Abstract
To identify surface-accessible residues and monitor conformational changes of the type I inositol 1,4,5-trisphosphate receptor protein in membranes, we have introduced 10 cysteine substitutions into the N-terminal ligand-binding domain. The reactivity of these mutants with progressively larger maleimide-polyethylene glycol derivatives (MPEG) was measured using a gel shift assay of tryptic fragments. The results indicate that the mutations fall into four categories as follows: sites that are highly accessible based on reactivity with the largest 20-kDa MPEG (S2C); sites that are moderately accessible based on reactivity only with 5-kDa MPEG (S6C, S7C, A189C, and S277C); sites whose accessibility is markedly enhanced by Ca2+ (S171C, S277C, and A575C); and sites that are inaccessible irrespective of incubation conditions (S217C, A245C, and S436C). The stimulation of accessibility induced by Ca2+ at the S277C site occurred with an EC50 of 0.8 μm and was mimicked by Sr2+ but not Ba2+. Inositol 1,4,5-trisphosphate alone did not affect reactivity of any of the mutants in the presence or absence of Ca2+. The data are interpreted using crystal structures and EM reconstructions of the receptor. Our data identify N-terminal regions of the protein that become exposed upon Ca2+ binding and suggest possible orientations of the suppressor and ligand-binding domains that have implications for the mechanism of gating of the channel.
Inositol 1,4,5-trisphosphate receptors (IP3R)2 are ligand-gated channels important in Ca2+ signaling triggered by diverse cellular stimuli (1). Three different isoforms are present that share 60-70% sequence homology (2-4). The type I isoform is also subject to alternative splicing at three sites (5). IP3R channels are tetrameric with each monomer organized into four distinct domains as follows: an N-terminal suppressor domain, a core ligand-binding domain (LBD), a regulatory domain, and a C-terminal channel domain (2-4). Deletion mutagenesis mapped the suppressor domain to amino acids 1-223, and IP3 binding studies showed that this domain reduces the IP3 binding affinity for the receptor (6). The crystal structure of the suppressor domain shows that it folds into a variant of a β-trefoil structure (7). The core LBD (amino acids 224-604) has also been crystallized and shown to fold into two distinct domains: a β-trefoil and a α-helical domain, with residues from both domains contributing to the IP3-binding site. The regulatory domain spans amino acids 600-2220 and contains sites for numerous channel modulators (2). Ca2+ release from the receptor occurs in the channel domain (amino acids 2250-2700) consisting of the six transmembrane (TM) segments, of which the TM5 and TM6 form the channel pore (8, 9).
The two principal modulators of the channel are IP3 and Ca2+. Conformational changes induced by these ligands are likely to be critical to the mechanism of channel function. IP3 binding in the N-terminal domain results in channel gating in the C-terminal domain. Conformational changes resulting from IP3 binding have been reported in N-terminal fusion proteins utilizing gel filtration analysis (10), FRET assays (11), or a variety of biophysical techniques (12). The IP3R is cleaved by trypsin into five major fragments, and several studies have previously shown that noncovalent interactions exist between the N-terminal trypsin fragment I and the C-terminal trypsin fragment V (13, 14). Recently, the site of interaction in the C-terminal domain was mapped to the TM4-TM5 linker (amino acids 2418-2437), and mutations within this linker were shown to prevent the C- and N-terminal domain interaction, reduce channel activity, and augment IP3 binding (15). Conformational changes in the LBD upon IP3 binding were hypothesized to induce a movement of the TM4-TM5 linker leading to channel opening (16).
Ca2+ regulates IP3R channels in a biphasic manner, with low concentrations being stimulatory and high concentrations being inhibitory (17). The location of the activatory and inhibitory sites and the mechanism for Ca2+ effects have not been firmly established (18). Structural changes induced by Ca2+ have been observed in EM studies of the detergent-solubilized, purified IP3R from mouse cerebellum. The presence of Ca2+ caused a transition from a compact square structure to a more extended windmill conformation (19). However, in another EM study the receptor had adopted the more extended structure even in the absence of Ca2+ (20). Ca2+ has also been shown to have effects on an isolated N-terminal fusion protein (amino acids 1-604) that is consistent with enhanced rotational flexibility of the three independently folded domains around two flexible linkers (21).
Almost all studies on the conformation of IP3Rs have utilized fusion proteins or isolated detergent-purified receptor. Our knowledge on IP3Rs would benefit from the application of methods that might provide information on structure and conformational dynamics of the tetrameric, full-length receptor in its native membrane environment. In this study, we have utilized the thiol-reacting agent, maleimide polyethylene glycol (MPEG), to gain information on the accessibility of endogenous and mutant thiol groups in the protein. Previously, we showed that only tryptic fragments I and III of the type I IP3R contained highly reactive cysteines capable of reacting with large MPEGs (22). Furthermore, the reactivity of trypsin fragment I was observed only in the SI(+) splice variant (22). The absence of MPEG reactivity in the SI(-) splice form provided a null background into which cysteine substitution mutants could be introduced. This was carried out in this study, and the relative accessibility of these mutant cysteines was determined using MPEG molecules of various sizes (MPEG-2, -5, and -20 kDa). We have focused our study to fragments I and II that encompass the suppressor domain and LBD because crystal structures for these domains are available (7, 23). The MPEG reactivity experiments were performed in the absence and presence of Ca2+ and/or IP3 to determine whether accessibility of MPEG is altered by modulation of the receptor. Changes in accessibility are interpreted as reflecting conformational changes of the protein in native membranes. The data are discussed in relation to the available structural models of the IP3R.
EXPERIMENTAL PROCEDURES
Materials—MPEGs of 2, 5, and 20 kDa were obtained from Nektar Therapeutics (San Carlos, CA). Dithiothreitol (DTT) was purchased from Sigma. 45Ca2+ was purchased from Amer-sham Biosciences. All other chemicals and reagents were purchased from Fisher.
Antibodies—Previously characterized Abs against rat type I IP3R used in this study were as follows: tryptic fragment I Ab to amino acids 326-341 designated as NT-1 (22) and tryptic fragment II Ab to amino acids 401-414 designated as KEEK (22). An additional Ab against trypsin fragment II was raised against amino acids 501-517 and was designated N3 (13).
Expression Constructs—cDNA encoding rat type I IP3R SI(-)/SII(+)/SIII(+) splice variant in pCMV3 was the gift of Dr. Thomas Sudhof (24). The cDNA encoding the rat type I IP3R SI(+) variant was the gift of Dr. Gregory Mignery (Department of Physiology, Loyola University, Chicago, IL) (25). Cysteine substitutions at positions Ser-2, Ser-6, Ser-7, Ser-171, Ala-189, Ser-217, Ala-245, and Ser-277 were generated using the QuikChange mutagenesis system (Stratagene, La Jolla, CA) using a template of IP3R SI(-) in pBluescript. The mutations were transferred to the full-length receptor using KpnI restriction sites. The cysteine substitutions at positions Ser-436 and Ala-575 were made with the QuikChange kit using the pGEX-LBD plasmid encoding amino acids 1-601 of the SI(-) splice variant of the LBD. The segment containing the mutations between amino acids 420 and 606 was amplified using PCR primers containing flanking KpnI and XhoI sites. This fragment was introduced into a full-length type I IP3R containing a “silent” XhoI site (26).
Cell Culture and Transient Transfection—COS-7 cells were grown on 150-mm plates (Sarstedt) in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% fetal bovine serum (Invitrogen), 0.1 mg/ml streptomycin (Invitrogen), and 100 IU/ml penicillin (Invitrogen) until 70-80% confluent. 15 μg of IP3R DNA was transfected into COS cells containing Dulbecco's modified Eagle's medium without serum using LT-1 (Mirus) and NovaFECTOR (VennNova, Inc.) at a lipid to DNA ratio of 1:1. After 24 h, serum containing Dulbecco's modified Eagle's medium was added, and cells were allowed to grow for 48-72 h.
Preparation of Microsomes—Cells were washed twice with ice-cold phosphate-buffered saline and scraped into a sucrose-based buffer containing 0.5 m sucrose, 20 mm Tris-HCl, pH 7.8, 50 μm EGTA and protease inhibitor mixture tablet (Roche Applied Science). Cells were lysed by passage through a 26.5-gauge needle 10 times. The lysates were spun at 2,500 rpm for 5 min to remove debris, and the supernatants were spun at 40,000 rpm for 50 min. The vesicle pellets were resuspended in storage buffer (0.5 m sucrose, 20 mm Tris-HCl, pH 7.8, and 50 μm EGTA). The vesicles were used fresh or stored at -80 °C for further use.
Reaction with MPEG and Trypsin Digestion—MPEG reactions were carried out as described previously (22) with few modifications. Briefly, microsome preparations were incubated at a final protein concentration of 0.5 mg/ml in a buffer containing 120 mm NaCl, 20 mm Tris-HCl, pH 7.2, and 0.5 mm HEDTA in the presence or absence of MPEG-2, MPEG-5, or MPEG-20. Incubations were carried out at room temperature for 5 min. Analysis of the kinetics of reactivity of selected mutants (S2C and S6C) showed that the reaction was complete within 15-20 min (data not shown). A time point of 5 min was selected as suitable to observe any effects of Ca2+ or IP3 on the reaction process. The reaction was stopped by the addition of 20 mm DTT. Trypsin digestion was carried out subsequent to MPEG reaction at a final concentration of 6 μg/ml for 5 min at room temperature. Titrations with different concentrations of Ca2+ were carried out by addition of 10× stock solutions of Ca2+ buffers containing (final concentration) 5 mm Tris/Hepes, pH 7.5, 0.5 mm HEDTA, and different concentrations of CaCl2 to achieve the indicated concentrations of free Ca2+ as determined by calibration with the fluorescent dyes Calcium Orange and Calcium Green-5N (Invitrogen). The Kd values used in these calculations were 0.22 and 14 μm. respectively. Where Sr2+ and Ba2+ were used, the free divalent cation concentrations were calculated using the program MAXC, available on line. All experiments were repeated three times from three separate microsome preparations.
Electrophoresis and Immunoblotting—For protein expression, microsomes were run on 5% SDS-polyacrylamide gels. For MPEG experiments, microsomes were run on either 10% (MPEG-5 and -20) or 12% 16-cm gels (MPEG-2). Gels were transferred to nitrocellulose membranes and blocked in a 10% milk solution in Tris-buffered containing 0.1% Tween 20. Membranes were incubated with affinity-purified primary antibodies NT-1 (1:5000) or KEEK (1:1000) for 2 h and anti-rabbit secondary horseradish peroxidase antibody for 1 h. Membranes were developed with chemiluminescent substrates (Pierce). In cases where a blot was probed sequentially with more than one antibody, the nitrocellulose was stripped at 65 °C for 30 min in stripping buffer (2% SDS, 100 mm β-mercaptoethanol, 62.5 mm Tris-HCl, pH 6.8) before probing with the next antibody. All immunoblots shown are representative of three or more experiments.
45Ca2+ Flux Assays—Microsomal membranes were prepared 48 h post-transfection. The measurements of the channel activity of wild-type or mutant IP3Rs required the co-transfection of SERCA2b and were performed as described previously (27).
RESULTS
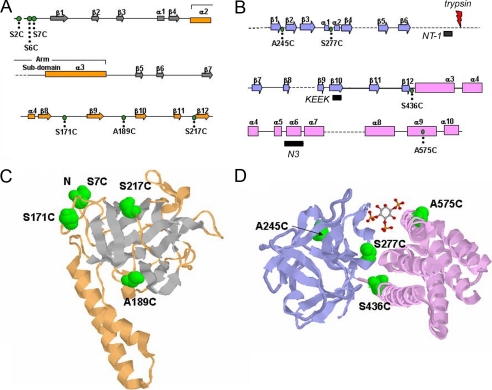
Reactivity of Cysteine Substitution Mutants with MPEG-5—Fig. 1 shows the location of the 10 cysteine substitution mutants used in this study. Their positions within the primary sequence (Fig. 1, A and B) and within the published crystal structure of the suppressor and core LBD (Fig. 1, C and D) are indicated. Because the crystal structures do not provide information on the accessibility of residues in the native tetrameric receptor, the choice of residues to mutate was largely empirical. With the exception of Ala-575, all the selected residues were in loops located between secondary structures. In addition, the Ser-436 residue is the known site of phosphorylation by ERK2 mitogen-activated protein kinase (MAPK) (28, 29), and the Ser-217 residue, when mutated to phenylalanine in Drosophila IP3R, enhances the IP3 sensitivity of channel function (30). All the mutants expressed normally when transiently transfected into COS-7 cells (see individual figures). The functional responsiveness of the mutants was measured with saturating doses of IP3 using 45Ca2+ flux assays performed on microsomes prepared from transfected cells (supplemental Fig. S1). Only the A189C mutant was found to be functionally inactive.
FIGURE 1.
Location of the cysteine-substituted residues in the IP3R crystal structure. Linear representation of the secondary structure of the suppressor domain (A) and the ligand-binding domain (B) (adapted from Refs. 7, 23). The locations of cysteine substitution mutants are labeled as green circles. Amino acid epitopes for fragment I-specific (NT-1) and fragment II-specific (KEEK) antibodies are depicted as black bars, and the trypsin cleavage site is indicated in red (B). C, crystal structure of the IP3R suppressor domain (Protein Data Bank code 1XZZ) depicting cysteine mutants in space fill format (green). The first residue in the crystal structure is serine 7, and therefore the locations of serines 2 and 6 are not shown. D, crystal structure of the ligand-binding domain (Protein Data Bank code 1 N 4 K) is shown with the location of the mutants. The β-trefoil domain is shown in blue, and the α-helical domain is shown in pink. The IP3 ligand is shown in ball and stick format.
Accessibility of the various cysteine mutants was determined in microsomes prepared in the absence of thiol reductants and incubated with 0.5 mm MPEG-5 for 5 min in the presence or absence of Ca2+ (2.2 μm) and/or IP3 (10 μm). Reaction of sites located within the ∼40-kDa trypsin fragment I band was observed by gel shifts detected by immunoblotting with NT-1 Ab (Fig. 2A). Similarly, reaction of sites within the 64-kDa trypsin fragment II was detected by immunoblotting with KEEK Ab (Fig. 2B). As observed previously, the eight endogenous cysteine residues in fragment I of the wild-type SI(-)typeIIP3R did not react with MPEG-5 (22) (Fig. 2A, top panel, compare lanes 1 and 2). Similarly, we observed no reactivity for the S2C, S171C, S217C, and A245C mutants indicating that these sites are also inaccessible to MPEG-5. By contrast, the S6C, S7C, A189C, and S277C sites were reactive indicating that these locations are exposed in the IP3 R structure. Ca2+, which is known to induce large conformational changes in the detergent-solubilized purified protein (31), would be expected to induce changes in accessibility at some sites. The inclusion of Ca2+ did not enhance the reactivity of endogenous cysteines in fragment I of the wild-type SI(-) receptor (Fig. 2A, top panel, compare lanes 2 and 3). A Ca2+-induced enhancement of reactivity was seen for the S6C, S7C, and A189C mutants. Analysis of the time course of reactivity of the S6C mutant indicated that the effect of Ca2+ was primarily to accelerate the rate of the reaction with MPEG-5 (data not shown). The most dramatic change was observed for the S171C mutant that showed substantial reactivity with MPEG-5 only in the presence of Ca2+. The pattern of MPEG-5 reactivity for wild-type and all mutant receptors was not altered by 10 μm IP3, either added alone (Fig. 2A, lane 4) or in combination with Ca2+ (lane 5).
FIGURE 2.
MPEG-5 kDa reactivity of fragments I and II. A, microsome containing type I IP3R SI(-) or the cysteine substitution mutants were incubated with 500 μm MPEG-5 kDa for 5 min in the presence or absence of 2.2 μm Ca2+, 10 μm IP3, or both. The reaction was stopped by addition of 20 mm DTT, and samples were subsequently digested with 6 μg/ml trypsin for 5 min to generate IP3R tryptic fragments. Protein samples were run on 10% SDS-PAGE, and tryptic fragments were immunoblotted with NT-I antibody. Tryptic fragment I (∼40 kDa) is indicated by the open triangle, and shifted bands are marked by closed triangles. B, reactivity of MPEG-5 kDa with fragment II cysteine substitution mutants (S436C and A575C). Experiments were performed as indicated above. The fragment II band (open triangle) appears at ∼67 kDa as detected by immunoblotting with KEEK and or/N3 antibody. The results shown are representative of three or more independent experiments.
The interpretation of the reactivity of the S436C and A575C mutants located in fragment II was complicated by the background reactivity of the wild-type SI(-), which showed multiple shifted bands (Fig. 2B, compare lanes 1 and 2). This was not observed in our previous study (22). The reason for this discrepancy is not known but may be related to the weak reactivity of the previously used batch of KEEK Ab. The data shown in Fig. 2B were obtained with a new batch of higher titer KEEK-Ab and were confirmed with a separate Ab directed at a different epitope in fragment II (N3 Ab; data not shown). Neither the S436C nor the A575C mutant showed any additional MPEG-5 reactivity above background under any incubation conditions.
Reactivity of Cysteine Substitution Mutants with MPEG-20—The degree of accessibility of those residues that reacted with MPEG-5 was examined using the much larger MPEG-20 derivative. Fig. 3A shows that the Ser-6, Ser-7, Ala-189, and Ser-277 sites, which reacted with MPEG-5 in the absence of Ca2+, did not react with MPEG-20 under the same conditions. The S171 site, which reacted with MPEG-5 only in the presence of Ca2+, also did not react with MPEG-20 under the same conditions. The only site that retained reactivity to MPEG-20 was at Ser-277, but only in the presence of Ca2+ (Fig. 3A, bottom panel, lanes 3 and 5). The Ca2+-dependent changes in MPEG-20 reactivity of the S277C site were used as a probe to examine the Ca2+ concentration dependence of the effects (Fig. 3B). Half-maximal effects of Ca2+ were observed at 0.8 μm, and the effects were not significantly affected by the presence of 10 μm IP3 .Sr2+ mimicked the effect of Ca2+, albeit at higher concentrations (Fig. 3C). Ba2+ was relatively ineffective. This specificity to divalent cations is similar to that reported for an activatory modulatory site on rat liver IP3Rs (32).
FIGURE 3.
MPEG-20 kDa reactivity of fragments I. A, MPEG-5 kDa reactive cysteine mutants (S6C, S7C, S171C, A189C, and S277C) were screened for reactivity with the larger MPEG-20 kDa. Experiments were performed as stated in Fig. 2A. B, dose-dependent Ca2+ effect on MPEG-20 kDa reactivity of S277C. Incubations were performed in the presence (closed circle) or absence (open circle) of 10 μm IP3 at the indicated free [Ca2+] buffered with HEDTA. The data are the means ± S.E. of three experiments. C, increasing concentrations (1, 10, and 100 μm) of Sr2+ (lanes 3-5) and Ba2+ (lanes 6-8) were substituted for Ca2+ (lane 2) during the MPEG-20 kDa experiments in S277C. Lane 1 represents the absence of MPEG. Immunoblots were detected with NT-1 antibody. The results shown are representative of three independent experiments.
Reactivity of Endogenous and Mutant Cysteines with MPEG-2—The analysis of the IP3R reactivity with the smaller 2-kDa MPEG derivative was complicated by two factors. First, resolution of the smaller shifts in reactivity required analysis on larger (16 cm) SDS-polyacrylamide gels. Second, the smaller MPEG-2 formed adducts with endogenous as well as cysteine substitution mutants and therefore gave a more complex pattern of bands. This is illustrated for selected IP3R constructs in Fig. 4. The wild-type SI(-) showed reactivity at one endogenous site that was enhanced in the presence of Ca2+ (Fig. 4A, top panel, lanes 2 and 3). A weaker additional reactivity at a second endogenous site was observed in the presence of Ca2+ at higher exposures of the blot (data not shown). The S2C, S217C, and A245C mutants, which were nonreactive with MPEG-5, were also nonreactive with MPEG-2 as judged by the presence of the same background bands observed with wild-type SI(-) (Fig. 4A, 2nd panel, and data not shown for S217C and A245C). The data on MPEG-2 reactivity of the S436C and A575C also confirmed a lack of reactivity above the wild-type background in the absence of Ca2+, although the A575C site reacted with MPEG-2 in the presence of Ca2+ (Fig. 4B, 3rd panel, lanes 3 and 5). The behavior of the S171C and S277C sites with MPEG-2 was similar to that observed with MPEG-5. Notably, the S171C site reacted only in the presence of Ca2+, whereas the S277C was constitutively reactive. In both cases, the presence of Ca2+ generated a prominent doublet of markedly shifted bands (Fig. 4A, lower two panels, lane 3). The number of shifted bands is consistent with the presence of at least three reactive sites in the presence of Ca2+ encompassing two endogenous sites and a third contributed by the mutant cysteine (see schematic in Fig. 4A). Occupation of the mutant cysteine in combination with one or both endogenous sites would produce a doublet of shifted bands. The data suggest that the use of sufficiently small MPEG derivatives may allow visualization of the exposure of endogenous cysteines during Ca2+-associated conformational changes. Although these endogenous sites were not located in the present study, the apparently additive nature of the shifts suggests that the reactive sites are sufficiently separated to avoid steric interference when multiple sites are occupied.
FIGURE 4.
MPEG-2 kDa reactivity of fragments I and II. A, microsomes containing IP3R1 SI(-) or fragment I cysteine substitution mutants were incubated with 500 μm MPEG-2 kDa, and experiments were performed as stated in Fig. 2A with a slight modification. The protein samples were run on 12% SDS-PAGE 16-cm gels to obtain proper separation of MPEG reactive bands. An interpretation of the banding pattern for the S171C mutant is shown as a schematic assuming occupation of two endogenous and one mutant site. B, same experiment repeated with fragment II mutants, but immunoblotted with the KEEK and/or N3 antibody. The results shown in the figure are representative of three or more independent experiments.
Reactivity of Cysteine Substitution Mutants with MPEG Derivatives in DTT-treated Membranes—A lack of reactivity of the S2C mutant with MPEG-5 (Fig. 2) and MPEG-2 (Fig. 4A) could indicate that the N-terminal region is buried within the native receptor and is therefore occluded. This appears inconsistent with previous observations that showed that N-terminally tagged green fluorescent protein constructs were functionally active (29) and that N-terminally epitope-tagged IP3Rs can be readily immunoprecipitated (33). Similarly, the lack of reactivity of the S436C position is surprising because this site lies within a flexible linker region between two domains (23) and is the site phosphorylated by ERK2, a 42-kDa enzyme (29). Our routine preparation method for membranes avoids the use of thiol reductants in buffers so as to avoid compromising subsequent reaction with MPEGs. It is therefore possible that highly reactive cysteines may already have been modified and are therefore unavailable for MPEG reaction. To test this hypothesis, membranes were prepared in the presence of 1 mm DTT. The wild-type SI(-) DTT-treated membranes did not react with MPEG-5 or -20 as observed for untreated membranes (Fig. 5, A and B, top panels, lane 2). However, a shifted band was observed with MPEG-5 in the presence of Ca2+ (Fig. 5A, top panel, lanes 3 and 5) indicating that an endogenous thiol is available for limited reaction in DTT-treated membranes. In contrast to the data observed in Fig. 2 with untreated membranes, the S2C mutant reacted with MPEG-5 and MPEG-20 in DTT-treated membranes (Fig. 5, A and B, 2nd panel). Reactivity of the S2C position with MPEG-20 was enhanced in the presence of Ca2+ (Fig. 5B, 2nd panel, lanes 3 and 5). In contrast to the S2C mutant, the S436C mutant did not react with MPEG-5 (Fig. 5A, compare with wild-type SI(-)) or MPEG-20 (Fig. 5B) in DTT-treated membranes. The behavior of other mutants that were unreactive with MPEG-5 (S217C and A245C) was also not altered by DTT treatment (data not shown).
FIGURE 5.
Reactivity of MPEG-5 and -20 kDa in DTT-treated microsomes. Microsomes were prepared in the presence of 1 mm DTT and treated with MPEG-5 (A) or MPEG-20 (B) as indicated above. The wild-type I IP3R SI(-) was used as the control. Fragment (Frag) I was detected with NT-1 Ab, and fragment II was detected with N3 Ab.
DISCUSSION
Fig. 6A summarizes the experimental observations made in this study regarding accessibility of the indicated 10 cysteine substitution mutants to MPEG derivatives of various sizes. The schematic in Fig. 6A locates these sites within the three main structural domains identified in the N-terminal region of the receptor, notably, the suppressor domain and the core LBD made up of the β-trefoil and α-helical domains. The results indicate that the mutants fall into four categories as follows: sites that are highly accessible, sites that are moderately accessible, sites whose accessibility is enhanced by Ca2+, and sites that are inaccessible irrespective of incubation conditions.
FIGURE 6.
Summary of data and model of proposed location of reactive cysteines in the three-dimensional structure of the receptor. A shows the three independently folded domains that constitute the N-terminal segment of the receptor and the location of the 10 cysteine substitution mutants used in this study. The accessibility to each site in the presence or absence of Ca2+ is indicated by the colored spheres of different sizes. The data with MPEG-2 have been omitted for clarity, but it was verified that all sites accessible to MPEG-5 are also accessible to MPEG-2. Reactivity at the S2C site was observed only when membranes are prepared in the presence of DTT. B, cross-section through two subunits of the receptor according to the EM reconstruction of da Fonseca et al. (43) has been used to illustrate a model of the localization of MPEG-reactive sites. For additional details see text. C, crystal structure of the suppressor domain (Protein Data Bank code 1XZZ) and core LBD (Protein Data Bank code 1N4K) were docked using the program GRAMM. The docked structure was orientated with the MPEG-reactive sites in the suppressor domain facing the surface of the central density. The figure is for illustration only and does not attempt to rigorously fit the crystal structures into the EM density. A side view and a view from the cytosol are shown.
The S2C position was the most highly accessible of the sites tested based on partial reactivity with MPEG-20 even in the absence of Ca2+. Indeed, when microsomal membranes were prepared from this mutant in the absence of DTT, this site was found to be refractory to MPEG reaction. This behavior was unique to the S2C position, and the nature of the blocking modification was not explored in this study. The possibility that the result arises because only the S2C site is sufficiently accessible to a large oxidoreductase enzyme seems unlikely because other highly accessible endogenous cysteines in trypsin fragment III (22) and the A2749C mutant at the C terminus (data not shown) remain accessible to MPEG-20 even in membranes prepared in the absence of DTT.
The distinction between sites accessible to MPEG-5 and MPEG-20 is presumably related to the relative sizes of the MPEG molecules and the exact location of the cysteine substitution mutants in the three-dimensional structure of the receptor. PEGs behave approximately like spherical molecules in aqueous solutions (34, 35), and PEG molecules of different sizes have been widely used to estimate the diameter of artificial and natural ion channels (36-38). Estimates of the hydrated diameter of PEG-5 and PEG-20 are 41-60 Å (39) and ∼130 Å (40), respectively. However, based on previous studies, MPEGs would not be expected to behave like rigid spheres because inherent flexibility may permit the molecules to penetrate into some spaces to a limited extent (41). Several groups have published EM reconstructions of detergent-solubilized purified IP3R (42-44). Although there are many differences in these studies, a basic structure with 4-fold symmetry and dimensions of ∼200 Å3 has been visualized. In the model published by da Fonseca et al. (43)., which has been described as resembling a flower, the central density (stigma) has been proposed to contain the N-terminal portion of the receptor (Fig. 6B) (43). The reactivity of the S2C site in DTT-treated membranes with MPEG-5 and with the larger MPEG-20 suggests that this residue is highly exposed and is therefore more likely to be situated at the exposed cytosolic face of the central density (Fig. 6B). In the crystal structure the position of serine 2 is not resolved, and the first N-terminal residue is serine 7. The latter residue, together with positions 171 and 189, are all on the same side of the suppressor domain. The S7C and A189C mutants are accessible to MPEG-5, and the S171C mutant becomes accessible in the presence of Ca2+. We suggest that the suppressor domain is oriented with the MPEG-5 reactive residues at the exposed surface of the central density (Fig. 6C). Deletion of the suppressor domain eliminates channel gating (45, 46). A proposed gating mechanism has suggested that there is direct contact of the suppressor domain with the TM4-TM5 loop in the channel domain (15). However, there is no direct evidence for such contacts and a surface disposition of the suppressor domain, at some distance from the channel domain, makes such contacts unlikely. Conformational changes in the suppressor domain may be transmitted to the channel indirectly, or direct interactions with the channel domain may involve other regions of the LBD that are in closer proximity. We have selected to interpret our data using the structure of da Fonseca et al. (43) because the assignments in this model accommodate the experimental observation of a close association of N- and C-terminal segments and that the ligand-binding sites in tetramers are closely spaced (47). However, others have proposed different locations of the ligand-binding sites in the EM structures (42, 44). It should be noted that the basic arguments favoring a location of the suppressor domain at the surface of the protein are independent of the EM model selected.
IP3 alone had no discernible effects on MPEG reactivity for any of the cysteine substitution mutants examined in this study. This is in line with previous observations indicating that IP3 elicits more subtle changes in the protein than those induced by Ca2+ (48). We cannot formally exclude the possibility that the inability to detect IP3-mediated conformational changes may be due to the requirement for some additional factor lacking in our specific experimental conditions. By contrast, Ca2+ had large effects on the MPEG reactivity of specific IP3R cysteine mutants. In the presence of Ca2+ the A575C position reacted with MPEG-2 (but not larger MPEGs), the S171C site reacted with MPEG-5 (but not MPEG-20), and the S277C site became accessible to MPEG-20 (Fig. 6A). Ca2+ also enhanced the reactivity of certain sites, e.g. S6C, S7C, A189C with MPEG-5 and S2C with MPEG-20 (Fig. 2A and Fig. 5B). EM studies on purified IP3Rs have revealed a large transition from a compact to a windmill conformation induced by low concentrations of Ca2+ (31). In the model of da Fonseca et al. (43), we suggest that the structural change may involve a wider separation of the side arms from the central density (Fig. 6B, right panel). In the crystal structure of the LBD, the Ser-277 position is located within the binding crevice occupied by IP3. Given this location, it is somewhat surprising that the S277C is so reactive with the larger MPEGs. We hypothesize that the IP3 binding pocket may be located at the narrowest constriction in the central density. This would be compatible with the close spacing of IP3-binding sites observed with IP3 dimers (19, 47) and would allow a molecule of the size of MPEG-5 to be accommodated in the cavity (Fig. 6B). The dramatic increase in reactivity of the S277C mutant with MPEG-20 in the presence of Ca2+ may be caused by the widening of the cavity resulting from the movement of the side arm densities. It is not immediately obvious from the model in Fig. 6B why residues located at the surface of the central density (e.g. S2C and S171C) should show an increase in accessibility in the presence of Ca2+. The S171C position is close to the N-terminal region in the crystal structure, so the effects of Ca2+ on these residues may have a common mechanism. These specific locations could also become less hindered because of the outward movement of the side arms. Alternatively, conformational changes in the N-terminal region itself could contribute to the results. Biophysical measurements on purified fusion proteins indicate that Ca2+ may promote the movement of the suppressor domain away from the binding core (21).
Although reaction of a particular site with MPEG is unequivocal, the failure to react under any conditions (e.g. S217C and A245C) could be due to many factors. The most obvious is that the site is located in the interior of the protein or at highly restricted positions in the central density. Even residues that are on the surface could fail to react if the side chain orientation of the cysteine is not appropriate. In addition, a lack of access could also be due to an associated protein that obscures a particular site. The S436C mutant, which is located in a small flexible linker between β-trefoil and α-helical domains (Fig. 6A), was not accessible to any of the MPEGs. This is surprising because Ser-436 is a site that is proposed to be phosphorylated by ERK2 (a 42-kDa protein) both in vitro and in vivo (28, 29). We were unable to observe phosphorylation of IP3R in COS cell microsomes incubated with purified ERK2 using a phosphospecific Ab (MPM-2) that has previously been used to recognize the consensus phosphorylation site at Ser-436 (29) (data not shown). Further work is required to resolve the issue of relative accessibility of Ser-436 in intact membranes.
This study is the first to use cysteine substitution mutagenesis to examine surface accessibility in IP3Rs. The use of MPEG as a means of tagging accessible cysteines was originally introduced in studies of potassium channels (49) and has subsequently been employed in other experimental systems (50-52). A particular advantage of this methodology, as applied to IP3Rs, is that it yields structural information on the tetrameric, full-length receptor in its native membrane environment. The fortuitous lack of reactivity of large MPEGs with the endogenous cysteines in the N-terminal segments of the SI(-)typeIIP3R circumvents the need to make cysteine-less versions of the receptor. No attempt was made in this study to rigorously fit the high resolution (1.8-2.2 Å) crystal structures of the suppressor and LBD domains (7, 23) into the low resolution (15-30 Å) EM structures (42-44) using the accessibility criteria obtained from the present MPEG studies as a guide. This approach awaits improved EM resolution, a greater certainty of the location of the N-terminal segment, and better consensus between different EM models. Nevertheless, the methodology has the potential to provide independent information on structure and conformational dynamics that will be valuable in building a more complete and accurate picture of this complex ion channel.
Supplementary Material
Acknowledgments
We thank Drs. Paula da Fonseca, Ed Morris, and Gyorgy Hajnoczky for their helpful comments on this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK34804 (to S. K. J.) and T32-AA07463 (training grant to G. A.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: IP3R, myo-inositol 1,4,5-trisphosphate receptor; IP3, myo-inositol 1,4,5-trisphosphate; LBD, ligand-binding domain; Ab, antibody; MPEG, methoxy-polyethylene glycol maleimide; DTT, dithiothreitol; HEDTA, N-(2-hydroxyethyl)ethylenediaminetriacetic acid; TM, transmembrane; PEG, polyethylene glycol.
References
- 1.Berridge, M. J., Bootman, M. D., and Roderick, H. L. (2003) Nat. Rev. Mol. Cell Biol. 4 517-529 [DOI] [PubMed] [Google Scholar]
- 2.Patel, S., Joseph, S. K., and Thomas, A. P. (1999) Cell Calcium 25 247-264 [DOI] [PubMed] [Google Scholar]
- 3.Mikoshiba, K. (2007) J. Neurochem. 102 1426-1446 [DOI] [PubMed] [Google Scholar]
- 4.Foskett, J. K., White, C., Cheung, K. H., and Mak, D. O. (2007) Physiol. Rev. 87 593-658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa, T., Okano, H., Furuichi, T., Aruga, J., and Mikoshiba, K. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 6244-6248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshikawa, F., Morita, M., Monkawa, T., Michikawa, T., Furuichi, T., and Mikoshiba, K. (1996) J. Biol. Chem. 271 18277-18284 [DOI] [PubMed] [Google Scholar]
- 7.Bosanac, I., Yamazaki, H., Matsu-ura, T., Michikawa, T., Mikoshiba, K., and Ikura, M. (2005) Mol. Cell 17 193-203 [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Franco, J., Galvan, D., Mignery, G., and Fill, M. (1999) J. Gen. Physiol. 114 243-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph, S. K., Boehning, D., Pierson, S., and Nicchitta, C. V. (1997) J. Biol. Chem. 272 1579-1588 [DOI] [PubMed] [Google Scholar]
- 10.Mignery, G. A., and Sudhof, T. C. (1990) EMBO J. 9 3893-3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanimura, A., Nezu, A., Morita, T., Turner, R. J., and Tojyo, Y. (2004) J. Biol. Chem. 279 38095-38098 [DOI] [PubMed] [Google Scholar]
- 12.Boehning, D., Patterson, R. L., Sedaghat, L., Glebova, N. O., Kurosaki, T., and Snyder, S. H. (2003) Nat. Cell Biol. 5 1051-1061 [DOI] [PubMed] [Google Scholar]
- 13.Yoshikawa, F., Iwasaki, H., Michikawa, T., Furuichi, T., and Mikoshiba, K. (1999) J. Biol. Chem. 274 316-327 [DOI] [PubMed] [Google Scholar]
- 14.Joseph, S. K., Pierson, S., and Samanta, S. (1995) Biochem. J. 307 859-865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schug, Z. T., and Joseph, S. K. (2006) J. Biol. Chem. 281 24431-24440 [DOI] [PubMed] [Google Scholar]
- 16.Schug, Z. T., da Fonseca, P. C., Bhanumathy, C. D., Wagner, L., Zhang, X., Bailey, B., Morris, E. P., Yule, D. I., and Joseph, S. K. (2007) J. Biol. Chem. 283 2939-2948 [DOI] [PubMed] [Google Scholar]
- 17.Bezprozvanny, I., Watras, J., and Ehrlich, B. E. (1991) Nature 351 751-754 [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Cobo, M., Gingalewski, C., Drujan, D., and De Maio, A. (1999) Cytokine 11 216-224 [DOI] [PubMed] [Google Scholar]
- 19.Hamada, K., Miyata, T., Mayanagi, K., Hirota, J., and Mikoshiba, K. (2002) J. Biol. Chem. 277 21115-21118 [DOI] [PubMed] [Google Scholar]
- 20.Jiang, Q. X., Thrower, E. C., Chester, D. W., Ehrlich, B. E., and Sigworth, F. J. (2002) EMBO J. 21 3575-3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan, J., Whitten, A. E., Jeffries, C. M., Bosanac, I., Mal, T. K., Ito, J., Porumb, H., Michikawa, T., Mikoshiba, K., Trewhella, J., and Ikura, M. (2007) J. Mol. Biol. 373 1269-1280 [DOI] [PubMed] [Google Scholar]
- 22.Joseph, S. K., Nakao, S. K., and Sukumvanich, S. (2005) Biochem. J. 393 575-582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosanac, I., Alattia, J. R., Mal, T. K., Chan, J., Talarico, S., Tong, F. K., Tong, K. I., Yoshikawa, F., Furuichi, T., Iwai, M., Michikawa, T., Mikoshiba, K., and Ikura, M. (2002) Nature 420 696-700 [DOI] [PubMed] [Google Scholar]
- 24.Ramos-Franco, J., Fill, M., and Mignery, G. A. (1998) Biophys. J. 75 834-839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mignery, G. A., Newton, C. L., Archer, B. T., III, and Sudhof, T. C. (1990) J. Biol. Chem. 265 12679-12685 [PubMed] [Google Scholar]
- 26.Joseph, S. K., Brownell, S., and Khan, M. T. (2005) Cell Calcium 38 539-546 [DOI] [PubMed] [Google Scholar]
- 27.Boehning, D., and Joseph, S. K. (2000) J. Biol. Chem. 275 21492-21499 [DOI] [PubMed] [Google Scholar]
- 28.Bai, G. R., Yang, L. H., Huang, X. Y., and Sun, F. Z. (2006) Biochem. Biophys. Res. Commun. 348 1319-1327 [DOI] [PubMed] [Google Scholar]
- 29.Lee, B., Vermassen, E., Yoon, S. Y., Vanderheyden, V., Ito, J., Alfandari, D., De Smedt, H., Parys, J. B., and Fissore, R. A. (2006) Development (Camb.) 133 4355-4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srikanth, S., Wang, Z., Tu, H., Nair, S., Mathew, M. K., Hasan, G., and Bezprozvanny, I. (2004) Biophys. J. 86 3634-3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamada, K., Terauchi, A., and Mikoshiba, K. (2003) J. Biol. Chem. 278 52881-52889 [DOI] [PubMed] [Google Scholar]
- 32.Marshall, I. C. B., and Taylor, C. W. (1994) Biochem. J. 301 591-598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasri, N. N., Bultynck, G., Smyth, J., Szlufcik, K., Parys, J. B., Callewaert, G., Missiaen, L., Fissore, R. A., Mikoshiba, K., and De Smedt, H. (2004) Mol. Pharmacol. 66 276-284 [DOI] [PubMed] [Google Scholar]
- 34.Merzlyak, P. G., Yuldasheva, L. N., Rodrigues, C. G., Carneiro, C. M., Krasilnikov, O. V., and Bezrukov, S. M. (1999) Biophys. J. 77 3023-3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peyronnet, O., Nieman, B., Genereux, F., Vachon, V., Laprade, R., and Schwartz, J. L. (2002) Biochim. Biophys. Acta 1567 113-122 [DOI] [PubMed] [Google Scholar]
- 36.Ternovsky, V. I., Okada, Y., and Sabirov, R. Z. (2004) FEBS Lett. 576 433-436 [DOI] [PubMed] [Google Scholar]
- 37.Sabirov, R. Z., and Okada, Y. (2004) Biophys. J. 87 1672-1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desai, S. A., and Rosenberg, R. L. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 2045-2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howorka, S., Movileanu, L., Lu, X., Magnon, M., Cheley, S., Braha, O., and Bayley, H. (2000) J. Am. Chem. Soc. 122 2411-2416 [Google Scholar]
- 40.Lin, J. K., Ladisch, M. R., Patterson, J. A., and Noller, C. H. (1987) Biotechnol. Bioeng. 22 976-981 [DOI] [PubMed] [Google Scholar]
- 41.Movileanu, L., Cheley, S., Howorka, S., Braha, O., and Bayley, H. (2001) J. Gen. Physiol. 117 239-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato, C., Hamada, K., Ogura, T., Miyazawa, A., Iwasaki, K., Hiroaki, Y., Tani, K., Terauchi, A., Fujiyoshi, Y., and Mikoshiba, K. (2004) J. Mol. Biol. 336 155-164 [DOI] [PubMed] [Google Scholar]
- 43.da Fonseca, P. C., Morris, S. A., Nerou, E. P., Taylor, C. W., and Morris, E. P. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 3936-3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serysheva, I. I., Bare, D. J., Ludtke, S. J., Kettlun, C. S., Chiu, W., and Mignery, G. A. (2003) J. Biol. Chem. 278 21319-21322 [DOI] [PubMed] [Google Scholar]
- 45.Uchida, K., Miyauchi, H., Furuichi, T., Michikawa, T., and Mikoshiba, K. (2003) J. Biol. Chem. 278 16551-16560 [DOI] [PubMed] [Google Scholar]
- 46.Bultynck, G., Szlufcik, K., Kasri, N. N., Assefa, Z., Callewaert, G., Missiaen, L., Parys, J. B., and De Smedt, H. (2004) Biochem. J. 381 87-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riley, A. M., Morris, S. A., Nerou, E. P., Correa, V., Potter, B. V., and Taylor, C. W. (2002) J. Biol. Chem. 277 40290-40295 [DOI] [PubMed] [Google Scholar]
- 48.Taylor, C. W., da Fonseca, P. C., and Morris, E. P. (2004) Trends Biochem. Sci. 29 210-219 [DOI] [PubMed] [Google Scholar]
- 49.Lu, J., and Deutsch, C. (2001) Biochemistry 40 13288-13301 [DOI] [PubMed] [Google Scholar]
- 50.Bauer, P. J., and Krause, E. (2005) Biochemistry 44 1624-1634 [DOI] [PubMed] [Google Scholar]
- 51.Guo, Z. Y., Chang, C. C., Lu, X., Chen, J., Li, B. L., and Chang, T. Y. (2005) Biochemistry 44 6537-6546 [DOI] [PubMed] [Google Scholar]
- 52.Howorka, S., Sara, M., Wang, Y., Kuen, B., Sleytr, U. B., Lubitz, W., and Bayley, H. (2000) J. Biol. Chem. 275 37876-37886 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.