Abstract
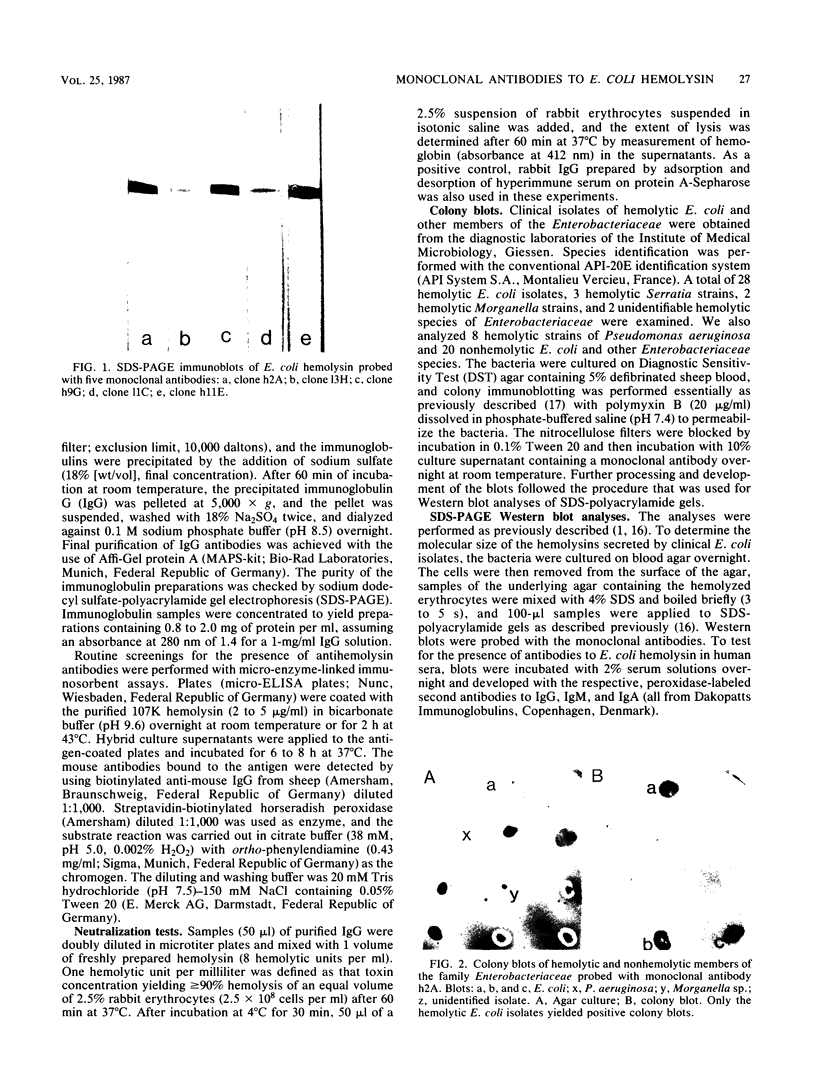
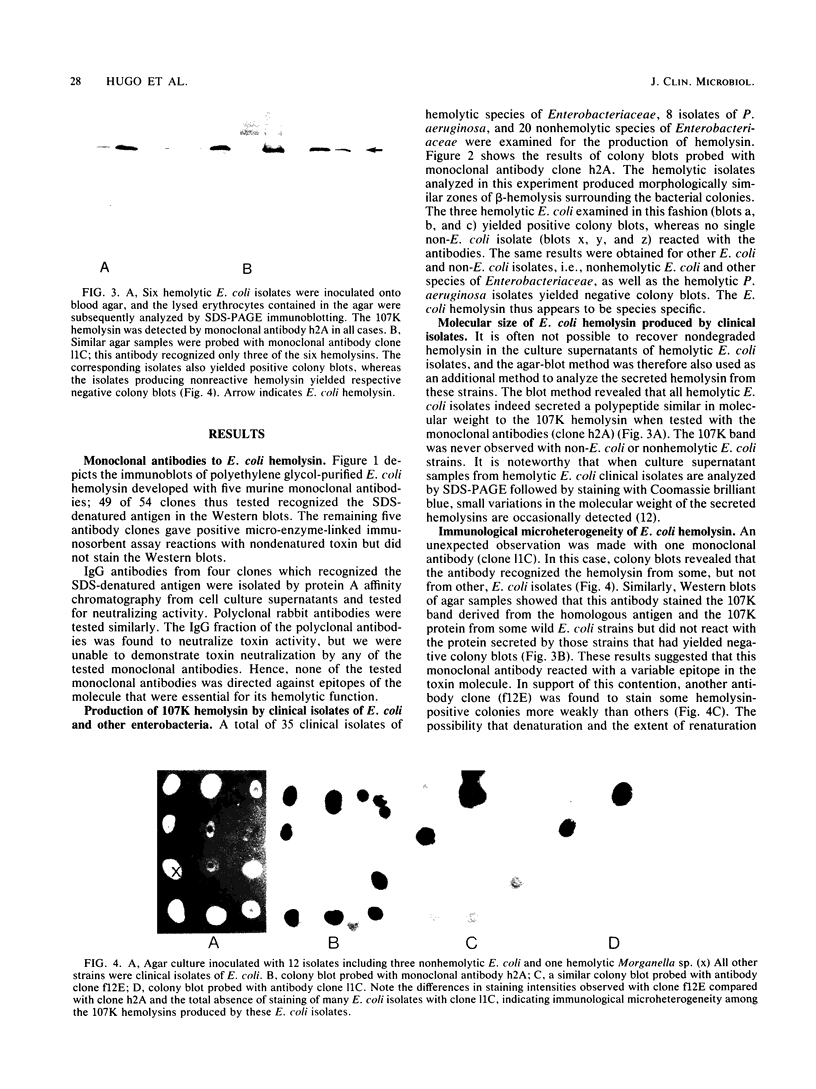
Murine monoclonal antibodies were generated against the 107,000-dalton hemolysin encoded by the hemolytic determinant from Escherichia coli LE 2001, and colony blotting was used to assay for production of the hemolysin by 35 hemolytic strains of E. coli and other hemolytic members of the family Enterobacteriaceae of clinical origin. All hemolytic E. coli strains gave positive reactions with two monoclonal antibodies. In contrast, none of the hemolytic, non-E. coli isolates yielded positive colony blots. In addition, Western blotting showed that the hemolysins produced by all clinical E. coli isolates had a similar molecular weight of about 107,000. Discrete antigenic variation may occur in the molecule, since a third monoclonal antibody did not react with the hemolysin from a number of wild-type E. coli strains. Western blot analysis was used to assess the presence of immunoglobulin G (IgG), IgA, and IgM antibodies to E. coli hemolysin in human sera. All 20 of the tested sera from healthy adults contained antibodies to the toxin, with various constellations among the antibody classes. In contrast, sera from five of eight infants aged 8 to 36 months contained no antihemolysin antibodies. We conclude that the 107,000-dalton hemolysin of E. coli is a widespread immunogen that is produced by most or all hemolytic E. coli strains in the human host.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by pore-forming bacterial cytolysins. Microb Pathog. 1986 Feb;1(1):5–14. doi: 10.1016/0882-4010(86)90027-6. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke E. M., Ewins S. P. Properties of strains of Escherichia coli isolated from a variety of sources. J Med Microbiol. 1975 Feb;8(1):107–111. doi: 10.1099/00222615-8-1-107. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Lee E. Y., Welch R. A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985 Jul;163(1):88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried F. A., Vermeulen C. W., Ginsburg M. J., Cone C. M. Etiology of pyelonephritis: further evidence associating the production of experimental pyelonephritis with hemolysis in Escherichia coli. J Urol. 1971 Sep;106(3):351–354. doi: 10.1016/s0022-5347(17)61286-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Carreró M. I., Zabala J. C., de la Cruz F., Ortiz J. M. Purification of alpha-hemolysin from an overproducing E. coli strain. Mol Gen Genet. 1985;199(1):106–110. doi: 10.1007/BF00327518. [DOI] [PubMed] [Google Scholar]
- Hacker J., Hughes C., Hof H., Goebel W. Cloned hemolysin genes from Escherichia coli that cause urinary tract infection determine different levels of toxicity in mice. Infect Immun. 1983 Oct;42(1):57–63. doi: 10.1128/iai.42.1.57-63.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C., Hacker J., Roberts A., Goebel W. Hemolysin production as a virulence marker in symptomatic and asymptomatic urinary tract infections caused by Escherichia coli. Infect Immun. 1983 Feb;39(2):546–551. doi: 10.1128/iai.39.2.546-551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo F., Jenne D., Bhakdi S. Monoclonal antibodies against neoantigens of the terminal C5b-9 complex of human complement. Biosci Rep. 1985 Aug;5(8):649–658. doi: 10.1007/BF01116996. [DOI] [PubMed] [Google Scholar]
- Mackman N., Holland I. B. Functional characterization of a cloned haemolysin determinant from E. coli of human origin, encoding information for the secretion of a 107K polypeptide. Mol Gen Genet. 1984;196(1):129–134. doi: 10.1007/BF00334104. [DOI] [PubMed] [Google Scholar]
- Mackman N., Nicaud J. M., Gray L., Holland I. B. Genetical and functional organisation of the Escherichia coli haemolysin determinant 2001. Mol Gen Genet. 1985;201(2):282–288. doi: 10.1007/BF00425672. [DOI] [PubMed] [Google Scholar]
- Mackman N., Nicaud J. M., Gray L., Holland I. B. Secretion of haemolysin by Escherichia coli. Curr Top Microbiol Immunol. 1986;125:159–181. doi: 10.1007/978-3-642-71251-7_10. [DOI] [PubMed] [Google Scholar]
- Nicaud J. M., Mackman N., Gray L., Holland I. B. Regulation of haemolysin synthesis in E. coli determined by HLY genes of human origin. Mol Gen Genet. 1985;199(1):111–116. doi: 10.1007/BF00327519. [DOI] [PubMed] [Google Scholar]
- Parrisius J., Bhakdi S., Roth M., Tranum-Jensen J., Goebel W., Seeliger H. P. Production of listeriolysin by beta-hemolytic strains of Listeria monocytogenes. Infect Immun. 1986 Jan;51(1):314–319. doi: 10.1128/iai.51.1.314-319.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strockbine N. A., Marques L. R., Holmes R. K., O'Brien A. D. Characterization of monoclonal antibodies against Shiga-like toxin from Escherichia coli. Infect Immun. 1985 Dec;50(3):695–700. doi: 10.1128/iai.50.3.695-700.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., MacLaren D. M., de Graaff J. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect Immun. 1983 Oct;42(1):245–249. doi: 10.1128/iai.42.1.245-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., Vogel M., Goebel W. Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol. 1983 Apr;154(1):200–210. doi: 10.1128/jb.154.1.200-210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Falkow S. Characterization of Escherichia coli hemolysins conferring quantitative differences in virulence. Infect Immun. 1984 Jan;43(1):156–160. doi: 10.1128/iai.43.1.156-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch J. F., Postma P., Koopman P. A., de Graaff J., MacLaren D. M., van Brenk D. G., Guinée P. A. Virulence of urinary and faecal Escherichia coli in relation to serotype, haemolysis and haemagglutination. J Hyg (Lond) 1982 Jun;88(3):567–577. doi: 10.1017/s002217240007042x. [DOI] [PMC free article] [PubMed] [Google Scholar]







