt, A., Simpson, D. A., Demtroder, K., Orntoft, T., Ayuso, C., Kenna, P. F., Farrar, G. J., and Humphries, P. (2002) Hum. Mol. Genet. 11 547-557 [DOI] [PubMed] [Google Scholar]
- 11.Hedstrom, L. (2008) Nucleosides Nucleotides Nucleic Acids 27 839-849 [DOI] [PubMed] [Google Scholar]
- 12.Nimmesgern, E., Black, J., Futer, O., Fulghum, J. R., Chambers, S. P., Brummel, C. L., Raybuck, S. A., and Sintchak, M. D. (1999) Protein Expression Purif. 17 282-289 [DOI] [PubMed] [Google Scholar]
- 13.Gan, L., Petsko, G. A., and Hedstrom, L. (2002) Biochemistry 41 13309-13317 [DOI] [PubMed] [Google Scholar]
- 14.Mortimer, S. E., and Hedstrom, L. (2005) Biochem. J. 390 41-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowne, S. J., Sullivan, L. S., Mortimer, S. E., Hedstrom, L., Zhu, J., Spellicy, C. J., Gire, A. I., Hughbanks-Wheaton, D., Birch, D. G., Lewis, R. A., Heckenlively, J. R., and Daiger, S. P. (2006) Investig. Ophthalmol. Vis. Sci. 47 34-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Townley, R., and Shapiro, L. (2007) Science 315 1726-1729 [DOI] [PubMed] [Google Scholar]
- 17.Kemp, B. E. (2004) J. Clin. Investig. 113 182-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pimkin, M., and Markham, G. D. (2008) Mol. Microbiol. 68 342-359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neidhardt, F. C., Bloch, P. L., and Smith, D. F. (1974) J. Bacteriol. 119 736-747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, J. H. (1992) A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 21.Sambrook, J., and Russell, D. W. (2001) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 22.Datsenko, K. A., and Wanner, B. L. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 6640-6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bower, S. G., Harlow, K. W., Switzer, R. L., and Hove-Jensen, B. (1989) J. Biol. Chem. 264 10287-10291 [PubMed] [Google Scholar]
- 24.Bochner, B. R., and Ames, B. N. (1982) J. Biol. Chem. 257 9759-9769 [PubMed] [Google Scholar]
- 25.Juang, R. H., McCue, K. F., and Ow, D. W. (1993) Arch. Biochem. Biophys. 304 392-401 [DOI] [PubMed] [Google Scholar]
- 26.Petersen, C. (1999) J. Biol. Chem. 274 5348-5356 [DOI] [PubMed] [Google Scholar]
- 27.Bochner, B. R., Gadzinski, P., and Panomitros, E. (2001) Genome Res. 11 1246-1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori, H., Iida, A., Teshiba, S., and Fujio, T. (1995) J. Bacteriol. 177 4921-4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawasaki, H., Shimaoka, M., Usuda, Y., and Utagawa, T. (2000) Biosci. Biotechnol. Biochem. 64 972-979 [DOI] [PubMed] [Google Scholar]
- 30.Nygaard, P. (1983) in Metabolism of Nucleotides, Nucleosides and Nucleobases in Microorganisms (Munch-Petersen, A., ed) pp. 27-93, Academic Press, London
- 31.Jochimsen, B. U., Hove-Jensen, B., Garber, B. B., and Gots, J. S. (1985) J. Gen. Microbiol. 131 245-252 [DOI] [PubMed] [Google Scholar]
- 32.Kelln, R. A., Kinahan, J. J., Foltermann, K. F., and O'Donovan, G. A. (1975) J. Bacteriol. 124 764-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunter, J. H., Thomas, E. C., Lengefeld, N., Kruger, S. J., Worton, L., Gardiner, E. M., Jones, A., Barnett, N. L., and Whitehead, J. P. (2008) Int. J. Biochem. Cell Biol. 40 1716-1728 [DOI] [PubMed] [Google Scholar]
- 34.Kennan, A., Aherne, A., and Humphries, P. (2005) Trends Genet. 21 103-110 [DOI] [PubMed] [Google Scholar]
- 35.Aherne, A., Kennan, A., Kenna, P. F., McNally, N., Lloyd, D. G., Alberts, I. L., Kiang, A. S., Humphries, M. M., Ayuso, C., Engel, P. C., Gu, J. J., Mitchell, B. S., Farrar, G. J., and Humphries, P. (2004) Hum. Mol. Genet. 13 641-650 [DOI] [PubMed] [Google Scholar]
- 36.McLean, J. E., Hamaguchi, N., Belenky, P., Mortimer, S. E., Stanton, M., and Hedstrom, L. (2004) Biochem. J. 379 243-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu, D., Cobb, G., Spellicy, C. J., Bowne, S. J., Daiger, S. P., and Hedstrom, L. (2008) Arch. Biochem. Biophys. 472 100-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mortimer, S. E., Xu, D., McGrew, D., Hamaguchi, N., Lim, H. C., Bowne, S. J., Daiger, S. P., and Hedstrom, L. (2008) J. Biol. Chem. 283 36354-36360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan, J., Fowler, W. U., Kimball, E., Lu, W., and Rabinowitz, J. D. (2006) Nat. Chem. Biol. 2 529-530 [DOI] [PubMed] [Google Scholar]
- 40.Roy-Burman, S., and Visser, D. W. (1975) J. Biol. Chem. 250 9270-9275 [PubMed] [Google Scholar]
- 41.Stayton, M. M., Rudolph, F. B., and Fromm, H. J. (1983) Curr. Top. Cell Regul. 22 103-141 [DOI] [PubMed] [Google Scholar]
- 42.Gilbert, H. J., Lowe, C. R., and Drabble, W. T. (1979) Biochem. J. 183 481-494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pettersen, E. F., Goddard, T. D., Huang, C. C., Couch, G. S., Greenblatt, D. M., Meng, E. C., and Ferrin, T. E. (2004) J. Comput. Chem. 25 1605-1612 [DOI] [PubMed] [Google Scholar]
- 44.Baba, T., Ara, T., Hasegawa, M., Takai, Y., Okumura, Y., Baba, M., Datsenko, K. A., Tomita, M., Wanner, B. L., and Mori, H. (2006) Mol. Syst. Biol. 2 2006 0008 [DOI] [PMC free article] [PubMed] [Google Scholar]