Abstract
TDP-43 (43-kDa TAR DNA-binding domain protein) is a major constituent of ubiquitin-positive cytoplasmic aggregates present in neurons of patients with fronto-temporal lobular dementia and amyotrophic lateral sclerosis (ALS). The pathologic significance of TDP-43 aggregation is not known; however, dominant mutations in TDP-43 cause a subset of ALS cases, suggesting that misfolding and/or altered trafficking of TDP-43 is relevant to the disease process. Here, we show that the presenilin-binding protein ubiquilin 1 (UBQLN) plays a role in TDP-43 aggregation. TDP-43 interacted with UBQLN both in yeast and in vitro, and the carboxyl-terminal ubiquitin-associated domain of UBQLN was both necessary and sufficient for binding to polyubiquitylated forms of TDP-43. Overexpression of UBQLN recruited TDP-43 to detergent-resistant cytoplasmic aggregates that colocalized with the autophagosomal marker, LC3. UBQLN-dependent aggregation required the UBQLN UBA domain, was mediated by non-overlapping regions of TDP-43, and was abrogated by a mutation in UBQLN previously linked to Alzheimer disease. Four ALS-associated alleles of TDP-43 also coaggregated with UBQLN, and the extent of aggregation correlated with in vitro UBQLN binding affinity. Our findings suggest that UBQLN is a polyubiquitin-TDP-43 cochaperone that mediates the autophagosomal delivery and/or proteasome targeting of TDP-43 aggregates.
Amyotrophic lateral sclerosis (ALS)5 is a progressive, typically fatal, neurodegenerative disease that targets motor neurons (1). The cumulative lifetime risk for ALS is ∼1 in 1000, which is comparable with the occurrence rate of multiple sclerosis (1). However, only about 3,000 cases of ALS are observed at any given time in the United States, because of the low five-year median survival rate (20%) for this disease. There are currently no effective treatments to halt the course of ALS.
Approximately 10% of ALS cases, termed familial ALS (fALS), have a clear genetic link. Dominant mutations in the Cu,Zn-superoxide dismutase 1 (SOD1) are responsible for ∼20% of fALS (2). Well over 100 different disease-associated mutations in SOD1 have been identified in humans (3). The mutations occur throughout the SOD1 open reading frame and do not alter the catalytic properties of the SOD1 enzyme per se (4). Instead, mutant SOD1 proteins are thought to acquire an abnormal fold that confers a toxic gain-of-function activity (4). Multiple cellular processes, including mitochondrial function, axon transport, and glutamate transporter function are adversely affected by toxic SOD1 mutants (5-10). Remarkably, mouse models of SOD1-induced ALS have shown that it is not a neuron-autonomous disease; expression of toxic SOD1 mutants in non-neuronal glial cells is sufficient to induce neuron pathology, whereas expression of toxic SOD1 mutants in motor neurons is insufficient for full expression of the disease phenotype (11, 12). The SOD1 mouse model has been used to test candidate ALS therapeutics, including gene therapies directed toward silencing toxic SOD1 alleles in fALS (13-15).
Sporadic ALS (sALS), representing 90-95% of all ALS cases, occurs in the absence of a family history of disease, but follows a clinical course that is similar to SOD1 ALS. A hallmark of sALS is the presence of ubiquitin (Ub)-positive cytosolic aggregates in degenerating motor neurons (16). Recently, Neumann et al. (17) demonstrated that the 43-kDa human immunodeficiency virus transactivating region DNA-binding domain protein (TDP-43) is a major protein component of ubiquitin-positive aggregates in patients with sALS or ubiquitin-positive fronto-temporal lobular dementia. First identified as a binding factor of the long-terminal repeat region of the human immunodeficiency virus genome, TDP-43 is a nuclear RNA-binding protein that regulates splicing of the cystic fibrosis transmembrane conductance regulator mRNA (18-20). Inactivation of TDP-43 through RNA interference caused enhanced transcription of cyclin-dependent kinase 6, hyperphosphorylation of the pRb tumor suppressor, and nuclear defects (21). Furthermore, although it is unclear how TDP-43 accumulates in the cytoplasm of degenerating neurons in ALS and ubiquitin-positive fronto-temporal lobular dementia, Winton et al. (22) found that mutation of candidate nuclear localization sequences resulted in accumulation of cytoplasmic TDP-43 aggregates in mammalian cells. TDP-43 also forms toxic aggregates when heterologously overexpressed in yeast (23).
The significance of TDP-43 aggregates has been validated by several recent studies demonstrating that mutations in the TARDBP gene locus encoding TDP-43 cause a subset of fALS and sALS cases (24, 25). Many of the identified disease-associated mutations are clustered in the carboxyl terminus of TDP-43 and may affect its stability and/or folding. Considered together, the current findings suggest that the molecular neuropathogenesis of sALS with TDP-43 aggregates may be conceptually similar to other neurodegenerative proteinopathies, including Alzheimer disease (AD), Parkinson disease, and Huntington disease (26). Furthermore, accumulation of TDP-43 within Ub-positive insoluble aggregates implies that the Ub-proteasome pathway plays an important role in TDP-43-associated pathology. In this report, we provide evidence that ubiquilin 1 (UBQLN), a proteasome targeting factor implicated in the etiology of AD, is potentially linked to sALS through an association with TDP-43. The association of UBQLN with topologically distinct protein aggregates in sALS and AD suggests a common mechanistic link between these neurodegenerative conditions.
EXPERIMENTAL PROCEDURES
Yeast Two-hybrid Screening and DNA Construction—We screened a human fetal brain cDNA library (Matchmaker™GAL4 Two-Hybrid System3, Clontech) using a full-length TDP-43 cDNA cloned into XmaI and BamHI sites of the bait vector pGBKT7 (Clontech). Approximately 7.5 × 106 cDNA clones, which represents over 2-fold coverage of the library, were screened using standard procedures recommended by the Clontech user manual and Saccharomyces cerevisiae strain AH109 as a cotransformation host. Primary positives were selected for growth on SD (minimal synthetic dropout medium)/-Leu/-Trp plates containing the chromogenic substrate for α-galactosidase, X-α-Gal (5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside, from Labscientific). Individual positive colonies were re-streaked onto SD/-Ade/-His/-Leu/-Trp/X-α-Gal plates and the surviving secondary clones were chosen for further study. Individual clones were grown in liquid culture and yeast plasmid DNA isolated using Zymoprep I™ Yeast Plasmid Minipreparation Kit from Zymo Research. Purified yeast shuttle plasmids were transformed in Escherichia coli and re-isolated prior to DNA sequence analysis. cDNA clones for human full-length TDP-43 and UBQLN were obtained from Open Biosystems. The open reading frame of TDP-43 (amino acids 1-414) was PCR amplified using expand Hi-Fi DNA polymerase (Roche Applied Science). For the mammalian expression system, TDP-43 cDNA was cut from pGBKT7 vector and subcloned into pCMV-HA vector (Clontech) through SfiI and SalI restriction sites. PCR-generated TDP-43 deletion mutants in this vector were constructed. The open reading frame of human UBQLN (amino acids 1-589) was PCR amplified as mentioned above and subcloned into pCMV-Myc vector for expression in the mammalian tissue culture system. Myc-UBQLNΔUBA (amino acids 1-512) was made through the introduction of a termination codon upstream of the UBA domain of full-length Myc-UBQLN by site-directed mutagenesis using Pfu Turbo DNA polymerase (Stratagene). Similarly, human UBQLN and its truncation mutants were PCR amplified and subcloned into pGEX-5X-2 for glutathione S-transferase (GST) fusion protein expression. Full-length UBQLN and UBQLNΔUBL (amino acids 131-589) were subcloned into the BamHI and XhoI sites of pGEX-5X-2, whereas the GST-UBQLNΔUBA mutant was made by introducing a translation termination mutation after codon 512. A fragment encoding only the UBL domain (amino acids 1-130) was subcloned into BamHI and SmaI sites and the UBA domain cDNA fragment (encoding amino acids 540-589) was subcloned into the EcoRI and XhoI of pGEX-5X-2. Amino- and carboxyl-terminal fusions of TDP-43 to GFP were generated by subcloning full-length TDP-43 PCR products into the HindIII-BamHI sites of pEGFP-C1 (GFP-TDP-43) or pEGFP-N1 (TDP-43-GFP), respectively. GFP-LC3 was obtained from Dr. Gary Chiang (Burnham Institute) and has been described (27).
Antibodies—Antibody suppliers included: Santa Cruz (α-Myc, α-CREB1), Sigma (horseradish peroxidase conjugated α-GST), Roche Applied Science (α-HA), Upstate (α-ATF1) ProteinTech Group (α-TDP-43), Zymed Laboratories Inc. (α-UBQLN, clone 3D5E2), Abcam (α-UBQLN, rabbit polyclonal), and BioMol International (mono- and polyubiquitylated conjugates, clone FK2). The secondary antibody suppliers included: Jackson (horseradish peroxidase-conjugated goat α-rabbit IgG), GE Healthcare (horseradish peroxidase-conjugated sheep α-mouse IgG), and Caltag Laboratories (fluorescein isothiocyanate-conjugated goat α-rabbit IgG, Cy3-conjugated goat α-rabbit IgG, fluorescein isothiocyanate-conjugated goat α-mouse IgG, and Cy3-conjugated goat α-mouse IgG).
Cell Culture and Transfections—HeLa cells and HEK 293T cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. PC-12 cells were maintained in Dulbecco's modified Eagle's medium containing 5% fetal bovine serum and 10% horse serum. HeLa cells were transfected with FuGENE 6 (Roche Applied Science) according to the manufacturer's instructions. For immunofluorescence experiments, HeLa cells were seeded on coverslips and a total of 0.8 μg of plasmid DNA was introduced into each well of a 12-well plate. HEK 293T cells were transfected with the calcium phosphate method and a total of 2 or 3 μg of plasmid DNA was introduced into each 6-well plate or 60-mm cell culture dish. Concanamycin A (Sigma) was prepared as a 10 mm stock solution in dimethyl sulfoxide and used at a final concentration of 50 μm.
Protein Analysis—HEK 293T or HeLa cell pellets were collected by centrifugation, washed with phosphate-buffered saline, and incubated with ice-cold cell lysis buffer (50 mm Tris buffer, pH 7.50, 100 mm NaCl, 10% glycerol, 1% Nonidet P-40, 2 mm MgCl2, 3 mm EDTA) containing a protease/phosphatase inhibitor mixture (50 mm β-glycerophosphate, 10 mm sodium fluoride, 20 nm microcystin, 10 μg/ml leupeptin, 5 μg/ml aprotinin, 5 μg/ml pepstatin A, 0.2 mm phenylmethylsulfonyl fluoride) for 15 min. Cell extracts were then clarified by centrifugation at 20,000 × g for 10 min. The soluble fraction was transferred to a new tube. An insoluble fraction was prepared by washing the pellet once in lysis buffer followed by boiling in 1× SDS-PAGE sample buffer. For TDP-43 ubiquitylation assays, transfected HeLa cells were treated with 10 μm MG-132 for 4 h. The cells were lysed in a solution containing 0.15 m Tris-HCl, pH 6.7, 5% SDS, and 30% glycerol, and incubated at 95 °C for 15 min. The lysate was diluted 1:20 in lysis buffer containing protease and phosphatase inhibitors and N-ethylmaleimide. Two micrograms of α-TDP-43 was added to the lysate, incubated for 1 h at 4 °C with gentle inversion mixing, after which protein A-Sepharose was added. After incubation for 16 h, the beads were collected and washed four times with lysis buffer. Immunoprecipitated proteins were analyzed by SDS-PAGE and immunoblot using α-HA antibodies.
GST Pull-down Assays—GST fusion proteins were produced in E. coli strain BL21 (Invitrogen) transformed with the appropriate plasmids. Protein expression was induced with 0.1 mm isopropyl 1-thio-β-d-galactopyranoside for 4 h at 30 °C. Pelleted bacteria were resuspended in chilled PBS containing protease and phosphatase inhibitor mixture and N-ethylmaleimide. The mixture was immediately sonicated on ice for a total time of 1 min using a 20-s pulse length and 20% amplitude setting using a Branson Digital Sonifier. Samples were clarified by centrifugation at 20,000 × g for 30 min. After clarification, the samples were incubated with glutathione-Sepharose 4B beads (GE Healthcare) for 1 h at 4 °C. Binding, washing, and elution of the GST fusion proteins was carried out according to the manufacturer's instructions. For GST pull-down experiments, HEK 293T cells were transfected with plasmid DNA encoding wild-type or mutant HA-TDP-43 expression plasmids. Forty-eight hours after transfection, cell pellets were extracted with lysis buffer and 500 μg of each clarified extract was incubated with GST or GST-UBQLN fusion proteins (20 μg) that had been prebound to GSH-agarose beads. After inversion mixing for 4 h at 4 °C, the beads were washed three times with lysis buffer and boiled in 1× SDS-PAGE sample buffer. Samples were resolved by 10% SDS-PAGE, transferred onto polyvinylidene difluoride membrane, and blotted using α-HA antibodies to detect affinity purified HA-TDP-43. Fusion protein levels were determined by immunoblotting with α-GST antibodies.
Immunofluorescence Microscopy—Immunostaining and immunofluorescence microscopy were carried out as previously described (28). HeLa cells were grown on glass coverslips and following transfections and fixation with paraformaldehyde, processed for immunostaining with the indicated antibodies. Unless otherwise stated, all primary antibodies were used at a concentration of 1 μg/ml. Fluorescein isothiocyanate and Cy3-conjugated secondary antibodies were used at a dilution of 2 μg/ml from 500 μg/ml stock solutions. Confocal images were generated using a Bio-Rad Radiance 2100 MP Rainbow scanning laser microscope at the W.M. Keck Laboratory for Biological Imaging at the University of Wisconsin, Madison, WI. For TDP-43 particle size analysis, HeLa cells were cotransfected with UBQLN and wild-type, D169G, or G37V TDP-43, and immunofluorescence performed as described. For each condition, ∼25-30 ×40 images were combined into a master file, which contained a total of 125-150-transfected cells. Adobe Photoshop was used to isolate cytoplasmic staining by deletion of areas of overlap with 4′,6-diamidino-2-phenylindole. The Particle Analysis function of ImageJ was used to count aggregates, defined as a particle of at least 10 square pixels and displaying at least 80% circularity. Significance between groups was determined using a two-tailed Student's t test.
RESULTS
UBQLN Is a TDP-43-interacting Protein—We initiated a yeast two-hybrid screen to identify candidate TDP-43-interacting proteins. A screen of ∼7.5 × 106 clones from a human fetal brain cDNA library using full-length human TDP-43 cDNA as bait identified a nearly full-length cDNA clone of Ubiquilin 1 (UBQLN) as a candidate TDP-43 interacting protein (Fig. 1A). UBQLN belongs to a family of proteins defined by the presence of amino-terminal ubiquitin-like (UBL) and carboxyl-terminal ubiquitin-associated (UBA) domains (Fig. 1B). UBL-UBA domain proteins, such as RAD23 and S. cerevisiae DSK2, participate in the shuttling of ubiquitylated proteins to the proteasome (29, 30). Mammals encode three additional UBQLN proteins. UBQLN2 and UBQLN3 display 76 and 46% identity, respectively, to UBQLN1 (31, 32). UBQLN4/A1U is a more distantly related family member (33). Of interest to us, UBQLN binds to Presenilin 1 (PS1) and Presenilin 2 (PS2) gene products that are mutated in inherited forms of AD (34-37). PS1 and PS2 are components of the membrane-associated γ-secretase protease complex that cleaves the amyloid precursor protein into non-toxic Aβ-(1-40) and toxic Aβ-(1-42) peptides (35, 38). Intriguingly, a mutation that affects the splicing of the UBQLN mRNA has been associated with increased AD risk (39). Given the links between UBQLN and neurodegenerative disease, we explored its potential functional links to TDP-43.
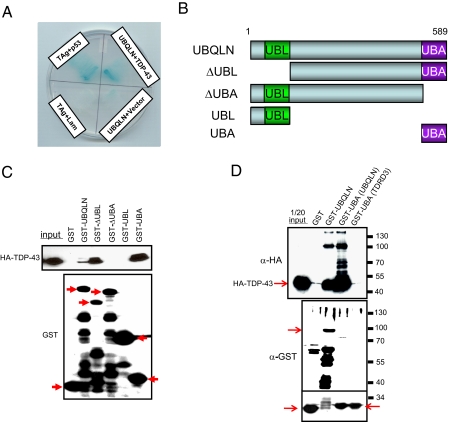
FIGURE 1.
TDP-43 interacts with UBQLN. A, two-hybrid interaction between TDP-43 and UBQLN in yeast. Yeast were cotransformed with shuttle vectors encoding TDP-43 and UBQLN (or empty vector; (see “Experimental Procedures”). Growth on His-, Leu-, and Trp-deficient media and α-galactosidase activity was measured 1 week after cotransformation. Yeast were cotransformed with SV40 large T antigen (TAg) and p53 as a positive control. B, schematic depiction of UBQLN domains and deletion mutants used for TDP-43 binding assays. UBL (ubiquitin-like) and UBA (ubiquitin-associated) domains are shown. C, TDP-43 interacts with the UBQLN UBA domain. Purified GST fusions of full-length UBQLN or the indicated UBQLN deletion mutants were incubated with 500 μg of HEK 293T cell extract containing HA-TDP-43. Bound proteins were analyzed by SDS-PAGE and immunoblotting with the indicated antibodies. For panels C and D, the “input” lane contains 1/20th of the total cell extract used for the pull-down experiments. Arrows indicate the positions of the various intact GST-UBQLN fusion proteins relative to lower molecular weight degradation products. D, the UBQLN UBA domain interacts with high molecular weight forms of TDP-43. HeLa cells were transfected with HA-TDP-43 plasmid DNA and soluble fractions incubated with the indicated GST fusion proteins. Bound proteins were analyzed by immunoblotting with α-HA and equal levels of fusion protein confirmed by immunoblotting with α-GST.
To validate the two-hybrid screening results, we tested whether HA-tagged TDP-43 bound to a panel of GST-UBQLN fusion proteins. Both full-length UBQLN, as well as a deletion mutant of UBQLN lacking the UBL domain, interacted with HA-TDP-43 in vitro (Fig. 1C). Deletion of the UBQLN UBA domain abrogated HA-TDP-43 binding, whereas the isolated UBA domain, but not the UBL domain, bound TDP-43 in the GST pull-down assay (Fig. 1C). These findings support the two-hybrid interaction between TDP-43 and UBQLN and suggest that the UBA domain of UQBLN directly binds to TDP-43. Furthermore, we consistently observed that UBQLNΔUBL interacted more robustly with HA-TDP-43 than wild-type UBQLN, suggesting that the UBL domain antagonizes the UBQLN-TDP-43 association. Consistent with this idea, addition of increasing amounts of GST-UBL reduced the amount of HA-TDP-43 that was affinity purified using the GST-UBA fusion protein (Fig. S1). Together these findings demonstrate that TDP-43 and UBQLN interact in vitro and that the UBA domain of UBQLN is sufficient to mediate the interaction.
UBQLN Associates with Polyubiquitylated TDP-43—The fact that the UBA domain is sufficient to interact with TDP-43 suggested that this motif may directly bind to ubiquitylated lysines in TDP-43. To begin testing this hypothesis, we performed additional GST-UBA pull-down experiments, utilizing increased amounts of cell extracts prepared in the presence of the ubiquitin-specific protease inhibitor, N-ethylmaleimide. Under these conditions both full-length GST-UBQLN and the GST-UBA fragment bound HA-TDP-43; however, GST-UBA affinity purified ∼10-fold more HA-TDP-43 than did GST-UBQLN (Fig. 1D). In addition to the predominant 45-kDa TDP-43 band, GST-UBA affinity purified several higher molecular mass forms of TDP-43 that differed in molecular mass by ∼8 kDa. The ladder-like TDP-43 banding pattern strongly suggested that the higher molecular weight forms represented ubiquitylated forms of TDP-43. On the other hand, the UBA domain of an unrelated protein, TDRD3, did not affinity purify TDP-43 or its putative ubiquitylated forms from HeLa cell extract (Fig. 1D). Blotting with an α-Ub antibody confirmed the presence of ubiquitylated proteins in the GST-UBA pull-down, although it was not possible to conclude that the individual species represent ubiquitylated forms of TDP-43 in this experiment (data not shown).
UBQLN Potentiates TDP-43 Aggregation—To begin functional assessment of the TDP-43-UBQLN interaction, we first attempted to coimmunoprecipitate the proteins from extracts of HeLa cells. However, coexpression of the proteins led to drastically reduced detergent solubility of TDP-43 and we were unable to establish conditions under which the proteins coimmunoprecipitated (data not shown). To investigate this phenomenon further, we compared the localization patterns of the TDP-43 and UBQLN in HeLa cells. Upon overexpression, HA-TDP-43 exhibited a predominantly diffuse nuclear localization pattern, similar to that of the endogenous protein (Fig. 2A, top panel). However, ∼20% of the cells displayed one or more intensely staining foci that were localized to either the nucleus (Fig. 2A, middle panel) or cytoplasm (Fig. 2A, bottom panel). These TDP-43 patterns have been described in the literature (22).
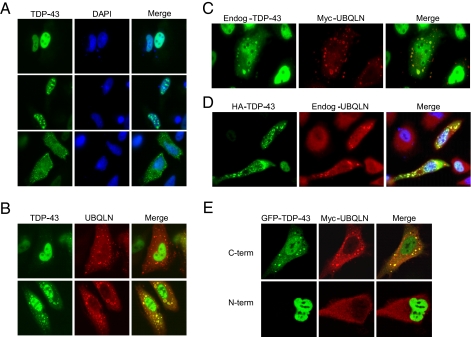
FIGURE 2.
UBQLN stimulates TDP-43 aggregation. A, localization patterns of HA-TDP-43 in HeLa cells. HeLa cells were transfected with a HA-TDP-43 expression plasmid and then stained with α-TDP-43 antibodies 48 h later. Three common staining patterns are shown: diffuse-nuclear (top), aggregate-nuclear (middle), and aggregate-cytoplasmic (bottom). B, effects of UBQLN overexpression on TDP-43 localization. HeLa cells were cotransfected with HA-TDP-43 and Myc-UBQLN expression plasmids and then immunostained 48 h later with α-Myc (red) and α-TDP-43 (green) antibodies. Two types of TDP-43 localization patterns are shown. C, colocalization of endogenous TDP-43 with Myc-UBQLN. HeLa cells were transfected with a Myc-UBQLN expression plasmid and then immunostained with α-TDP-43 and α-Myc antibodies. D, overexpression of TDP-43 recruits endogenous UBQLN to cytoplasmic aggregates. HeLa cells were transfected with HA-TDP-43 and then stained with rabbit α-TDP-43 and mouseα-UBQLN antibodies. E, colocalization analysis of UBQLN and GFP-tagged TDP-43 fusions. HeLa cells were cotransfected with Myc-UBQLN and TDP-43 containing either a carboxyl-terminal (C-term) or amino-terminal (N-term) GFP tag. The transfected cells were then immunostained with α-Myc (red) to visualize Myc-UBQLN.
Coexpression of UBQLN led to a dramatic increase in both the number and intensity of the cytoplasmic HA-TDP-43 aggregates. Although ∼30% of the cotransfected cells still exhibited a diffuse nuclear TDP-43 staining pattern (Fig. 2B, top panel), the remainder of the cells displayed cytoplasmic TDP-43 aggregates, which were extensively colocalized with UBQLN (Fig. 2B, bottom panel). Although nuclear staining of TDP-43 was still apparent in Myc-UBQLN-positive cells, in many cells the bulk of the TDP-43 immunostaining signal was localized to cytoplasmic aggregates. Finally, the recruitment to UBQLN-positive aggregates was specific for TDP-43, as UBQLN did not recruit coexpressed CREB (cyclic AMP response element-binding protein), ATF1 (activating transcription factor 1), and GFP to aggregates (Fig. S2A). The formation of UBQLN-TDP-43 aggregates was also observed in transiently transfected, neuron-like, PC12 pheochromoctyoma cells (Fig. S2B). These combined findings indicate that UBQLN promotes cytoplasmic aggregation of TDP-43 in intact cells.
Although endogenous UBQLN and TDP-43 were not colocalized in HeLa cells, we wished to test whether overexpression of UBQLN altered the subcellular localization pattern of endogenously expressed TDP-43. Consistent with this possibility, we observed that a fraction of UBQLN-positive cells (∼5%), but not UBQLN-negative cells, demonstrated cytoplasmic aggregates of endogenous TDP-43 (Fig. 2C). Although the number and intensity of endogenous TDP-43 aggregates was reduced in comparison to cells that also overexpressed HA-TDP-43, these findings suggested that endogenous TDP-43 is recruited to aggregates under conditions of UBQLN overexpression. The reciprocal HA-TDP-43 overexpression experiment revealed that endogenous UBQLN is recruited to TDP-43-positive aggregates (Fig. 2D). Thus, TDP-43 and UBQLN coaggregate within cytoplasmic structures when either binding partner is overexpressed. Finally, we also tested the colocalization between UBQLN and a pair of GFP-tagged TDP-43 fusion proteins containing the GFP moiety at either the amino or carboxyl terminus. TDP-43-GFP containing a carboxyl-terminal GFP tag formed cytosolic aggregates and colocalized with Myc-UBQLN in HeLa cells (Fig. 2E, top panel). On the other hand, GFP-TDP-43 containing an amino-terminal tag was localized almost exclusively within the nucleus and failed to coaggregate with Myc-UBQLN (Fig. 2E, bottom panel). Thus, the presence of a large amino-terminal tag altered the subcellular localization properties of TDP-43.
TDP-43 Aggregation Requires the UBA Domain of UBQLN and Is Mediated by Non-overlapping Regions of TDP-43—Based on the above findings we tested the hypothesis that UBQLN-dependent aggregation of TDP-43 required the UBQLN UBA domain. Consistent with this idea, the UBQLNΔUBA protein exhibited a diffuse cytoplasmic localization pattern that was distinct from the punctate staining profile exhibited by wild-type UBQLN, and there was virtually no colocalization between UBQLNΔUBA and TDP-43 in HeLa cells (Fig. 3A). On the other hand, deletion of the UBL domain did not prevent colocalization between UBQLN and TDP-43 (Fig. 3A), which supports the GST pull-down results shown in Fig. 1C.
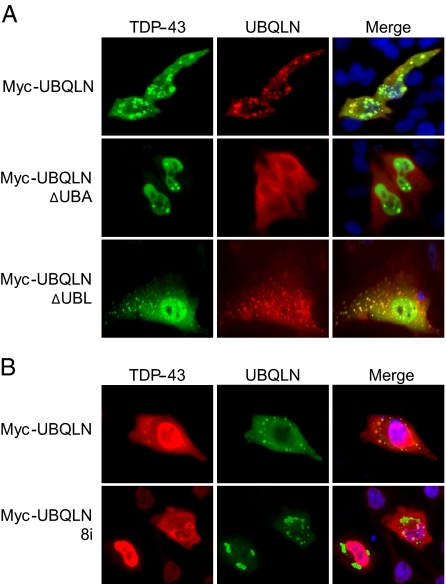
FIGURE 3.
The UBA domain of UBQLN is required for its coaggregation with TDP-43. A, HeLa cells were cotransfected with HA-TDP-43 and either wild-type Myc-UBQLN (top panel) or Myc-UBQLN containing deletions in either the UBA (middle panel) or UBL (bottom panel) domains. The transfected cells were immunostained with α-Myc and α-TDP-43 antibodies. B, the AD-associated UBQLN-8i mutant does not coaggregate with TDP-43. HeLa cells were cotransfected with HA-TDP-43 and wild-type or exon 8-deleted (8i) UBQLN. The transfected cells were stained with α-TDP-43 and α-Myc antibodies.
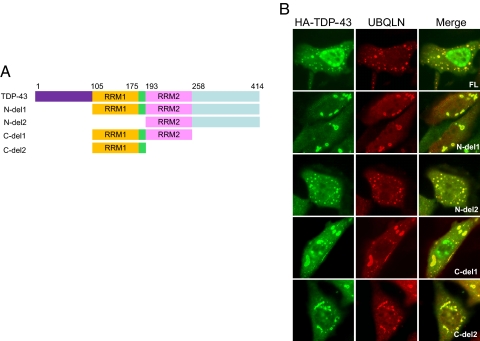
A splice site mutation in the UBQLN gene that results in deletion of exon 8 (UBQLN-8i) has previously been linked to AD and shown to exacerbate neurodegeneration in Drosophila (40). The UBQLN-8i mutant lacks a portion of a centrally located STI1 repeat, first identified in the cochaperone STI1, but retains the carboxyl-terminal UBA domain (41). UBQLN-8i is overexpressed in AD and appears to function dominantly (42). Given these results, we tested whether UBQLN-8i could colocalize with TDP-43 in transiently transfected HeLa cells. Whereas wild-type UBQLN extensively colocalized with TDP-43 in cytoplasmic aggregates, cytoplasmic coaggregation between TDP-43 and UBQLN-8i was rarely observed, even in cells where both proteins were clearly overexpressed (Fig. 3B). Unlike UQBLNΔUBA, UBQLN-8i retained its ability to form large cytoplasmic aggregates in the absence of TDP-43, indicating that its failure to colocalize is not a consequence of defective aggregate targeting. These findings indicate that an AD-associated mutation in UBQLN is diminished in its ability to interact with TDP-43. Finally, a panel of four TDP-43 deletion mutants was also tested for colocalization with UBQLN in HeLa cells (Fig. 4A). We found that each of the four TDP-43 mutants tested colocalized to varying degrees with Myc-UBQLN in transiently transfected cells (Fig. 4B). The fact that two of these fragments, TDP-43 N-del2 (amino acids 193-414) and TDP-43 C-del2 (amino acids 105-192), are entirely non-overlapping suggests that UBQLN may recognize multiple motifs on TDP-43.
FIGURE 4.
UBQLN coaggregates with non-overlapping fragments of TDP-43. A, schematic depiction of TDP-43 deletion mutants. B, colocalization analysis of UBQLN with TDP-43 deletion mutants. HeLa cells were cotransfected with wild-type or mutant HA-TDP-43 plasmids and Myc-UBQLN and stained with α-TDP-43 and α-UBQLN antibodies. Representative images (×100) for each mutant are shown.
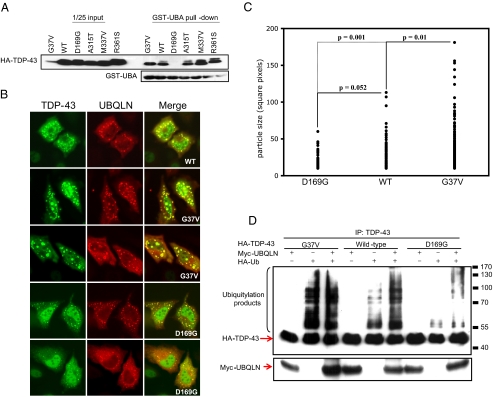
UBQLN-binding Affinity Correlates with Aggregation Severity of TDP-43 Mutants—To date, 21 dominant ALS-associated mutations in the TDP-43 open reading frame have been identified (22, 24, 25, 43-46). Although the structural impact of individual TDP-43 mutations has not been defined, it is reasonable to predict that disease-associated TDP-43 mutants possess altered folding properties that promote their aggregation. We tested four of these mutants (TDP-43D169G, TDP-43A315T, TDP-43M337V, and TDP-43R361S), as well as another TDP-43 mutant containing a non-conservative Gly to Val substitution at codon 37 (TDP-43G37V), for interaction with UBQLN in GST-UBA pulldown experiments. Three of the four ALS-associated TDP-43 mutants (A315T, M337V, R361S) bound to GST-UBA with comparable apparent affinity (Fig. 5A). However, TDP-43D169G showed reduced binding in comparison to wild-type TDP-43. On the other hand, TDP-43G37V showed a 5-10-fold enhanced binding to GST-UBA in comparison to wild-type TDP-43 when the pull-down results were normalized for the expression of soluble HA-TDP-43 (Fig. 5A and data not shown). The enhanced binding of TDP-43G37V to the UBQLN UBA domain correlated with the formation of characteristically larger aggregates in comparison to wild-type TDP-43 (Figs. 5, B and C, and supplemental S3). In contrast to TDP-43G37V, TDP-43D169G formed smaller aggregates and the overall frequency of cells displaying aggregates was reduced in comparison to wild-type TDP-43 and TDP-43G37V (Figs. 5, B and C, and supplemental S3). Thus, the aggregation of TDP-43 mutants roughly correlated with their respective affinities for the UBQLN UBA domain in vitro.
FIGURE 5.
ALS-associated TDP-43 mutants coaggregate with UBQLN. A, ALS-associated TDP-43 mutants interact with the UBQLN UBA domain. GST-UBA pull-down assays were performed using HeLa cell extracts containing wild-type HA-TDP-43 or the indicated HA-TDP-43 point mutants. B, localization patterns of TDP-43 point mutants. HeLa cells were cotransfected with Myc-UBQLN and wild-type HA-TDP-43 or the indicated HA-TDP-43 point mutants. C, particle size analysis of transfected cells displaying HA-TDP-43 aggregates in panel B was established for the wild-type and mutant proteins. Approximately 150 aggregates were plotted for each sample (see “Experimental Procedures”). D, polyubiquitylation of wild-type and mutant TDP-43. Wild-type HA-TDP-43 or the indicated HA-TDP-43 point mutants were immunoprecipitated (IP) under denaturing conditions from extracts of transfected HeLa cells with α-TDP-43 antibodies followed by immunoblot using α-HA and α-Myc antibodies. The positions of molecular weight standards are indicated on the right side of the panel.
The differential association of TDP-43 mutants with the UBQLN UBA domain could reflect intrinsic binding differences or altered ubiquitylation of the proteins. To distinguish between these possibilities, we cotransfected HeLa cells with HA-TDP-43 and HA-Ub in the absence or presence of UBQLN and then examined ubiquitylation profiles of the immunoprecipitated TDP-43 proteins. This analysis revealed that immunoprecipitated TDP-43G37V exhibited more robust polyubiquitin chains than wild-type TDP-43, whereas the fraction of polyubiquitylated TDP-43D169G was reduced in comparison to wild-type TDP-43 (Fig. 5D). Thus, differential ubiquitylation may underlie the differences in UBA domain affinity between these three alleles. In addition, this finding suggests that the extent of TDP-43 ubiquitylation is most likely a critical determinant of its recruitment to UBQLN-positive aggregates.
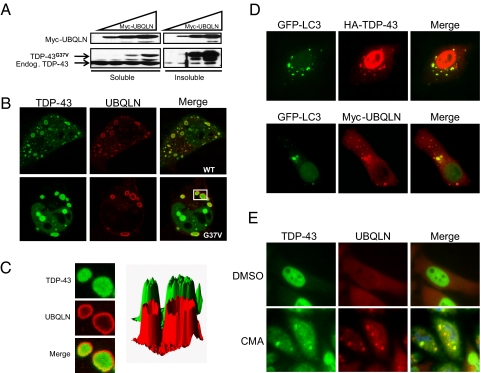
UBQLN Targets TDP-43 to Autophagosomes—The robust, UBQLN-dependent aggregation of TDP-43G37V provided a model to investigate the biochemical properties and architecture of these structures. When coexpressed with UBQLN, the vast majority of TDP-43G37V partitioned to a detergent-resistant cellular fraction, which is consistent with the propensity of this mutant to form large cytosolic aggregates (Fig. 6A). Confocal microscopic analysis of cells displaying the largest TDP-43G37V aggregates revealed that in many instances the localization patterns of TDP-43G37V and UBQLN within aggregates were not identical. In these cells, UBQLN appeared to be excluded from the center of the largest TDP-43G37V aggregates and instead exhibited a ring-like localization pattern around the periphery of the aggregates (Fig. 6B). A pixel density analysis of these aggregates more clearly revealed the distinct colocalization profile between TDP-43 and UBQLN within these vesicle-like structures (Fig. 6C).
FIGURE 6.
UBQLN targets TDP-43 to autophagosomes. A, UBQLN-TDP-43G37V aggregates are insoluble. HeLa cells were transfected with a fixed amount of plasmid DNA encoding HA-TDP-43G37V and increasing amounts of plasmid DNA encoding Myc-UBQLN. Empty pCMV-Myc vector was added so that a total of 5 μg of DNA was used for each transfection. HeLa cells were fractionated into detergent-soluble and -insoluble fractions, resolved by SDS-PAGE, and the levels of HA-TDP-43G37V, endogenous TDP-43, and Myc-UBQLN were measured by immunoblotting with α-TDP-43 and α-Myc antibodies. B, UBQLN decorates the periphery of TDP-43 aggregates. Confocal images of HeLa cells coexpressing Myc-UBQLN and wild-type TDP-43 (top) or TDP-43G37V (bottom). C, enlargement and pixel density analysis of the boxed aggregates shown in panel B. D, HeLa cells were cotransfected with HA-TDP-43, Myc-UBQLN, and GFP-LC3 and immunostained with α-HA (top) or α-Myc (bottom) antibodies. Representative images are shown. E, HeLa cells were treated with 50 μm concanamycin A (CMA) for 4 h and immunostained with α-TDP-43 and α-UBQLN antibodies.
The vesicular appearance of TDP-43-UBQLN aggregates was reminiscent of autophagosomes, which are membrane-enclosed structures that target cytoplasmic proteins and organelles for lysosomal degradation (47). To test this idea, we cotransfected HeLa cells with Myc-UBQLN, HA-TDP-43, and GFP-tagged LC3 (microtubule-associated protein 1 light chain 3), which targets to autophagosomal membranes upon carboxyl-terminal lipidation with phosphatidyleth-anolamine (27, 48). Pairwise comparison revealed extensive, but not complete overlap between GFP-LC3 and either TDP-43 or UBQLN (Fig. 6D).
The above results suggested that UBQLN regulates the targeting of TDP-43 to autophagosomal structures. Based on this finding, we hypothesized that stress insults that interfere with autophagic clearance would stimulate the coaggregation of UBQLN and TDP-43. To test this hypothesis, we treated HeLa cells with concanamycin A, an inhibitor of vacuoloar-type ATPases that blocks autophagosomal acidification and cargo proteolysis (49, 50). Concanamycin A treatment of HeLa cells induced cytoplasmic aggregates that contained endogenous TDP-43 and endogenous UBQLN (Fig. 6E). This result supports the conclusion that UBQLN and TDP-43 colocalize in autophagosomes and establishes conditions under which the endogenous proteins colocalize when expressed at physiologic levels.
DISCUSSION
UBQLN and related UBL-UBA domain proteins, such as RAD23, are believed to function as proteasome targeting factors (29, 34, 36, 51). Previous studies demonstrated that UBQLN forms aggresome-like structures when coexpressed with presenilins or polyglutamine proteins and that UBQLN colocalizes with endosomal and proteasomal markers (34, 35, 52, 53). UBQLN interacts with the S5a subunit of the 19 S proteasome cap in a UBL domain-dependent manner (53, 54) and its overexpression results in the accumulation of several proteins, including GABAA, PS1/PS2, p53, amyloid precursor protein, IκBα, and Kaposi sarcoma-associated Herpesvirus protein K7 (52, 55-58). A general model that has emerged from these studies is that UBQLN and related UBL-UBA domain proteins, such as RAD23, regulate protein homeostasis by guiding ubiquitylated substrates to the proteasome via UBA-polyubiquitin interactions (29, 34, 36, 51). Our findings that UBQLN binds to ubiquitylated TDP-43 and stimulates TDP-43 aggregation are generally consistent with this view.
The UBA domain of UBQLN was both necessary and sufficient for its interaction with TDP-43 in vitro and was required for colocalization with TDP-43 in cytosolic aggregates. The UBQLN UBA domain binds poly-Ub chains (36), which implies that the interaction between TDP-43 and UBQLN may require prior ubiquitylation of TDP-43. Consistent with this idea, the UBA domain of UBQLN affinity purified higher molecular mass forms of TDP-43 that most likely represent ubiquitylated forms of the protein. A presumed folding mutant of TDP-43, TDP-43G37V, formed large aggregates with UBQLN, bound robustly to the UBQLN UBA domain, and showed extensive polyubiquitin conjugates in intact cells, suggesting that the extent of TDP-43 ubiquitylation dictates its interaction with UBQLN. The UBA domain of UBQLN was also shown to be important for its binding to presenilins, although mutation of candidate Lys residues failed to reveal direct requirement for presenilin ubiquitylation (59). The requirement of individual TDP-43 Lys residues for UBQLN interaction is not established. However, because non-overlapping amino-terminal and carboxyl-terminal fragments of TDP-43 coaggregated with UBQLN in transiently transfected HeLa cells, we suggest that TDP-43 possesses multiple UBQLN binding surfaces and/or multiple ubiquitylated lysine residues capable of associating with the UBA domain. Finally, it is also formally possible that the UBQLN-TDP-43 interaction detected in vitro is indirect.
With one exception, ALS-associated mutations in TDP-43 did not grossly alter its association with UBQLN in comparison to wild-type TDP-43; however, TDP-43D169G showed dramatically reduced binding to the UBQLN UBA domain in vitro, and attenuated coaggregation with UBQLN in intact cells. TDP-43D169G also exhibited reduced polyubiquitin chain formation relative to wild-type TDP-43 and TDP-43G37V, which may explain its reduced UBQLN-binding affinity (Fig. 6). Although the importance of this finding is not known, D169G lies within the first RNA recognition motif and it is possible that this disease-associated mutation alters TDP-43 activity independent of gross alterations in its folding. In this regard, it will be important to test whether binding to UBQLN correlates with, or contributes to, TDP-43-associated disease. Thus far we have been unable to detect specific UBQLN immunoreactivity in affected spinal cord sections from ALS patients using commercially available UBQLN antibodies.6 However, UBQLN has been identified in neurofibrillary tangles of AD and Parkinson disease patients, indicating its presence within aggregates may link different neurodegenerative states (35).
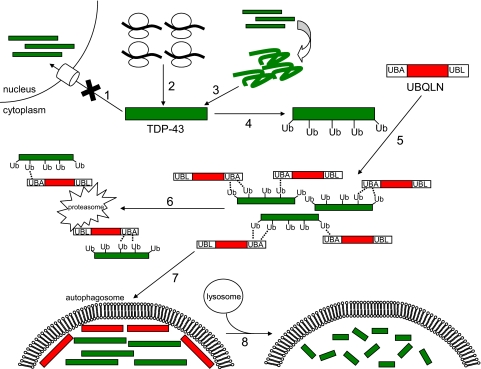
The biochemical fate of TDP-43-UBQLN aggregates is unclear, but a subset of these inclusions resembles autophagosomes: membrane-enclosed structures that form around large aggregates and organelles, targeting them for lysosomal degradation. The degradative process of autophagy has gained notoriety as a protective and therapeutically inducible response to neurodegenerative proteinopathies such as Huntington disease (60). In the present study we found that UBQLN decorates the periphery of TDP-43 aggregates and that UBQLN and TDP-43-containing aggregates colocalize with the autophagosome marker, LC3 (Fig. 6). Published studies have shown that UBQLN suppressed polyglutamine-induced toxicity in animal and cell culture models (61) and, combined with findings showing that autophagy is protective in animal models of Huntington disease (62-66), it is possible that UBQLN-dependent sequestration of TDP-43 in autophagosomes is cytoprotective. A model depicting the role of UBQLN in the targeting of TDP-43 to proteasome or lysosomal pathways is shown in Fig. 7.
FIGURE 7.
Speculative model depicting the potential role of UBQLN in TDP-43 trafficking. In this model TDP-43 accumulates in the cytoplasm either due to impaired nuclear import (1), overexpression (2), or misfolding (3), possibly as a result of ALS-associated point mutations. After ubiquitylation of TDP-43 (4), cytosolic UBQLN binds (5) via the interaction of its UBA domain with ubiquitin on TDP-43. UBQLN potentially then aids in delivering accumulated TDP-43 to the proteasome (6) via its UBL domain. Alternatively, UBQLN may target TDP-43 to autophagosomes (7), where it is degraded by the lysosomal pathway (8).
Aggregation of TDP-43 is emerging as a common feature of sALS, even though the frequency of TARDBP gene mutations in sALS appears to be relatively low (24, 43). It is therefore reasonable to expect that alterations in pathways controlling TDP-43 synthesis, degradation, and intracellular trafficking may underlie a significant proportion of ALS cases. Of potential interest in this regard, a genetic interval spanning 9q21-22, and containing the UBQLN1 gene, has been linked to a small number of inherited cases of ALS with ubiquitin-positive fronto-temporal lobular dementia (67). In addition, an AD-associated mutant of UBQLN failed to efficiently recruit TDP-43 to cytosolic aggregates (Fig. 3). Given the emerging biochemical links between UBQLN and TDP-43, the potential involvement of UBQLN in ALS merits additional study.
Supplementary Material
Acknowledgments
We thank Lance Rodenkirch at the W. M. Keck Laboratory for Biological Imaging and Dr. David Wassarman for helpful comments.
This work was supported, in whole or in part, by National Institutes of Health Grant CA124722. This work was also supported by the American Cancer Society and a Shaw Scientist Award (to R. S. T.) from the Greater Milwaukee Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S3.
Footnotes
The abbreviations used are: ALS, amyotrophic lateral sclerosis; TDP-43, 43-kDa TAR DNA-binding domain protein; UBQLN, ubiquilin 1; AD, Alzheimer disease; Ub, ubiquitin; fALS, familial amyotrophic lateral sclerosis; SOD1, superoxide dismutase 1; sALS, sporadic amyotrophic lateral sclerosis; GST, glutathione S-transferase; GFP, green fluorescent protein; HA, hemagglutinin; HEK, human embryonic kidney; PS, presenilin; LC3, light chain 3; UBL, amino-terminal ubiquitin-like domain; UBA, carboxyl-terminal ubiquitin-associated domain.
K. Hanson and R. Tibbetts, unpublished observation.
References
- 1.Mitsumoto, H., Chad, D. A., and Pioro, E. P. (1998) Amyotrophic Lateral Sclerosis, Davis, Philadelphia, PA
- 2.Rosen, D. R., Siddique, T., Patterson, D., Figlewicz, D. A., Sapp, P., Hentati, A., Donaldson, D., Goto, J., O'Regan, J. P., Deng, H. X., et al. (1993) Nature 362 59-62 [DOI] [PubMed] [Google Scholar]
- 3.Andersen, P. M., Sims, K. B., Xin, W. W., Kiely, R., O'Neill, G., Ravits, J., Pioro, E., Harati, Y., Brower, R. D., Levine, J. S., Heinicke, H. U., Seltzer, W., Boss, M., and Brown, R. H., Jr. (2003) Amyotroph. Lateral Scler. Other Motor Neuron Disord. 4 62-73 [DOI] [PubMed] [Google Scholar]
- 4.Boillee, S., Vande Velde, C., and Cleveland, D. W. (2006) Neuron 52 39-59 [DOI] [PubMed] [Google Scholar]
- 5.Cassina, P., Cassina, A., Pehar, M., Castellanos, R., Gandelman, M., de Leon, A., Robinson, K. M., Mason, R. P., Beckman, J. S., Barbeito, L., and Radi, R. (2008) J. Neurosci. 28 4115-4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki, S., Warita, H., Abe, K., and Iwata, M. (2005) Acta Neuropathol. 110 48-56 [DOI] [PubMed] [Google Scholar]
- 7.Maragakis, N. J., and Rothstein, J. D. (2006) Nat. Clin. Pract. Neurol. 2 679-689 [DOI] [PubMed] [Google Scholar]
- 8.Williamson, T. L., and Cleveland, D. W. (1999) Nat. Neurosci. 2 50-56 [DOI] [PubMed] [Google Scholar]
- 9.Vande Velde, C., Garcia, M. L., Yin, X., Trapp, B. D., and Cleveland, D. W. (2004) Neuromolecular Med. 5 193-203 [DOI] [PubMed] [Google Scholar]
- 10.Borchelt, D. R., Wong, P. C., Becher, M. W., Pardo, C. A., Lee, M. K., Xu, Z. S., Thinakaran, G., Jenkins, N. A., Copeland, N. G., Sisodia, S. S., Cleveland, D. W., Price, D. L., and Hoffman, P. N. (1998) Neurobiol. Dis. 5 27-35 [DOI] [PubMed] [Google Scholar]
- 11.Clement, A. M., Nguyen, M. D., Roberts, E. A., Garcia, M. L., Boillee, S., Rule, M., McMahon, A. P., Doucette, W., Siwek, D., Ferrante, R. J., Brown, R. H., Jr., Julien, J. P., Goldstein, L. S., and Cleveland, D. W. (2003) Science 302 113-117 [DOI] [PubMed] [Google Scholar]
- 12.Boillee, S., Yamanaka, K., Lobsiger, C. S., Copeland, N. G., Jenkins, N. A., Kassiotis, G., Kollias, G., and Cleveland, D. W. (2006) Science 312 1389-1392 [DOI] [PubMed] [Google Scholar]
- 13.Towne, C., Raoul, C., Schneider, B. L., and Aebischer, P. (2008) Mol. Ther. 16 1018-1025 [DOI] [PubMed] [Google Scholar]
- 14.Raoul, C., Abbas-Terki, T., Bensadoun, J. C., Guillot, S., Haase, G., Szulc, J., Henderson, C. E., and Aebischer, P. (2005) Nat. Med. 11 423-428 [DOI] [PubMed] [Google Scholar]
- 15.Ralph, G. S., Radcliffe, P. A., Day, D. M., Carthy, J. M., Leroux, M. A., Lee, D. C., Wong, L. F., Bilsland, L. G., Greensmith, L., Kingsman, S. M., Mitrophanous, K. A., Mazarakis, N. D., and Azzouz, M. (2005) Nat. Med. 11 429-433 [DOI] [PubMed] [Google Scholar]
- 16.Ikeda, K., and Tsuchiya, K. (2004) Neuropathology 24 117-124 [DOI] [PubMed] [Google Scholar]
- 17.Neumann, M., Sampathu, D. M., Kwong, L. K., Truax, A. C., Micsenyi, M. C., Chou, T. T., Bruce, J., Schuck, T., Grossman, M., Clark, C. M., McCluskey, L. F., Miller, B. L., Masliah, E., Mackenzie, I. R., Feldman, H., Feiden, W., Kretzschmar, H. A., Trojanowski, J. Q., and Lee, V. M. (2006) Science 314 130-133 [DOI] [PubMed] [Google Scholar]
- 18.Ayala, Y. M., Pantano, S., D'Ambrogio, A., Buratti, E., Brindisi, A., Marchetti, C., Romano, M., and Baralle, F. E. (2005) J. Mol. Biol. 348 575-588 [DOI] [PubMed] [Google Scholar]
- 19.Buratti, E., and Baralle, F. E. (2001) J. Biol. Chem. 276 36337-36343 [DOI] [PubMed] [Google Scholar]
- 20.Buratti, E., Brindisi, A., Pagani, F., and Baralle, F. E. (2004) Am. J. Hum. Genet. 74 1322-1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayala, Y. M., Misteli, T., and Baralle, F. E. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 3785-3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winton, M. J., Igaz, L. M., Wong, M. M., Kwong, L. K., Trojanowski, J. Q., and Lee, V. M. (2008) J. Biol. Chem. 283 13302-13309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, B. S., McCaffery, J. M., Lindquist, S., and Gitler, A. D. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 6439-6444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabashi, E., Valdmanis, P. N., Dion, P., Spiegelman, D., McConkey, B. J., Vande Velde, C., Bouchard, J. P., Lacomblez, L., Pochigaeva, K., Salachas, F., Pradat, P. F., Camu, W., Meininger, V., Dupre, N., and Rouleau, G. A. (2008) Nat. Genet. 40 572-574 [DOI] [PubMed] [Google Scholar]
- 25.Sreedharan, J., Blair, I. P., Tripathi, V. B., Hu, X., Vance, C., Rogelj, B., Ackerley, S., Durnall, J. C., Williams, K. L., Buratti, E., Baralle, F., de Belleroche, J., Mitchell, J. D., Leigh, P. N., Al-Chalabi, A., Miller, C. C., Nicholson, G., and Shaw, C. E. (2008) Science 319 1668-1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forman, M. S., Trojanowski, J. Q., and Lee, V. M. (2007) Curr. Opin. Neurobiol. 17 548-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kabeya, Y., Mizushima, N., Ueno, T., Yamamoto, A., Kirisako, T., Noda, T., Kominami, E., Ohsumi, Y., and Yoshimori, T. (2000) EMBO J. 19 5720-5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakasai, R., and Tibbetts, R. (2008) J. Biol. Chem. 283 13549-13555 [DOI] [PubMed] [Google Scholar]
- 29.Ron, D., Chen, C. H., Caldwell, J., Jamieson, L., Orr, E., and Mochly-Rosen, D. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 839-843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao, H., and Sastry, A. (2002) J. Biol. Chem. 277 11691-11695 [DOI] [PubMed] [Google Scholar]
- 31.Conklin, D., Holderman, S., Whitmore, T. E., Maurer, M., and Feldhaus, A. L. (2000) Gene (Amst.) 249 91-98 [DOI] [PubMed] [Google Scholar]
- 32.Kim, T. Y., Kim, E., Yoon, S. K., and Yoon, J. B. (2008) Biochem. Biophys. Res. Commun. 369 741-746 [DOI] [PubMed] [Google Scholar]
- 33.Davidson, J. D., Riley, B., Burright, E. N., Duvick, L. A., Zoghbi, H. Y., and Orr, H. T. (2000) Hum. Mol. Genet. 9 2305-2312 [DOI] [PubMed] [Google Scholar]
- 34.Regan-Klapisz, E., Sorokina, I., Voortman, J., de Keizer, P., Roovers, R. C., Verheesen, P., Urbe, S., Fallon, L., Fon, E. A., Verkleij, A., Benmerah, A., and van Bergen en Henegouwen, P. M. (2005) J. Cell Sci. 118 4437-4450 [DOI] [PubMed] [Google Scholar]
- 35.Mah, A. L., Perry, G., Smith, M. A., and Monteiro, M. J. (2000) J. Cell Biol. 151 847-862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko, H. S., Uehara, T., Tsuruma, K., and Nomura, Y. (2004) FEBS Lett. 566 110-114 [DOI] [PubMed] [Google Scholar]
- 37.Massey, L. K., Mah, A. L., and Monteiro, M. J. (2005) Biochem. J. 391 513-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selkoe, D. J., and Wolfe, M. S. (2007) Cell 131 215-221 [DOI] [PubMed] [Google Scholar]
- 39.Slifer, M. A., Martin, E. R., Haines, J. L., and Pericak-Vance, M. A. (2005) N. Engl. J. Med. 352 2752-2753 [DOI] [PubMed] [Google Scholar]
- 40.Ganguly, A., Feldman, R. M., and Guo, M. (2008) Hum. Mol. Genet. 17 293-302 [DOI] [PubMed] [Google Scholar]
- 41.Ford, D. L., and Monteiro, M. J. (2006) Biochem. J. 399 397-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertram, L., Hiltunen, M., Parkinson, M., Ingelsson, M., Lange, C., Ramasamy, K., Mullin, K., Menon, R., Sampson, A. J., Hsiao, M. Y., Elliott, K. J., Velicelebi, G., Moscarillo, T., Hyman, B. T., Wagner, S. L., Becker, K. D., Blacker, D., and Tanzi, R. E. (2005) N. Engl. J. Med. 352 884-894 [DOI] [PubMed] [Google Scholar]
- 43.Rutherford, N. J., Zhang, Y. J., Baker, M., Gass, J. M., Finch, N. A., Xu, Y. F., Stewart, H., Kelley, B. J., Kuntz, K., Crook, R. J., Sreedharan, J., Vance, C., Sorenson, E., Lippa, C., Bigio, E. H., Geschwind, D. H., Knopman, D. S., Mitsumoto, H., Petersen, R. C., Cashman, N. R., Hutton, M., Shaw, C. E., Boylan, K. B., Boeve, B., Graff-Radford, N. R., Wszolek, Z. K., Caselli, R. J., Dickson, D. W., Mackenzie, I. R., Petrucelli, L., and Rademakers, R. (2008) PLoS Genet. 4 e1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cairns, N. J., Neumann, M., Bigio, E. H., Holm, I. E., Troost, D., Hatanpaa, K. J., Foong, C., White, C. L., 3rd, Schneider, J. A., Kretzschmar, H. A., Carter, D., Taylor-Reinwald, L., Paulsmeyer, K., Strider, J., Gitcho, M., Goate, A. M., Morris, J. C., Mishra, M., Kwong, L. K., Stieber, A., Xu, Y., Forman, M. S., Trojanowski, J. Q., Lee, V. M., and Mackenzie, I. R. (2007) Am. J. Pathol. 171 227-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhnlein, P., Sperfeld, A. D., Vanmassenhove, B., Van Deerlin, V., Lee, V. M., Trojanowski, J. Q., Kretzschmar, H. A., Ludolph, A. C., and Neumann, M. (2008) Arch. Neurol. 65 1185-1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daoud, H., Valdmanis, P. N., Kabashi, E., Dion, P., Dupre, N., Camu, W., Meininger, V., and Rouleau, G. A. (2009) J. Med. Genet., in press [DOI] [PubMed]
- 47.Rubinsztein, D. C. (2007) Neuron 54 854-856 [DOI] [PubMed] [Google Scholar]
- 48.Tanida, I., Yamaji, T., Ueno, T., Ishiura, S., Kominami, E., and Hanada, K. (2008) Autophagy 4 131-134 [DOI] [PubMed] [Google Scholar]
- 49.Drose, S., Bindseil, K. U., Bowman, E. J., Siebers, A., Zeeck, A., and Altendorf, K. (1993) Biochemistry 32 3902-3906 [DOI] [PubMed] [Google Scholar]
- 50.Kataoka, T., Takaku, K., Magae, J., Shinohara, N., Takayama, H., Kondo, S., and Nagai, K. (1994) J. Immunol. 153 3938-3947 [PubMed] [Google Scholar]
- 51.Funakoshi, M., Sasaki, T., Nishimoto, T., and Kobayashi, H. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 745-750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Massey, L. K., Mah, A. L., Ford, D. L., Miller, J., Liang, J., Doong, H., and Monteiro, M. J. (2004) J. Alzheimer's Dis. 6 79-92 [DOI] [PubMed] [Google Scholar]
- 53.Heir, R., Ablasou, C., Dumontier, E., Elliott, M., Fagotto-Kaufmann, C., and Bedford, F. K. (2006) EMBO Rep. 7 1252-1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walters, K. J., Kleijnen, M. F., Goh, A. M., Wagner, G., and Howley, P. M. (2002) Biochemistry 41 1767-1777 [DOI] [PubMed] [Google Scholar]
- 55.Bedford, F. K., Kittler, J. T., Muller, E., Thomas, P., Uren, J. M., Merlo, D., Wisden, W., Triller, A., Smart, T. G., and Moss, S. J. (2001) Nat. Neurosci. 4 908-916 [DOI] [PubMed] [Google Scholar]
- 56.Kleijnen, M. F., Shih, A. H., Zhou, P., Kumar, S., Soccio, R. E., Kedersha, N. L., Gill, G., and Howley, P. M. (2000) Mol. Cell 6 409-419 [DOI] [PubMed] [Google Scholar]
- 57.Feng, P., Scott, C. W., Cho, N. H., Nakamura, H., Chung, Y. H., Monteiro, M. J., and Jung, J. U. (2004) Mol. Cell Biol. 24 3938-3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gross, G. G., Feldman, R. M., Ganguly, A., Wang, J., Yu, H., and Guo, M. (2008) PLoS ONE 3 e2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ford, D. L., and Monteiro, M. J. (2007) Biochemistry 46 8827-8837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubinsztein, D. C., Gestwicki, J. E., Murphy, L. O., and Klionsky, D. J. (2007) Nat. Rev. Drug Discov. 6 304-312 [DOI] [PubMed] [Google Scholar]
- 61.Wang, H., Lim, P. J., Yin, C., Rieckher, M., Vogel, B. E., and Monteiro, M. J. (2006) Hum. Mol. Genet. 15 1025-1041 [DOI] [PubMed] [Google Scholar]
- 62.Ravikumar, B., Vacher, C., Berger, Z., Davies, J. E., Luo, S., Oroz, L. G., Scaravilli, F., Easton, D. F., Duden, R., O'Kane, C. J., and Rubinsztein, D. C. (2004) Nat. Genet. 36 585-595 [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto, A., Cremona, M. L., and Rothman, J. E. (2006) J. Cell Biol. 172 719-731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams, A., Sarkar, S., Cuddon, P., Ttofi, E. K., Saiki, S., Siddiqi, F. H., Jahreiss, L., Fleming, A., Pask, D., Goldsmith, P., O'Kane, C. J., Floto, R. A., and Rubinsztein, D. C. (2008) Nat. Chem. Biol. 4 295-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sarkar, S., Perlstein, E. O., Imarisio, S., Pineau, S., Cordenier, A., Maglathlin, R. L., Webster, J. A., Lewis, T. A., O'Kane, C. J., Schreiber, S. L., and Rubinsztein, D. C. (2007) Nat. Chem. Biol. 3 331-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winslow, A. R., and Rubinsztein, D. C. (2008) Biochim. Biophys. Acta 1782 723-729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hosler, B. A., Siddique, T., Sapp, P. C., Sailor, W., Huang, M. C., Hossain, A., Daube, J. R., Nance, M., Fan, C., Kaplan, J., Hung, W. Y., McKenna-Yasek, D., Haines, J. L., Pericak-Vance, M. A., Horvitz, H. R., and Brown, R. H., Jr. (2000) J. Am. Med. Assoc. 284 1664-1669 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.