Abstract
Ezrin, encoded by VIL2, is a membrane-cytoskeletal linker protein that has been suggested to be involved in tumorigenesis. Ezrin expression in esophageal squamous cell carcinoma (ESCC) was described recently, but its clinical significance and the molecular mechanism underlying its regulated expression remain unclear. Thus, we retrospectively evaluated ezrin expression by immunohistochemistry in a tissue microarray representing 193 ESCCs. Ezrin overexpression in 90 of 193 tumors (46.6%) was associated with poor survival (p = 0.048). We then explored the mechanism by which ezrin expression is controlled in ESCC by assessing the transcriptional regulatory regions of human VIL2 by fusing deletions or site-directed mutants of the 5′-flanking region of the gene to a luciferase reporter. We found that the region -87/-32 containing consensus Sp1 (-75/-69) and AP-1 (-64/-58) binding sites is crucial for VIL2 promoter activity in esophageal carcinoma cells (EC109) derived from ESCC. AP-1 is comprised of c-Jun and c-Fos. Electrophoretic mobility shift and chromatin immunoprecipitation experiments demonstrated that Sp1 and c-Jun bound specifically to their respective binding sites within the VIL2 promoter. In addition, transient expression of Sp1, c-Jun, or c-Fos increased ezrin expression and VIL2 promoter activity. Use of selective inhibitors revealed that VIL2 transactivation required the MEK1/2 signal transduction pathway but not JNK or p38 MAPK. Taken together, we propose a possible signal transduction pathway whereby MEK1/2 phosphorylates ERK1/2, which phosphorylates Sp1 and AP-1 that in turn bind to their respective binding sites to regulate the expression of human VIL2 in ESCC cells.
Ezrin (villin 2), encoded by VIL2, is a membrane-cytoskeleton linker protein belonging to the ezrin-radixin-moesin family (1). By linking the cytoplasmic face of the plasma membrane to the actin cytoskeleton, ezrin acts as both a structural scaffold and a platform for the transmission of signals in response to extracellular cues (2). Ezrin is involved in a wide variety of cellular processes such as adhesion (3), survival (4), motility (5), and signal transduction (6-8). Furthermore, recent biochemical and functional data have identified a novel role for ezrin in the control of cyclin A gene transcription and endothelial cell proliferation (9).
Ezrin is often aberrantly expressed in human cancers. There is a relationship between high expression of ezrin and metastatic potential of some carcinomas, including hepatocellular carcinoma (10), lung cancer (11), breast carcinoma (12), pancreatic adenocarcinoma (13), and endometrial cancer (14). We also have demonstrated that ezrin is overexpressed in a malignantly transformed esophageal epithelial cell line compared with an immortalized cell line (15). Our more recent study on esophageal squamous cell carcinoma (ESCC)3 samples showed that ezrin tends to translocate from the plasma membrane to the cytoplasm in the progression from normal epithelium to invasive carcinoma of the esophagus (16). Moreover, both in vivo and in vitro experiments suggest that ezrin may affect tumor formation and tumor invasiveness directly (17). These findings of ezrin up-regulation associated with epithelial tumor metastasis and invasion make ezrin a potentially new prognostic marker and/or therapeutic target for some carcinomas (12, 18, 19).
Although much is known about how ezrin functions, there have been few reports about how ezrin expression is regulated. It has been reported that human ezrin expression can be regulated by cytokines, interleukin 2 (IL-2), IL-8, IL-10, and insulin-like growth factor 1 inhibit ezrin expression in human colon cancer cells, whereas epidermal growth factor and IL-11 increase cellular ezrin levels (20). Moreover, tumor necrosis factor-α treatment of human endothelial cells elevates ezrin expression (9). In disseminated osteosarcoma, ezrin is strongly stained by immunohistochemistry and has been proposed as a crucial factor for osteosarcoma metastasis (21). Ogino et al. (22) demonstrated a high level of ezrin mRNA expression in an osteosarcoma biopsy sample with lung metastasis, which was compatible with previous reports analyzing ezrin protein levels (21). These data suggest that ezrin levels are controlled at the transcriptional level. Stable transformation of Rat-1 fibroblasts by Fos results in increased expression of ezrin (23, 24). Mouse ezrin expression correlates with Six1 expression in rhabdomyosarcoma (25). Six1, a homeodomain-containing transcription factor required for skeletal muscle development, can bind to the mouse Vil2 promoter between -1106 and -870, a region containing the MEF3-like motif TTCAGGA, and regulate ezrin expression (26). Sequence alignment showed that the 5′-flanking regions of human VIL2 and mouse Vil2 are highly diverged (supplemental Fig. S1). Also the MEF3-like motif TTCAGGA present in the mouse sequence does not exist in the human VIL2 promoter. These sequence differences imply that the transcriptional regulation mechanism probably differs between human VIL2 and mouse Vil2. However, no study has addressed the transcriptional regulation of human VIL2, the key transcriptional regulatory regions of the gene, or the regulatory mechanisms governing its expression.
In the present study, we evaluated the clinical significance of ezrin overexpression in ESCCs and explored the importance of DNA sequence elements, transcription factors, and the mitogen-activated protein kinase (MAPK) signal transduction pathway in regulating ezrin expression in human esophageal carcinoma cells (EC109 cells), which are derived from ESCC (27). We found that ezrin overexpression in ESCCs was associated with decreased survival and that consensus transcription factor Sp1 (-75/-69) and AP-1 (-64/-58) binding sites are essential for human VIL2 promoter activity. We further demonstrated that the cooperativity of Sp1 and AP-1 (c-Jun/c-Fos heterodimer) regulate VIL2 promoter activity and ezrin expression and that mitogen-activated protein kinase kinase (MEK1/2) and extracellular signal-regulated kinase (ERK1/2) are upstream kinases that control human VIL2 transcriptional activation in ESCC cells.
EXPERIMENTAL PROCEDURES
Materials—Expression plasmid CMV-Sp1 was kindly provided by Dr. Guntram Suske (Philips University, Marburg, Germany). Plasmid pcDNA3 was purchased from Invitrogen. Plasmids pGL3-Basic and pRL-TK, recombinant human (rh) proteins rhSp1 and rhAP-1 (c-Jun), and kinase inhibitors U0126, PD98059, and SB203580 were purchased from Promega (Madison, WI). c-Jun N-terminal kinase (JNK) inhibitor II SP600125 was purchased from Calbiochem (La Jolla, CA). Antibodies against Sp1, c-Jun, c-Fos, phospho-ERK1/2, ERK1/2, and ezrin, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). β-Actin was purchased from Sigma. All other reagents were of analytical grade.
Tissue Specimens and Immunohistochemical Staining—Surgically removed tumors embedded in paraffin wax blocks from 193 ESCC cases were retrieved from the archives of the Department of Pathology of the Central Hospital of Shantou City, China. The cases were received between 1987 and 1997. The cases were selected to build tissue microarrays as described (28) and were included in this study only if a follow-up was obtained and clinical data were available. Mean age at surgery was 53 years (range 35-70), and 127 patients were male and 66 were female. The SuperPicTure Polymer Detection kit and Liquid Substrate kit (Invitrogen) were used to conduct immunohistochemistry according to the manufacturer's instructions. Staining was scored using the following scale: 0, no staining; 1+, minimal staining; 2+, moderate staining in at least 20% of cells; 3+, strong staining in at least 50% of cells. Cases scored as 0, 1+, or 2+ were classified as “non-overexpression,” and cases with 3+ staining were classified as “overexpression.” The study was approved by the ethical committee of the Central Hospital of Shantou City and the Medical College of Shantou University, and written informed consent was obtained from all surgical patients to use resected samples for research.
Expression Vectors and Reporter Gene Constructs—The human VIL2 5′-flanking region plus 134 bp of transcribed human VIL2 sequence was generated by PCR using the following primers: Fezr, 5′-CGGGGTACCA-1759GTGAATGCTGTTGCTGCTCGTCTGGAAG-3′ (KpnI site underlined; position -1759 is indicated); Rezr, 5′-CCCAAGCT+134TTCGGTTTCTGGTGAGTATCCTCGATCCC-3′ (HindIII site underlined; translation initiation site for the ezrin protein occurs at +135). The amplified fragment from the genomic DNA of EC109 cells was digested with KpnI/HindIII and inserted into the KpnI/HindIII sites of pGL3-basic, and the resulting plasmid was named pGLB-hE(-1759/+134). The luciferase reporter plasmids, pGLB-hE(-324/+134), pGLB-hE(-890/+134), pGLB-hE(-696/+134), pGLB-hE(-213/+134), pGLB-hE(-146/+134), pGLB-hE(-97/+134), pGLB-hE(-87/+134), and pGLB-hE(-32/+134) were generated from pGLB-hE(-1759/+134) using the Erase-a-Base® System (Promega). Site-directed mutagenesis to obtain sequences (-87/+134)Sm, (-87/+134)Am, and (-87/+134)SAm was performed by PCR using primer “Rezr” along with the following primers: Fezr-Sm, 5′--83GCAGTGCTAATATTTGCGCTGACTCACCCGGGCCCG-3′; Fezr-Am, 5′--83GCAGTGCTGGGCGGGGCGCGTCGGATCCCGGGCCCGGGCTGGCCGGTTC-3′; or Fezr-SAm, 5′--83GCAGTGCTAATATTTGCGCGTCGGATCCCGGGCCCGGGCTGGCCGG-3′ (position -83 is indicated; mutated bases are underlined). The amplified fragments obtained using Pfu DNA polymerase (Promega) were digested with HindIII and inserted into the SmaI/HindIII sites of pGL3-basic and were named pGLB-hE(-87/+134)Sm, pGLB-hE(-87/+134)Am, and pGLB-hE(-87/+134)SAm; in the constructs the sequence upstream of the sense primer was GCCC, which is the same sequence as -87/-84 of the human VIL2 5′-flanking region. The c-Fos and c-Jun expression vectors were constructed by cloning full-length c-Fos or c-Jun cDNA in the pcDNA3 plasmid. Primers for c-Fos were 5′-CCAAGCTTACCGCCACGATGATGTTCTC-3′ (HindIII site underlined) and 5′-CGGGATCCTTCCCTGCCCCCTCACA-3′ (BamHI site underlined). Primers for c-Jun were 5′-CCAAGCTTTGACGGACTGTTCTATGACTGC-3′ (HindIII site underlined) and 5′-CGGGATCCCGACGGTCTCTCTTCAAAATGT-3′ (BamHI site underlined).
Cell Culture and Transfection—EC109 cells were maintained in 199 medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) at 37 °C in a 5% CO2 environment. For transfection, cells were seeded in 96-well plates at 1.5 × 105 cells/ml, grown to 50-80% confluency and transfected with the plasmids described above using Superfect Transfection Reagent (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Following transfection, cells were incubated for another 48 h before being harvested for the luciferase assay or gene expression assay. Alternatively, after transfection for 24 h, cells were treated with a kinase inhibitor for another 24 h before being harvested.
Luciferase Assay—Transfected cells were harvested in Passive Lysis Buffer (Promega) and the cell lysates analyzed for luciferase activity with the Dual Luciferase Reporter Assay System (Promega) according to the manufacturer's recommendations.
Electrophoretic Mobility Shift Assay (EMSA)—Nuclear extracts from human esophageal cancer cells were produced according to standard methods (29). Extracts were aliquoted and stored at -70 °C. Equimolar amounts of complementary, single-stranded oligonucleotides were annealed and labeled with digoxigenin (DIG)-ddUTP by terminal transferase using a DIG Gel Shift kit (Roche). The oligonucleotide probes used in EMSA were as follows: probe W, 5′-GCCCGCAGTGCTGGGCGGGGCGCTGACTCACCCGGGCCCGGG-3′ (wild-type sequence); probe Sm, 5′-GCCCGCAGTGCTAATATTTGCGCTGACTCACCCGGGCCCGGG-3′ (mutated Sp1 binding site underlined); probe Am, 5′-GCCCGCAGTGCTGGGCGGGGCGCGTCGGATCCCGGGCCCGGG-3′ (mutated AP-1 binding site underlined); and probe SAm, 5′-GCCCGCAGTGCTAATATTTGCGCGTCGGATCCCGGGCCCGGG-3′ (both mutated Sp1 and AP-1 binding sites underlined). These probes corresponded to the human VIL2 5′-flanking sequence from -87 to -46. Each binding mixture (20 μl) for EMSA contained 5-10 μg of nuclear extract or 0.2-0.3 μg of recombinant protein, 20 mm HEPES (pH 7.9), 1 mm EDTA (pH 8.0), 1 mm dithiothreitol, 10 mm (NH4)2SO4, 0.2% (w/v) Tween 20, 30 mm KCl, 1 μg poly[d(I-C)], 0.1 μg of poly-l-lysine, and 0.05 pmol of labeled double-stranded oligonucleotide probe. Samples were incubated at room temperature for 30 min, and complexes were analyzed by electrophoresis on 6% non-denaturing polyacrylamide gels (acrylamide/bis-acrylamide ratio of 29:1) in 0.5× TBE at 80 V for 180 min at 4 °C. The gels were then transferred to a positively charged nylon membrane. Alkaline phosphatase-conjugated anti-digoxigenin antibody and chemiluminescent substrate were used to detect digoxigenin (Dig Gel Shift kit), and immunoreactive bands were photographed and analyzed by a Flu- orChemTMIS-8900 (Alpha Innotech, San Leandro, CA). For analysis of blocking of Sp1 binding to DNA, DNA probes were preincubated for 1 h at 4 °C with increasing concentrations of mithramycin A (0.1, 1, or 10 μm) before use in binding reactions. In specific competition experiments, a 100-fold molar excess of unlabeled oligonucleotide was added to the binding reactions.
Chromatin Immunoprecipitation (ChIP)—EC109 cells were grown to 70-80% confluency on three 15-cm plates (∼4.5 × 107 cells per plate). Chromatin immunoprecipitation was carried out using the ChIP-IT kit (Active Motif, Carlsbad, CA). Briefly, the enzymatically sheared chromatin was precleared with protein G beads and an aliquot saved as a positive control (input DNA). Aliquots of the precleared sheared chromatin were then immunoprecipitated using 2 μg of antibodies against IgG, Sp1, c-Jun, or c-Fos. The resulting DNA was used for PCR analysis, and the amplified DNA fragments were visualized on an agarose gel. PCR of immunoprecipitated DNA were carried out using human VIL2 promoter-specific primers and negative control primers flanking a region of genomic DNA between GAPDH and the chromosome condensation-related SMC-associated protein gene. The human VIL2 promoter-specific primers were 5′-CTCCCCATGCCCGCAGTGCT-3′ (VIL2 -95/-76 sequence) and 5′-GGTGAGTATCCTCGATCCCCGAAAA-3′ (VIL2 +123/+99 sequence). The negative control primers were 5′-ATGGTTGCCACTGGGGATCT-3′ and 5′-TGCCAAAGCCTAGGGGAAGA-3′.
Real-time Reverse Transcription (RT)-Polymerase Chain Reaction—Total cellular RNA was extracted from EC109 cells with TRIzol reagent (Invitrogen) and reverse transcribed to cDNA using the PrimeScript™ RT-PCR kit (TaKaRa, Dalian, China). The real-time RT-PCR assay was carried out with the Rotor-Gene 6000 system (Corbett Life Science, Sydney, Australia) using SYBR® Premix Ex Taq™ (TaKaRa) according to the manufacturer's instructions. All PCRs were done in triplicate. The sequences of VIL2 primers were designed based on the human VIL2 mRNA sequence (GenBank accession number NM_003379) as follows: VIL2 forward, 5′-AGCGCATCACTGAGGCAGAG-3′, and reverse, 5′-GCCGCAGCGTCTTGTACTTG-3′. The sequences of GAPDH primers were designed based on the human GAPDH mRNA sequence (GenBank accession number NM_002046) as follows: GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′. The absolute levels of mRNAs of VIL2 were normalized to that of GAPDH mRNA. The relative value from the vehicle-treated control group was considered equal to one arbitrary unit.
Western Blot Analysis—Whole cell protein extracts were boiled for 5 min with Laemmli buffer and subjected to 12% SDS-polyacrylamide gel electrophoresis using standard methodology. Proteins were then transferred electrophoretically onto a polyvinylidene difluoride membrane (Immobilon, pore size 0.45 μm, Millipore, Bedford, MA) using a constant voltage of 60 V for 120 min. The membranes were then blocked in 5% nonfat milk in phosphate-buffered saline containing 0.1% Tween 20 for 1 h at room temperature followed by the addition of the primary antibody for 1 h at room temperature. The membranes were then washed and incubated with a secondary antibody coupled to horseradish peroxidase for 1 h at room temperature. Antigen-antibody complexes were detected by Western blot luminol reagent (Santa Cruz Biotechnology).
Statistical Analysis—Data analysis was performed using SPSS 13.0 (SPSS, Inc., Chicago, IL). A two-tailed independent-sample t test was used to determine the significance of differences between groups. Differences were considered statistically significant at p < 0.05. Data are plotted as mean ± S.D. Survival was assessed by Kaplan-Meier analysis, and the log-rank score was used to determine statistical significance.
RESULTS
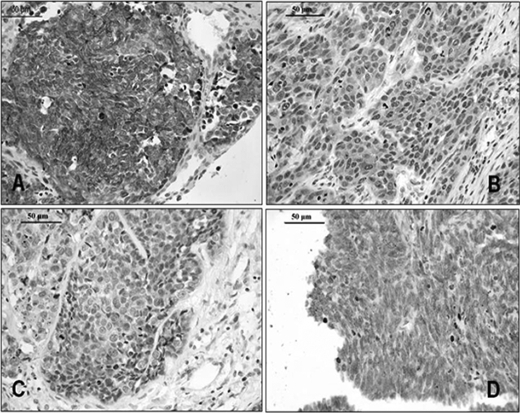
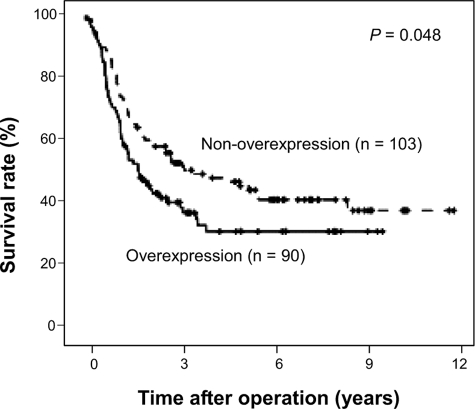
Ezrin Overexpression in ESCCs Is Associated with Poor Survival—Our recent study on ESCC samples showed that ezrin tends to translocate from the plasma membrane to the cytoplasm in the progression from normal epithelium to invasive carcinoma of the esophagus (16). Here, we confirmed this finding and further evaluated clinical significance. Ezrin immunoreactivity in normal esophageal epithelial tissue was weak to moderate in the membrane and cytoplasm (data not shown). However, ESCC tumors displayed one of four distinct immunostaining phenotypes (Fig. 1): intense diffuse membranous and/or cytoplasmic staining (Fig. 1A), moderate cytoplasmic staining (Fig. 1B), weak cytoplasmic staining (Fig. 1C), or no staining (Fig. 1D). Ezrin overexpression was defined as intense diffuse cytoplasmic and/or plasma membrane staining in >50% of tumor cells. In all, 90 of 193 tumors were designated as ezrin overexpressors. Kaplan-Meier survival analysis (Fig. 2) demonstrated that ezrin overexpression was associated with decreased survival (p = 0.048).
FIGURE 1.
Immunohistochemical staining of ezrin in ESCC. A, a case with 3+ staining. B, a case with 2+ staining. C, a case with 1+ staining. D, a negative-staining case. Bar = 50 μm.
FIGURE 2.
Kaplan-Meier estimates of the survival by ezrin status. The survival rate for patients overexpressing ezrin was significantly lower than that for patients not overexpressing ezrin.
Consensus Sp1 (-75/-69) and AP-1 (-64/-58) Binding Sites Are Essential for Human VIL2 Promoter Activity—To better understand the transcriptional regulation of ezrin in ESCCs, 1759 bp of the 5′-flanking region of human VIL2 and 134 bp of the transcribed sequence were cloned from EC109 cells (derived from ESCC) and sequenced (GenBank accession number EF184645). NCBI BLAST analysis (www.ncbi.nlm.nih.gov/BLAST) showed that the cloned fragment had 99% identity with the corresponding segment of human VIL2 AL589931. The CpGPlot Program (www.ebi.ac.uk/emboss/cpgplot) revealed this fragment to be highly GC rich, with CpG islands located at -1564/-581, -558/-303, and -203/+77 (supplemental Fig. S2). Potential transcription factor binding sites within human VIL2 were identified through gene-regulation (www.gene-regulation.com/pub/programs/alibaba2). The analysis revealed multiple potential transcription factor binding sites in this fragment, many of them overlapping, and one site that is a putative target of several factors (supplemental Fig. S3). Moreover, the human VIL2 promoter region lacks a typical TATA box but contains numerous potential Sp1 binding sites, as is common with many GC-rich promoters (30).
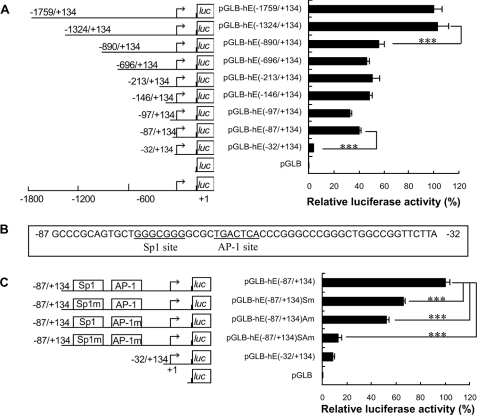
Transient transfection of EC109 cells showed that the 5′-flanking region of human VIL2 (∼1.8 kb) could drive transcription of a luciferase reporter. To localize the regulatory region of promoter activity, a series of 5′-deletion mutants was constructed and analyzed. In EC109 cells, the -1324/+134 region of VIL2 directed maximum luciferase activity (Fig. 3A). The region -87/+134 had considerable reporter activity. Sequence deletion from -1324 to -890 caused an ∼50% reduction in luciferase activity, whereas 5′-deletion from -87 to -32 nearly abolished the activity. When compared with region -890/+134, further deletions, i.e. -696/+134, -213/+134, -146/+134, -97/+134, and -87/+134, did not markedly change the reporter activity. These data suggest that the region -1324/-890 positively regulates transcription and that the region -87/-32 regulates the promoter activity of human VIL2 in EC109 cells.
FIGURE 3.
Transcriptional regulatory region of the human VIL2 5′-flanking region in EC109 cells. A, localization of the transcriptional regulatory region of human VIL2 by 5′-deletion analysis. Schematic representation of the VIL2 promoter 5′-deletion constructs used for transient transfections is shown on the left. 5′-Deletion constructs were cotransfected with pRL-TK into EC109 cells. Luciferase activity (right) was normalized to Renilla luciferase activity and then shown relative to that of EC109 cells transfected with pGLB-hE(-1759/+134), which was set to 100%. B, sequence of the -87/-32 region of human VIL2. The Sp1 binding site (-75/-69) and AP-1 binding site (-64/-58) are underlined. C, identification of functional sites within the human VIL2 promoter region. Schematic representation of the VIL2 promoter site-directed mutagenesis constructs used for transient transfections is shown on the left. Site-directed mutagenesis constructs were cotransfected with pRL-TK into EC109 cells. Luciferase activity (right) was normalized to Renilla luciferase activity and then shown relative to that of EC109 cells transfected with pGLB-hE(-87/+134), which was set to 100%. Each value represents the mean ± S.D. The data are representative of at least two independent experiments. Transfections were carried out in triplicate for each experiment. ***, p < 0.001.
To further investigate the role of specific 5′-flanking regions in the promoter activity of human VIL2, two candidate sites were chosen: a consensus Sp1 binding site (-75/-69, GGGCGGG) and a consensus AP-1 binding site (-64/-58, TGACTCA) (Fig. 3B). Constructs containing 87 bp of VIL2 5′-flanking region with site-directed mutations in the Sp1 site and/or the AP-1 were used for transfection, and expression of the constructs was detected by luciferase assays in EC109 cells. The Sp1 and AP-1 sites were essential for efficient luciferase activity; when either site was mutated, luciferase activity was reduced by 30-60% (Fig. 3C), and when both sites were simultaneously mutated the luciferase activity was greatly reduced. Similar data were obtained using cervical carcinoma HeLa cells (supplemental Fig. S4). These results imply that the Sp1 (-75/-69) and AP-1 sites (-64/-58) are essential for human VIL2 promoter activity in EC109 and HeLa cells.
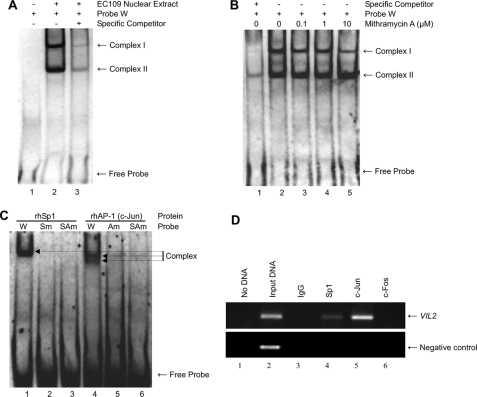
Interaction between Sp1 and the Sp1 Site (-75/-69) and between AP-1 and the AP-1 Site (-64/-58) of VIL2—Because the region between -87 and -32 seemed to be critical for VIL2 promoter function, we assessed the binding of certain factors to that sequence (Fig. 4). EC109 cell nuclear extracts were incubated with a labeled oligonucleotide (probe W) containing the wild-type Sp1 and AP-1 site sequences (i.e. spanning from -87 to -46 of the VIL2 promoter). This binding reaction generated two complexes (complex I and complex II), whereas no complex was formed in the absence of nuclear extract (Fig. 4A).
FIGURE 4.
Binding activity of the human VIL2 promoter region. A, binding of nuclear extract proteins from EC109 cells to the -87/-46 sequence of human VIL2. Digoxigenin-labeled probe W was incubated without (lane 1) or with (lanes 2 and 3) nuclear extract prepared from EC109 cells and then analyzed by EMSA. Unlabeled probe W, as a specific competitor, was used at 100-fold molar excess (lane 3). B, effect of mithramycin A on binding of nuclear extract from EC109 cells to probe W. Digoxigenin-labeled probe W was preincubated with increasing concentrations of mithramycin A (0.1, 1, or 10 μm) for 1 h at 4 °C before addition to the EMSA reaction. Specific competitor was added at 100-fold molar excess (lane 1). C, binding specificity of rhSp1 and rhAP-1 (c-Jun) for the Sp1 and AP-1 sites. Recombinant protein rhSp1 or rhAP-1 (c-Jun) (0.2-0.3 μg) was incubated with probe W, probe Sm, probe Am, or probe SAm, and then analyzed by EMSA. D, association of Sp1 and AP-1 with the human VIL2 promoter in cells using the ChIP assay. Cross-linked chromatin isolated from EC109 cells was immunoprecipitated with anti-Sp1 (lane 4), anti-c-Jun (lane 5), or anti-c-Fos (lane 6). The associated chromosomal DNA fragments were amplified with a human VIL2 promoter-specific primer pair resulting in a 218-bp fragment or a negative control primer pair resulting in a 174-bp fragment. The control reaction (no DNA, lane 1), chromosomal DNA input (lane 2, described under “Experimental Procedures”), and ChIP DNA with nonspecific IgG (lane 3) were subjected to the same PCR amplification. PCR products were separated on a 2% agarose gel containing ethidium bromide and detected via ultraviolet illumination.
We next analyzed complex formation in the presence of mithramycin A, a Sp1-specific chemical inhibitor that binds to the promoter GC box and blocks Sp1 or other Sp family proteins from binding (31). In EMSAs, mithramycin A inhibited the formation of complex I in a dose-dependent manner but did not affect complex II formation (Fig. 4B), suggesting that complex I formation may require Sp1 binding to its site.
To assess the specificity of Sp1 and AP-1 binding to their respective sites, recombinant proteins rhSp1 and rhAP-1 (c-Jun) were individually incubated with probe W, probe Sm, probe Am, or probe SAm. A complex formed in the presence of rhSp1 and probe W, but no complex formed with probes Sm or SAm in which the Sp1 site (-75/-69) is mutated (Fig. 4C). Similarly, two distinct complexes formed in the presence of rhAP-1 (c-Jun) with probe W, but no complex formed with probes Am or SAm in which the AP-1 site (-64/-58) is mutated. These results suggest that Sp1 and AP-1 bind specifically to their respective sites.
To investigate whether Sp1 and AP-1 bind the VIL2 promoter region in EC109 cells, we performed a ChIP assay. Immunoprecipitated chromosomal DNA was subjected to PCR using primers designed to amplify the VIL2 promoter region harboring the Sp1 and AP-1 binding sites or negative control primers flanking a region of genomic DNA between GAPDH and the gene encoding chromosome condensation-related SMC-associated protein. Sp1 and c-Jun indeed bound to the VIL2 promoter region containing the Sp1 and AP-1 sites (Fig. 4D), whereas c-Fos did not.
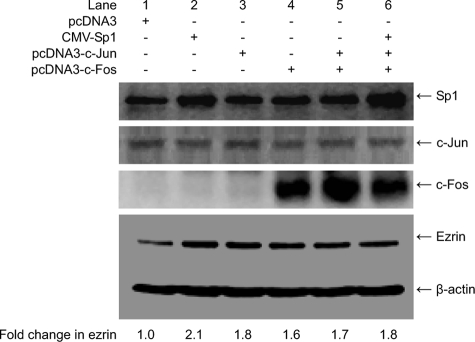
Transient Expression of Sp1, c-Jun, or c-Fos Up-regulates Ezrin Expression—To explore the effect of transcription factors Sp1, c-Jun, and c-Fos on ezrin expression, EC109 cells were transfected or cotransfected with expression vectors CMV-Sp1, pcDNA3-c-Jun, or/and pcDNA3-c-Fos. Backbone vector pcDNA3 was used as a negative control. Total protein was extracted for analysis of Sp1, c-Jun, c-Fos, and ezrin expression by Western blotting. Transfection with CMV-Sp1 (Fig. 5, lanes 2 and 6) or pcDNA3-c-Fos (lanes 4-6) increased Sp1 or c-Fos expression; in contrast, transfection with pcDNA3-c-Jun (lanes 3, 5, and 6) did not visibly change c-Jun expression. Interestingly, ezrin expression was increased in all transfectants and cotransfectants. These data indicate that Sp1, c-Jun, and c-Fos up-regulate ezrin expression in EC109 cells. However, compared with transfections involving a single plasmid encoding Sp1, c-Jun, or c-Fos, ezrin expression did not increase substantially upon co-transfection with c-Jun/c-Fos or Sp1/c-Jun/c-Fos plasmids.
FIGURE 5.
Transient expression of Sp1, c-Jun, orc-Fos up-regulates ezrin expression in EC109 cells. EC109 cells were transfected without (-) or with 600 ng (+) of transcription factor expression vectors pcDNA3, CMV-Sp1, pcDNA3-c-Jun, or/and pcDNA3-c-Fos. Totalprotein from EC109 cells was collected and analyzed by Western blotting using 20 μg of protein/lane. β-Actin is shown as a loading control. Fold-change in ezrin indicates the ratio of band intensity of ezrin to β-actin. Experiments were repeated two to three times with similar results.
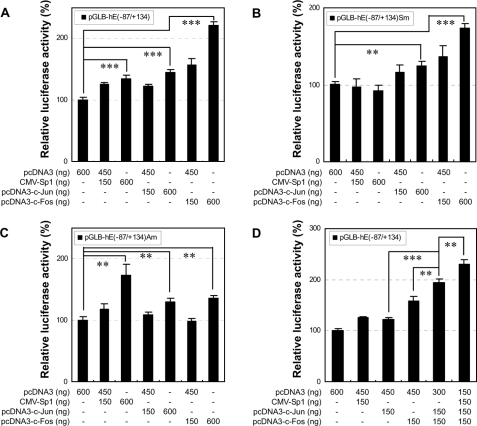
Sp1 and AP-1 Regulate Human VIL2 Promoter Activity through Their Respective Sp1 and AP-1 Sites—To define the respective roles of Sp1, c-Jun, and c-Fos in VIL2 transactivation, and to examine whether transactivation occurs through the respective Sp1 and AP-1 sites, EC109 cells were cotransfected with the CMV-based transcription factor expression vectors (CMV-Sp1, pcDNA3-c-Jun, and pcDNA3-c-Fos) or control vector pcDNA3 in combination with the following human VIL2 promoter constructs: pGLB-hE(-87/+134), in which the VIL2 promoter region contained both the Sp1 (-75/-69) and AP-1 (-64/-58) sites; pGLB-hE(-87/+134)Sm, containing a mutated Sp1 site; or pGLB-hE(-87/+134)Am, containing a mutated AP-1 site. The effect of backbone vectors pCMV and pcDNA3 on expression from the VIL2 promoter constructs was not significant (data not shown). Transfection with the expression vector for Sp1, c-Jun, or c-Fos, however, significantly increased the VIL2 promoter-directed luciferase expression (Fig. 6A, p < 0.001). Additionally, transfection with the Sp1 vector alone increased the luciferase expression directed by the VIL2 promoter containing the Sp1 site (Fig. 6, A and C) but not that directed by the promoter with the mutated Sp1 site (Fig. 6B). Transfection with the c-Fos vector stimulated stronger transactivation of the VIL2 promoter containing the AP-1 site (Fig. 6, A and B) compared with the construct with the mutated AP-1 site (Fig. 6C). However, transfection with the c-Jun vector had a similar effect on VIL2 promoter activity regardless of the status of the AP-1 site (Fig. 6, A-C). These results indicate that Sp1 regulates VIL2 promoter activity through the Sp1 site and c-Fos regulates the promoter activity with partial AP-1 site specificity. Considering the important function of the AP-1 site in VIL2 promoter activity and the specificity of c-Jun binding to the AP-1 site (Fig. 4, C and D), we deduced that c-Jun and c-Fos combine to form AP-1 heterodimer binding to the AP-1 site to regulate human VIL2 promoter activity and that c-Fos is the dominant partner in the dimer with regard to VIL2 transactivation.
FIGURE 6.
Effect of transient expression of Sp1, c-Jun, and c-Fos on human VIL2 promoter activity in EC109 cells. Constructs pGLB-hE(-87/+134) (A and D), pGLB-hE(-87/+134)Sm (B), or pGLB-hE(-87/+134)Am (C) were cotransfected into EC109 cells with pRL-TK and with the indicated amounts of pcDNA3, CMV-Sp1, pcDNA3-c-Jun, and/or pcDNA3-c-Fos. Luciferase activity was normalized to Renilla luciferase activity and then shown relative to that of cells cotransfected with pcDNA3 and pGLB-hE(-87/+134) (A and D), pGLB-hE(-87/+134)Sm (B), or pGLB-hE(-87/+134)Am (C), which was set at 100%. Each value represents the mean ± S.D. The data are representative of at least two independent experiments. Transfections were carried out in triplicate for each experiment. **, p < 0.01; ***, p < 0.001.
To further investigate the cooperation among Sp1, c-Jun, and c-Fos, EC109 cells were cotransfected with pGLB-hE(-87/+134) along with the transcription factor expression vectors indicated in Fig. 6D. Compared with individual transfection with c-Jun or c-Fos, co-transfection with c-Jun and c-Fos significantly increased VIL2 promoter activity (p < 0.01). In addition, co-transfection with all three vectors for Sp1, c-Jun, and c-Fos resulted in significantly greater VIL2 promoter activity compared with transfection with the two vectors for c-Jun and c-Fos (p < 0.01). These data suggest that c-Jun and c-Fos, as well as Sp1 and AP-1, act additively during VIL2 transactivation, which is compatible with the results for these factors in the site-directed mutagenesis experiments.
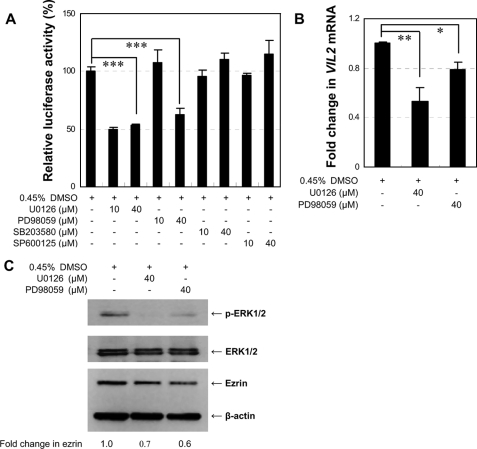
MEK/ERK1/2 Signaling Pathway Regulates VIL2 Promoter Activity and Ezrin Expression—ERK1/2, JNK, and p38 belong to a superfamily of serine-threonine kinases known as the MAPKs. These kinases participate in signal transduction pathways that control intracellular events, including acute responses to hormones and major developmental changes in organisms (32). To explore which MAPK activates the VIL2 promoter leading to VIL2 transcription via phosphorylation of Sp1 and AP-1, EC109 cells were cotransfected with constructs pGLB-hE(-87/+134) and pRL-TK. After 24 h of transfection, the cells were cultured for another 24 h in the absence or presence of the following MAPK inhibitors: U0126 (MEK1/2 inhibitor), PD98059 (MEK1/2 inhibitor), SB203580 (p38 MAPK inhibitor), or SP600125 (JNK inhibitor). The inhibitor concentrations (10 or 40 μm) used in these experiments did not affect cell viability (data not shown). U0126 (10 μm or 40 μm) or PD98059 (40 μm) significantly inhibited human VIL2 promoter activity (p < 0.001), whereas SB203580 or SP600125 did not (Fig. 7A). U0126 and PD98059 specifically block MEK1/2 activation and therefore inhibit the subsequent phosphorylation and activation of ERK1/2 (33, 34). These preliminary findings indicated that transactivation of the VIL2 promoter requires ERK1/2 and that p38 MAPK and JNK have no role in the activation.
FIGURE 7.
ERK1/2 inhibitors down-regulate VIL2 expression in EC109 cells. A, effect of MAPK inhibitors on human VIL2 promoter activity. Construct pGLB-hE(-87/+134) was transfected with pRL-TK into EC109 cells for 24 h before ERK1/2 inhibitors U0126 and PD98059, p38 inhibitor SB203580, JNK inhibitor SP600125, and/or DMSO were added to the cultures for another 24 h before harvesting the cells. Luciferase activity of pGLB-hE(-87/+134) was normalized to Renilla luciferase activity and then shown relative to that of cells transfected with the construct without inhibitors, which was set to 100%. Each value represents the mean ± S.D. The data are representative of at least two independent experiments. Transfections were carried out in triplicate for each experiment. B, real-time RT-PCR for VIL2 mRNA expression. EC109 cells were incubated with ERK1/2 inhibitors U0126, PD98059, and/or DMSO for 24 h before being harvested. VIL2 mRNA expression was analyzed by real-time RT-PCR, and expression was recorded as the fold-change normalized to mRNA from vehicle (DMSO)-treated cells. C, Western blot for phospho-ERK1/2 and ezrin expression. Total protein from EC109 cells treated as in B was analyzed by Western blotting using 25 μg of protein per lane. β-Actin is shown as a loading control. Fold-change in ezrin indicates the ratio of band intensity of ezrin to β-actin. All inhibitors used in the experiments were dissolved in DMSO, and the final concentration of DMSO in EC109 cell cultures was 0.45%. Experiments were done two to three times with similar results. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To further determine whether U0126 and PD98059 inhibit the phosphorylation of ERK1/2 to impact ezrin expression, EC109 cells were treated with these inhibitors (40 μm) for 24 h before being harvested. Total cellular RNA and proteins were extracted for real-time RT-PCR and Western blot analysis, respectively. The VIL2 mRNA level was significantly lower in MEK1/2 inhibitor-treated cells relative to DMSO-treated cells (Fig. 7B, p < 0.05). Moreover, U0126 and PD98059 not only inhibited the phosphorylation of ERK1/2 but also down-regulated the expression of ezrin protein (Fig. 7C). These results suggest that reduced phosphorylation of ERK1/2 decreases ezrin expression and VIL2 promoter activity and that ERK1/2 may be the upstream kinase that phosphorylates Sp1 and AP-1, which in turn activate human VIL2 transcription in EC109 cells.
DISCUSSION
Ezrin is often aberrantly expressed in human cancers and thus may be a potentially new prognostic marker and/or therapeutic target for some carcinomas (12, 18, 19). In this study, we also found that ezrin overexpression in ESCCs was associated with poor survival. Although it is well established that ezrin is implicated in many aspects of cancer cell biology (3-8), the transcription factors and signal transduction pathways that regulate VIL2 transcription remain poorly understood. Here, we showed that transcription factors Sp1 and AP-1 (c-Jun/c-Fos) can transactivate the VIL2 promoter through the Sp1 site and the adjacent AP-1 site, respectively, and that the MEK/ERK1/2 signaling pathway is implicated in this process.
Truncation experiments showed that the cloned human VIL2 5′-flanking sequence contains a positive regulatory region at -1324/-890 and a promoter regulatory region at -87/-32 (Fig. 3A). The human VIL2 promoter is a GC-rich TATA-less promoter. Similar promoters are found in many mammalian genes such as the human cystathionine β-synthase gene (35) and the human survivin gene (36). We studied the regulatory region for VIL2 promoter activity and showed that a consensus Sp1 binding site at -75/-69 and a consensus AP-1 binding site at -64/-58 contribute to VIL2 promoter activity in EC109 cells (Fig. 3C). Using Sp1 expression plasmid CMV-Sp1 and constructs containing the human VIL2 5′-flanking region with a wild-type or mutated Sp1 site, we found that the Sp1-mediated increase in VIL2 promoter activity depended on the Sp1 binding site (Fig. 6, A-C). Sp1 and Sp3 recognize consensus Sp1 binding sites with identical affinity (37). Sp1 is a general activator of transcription, whereas Sp3 can act as an activator or as a repressor of Sp1-mediated activation, depending on the cellular context (38). To determine whether Sp3 also has a role in VIL2 promoter activity, EC109 cells were transfected with plasmid CMV-Sp3; CMV-Sp3 slightly increased VIL2 promoter activity even in the presence of the mutated Sp1 site (data not shown), suggesting that Sp3 does not compete with Sp1 for binding to the Sp1 site at -75/-69 within the VIL2 promoter. Thus, only Sp1 mediates VIL2 transactivation through the Sp1 site.
Regulation of gene expression through the use of combinations of different transcription factors has been widely observed. Indeed, Sp1 can interact and functionally cooperate with other transcription factors, including nuclear factor-κB (39), Ets-1 (40), STAT1 (41), G-C homopolymer binding factors (42), CCAAT/enhancer binding protein β (43), and Egr-1 (44). Furthermore, the cooperativity between Sp1 and AP-1 in the transcriptional activation of human genes encoding leukocyte integrin CD11c (45), involucrin (46), and loricrin (47) has been reported. AP-1 is a sequence-specific transcription factor consisting of a heterodimer of proteins from the Jun family (c-Jun, JunD, and JunB) (48, 49) and the Fos family (c-Fos, FosB, Fra-1, and Fra-2) (50, 51). Moreover, members of the Jun family form homo- or heterodimers that recognize the AP-1 site (consensus DNA sequence TGAGTCA) or related sequences. In this study, luciferase expression experiments also showed that c-Jun with c-Fos, and Sp1 with AP-1 cooperated to activate the human VIL2 promoter (Fig. 6D). We did not examine whether the c-Jun/c-Fos or Sp1/AP-1 complex exists in EC109 cells, or whether the binding of one transcription factor to a VIL2 DNA element facilitated binding of other factors to their corresponding DNA elements; as such, the synergistic cooperation between c-Jun and c-Fos or between Sp1 and AP-1 needs further investigation. Previous studies have shown that the cooperation seems not to depend on the spacing between functional Sp1 and AP-1 binding sites (45, 46); one proposed concept to explain the interaction is that Sp1 induces a conformational change in the DNA that contributes to the activation, perhaps by enhancing AP-1 binding.
Sp1 and AP-1 are often final targets of signal-transducing kinase cascades, and upon phosphorylation they become activated and bind to their respective target promoters and trigger expression of the corresponding genes (52, 53). It has been reported that Sp1 can be phosphorylated by ERK1/2, JNK, and/or p38 MAPK (54, 55), depending on the target gene in different cells. AP-1 activity can be regulated by altering the expression of specific AP-1 components as well as by protein phosphorylation as a result of the activation of intracellular signaling cascades (56-58). For example, phosphorylation of c-Jun by UV-activated JNK permits c-Jun to bind to AP-1 sequences present in the c-Jun promoter (56). ERK1/2 can also phosphorylate c-Jun, but not as effectively as JNK (59). c-Fos was recently shown to be a target of ERK2 activity upon mitogenic stimulus (57). In addition, p38 MAPK also mediates stress-induced c-Fos phosphorylation that then activates transcription of specific genes (60).
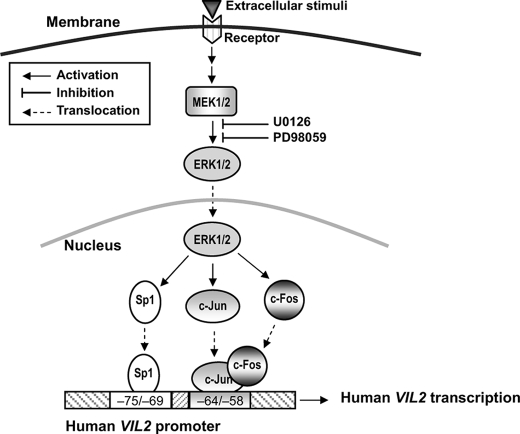
To understand the events upstream of the phosphorylation of Sp1 and AP-1 leading to VIL2 transcriptional activation in EC109 cells, we investigated the role of ERK1/2, JNK, and p38 MAPK using specific inhibitors. Analysis of human VIL2 promoter-luciferase constructs revealed that VIL2 transactivation requires the MEK/ERK1/2 signal transduction pathway but not JNK or p38 MAPK (Fig. 7A). Previous studies have shown that ERK1/2 can phosphorylate Sp1 (55), c-Jun (59), and c-Fos (57) in some cell types. Additionally, activation of MEK/ERK1/2 has been shown to involve the induction of various signaling pathways, depending on the stimulus and cell type. Several extracellular stimuli such as cytokines tumor necrosis factor-α, epidermal growth factor, insulin-like growth factor 1, and IL-1 modulate human ezrin expression (9, 20). Our present results showed that a MEK1/2 inhibitor not only inhibited the phosphorylation of ERK1/2 but also down-regulated the expression of VIL2 mRNA and ezrin protein (Fig. 7, B and C). Based on these results, we propose a possible signal transduction pathway for transactivation of VIL2 in EC109 cells whereby extracellular stimuli activate MEK1/2 and then MEK1/2 phosphorylates ERK1/2. Subsequently, phosphorylated ERK1/2 phosphorylates Sp1 and AP-1, which bind to their respective sites in the VIL2 promoter, resulting in VIL2 transcriptional activation (Fig. 8). Perhaps, this pathway is just one route by which ezrin expression is regulated in EC109 cells, as evidenced by the fact that CpG islands and enhancer sequences exist in the VIL2 5′-flanking region and that additive cooperation between Sp1 and AP-1 for VIL2 transactivation does not coincide with ezrin expression.
FIGURE 8.
Possible mechanism of human VIL2 transactivation by Sp1 and AP-1. Environmental stimuli induce MEK1/2 to phosphorylate ERK1/2. Phosphorylated ERK1/2 then phosphorylates Sp1 and AP-1 (c-Jun/c-Fos), which bind to their respective sites in the human VIL2 promoter, resulting in VIL2 transcriptional activation.
Supplementary Material
This work was supported by the National High Technology Research and Development Program of China Grant 2006AA02A403, National Natural Science Foundation of China Grants 30772485, 30672376, and 30570849, Specialized Research Fund for the Doctoral Program of Higher Education of China Grants 20050560002 and 20050560003, Guangdong Scientific Fund Key Items (7118419, 37788, and 05104541), Natural Science Foundation of Guangdong Province Grant 7301043, and the National Science Foundation for Post-doctoral Scientists of China Grant 20070410846. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S4.
Footnotes
The abbreviations used are: ESCC, esophageal squamous cell carcinoma; AP-1, activating protein-1; Sp1, specific protein-1; EMSA, electrophoretic mobility shift assay; MAPK, mitogen-activated kinase; ERK, extracellular signal-regulated kinase; MEK, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase; JNK, c-Jun N-terminal kinase; IL, interleukin; DMSO, dimethyl sulfoxide; RT, reverse transcription; ChIP, chromatin immunoprecipitation; CMV, cytomegalovirus; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; rh, recombinant human.
References
- 1.Algrain, M., Turunen, O., Vaheri, A., Louvard, D., and Arpin, M. (1993) J. Cell Biol. 120 129-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srivastava, J., Elliott, B. E., Louvard, D., and Arpin, M. (2005) Mol. Biol. Cell 16 1481-1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiscox, S., and Jiang, W. G. (1999) J. Cell Sci. 112 3081-3090 [DOI] [PubMed] [Google Scholar]
- 4.Gautreau, A., Poullet, P., Louvard, D., and Arpin, M. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 7300-7305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crepaldi, T., Gautreau, A., Comoglio, P. M., Louvard, D., and Arpin, M. (1997) J. Cell Biol. 138 423-434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louvet-Vallee, S. (2000) Biol. Cell 92 305-316 [DOI] [PubMed] [Google Scholar]
- 7.Pujuguet, P., Del Maestro, L., Gautreau, A., Louvard, D., and Arpin, M. (2003) Mol. Biol. Cell 14 2181-2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatzoglou, A., Ader, I., Splingard, A., Flanders, J., Saade, E., Leroy, I., Traver, S., Aresta, S., and Gunzburg, J. (2007) Mol. Biol. Cell 18 1242-1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishore, R., Qin, G., Luedemann, C., Bord, E., Hanley, A., Silver, M., Gavin, M., Goukassain, D., and Losordo, D. W. (2005) J. Clin. Investig. 115 1785-1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang, Y., Hu, M. Y., Wu, W. Z., Wang, Z. J., Zhou, K., Zha, X. L., and Liu, K. D. (2006) J. Cancer Res. Clin. Oncol. 132 685-697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng, X., Tannehill-Gregg, S. H., Nadella, M. V., He, G., Levine, A., Cao, Y., and Rosol, T. J. (2007) Clin. Exp. Metastasis 24 107-119 [DOI] [PubMed] [Google Scholar]
- 12.Elliott, B. E., Meens, J. A., SenGupta, S. K., Louvard, D., and Arpin, M. (2005) Breast Cancer Res. 7 R365-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akisawa, N., Nishimori, I., Iwamura, T., Onishi, S., and Hollingsworth, M. A. (1999) Biochem. Biophys. Res. Commun. 258 395-400 [DOI] [PubMed] [Google Scholar]
- 14.Ohtani, K., Sakamoto, H., Rutherford, T., Chen, Z., Kikuchi, A., Yamamoto, T., Satoh, K., and Naftolin, F. (2002) Cancer Lett. 179 79-86 [DOI] [PubMed] [Google Scholar]
- 15.Shen, Z. Y., Xu, L. Y., Chen, M. H., Li, E. M., Li, J. T., Wu, X. Y., and Zeng, Y. (2003) World J. Gastroenterol. 9 1182-1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng, H., Xu, L., Xiao, D., Zhang, H., Wu, X., Zheng, R., Li, Q., Niu, Y., Shen, Z., and Li, E. (2006) J. Histochem. Cytochem. 54 889-896 [DOI] [PubMed] [Google Scholar]
- 17.Xie, J. J., Xu, L. Y., Xie, Y. M., Zhang, H. H., Cai, W. J., Zhou, F., Shen, Z. Y., and Li, E. M. (2009) Int. J. Cancer, in press [DOI] [PubMed]
- 18.Yeh, T. S., Tseng, J. H., Liu, N. J., Chen, T. C., Jan, Y. Y., and Chen, M. F. (2005) Arch. Surg. 140 1184-1190 [DOI] [PubMed] [Google Scholar]
- 19.Kobel, M., Langhammer, T., Huttelmaier, S., Schmitt, W. D., Kriese, K., Dittmer, J., Strauss, H. G., Thomssen, C., and Hauptmann, S. (2006) Mod. Pathol. 19 581-587 [DOI] [PubMed] [Google Scholar]
- 20.Jiang, W. G., and Hiscox, S. (1996) Anticancer Res. 16 861-865 [PubMed] [Google Scholar]
- 21.Khanna, C., Wan, X., Bose, S., Cassaday, R., Olomu, O., Mendoza, A., Yeung, C., Gorlick, R., Hewitt, S. M., and Helman, L. J. (2004) Nat. Med. 10 182-186 [DOI] [PubMed] [Google Scholar]
- 22.Ogino, W., Takeshima, Y., Mori, T., Yanai, T., Hayakawa, A., Akisue, T., Kurosaka, M., and Matsuo, M. (2007) J. Pediatr. Hematol. Oncol. 29 435-439 [DOI] [PubMed] [Google Scholar]
- 23.Jooss, K. U., and Muller, R. (1995) Oncogene 10 603-608 [PubMed] [Google Scholar]
- 24.Lamb, R. F., Ozanne, B. W., Roy, C., McGarry, L., Stipp, C., Mangeat, P., and Jay, D. G. (1997) Curr. Biol. 7 682-688 [DOI] [PubMed] [Google Scholar]
- 25.Yu, Y., Khan, J., Khanna, C., Helman, L., Meltzer, P. S., and Merlino, G. (2004) Nat. Med. 10 175-181 [DOI] [PubMed] [Google Scholar]
- 26.Yu, Y., Davicioni, E., Triche, T. J., and Merlino, G. (2006) Cancer Res. 66 1982-1989 [DOI] [PubMed] [Google Scholar]
- 27.Pan, Q. G., and Xue, X. H. (1980) Zhonghua Zhong Liu Za Zhi 2 187-192 [PubMed] [Google Scholar]
- 28.Zhang, F. R., Tao, L. H., Shen, Z. Y., Lv, Z., Xu, L. Y., and Li. E. M. (2008) J. Histochem. Cytochem. 56 193-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook, J., and Russell, D. (2000) Molecular Cloning: A Laboratory Manual, 3rd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 30.Hapgood, J. P., Riedemann, J., and Scherer, S. D. (2001) Cell Biol. Int. 25 17-31 [DOI] [PubMed] [Google Scholar]
- 31.Blume, S. W., Snyder, R. C., Ray, R., Thomas, S., Koller, C. A., and Miller, D. M. (1991) J. Clin. Investig. 88 1613-1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearson, G., Robinson, F., Gibson, T. B., Xu, B., Karandikar, M., Berman, K., and Cobb, M. H. (2001) Endocr. Rev. 22 153-183 [DOI] [PubMed] [Google Scholar]
- 33.Ge, X., Fu, Y. M., and Meadows, G. G. (2002) Cancer Lett. 179 133-140 [DOI] [PubMed] [Google Scholar]
- 34.Dudley, D. T., Pang, L., Decker, S. J., Bridges, A. J., and Saltiel, A. R. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 7686-7689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ge, Y., Konrad, M. A., Matherly, L. H., and Taub, J. W. (2001) Biochem. J. 357 97-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu, R., Zhang, P., Huang, J., Ge, S., Lu, J., and Qian, G. (2007) Biochem. Biophys. Res. Commun. 356 286-292 [DOI] [PubMed] [Google Scholar]
- 37.Hagen, G., Muller, S., Beato, M., and Suske, G. (1994) EMBO J. 13 3843-3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suske, G. (1999) Gene (Amst.) 238 291-300 [DOI] [PubMed] [Google Scholar]
- 39.Perkins, N. D., Edwards, N. L., Duckett, C. S., Agranoff, A. B., Schmid, R. M., and Nabel, G. J. (1993) EMBO J. 12 3551-3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gegonne, A., Bosselut, R., Bailly, R. A., and Ghysdael, J. (1993) EMBO J. 12 1169-1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Look, D. C., Pelletier, M. R., Tidwell, R. M., Roswit, W. T., and Holtzman, M. J. (1995) J. Biol. Chem. 270 30264-30267 [DOI] [PubMed] [Google Scholar]
- 42.Bessereau, J. L., Mendelzon, D., LePoupon, C., Fiszman, M., Changeux, J. P., and Piette, J. (1993) EMBO J. 12 443-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, Y. H., Yano, M., Liu, S. Y., Matsunaga, E., Johnson, P. F., and Gonzalez, F. J. (1994) Mol. Cell. Biol. 14 1383-1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khachigian, L. M., Williams, A. J., and Collins, T. (1995) J. Biol. Chem. 270 27679-27686 [DOI] [PubMed] [Google Scholar]
- 45.Noti, J. D., Reinemann, B. C., and Petrus, M. N. (1996) Mol. Cell. Biol. 16 2940-2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banks, E. B., Crish, J. F., Welter, J. F., and Eckert, R. L. (1998) Biochem. J. 331 61-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang, S. I., and Steinert, P. M. (2002) J. Biol. Chem. 277 42268-42279 [DOI] [PubMed] [Google Scholar]
- 48.Ball, A. R., Bos, T. J., Loliger, C., Nagata, L. P., Nishimura, T., Su, H., Tsuchie, H., and Vogt, P. K. (1988) Cold Spring Harbor Symp. Quant. Biol. 53 687-693 [DOI] [PubMed] [Google Scholar]
- 49.Ryder, K., Lanahan, A., Perez-Albuerne, E., and Nathans, D. (1989) Proc. Natl. Acad. Sci. U. S. A. 86 1500-1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsui, M., Tokuhara, M., Konuma, Y., Nomura, N., and Ishizaki, R. (1990) Oncogene 5 249-255 [PubMed] [Google Scholar]
- 51.Zerial, M., Toschi, L., Ryseck, R. P., Schuermann, M., Muller, R., and Bravo, R. (1989) EMBO J. 8 805-813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chu, S., and Ferro, T. J. (2005) Gene (Amst.) 348 1-11 [DOI] [PubMed] [Google Scholar]
- 53.Cavigelli, M., Dolfi, F., Claret, F. X., and Karin, M. (1995) EMBO J. 14 5957-5964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D'Addario, M., Arora, P. D., and McCulloch, C. A. (2006) Gene (Amst.) 379 51-61 [DOI] [PubMed] [Google Scholar]
- 55.Benasciutti, E., Pagès, G., Kenzior, O., Folk, W., Blasi, F., and Crippa, M. P. (2004) Blood 104 256-262 [DOI] [PubMed] [Google Scholar]
- 56.Karin, M., Liu, Z., and Zandi, E. (1997) Curr. Opin. Cell Biol. 9 240-246 [DOI] [PubMed] [Google Scholar]
- 57.Monje, P., Marinissen, M. J., and Gutkind, J. S. (2003) Mol. Cell. Biol. 23 7030-7043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy, L. O., Smith, S., Chen, R. H., Fingar, D. C., and Blenis, J. (2002) Nat. Cell Biol. 4 556-564 [DOI] [PubMed] [Google Scholar]
- 59.Hibi, M., Lin, A., Smeal, T., Minden, A., and Karin, M. (1993) Genes Dev. 7 2135-2148 [DOI] [PubMed] [Google Scholar]
- 60.Tanos, T., Marinissen, M. J., Leskow, F. C., Hochbaum, D., Martinetto, H., Gutkind, J. S., and Coso, O. A. (2005) J. Biol. Chem. 280 18842-18852 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.