Abstract
Voltage-gated sodium channels initiate electrical signaling in excitable cells such as muscle and neurons. They also are expressed in non-excitable cells such as macrophages and neoplastic cells. Previously, in macrophages, we demonstrated expression of SCN8A, the gene that encodes the channel NaV1.6, and intracellular localization of NaV1.6 to regions near F-actin bundles, particularly at areas of cell attachment. Here we show that a splice variant of NaV1.6 regulates cellular invasion through its effects on podosome and invadopodia formation in macrophages and melanoma cells. cDNA sequence analysis of SCN8A from THP-1 cells, a human monocyte-macrophage cell line, confirmed the expression of a full-length splice variant that lacks exon 18. Immunoelectron microscopy demonstrated NaV1.6-positive staining within the electron dense podosome rosette structure. Pharmacologic antagonism with tetrodotoxin (TTX) in differentiated THP-1 cells or absence of functional NaV1.6 through a naturally occurring mutation (med) in mouse peritoneal macrophages inhibited podosome formation. Agonist-mediated activation of the channel with veratridine caused release of sodium from cationic vesicular compartments, uptake by mitochondria, and mitochondrial calcium release through the Na/Ca exchanger. Invasion by differentiated THP-1 and HTB-66 cells, an invasive melanoma cell line, through extracellular matrix was inhibited by TTX. THP-1 invasion also was inhibited by small hairpin RNA knockdown of SCN8A. These results demonstrate that a variant of NaV1.6 participates in the control of podosome and invadopodia formation and suggest that intracellular sodium release mediated by NaV1.6 may regulate cellular invasion of macrophages and melanoma cells.
In excitable tissues such as muscle and nerve, activation of voltage-gated sodium channels initiates electrical signaling through sodium influx coupled to membrane depolarization (1). During muscle contraction or synaptic vesicle release, depolarization is coupled to increases in cytosolic calcium from extracellular and intracellular stores. Increased cytosolic sodium due to entry of sodium through plasma membrane channels may also be sufficient to mobilize calcium from intracellular stores (2-5).
Non-excitable cells such as bone marrow-derived macrophages also express voltage-gated sodium channels (6-8). In human macrophages, variants of the neuronal channel, NaV1.6, and the cardiac channel, NaV1.5, are expressed intracellularly but not at the plasma membrane (6). NaV1.5 localizes to the macrophage late endosome in interferon-γ or lipolysaccharide-activated cells where it mediates endosomal acidification. NaV1.6 localizes to vesicles that are distributed throughout the cytoplasm and some of which are associated with the actin cytoskeleton. NaV1.6 has a less clear functional role in macrophages. Unlike NaV1.5, NaV1.6 is also expressed in unprimed human macrophages and in unprimed and primed mouse macrophages and microglia (7, 8). Many invasive neoplastic cell lines also express voltage-gated sodium channels, which may regulate their ability to metastasize (9).
Adhesion and movement of monocytes, macrophages, and invasive neoplasms are regulated in part by podosome assembly. Podosomes are specialized regions of the F-actin cytoskeleton that mediate local adhesion, invasion, and migration in specific cell types such as macrophages, bone osteoclasts, and other immune-derived cells (10-14). They are very dynamic structures that degrade and re-polymerize within seconds to minutes (15). Their formation is calcium-dependent (10, 16). In invasive neoplastic cells, these structures are called invadopodia and may be important determinants for invasiveness and metastasis (17-18).
Our goal here was to characterize the role of intracellular NaV1.6 in the regulation of the actin cytoskeleton. A splice variant of NaV1.6 that lacks exon 18 is expressed at a low level in fetal neurons and many non-neural tissues (19). We demonstrate the expression of this splice variant of SCN8A, the gene that encodes NaV1.6 in macrophages, and show that pharmacologic antagonism with tetrodotoxin (TTX),3 absence of a functional NaV1.6 due to a genetic mutation, or knockdown of NaV1.6 expression with shRNA impairs podosome formation in macrophages. Blockade of the channel also prevents cellular invasion of macrophages and melanoma cells through extracellular matrix. Activation of the channel by the voltage-gated sodium channel agonist veratridine leads to vesicular intracellular sodium release, uptake of sodium by the adjacent mitochondrial compartment, and release of mitochondrial calcium. These findings suggest that a variant of NaV1.6 contributes to the control of macrophage and melanoma cellular invasion through a signaling pathway that may link intracellular sodium channel activation to actin cytoskeleton dynamics.
EXPERIMENTAL PROCEDURES
Cells—THP-1 cells, a human premyelomonocytic leukemic cell line, were maintained in RPMI 1640 media supplemented with 10% fetal bovine serum (FBS), sodium pyruvate, and non-essential amino acids. Differentiation to a macrophage phenotype was induced by treatment with 12-O-tetradecanoylphorbol-13-acetate (10 ng/ml, 72 h). HTB-66 cells, an invasive human melanoma cell line, were grown in Dulbecco's modified Eagle's media supplemented with 10% FBS, sodium pyruvate, and non-essential amino acids.
Mice—Resident peritoneal macrophages were obtained from C57BL6/J, NaV1.6-deficient C3HeB/FeJ-Scn8amed/J mice, and wild-type littermates (Jackson Laboratories) by peritoneal lavage with serum supplemented RPMI. C3HeB/FeJ-Scn8amed/J mice and their control littermates were ∼14-21 days of age and were bred in our facility from heterozygous carriers of the med recessive gene. C57BL6/J mice were purchased from Jackson Laboratories and used at 6-8 weeks of age. Macrophages were isolated by centrifugation followed by isolation of mononuclear cells on a lymphocyte separation medium step gradient and adhesion to glass coverslips. Cells were plated at a density of 1 × 106/ml and maintained for 18-24 h in the same media as THP-1 cells. All animal studies were reviewed and approved by the Yale University School of Medicine Institutional Animal Care and Review Committee (IACUC).
SCN8A cDNA Sequence Analysis—A PCR-based approach was used to sequence SCN8A cDNA generated from THP-1. mRNA was isolated, reverse transcribed, and analyzed for expression of SCN8A by quantitative PCR as described previously (6). Three-way (“stitched”) PCR was used to generate a full-length coding PCR fragment for sequencing (exon 2-27). First, two separate PCR fragments were synthesized: one (2.52 kb) with the 2F and 15R primers and the other (3.36 kb) with 15F and 27R primers (see Table S1 for primer sequences). These two fragments were gel eluted and purified. Equal amounts of these fragments were used as a template for the three-way PCR using the 2F and 27R primers to amplify a 5.8-kb fragment. Cycle conditions were as follows: denaturation at 95 °C for 5 min × 1 cycle, denaturation at 95 °C for 30 s, annealing at 62 °C for 30 s, and extension at 68 °C for 7 min (×34 cycles), and further extension at 68 °C for 10 min × 1 cycle. Sequencing of the 5.8-kb PCR product was performed at the W.M. Keck facility at Yale University School of Medicine. The primers used for sequencing (“primer walking”) of this product are shown in Table S1. The products were sequenced in both the forward and reverse directions for confirmation. DNAS-TAR (Lasergene) was used for analysis of the sequence data.
Immunohistochemistry—Cells grown on multichambered glass coverslips were washed with PBS, fixed with 4% paraformaldehyde in PBS (10 min), and blocked in PBS containing 5% serum (goat or donkey, dependent on secondary antibody), 0.1% Triton X-100, 1% bovine serum albumin, and 0.04 mg/ml normal human IgG. Primary and secondary antibodies were diluted in blocking solution. Rabbit anti-NaV1.6 was obtained from Alomone Laboratories. Goat anti-gelsolin and mouse anti-dynamin II were from Santa Cruz Biotechnology and BD Biosciences, respectively. Secondary donkey antibodies conjugated to Alexa fluorophores, MitoTracker dye, and phalloidin-Alexa 350 and 488 were obtained from Invitrogen. 4′,6′-Diamidino-2-phenylindole was from Vector Laboratories. Images were obtained on a Zeiss Axiovert 200 fluorescent microscope with a ×63 objective (Zeiss Plan Apochromat, 1.4 oil).
Electron Microscopy—For fixation for regular epon embedding to show membrane preservation, samples were fixed in 2.5% gluteraldehyde in 0.1 m sodium cacodylate buffer, pH 7.4, with 3% sucrose for 1 h at room temperature. The samples were rinsed 3 times in sodium cacodylate rinse buffer and then pelleted in 2% agar. These were post-fixed in 1% osmium tetroxide for 1 h, en bloc stained in 2% uranyl acetate in maleate buffer, pH 5.2, for a further hour then rinsed, dehydrated, and infiltrated with epon resin and baked overnight at 60 °C. Hardened blocks were cut using a Leica UltraCut UCT. 60-nm sections were collected and stained using 2% uranyl acetate and lead citrate.
For sample fixation for immunoelectron microscopy, samples were fixed in 4% paraformaldehyde in 0.25 m Hepes for 1 h. Samples were rinsed in PBS and re-suspended in 10% gelatin, chilled, and trimmed to smaller blocks and placed in cryoprotectant of 2.3 m sucrose overnight on a rotor at 4 °C. They were transferred to aluminum pins and frozen rapidly in liquid nitrogen. The frozen block was trimmed on a Leica Cryo-EMUC6UltraCut and 75-nm thick sections were collected. The frozen sections were collected on a drop of sucrose, thawed, and placed on a nickel formvar/carbon-coated grid and floated in a dish of PBS ready for immunolabeling.
For immunolabeling of sections, grids were placed section side down on drops of 0.1 m ammonium chloride for 10 min to quench untreated aldehyde groups, then blocked for nonspecific binding in 1% fish skin gelatin in PBS for 20 min. Single labeled grids were incubated in either a primary antibody rabbit anti-Nav1.6, 1:50 (Alomone), or mouse anti-β-actin (Sigma), 1:200 dilutions, for 30 min. Controls were also done using rabbit IgG (Jackson) and mouse IgG (Jackson) at the same dilution. Secondary antibodies were either 12-nm anti-rabbit or anti-mouse colloidal gold (Jackson) for 30 min. Double labeling was performed as above but by combining the two primaries, rinsing, and then combining secondary direct gold anti-mouse 6 nm (actin) and anti-rabbit 12 nm (Nav1.6). All grids were rinsed in PBS, fixed using 1% gluteraldehyde for 5 min, and rinsed, transferring grids to a UA/methylcellulose drop for 10 min. Samples were all viewed on a FEI Tencai Biotwin TEM at 80 Kv. Images were taken using Morada CCD and iTEM (Olympus) software.
Podosome Quantitation—Differentiated THP-1 cells were treated with serum-free media for 4 h in the presence and absence of 300 nm TTX, a concentration that blocks TTX-sensitive sodium channels such as NaV1.6 but not TTX-resistant channels such as NaV1.5. They were then permeabilized in PBS containing 0.1% Triton X-100 for 20 min, incubated with phalloidin-Alexa 488 in 1% bovine serum albumin for an additional 20 min, and washed with PBS. Cells were counterstained with 4′,6′-diamidino-2-phenylindole and analyzed by fluorescent microscopy. Podosomes were identified manually with an event marker (Zeiss Axiovision 4.6.3 software) from images taken with a ×40 objective (LD Plan-Neofluar, 0.6).
Live Cell Imaging—For live cell imaging of sodium flux, 12-O-tetradecanoylphorbol-13-acetate-treated THP-1 cells plated on a Delta T4 cell culture plate (Bioptechs) were labeled with 4 μm Sodium Green and 1 μm Corona Red for 40 min at 37 °C in Hanks' buffered salt solution (HBSS). Cells were washed three times with HBSS and then monitored for fluorescence on a heated microscopy stage (37 °C) with a heated ×63 oil immersion objective on a Zeiss Axiovert 200 fluorescent microscope. Two color fluorescent images were taken in multiple Z-planes prior to and following a 1-min stimulation with veratridine (80 μm). Images were deconvoluted using Axiovision Three-dimensional Deconvolution software (Zeiss) and analyzed quantitatively with Axiovision 4.6.3 Automeasurement.
Fluorometry—For time-resolved fluorescence analysis of THP-1 cells or freshly isolated mouse peritoneal macrophages, cells were isolated by centrifugation, resuspended in serum-free HBSS at room temperature, and labeled with indicator dyes. For sodium flux measurements, cells were labeled with the ratiometric sodium indicator SBFI-AM (4 μm) for 40 min at room temperature in HBSS and then washed with assay buffer by three successive centrifugations. Assay buffer was either 135 mm NaCl, 4.5 mm KCl, 4 mm EGTA, 11 mm glucose, and 20 mm Hepes, pH 7.4, or the same buffer with NaCl replaced by 145 mm N-methyl-d-glucamine (NMDG). NMDG-containing solutions were titrated to pH 7.4 with HCl. Prior to and following veratridine stimulation (80 μm), the ratio of fluorescence intensities excited at 340/380 nm was monitored at an emission wavelength of 505 nm in a LS-50b spectrophotometer (PerkinElmer Life Sciences). For calibration of intracellular sodium, THP-1 cells in NMDG buffer were mixed with increasing concentrations of extracellular sodium (5, 15, 25, and 50 mm NaCl, balanced in a molar fashion with NMDG) following permeabilization of cells with 0.1% Triton X-100. At least four separate measurements were taken at the indicated concentration to generate a standard curve. By this method, the estimated intracellular sodium concentration was 11.15 ± 0.92 mm (n = 4) in the presence of NMDG buffer.
For Sodium Green fluorometric experiments, cells were labeled with 4 μm dye for 40 min and washed as described above. Samples were excited at 507 nm, and emission was monitored at 532 nm.
For calcium flux experiments, THP-1 cells were labeled with 1 μm Fluo-4 for 30 min at room temperature. Cells were washed by centrifugation three times with HBSS and then analyzed by fluorometry. In some experiments, cells were pre-treated with 4 mm EGTA or a combination of EGTA and 0.02 mm CGP-36517. Excitation was at 494 nm and emission at 516 nm.
For Fura-2 measurements in mouse peritoneal macrophages, cells were allowed to adhere to glass coverslips overnight, washed with HBSS, and labeled with Fura-2 (1 μm) for 30 min at 37 °C. Cells were washed and then incubated in fresh HBSS for an additional 30 min at 37 °C. Excitation was at 340 and 380 nm with ratiometric emission recorded at 510 nm. Calibration was performed using Ca-EGTA buffers in permeabilized cells.
Invasion and Migration Assays—Cellular invasion through a reconstituted basement membrane matrix (ECMatrix, Chemicon) was measured in a 96-well plate format. In the presence and absence of TTX (300 nm), cells were serum starved in 0.1% FBS for 24 h prior to plating on the matrix. 100,000 cells were added to each well in 0.1 ml of media with 0.1% FBS in the presence and absence of freshly added TTX and incubated at 37 °C for 24 h. For THP-1 cells, the lower chamber in the plate assay contained varying concentrations of human macrophage colony-stimulating factor (m-CSF) (25, 100, or 400 ng/ml) or no chemoattractant (control condition). THP-1 invasion also was examined in shRNA-treated cells at an m-CSF concentration of 100 ng/ml. For HTB-66 cells, the attractant was either 10% FBS in Dulbecco's modified Eagle's medium (experimental condition) or 0.1% FBS in Dulbecco's modified Eagle's medium (control condition). Cells that invaded through the matrix were dissociated, lysed, and detected by CyQuant GR dye (Invitrogen), using a fluorescent plate reader. Invasion was expressed in relative fluorescent units (RFU) and was determined by subtracting background invasion (control conditions) from that observed in the experimental conditions.
Migration was measured in a transwell assay (Chemicon) using a5-μm pore size. THP-1 cells were serum starved for 4 h in the presence and absence of TTX (300 nm) and then analyzed in the migration assay in the presence and absence of freshly added TTX. Human monocyte chemotractant protein (MCP-1) was used as the stimulus (7.5 ng/ml-250 ng/ml for 2 h). Quantitative analysis was performed as described above for invasion assays.
Small Hairpin RNA—THP-1 cells were transduced with shRNA lentiviral clones as previously described (6). The following five clones, specific for human SCN8A (encodes NaV1.6), were obtained from Sigma: SHVRSC-TRCN0000044488-92. Percent knockdown was determined by real time PCR (Cepheid) using TaqMan FAM-labeled primers (Applied Biosystems) (Table 1). Clones 89 and 92 were selected for functional assays because of knockdown >75% or absence of significant knockdown, respectively. Knockdown of NaV1.6 in THP clone 89 also was confirmed by Western blot using the anti-NaV1.6 subtype-specific antibody.
TABLE 1.
Real time PCR analysis of SCN8A expression
| Cell line | SCN8A Ct (threshold cycle) | GAPDH Ct | n |
|---|---|---|---|
| THP-1 differentiated | 36.84 ± 0.38 | 20.0 | 3 |
| THP-1 undifferentiated | 35.98 ± 0.93 | 20.0 | 7 |
| HTB-66 | 35.94 ± 0.49 | 20.0 | 7 |
| THP-1 Clone 89 (differentiated) | >39.74 (2 runs > 50 cycles) | 20.0 | 3 |
| THP-1 Clone 92 (differentiated) | 36.37 ± 0.24 | 20.0 | 3 |
Real time PCR analysis was performed as described under “Experimental Procedures.” Ct values (threshold cycle) were normalized using GAPDH expression as a control (Ct = 20.0). Expression of SCN8A, which encodes NaV1.6, was detected in only one out of three experiments for THP-1 Clone 89 (>50 cycles in two conditions). Knockdown was estimated to be >75%. THP-1 cells were not primed with either interferon-γ or lipopolysaccharide. Under these conditions, expression of SCN5A, which encodes NaV1.5, was not detected. Cycling conditions were the same for each cell line listed. Stage 1 was an initial denaturation at 95 °C for 120 s followed by Stage 2 with denaturation at 95 °C for 15 s and annealing/extension at 70 °C for 60 s for 50 cycles. All runs were performed on a Cepheid SmartCycler, and the conditions were selected per manufacturer's suggestions.
Statistics—Comparisons between groups were made using a Student's t test (unpaired, unequal variance) with a p < 0.05 considered statistically significant (Kaleidagraph 4.03, Synergy Software). Data are expressed as mean ± S.E.
Image Processing—Microscopic images were obtained using Axiovision software as described above, exported as TIFF files, compiled in composite figures using Adobe Illustrator, and exported as TIFF files.
RESULTS
cDNA Cloning of an SCN8A Transcript from THP-1 Cells Reveals a Splice Variant—cDNA sequence analysis of a full-length coding sequence for SCN8A from THP-1 cells demonstrated expression of the splice variant that lacks exon 18 (Figs. S1 and S2). This exon encodes a 41-amino acid portion of the IIIS3-IIIS4 transmembrane region of NaV1.6. This finding is consistent with prior studies of SCN8A because exon 18N, which was hypothesized to be a non-neuronal exon variant of SCN8A, encodes a stop codon within its sequence and, if expressed, would prevent the translation of a full-length NaV1.6 (19). A similar splice variant that would encode a truncated, but not full-length, variant of NaV1.6 has been described in another non-excitable cell type, astrocytes (20).
Some NaV1.6 Positive Vesicles Are Localized Adjacent to Melanoma Invadopodia and Macrophage Podosomes—Previously we demonstrated by immunofluorescence and immuno-EM that macrophage NaV1.6 appeared to localize to F-actin fiber bundles (6). Here we further analyzed the relationship between NaV1.6-positive membrane structures and the F-actin cytoskeleton in cells known to form invadopodia and podosomes. In the invasive human melanoma cell line, HTB-66, primary mouse peritoneal macrophages, and the human monocytic cell line, THP-1, differentiated with phorbol ester to a macrophage phenotype, NaV1.6-positive vesicles were observed throughout the cell but in some cellular regions they appeared to cluster around melanoma invadopodia and the core of macrophage podosome structures (Figs. 1A and 2, A and C). Unlike the previous study where NaV1.6/F-actin co-localization was demonstrated in a more longitudinal view in a migrating cell (6), these views are cross-sectional and, in a more detailed fashion, show the NaV1.6-positive regions surrounding the F-actin core rather than localizing to the core itself. In mouse macrophages and differentiated THP-1 cells, this vesicular localization was most intense around centrally located podosomes (Fig. 2, A and C). In addition, in differentiated THP-1 cells, there were clusters of mitochondria concentrated in podosome-dense regions (Fig. 2C). This latter image (Fig. 2C) is a deconvoluted image through only Z-planes that were podosome positive at the sites of cellular attachment.
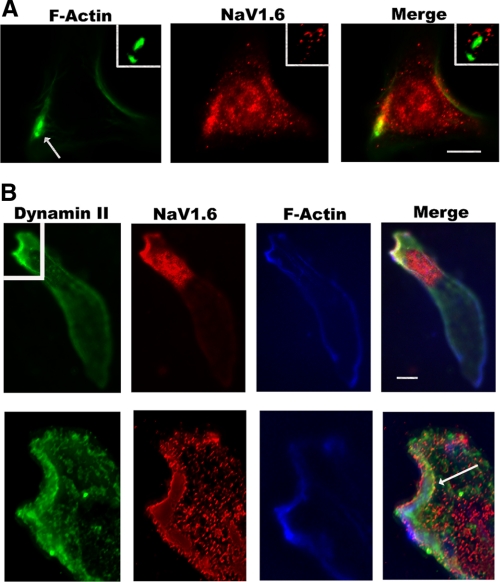
FIGURE 1.
NaV1.6-positive vesicles are present in high concentrations near invadopodia and the leading edge of HTB-66 cells, an invasive human melanoma cell line. A, NaV1.6-containing vesicles (middle panel, red) are distributed throughout HTB-66 cells, an invasive human melanoma cell line, but are observed in particularly high densities near F-actin (phalloidin-Alexa 488, left panel, green) positive invadopodia structures (arrow; detail in white box shown in upper right). Scale bar, 10 μm. B, at the leading edge of an invasive HTB-66 cell, NaV1.6-positive vesicles (left panel, red) co-localize and are localized to Dynamin II (far left panel, green) and F-actin-positive regions (phalloidin Alexa 350, right panel, blue). There is also a dense collection of Dynamin II-negative, NaV1.6-positive vesicles immediately below the leading edge. Scale bar, 10 μm. In more detailed deconvoluted images (bottom images) of the invadopodia region (detail from white box in upper left), all three proteins appear to be in adjacent compartments within this structure. There is a small degree of co-localization between Dynamin II and NaV1.6 at the leading edge (arrow).
FIGURE 2.
In macrophages, NaV1.6-positive vesicles localize to cellular regions rich in podosomes. A, phalloidin staining of F-actin (left panel, green) in a mouse peritoneal macrophage shows numerous podosomes (circular structures). Staining for NaV1.6 (middle panel, red) demonstrates positive staining of vesicular-type structures, some of which co-localize to the periphery of the F-actin-rich core of the podosome structure (merged image on the right). B, in a mouse peritoneal macrophage, NaV1.6-containing vesicles (red) also co-localize with the calcium-dependent, actin-regulatory protein gelsolin (green), particularly at the leading edge of the cell as compared with the trailing tail. C, a similar staining pattern was observed in differentiated THP-1 cells, a human monocytic cell line, as compared with mouse primary mouse macrophages (A). Centrally located podosomes (far left panel, green) were concentrated in regions rich in NaV1.6-positive vesicles (middle left panel, red) and mitochondria (stained with Mito-Tracker, middle right panel). Scale bar, 10 μm.
We also investigated the relationship of other actin regulatory proteins with NaV1.6. At the leading edge of a HTB-66 cell, NaV1.6-positive vesicles appeared to co-localize with or were adjacent to regions rich in F-actin and the GTPase Dynamin II, a known regulator of invadopodia and podosomes (21-22) (Fig. 1B, top). However, in a more detailed view of the invadopodia leading edge from the same cell using a deconvoluted image, there is some degree of co-localization between Dynamin II and NaV1.6 (arrow), but the three proteins can be seen to be tightly packed within the region in adjacent but distinct compartments (Fig. 1B, bottom). In primary mouse peritoneal macrophages treated with a chemotactic stimulus (mouse MIP-1α), NaV1.6-positive vesicles were also closely associated with gelsolin, a calcium-dependent regulator of actin polymerization (Fig. 2B). There was no strong co-localization of NaV1.6-positive vesicles with markers of endosomes (rab GTPases), lysosomes (LAMP-1), endoplasmic reticulum (calre-ticulin), or Golgi (golgin) (data not shown).
NaV1.6 Localizes to the Podosome Rosette Structure—We further analyzed the subcellular localization of NaV1.6 using immunoelectron microscopy in THP-1 cells that had been treated with m-CSF to enhance additional podosome and podosome rosette formation. As has been described previously using EM techniques, podosome rosettes appear as electron dense structures that appear circular when the cells are sectioned parallel to the substrate (23, 24). Each rosette structure contains several podosomes. Using epon-embedded sections without immuno-staining, podosome rosettes appeared as electron dense structures as described in the prior studies, but in contrast to those studies membrane structures could be identified within the rosette structure if membrane preservation techniques were used during tissue processing (Fig. 3, A and B). Immunostaining for NaV1.6 using cryo-EM demonstrated intensely positive staining within the rosette structure with much less staining outside of it (Fig. 3C). As expected, there was strong staining for actin within podosome rosettes (Fig. 3D). Double labeling for both NaV1.6 and actin revealed intense staining for both with the podosome rosette (Fig. 3E). Using cryo-EM for immunostaining, membrane structures are not as well preserved as in the epon-embedded sections shown in Fig. 3, A and B. Outside of the electron dense structure, the membrane structure can be better appreciated, and there appeared to be co-localization of single podosomes with small NaV1.6-positive vesicles that were ∼30-80 nm in diameter (Fig. 3E, arrows). These results suggested a potential role for NaV1.6 in podosome and invadopodia dynamics.
FIGURE 3.
Immuno-EM demonstrates localization of NaV1.6 to the electron dense podosome structure. The podosome rosette structure was analyzed in epon-embedded samples (A and B), and NaV1.6 and β-actin subcellular localization were analyzed by cryo-immuno-EM (C-E) in differentiated THP-1 that had been treated with m-CSF (50 ng/ml for 5 min) to enhance podosome and podosome rosette formation. Under these conditions, podosome rosettes appear as circular electron dense structures. A, low power view of a cell demonstrates a podosome rosette as an electron dense structure. Note the mitochondria adjacent to the structure. Scale bar, 1 μm. B, a higher power view shows preserved membrane structure within the rosette. Scale bar, 500 nm. C, cryo-immuno-EM revealed intense immuno-gold staining (black dots) for NaV1.6 within the electron dense podosome rosette structure with less intense staining elsewhere in the cell. Scale bar, 1 μm. D, the electron dense podosome structure also demonstrated intense staining for β-actin. Scale bar, 500 nm. E, double labeling for both NaV1.6 (larger 12-nm gold particles) and actin (smaller 6-nm gold particles) revealed co-localization within the electron dense podosome structure (lower left) and in small membrane vesicles (30-80 nm in diameter) outside of the rosette (arrows). Scale bar, 500 nm.
Absence of a Functional NaV1.6 in Mouse Peritoneal Macrophages Prevents Podosome Formation—To investigate whether or not NaV1.6 mediates podosome formation, we measured the density of podosomes in mouse peritoneal macrophages that express wild-type NaV1.6 channels and those that do not express functional NaV1.6 channels (med mouse) (25). Wild-type macrophages demonstrated a significantly greater number of podosomes per cell as compared with med macrophages (Fig. 4, A and B). There were 11.93 ± 5.64 podosomes per cell in wild-type cells and 1.23 ± 0.18 per cell in med macrophages (p < 0.0001).
FIGURE 4.
Absence of a functional NaV1.6 in mouse peritoneal macrophages or function block of the channel with tetrodotoxin in differentiated, human THP-1 cells reduces the number of podosomes. A and B, peritoneal macrophages were harvested from 2-week-old mice homozygous for the med mutation and healthy littermates. Following 1 day in culture, cells were stained for F-actin with Alexa 488 phalloidin. Wild-type cells (A) demonstrated more podosomes (left panels, green) as compared with macrophages from med mice (B). Cells were co-stained with 4′,6′-diamidino-2-phenylindole (DAPI) to reveal nuclei and cells without podosomes (A and B, merged images). As shown in the table below the micrographs, quantitative analysis revealed a statistically significant difference between the number of podosomes per cell in the two conditions. Scale bar, 20 μm. C and D, THP-1 cells were differentiated with phorbol ester for 48 h, incubated in serum-free media for an additional 4 h in the presence and absence of TTX (300 nm), harvested, and allowed to re-adhere to a glass coverslip for 1 h. Cells were then stained with phalloidin. Untreated cells (C) demonstrated a greater number of podosomes than those treated with TTX (D). Quantitative analysis (table below micrographs) revealed statistically significant reduction in the number of podosomes in TTX-treated cells. Scale bar, 10 μm.
Blockade of NaV1.6 with Tetrodotoxin Prevents Podosome Formation in Differentiated THP-1 Cells—We also measured the density of podosomes in the presence and absence of 300 nm TTX, a specific antagonist of voltage-gated sodium channels. This concentration of TTX functionally blocks TTX-sensitive channels such as NaV1.6 but not NaV1.5 (1, 6). In addition, NaV1.6, but not NaV1.5, is expressed in differentiated, but unprimed THP-1 cells (see Table 1 for real time PCR data on sodium channel expression), and a prior study did not demonstrate the expression of any other voltage-gated sodium channels in this cell line (6). Although exposure to TTX for 1 h did not have a significant effect, treatment with TTX for 4 h significantly reduced the number of podosomes in unprimed, differentiated THP-1 cells (Fig. 4, C and D). There were 10.94 ± 0.82 podosomes per cell in untreated cells and 1.37 ± 0.33 in the TTX condition (p < 0.0001).
Activation of NaV1.6 with the Channel Agonist Veratridine Leads to a Shift in Intracellular Sodium from Positively Charged Vesicular Compartments to Anionic Mitochondria—To assess how NaV1.6 could regulate intracellular signaling, we analyzed intracellular sodium flux in THP-1 cells following pharmacologic activation with veratridine, a selective voltage-gated sodium channel agonist. Cells were labeled with specific sodium indicators. Sodium Green was used to label positively charged vesicular compartments and the cytosol and Corona Red to label negatively charged mitochondria. Prior to stimulation with veratridine, Sodium Green staining of vesicles was more intense than mitochondrial sodium staining with Corona Red (Fig. 5, A and C). Following a brief stimulation with veratridine (1 min), the ratio of red to green staining increased dramatically (Fig. 5, B and D), demonstrating intracellular release of sodium from vesicular stores and rapid uptake of sodium from the cytosol by mitochondria. Quantitative analysis of these fluorescent shifts was statistically significant (Fig. 5, see table).
FIGURE 5.
Voltage-gated sodium channel activation by the agonist veratridine causes a shift in intracellular sodium from cationic vesicles and the cytosol to the mitochondria. A-D, differentiated, unprimed THP-1 cells, which express NaV1.6, but not NaV1.5, were labeled with Sodium Green and Corona Red and then stimulated for 1 min with veratridine. Following stimulation, there is a clear shift at low magnification from a green predominant staining pattern (A) to red (B), suggesting release of sodium from positively charged compartments and uptake of sodium from the cytosol by mitochondria. Scale bar, 20 μm. At higher magnification (C and D, detailed image of the cell (arrow) in A), separation of the compartments can be distinguished both prior to stimulation (C) and following channel activation (D). Scale bar, 2 μm. Quantitative analysis of these fluorescent shifts was statistically significant (table).
Fluorometric Analysis Also Demonstrates Intracellular Sodium Release in THP-1 Cells, Primary Mouse Macrophages, and Human Melanoma Cells—We confirmed these results by time-resolved fluorometry. In cells labeled with the ratiometric sodium indicator, SBFI-AM (sodium-binding benzofuran isopthalate), which like Sodium Green labels both positively charged vesicles and the cytosol (6), veratridine caused a rapid and persistent decrease in vesicular and cytosolic sodium levels in both the presence (tracing not shown) and absence of extracellular sodium (Fig. 6A). The peak change in fluorescent ratio was -0.62 ± 0.06 RFU (n = 5) in the presence of NaCl (135 mm NaCl, 4.5 mm KCl, 4 mm EGTA, 11 mm glucose, and 20 mm Hepes) and -0.45 ± 0.03 RFU (n = 4; differences not statistically significant) when the NaCl was replaced by NMDG (Fig. 5A). This latter change represented an intracellular shift of ∼9.12 ± 1.38 mm sodium from positively charged compartments and the cytosol to other cellular compartments such as mitochondria. TTX (300 nm) pre-treatment of cells inhibited this response (SBFI-NMDG-TTX, -0.13 ± 0.02 RFU, n = 4, p = 0.0001 as compared with SBFI-NMDG untreated) (Fig. 6B). Similar fluorometric results were obtained with SBFI-labeled mouse primary peritoneal macrophages (Fig. 6C), HTB-66 melanoma cells (Fig. 6D), and THP-1 cells labeled with Sodium Green (data not shown). In mouse peritoneal macrophages, the calculated veratridine-induced sodium shift was 8.48 ± 0.67 mm sodium (n = 3).
FIGURE 6.
The microscopic results for veratridine-induced sodium flux were confirmed by fluorometry. A, unprimed THP-1 cells were loaded with the ratiometric sodium dye, SBFI, which like Sodium Green predominantly labels the cytoplasm and positively charged organelles. Stimulation with veratridine resulted in a rapid decrease in sodium in those compartments in the absence of extracellular sodium (NaCl replaced by NMDG). The average peak change in fluorescent ratio values was -0.62 ± 0.06 RFU for the NaCl group (n = 5) (tracing not shown) and -0.45 ± 0.03 RFU for the NMDG condition (n = 4) (differences not statistically significant). The intracellular shift from SBFI-labeled compartments to other cellular compartments was calculated to be 9.12 ± 1.38 mm. B, TTX (300 nm) blocked the veratridine-induced response in THP-1 cells (SBFI-NMDG-TTX, -0.13 ± 0.02 RFU, n = 4, p = 0.0001 as compared with SBFI-NMDG-untreated). C, mouse primary peritoneal macrophages were loaded with SBFI and then stimulated with veratridine. A similar decrease was observed following veratridine stimulation as compared with THP-1 cells (-0.70 + 0.06 RFU, n = 3). The decrease was estimated to be 8.48 ± 0.67 mm sodium. D, in HTB-66 cells, an invasive human melanoma line, the veratridine-induced response was -0.40 RFU ± 0.03 (n = 3).
Intracellular Sodium Release Is Linked to Mitochondrial Calcium Release through the Mitochondrial Na/Ca Exchanger—Because calcium is an important regulator of podosome formation in macrophages and osteoclasts (10, 16), a related cell type found in bone, we also measured calcium flux in response to sodium channel activation by veratridine. Veratridine stimulation of THP-1 cells, loaded with the calcium indicator Fluo-4, resulted in a persistent rise in intracellular calcium in the presence of extracellular calcium (Fig. 7A). Buffering of extracellular calcium with EGTA transformed the response to a prolonged, but transient, response, suggesting that the persistent phase requires calcium activation of plasma membrane calcium channels (Fig. 7B). Treatment with an inhibitor of the Na/Ca mitochondrial exchanger, CGP-37157, significantly blocked the veratridine-induced calcium response (Fig. 7C). The peak response was 37.00 ± 3.21 RFU (n = 3) for EGTA alone and 7.33 ± 2.33 RFU in the presence of both EGTA and CGP-37157 (p = 0.002) (Fig. 7E).
FIGURE 7.

Veratridine activation of voltage-gated sodium channels leads to release of calcium from mitochondria mediated by the Na/Ca exchanger. A--E, THP-1 cells were loaded with the calcium indicator dye Fluo-4 and stimulated with veratridine. A, following a brief injection artifact, there is a delayed but sustained increase in cytosolic calcium in the presence of extracellular calcium. B, when extracellular calcium is buffered with EGTA, the peak response is similar but is not sustained and lasts for ∼2 min. C, this response was almost entirely blocked when the cells were pre-treated with an inhibitor of the mitochondrial Na/Ca exchanger, CGP-37157 in combination with EGTA. The injection response in the absence of cells is shown in D. E, the average peak response was 45.75 ± 9.23 RFU (n = 4) in the presence of extracellular calcium (far left). The peak response was 37.00 ± 3.21 (n = 3) for EGTA alone and 7.33 ± 2.33 (n = 3) in the presence of both EGTA and CGP-37157 (0.02 mm)(p = 0.002).
Absent Veratridine-induced Calcium Response in NaV1.6-deficient Macrophages—As compared with THP-1 cells, similar calcium responses were obtained with mouse peritoneal macrophages loaded with the ratiometric calcium indicator, Fura-2. With extracellular calcium buffered with EGTA, the intracellular calcium increase in response to veratridine was 0.15 ± 0.04 (change in fluorescent ratio) in peritoneal macrophages iso-lated from 2-3-week-old control littermates of NaV1.6-deficient med mice (Fig. 8A). The approximate change in intracellular calcium was 211 ± 51 nm. Peritoneal macrophages from NaV1.6-deficient med mice demonstrated either no response to veratridine or a slight negative response (-0.07 + 0.03 change in fluorescent ratio; p = 0.019) (Fig. 8B). As compared with macrophages isolated from 2-week-old mice, the calcium response to veratridine appeared to be slightly more robust in macrophages isolated from mature C57BL6/J mice (0.27 ± 0.07 change in fluorescent ratio; tracing not shown); however, as compared with 2-3-week-old control mice this difference was not statistically significant.
FIGURE 8.
NaV1.6-deficient mouse peritoneal macrophages lack a veratridine-induced calcium response. A, mouse peritoneal macrophages from 2-3-week-old littermates of NaV1.6-deficient med mice were labeled with the calcium indicator Fura-2 and then stimulated with veratridine (80 μm). With extracellular calcium buffered with EGTA, veratridine induced a transient increase in intracellular calcium (0.15 ± 0.04 change in fluorescent ratio). The approximate change in intracellular calcium was 211 ± 51 nm. B, in peritoneal macrophages from NaV1.6-deficient med mice, there was no response or a slight negative response (-0.07 ± 0.03, p = 0.019). Representative tracings are shown for both conditions.
m-CSF Stimulates Intracellular Sodium Release—To demonstrate a link between this intracellular signaling pathway and cellular motility, we examined the response of THP-1 cells to m-CSF, a known inducer of podosome formation in macrophages. In the absence of extracellular sodium (NMDG buffer), treatment of SBFI-labeled cells with m-CSF (10 ng/ml) resulted in a transient decrease in intracellular sodium in cationic compartments and the cytosol, which could be blocked by pre-treatment with TTX (300 nm) (Fig. 9, A and B). The peak response was 0.24 ± 0.6 RFU in the absence of TTX (n = 4). This response is notable considering the low m-CSF concentration used to elicit the response as opposed to the higher doses required for cellular invasion (see Fig. 11, below). The response is not prolonged as seen with veratridine, because veratridine prevents voltage-gated sodium channel inactivation.
FIGURE 9.
m-CSF, an inducer of podosome formation, stimulates a transient intracellular sodium flux that can be blocked by tetrodotoxin. A and B, THP-1 cells were labeled with the sodium indicator dye, SBFI, and then stimulated in the absence of extracellular sodium (NMDG buffer, see “Experimental Procedures”) with m-CSF (10 ng/ml), a known inducer of podosomes in monocyte-macrophages. m-CSF caused a transient decrease in sodium concentration that could be blocked by pre-treatment with TTX (300 nm). The peak response was 0.24 ± 0.6 RFU in the absence of TTX.
FIGURE 11.
Invasion of differentiated THP-1 cells and HTB-66 cells through extracellular matrix is blocked by tetrodotoxin, but migration is unaffected. Invasion of differentiated THP-1 and HTB-66 through reconstituted basement membrane was measured in a transwell assay. A, for THP-1 cells, in response to varying concentrations of m-CSF, TTX (300 nm) significantly inhibited invasion at all concentrations examined. Invasion, normalized to nonspecific invasion in the absence of stimulus, was 31,779 ± 10,798 RFU in the untreated group versus -5,080 ± 6,656 in the TTX group at 25 ng/ml m-CSF (n = 6; p = 0.019); 71,382 ± 12,209 versus 12,850 ± 12,406 at 100 ng/ml m-CSF (n = 6; p = 0.007); and 87,446 ± 15,216 versus 25,133 ± 17,752 at 400 ng/ml m-CSF (n = 6; p = 0.02). B, in a similar manner, TTX also blocked invasion of HTB-66 cells in response to 10% FBS. In the untreated condition invasion was 70,691 ± 14,700 RFU as compared with -10,244 + 9,302 (n = 5; p = 0.003). C, in contrast, migration of THP-1 cells through a 5-μm pore in a transwell assay was not affected by TTX treatment (300 nm). Varying concentrations of MCP-1 were used as the chemotractant (7.5 to 250 ng/ml for 2 h). Migration, normalized to nonspecific migration in the absence of stimulus, was 151,720 ± 19,816 RFU in the untreated group versus 183,610 ± 11,711 in the TTX group at 7.5 ng/ml MCP-1, 259,570 ± 79,161 versus 214,450 ± 36,795 at 15 ng/ml MCP-1, 255,780 ± 51,738 versus 267,820 ± 62,777 at 31 ng/ml MCP-1, 377,600 ± 127,550 versus 347,200 ± 138,330 at 62.5 ng/ml MCP-1, 325,860 ± 74,887 versus 267,590 ± 43,929 at 125 ng/ml MCP-1, and 197,100 ± 57,302 versus 239,610 ± 33,218 at 250 ng/ml MCP-1 (n = 4 for each condition, no differences were statistically significant). D, invasion also was assessed in shRNA knockdown THP-1 clones. SCN8A knockdown clone 89 THP-1 cells (3,126 ± 3,793 RFU, n = 6) showed almost complete absence of invasion through reconstituted basement membrane in response to m-CSF (100 ng/ml) as compared with control clone 92 THP-1 (65,716 ± 5,774 RFU, n = 6) and wild-type cells (42,053 ± 1,761 RFU, n = 6) (p < 0.0001 for clone 89 as compared with either clone 92 or wild type).
shRNA Knockdown of SCN8A, the Gene That Encodes NaV1.6, Inhibits m-CSF-induced Podosome Formation—Formation of podosomes in response to m-CSF and the morphology of the F-actin cytoskeleton were assessed in cells transduced with shRNA that knockdown expression of SCN8A, the gene that encodes NaV1.6 (THP-clone 89), and those that do not (THP-clone 92) (Table 1). NaV1.6 knockdown was confirmed by Western blot (Fig. 10A). Knockdown of SCN8A reduced podosome formation, and those podosomes that did form were smaller (Fig. 10, B and C). In addition, the overall staining for F-actin was reduced severely in the knockdown cells. The average densitometric sum per cell was 76.33 ± 13.96 RFU for control THP clone 92 cells and 3.66 ± 1.93 RFU for the knockdown THP clone 89 cells (p = 0.0005) (Fig. 10C).
FIGURE 10.
shRNA knockdown of SCN8A, the gene that encodes NaV1.6, prevents m-CSF induced podosome formation. shRNA clones were screened for their ability to knockdown expression of SCN8A (Table 1). Two clones, THP clone 89 and THP clone 92, were selected for further functional analysis based on their ability to either knockdown expression of SCN8A mRNA by >85% (clone 89) or by lack of gene knockdown (clone 92, control) (Table 1). A, decreased expression of NaV1.6 in clone 89 cells was confirmed by Western blot analysis. B and C, following a brief stimulation with m-CSF (5 min), podosome induction was measured by phalloidin staining. The podosomes identified in the knockdown cells were smaller and the overall F-actin content was decreased. Scale bar, 10 μm. D, the average densitometric sum per cell was 76.33 ± 13.96 RFU for control THP clone 92 cells and 3.66 ± 1.93 RFU for the knockdown THP clone 89 cells (p = 0.0005; n = 10-14 microscopic fields per condition).
Cellular Invasion through Extracellular Matrix Is Blocked by Tetrodotoxin in THP-1 and Melanoma Cells—We further assessed the invasive potential of THP-1 cells differentiated to a macrophage phenotype and HTB-66 melanoma cells in the presence and absence of TTX (300 nm). In response to varying concentrations of m-CSF (25, 100, and 400 ng/ml), TTX significantly reduced invasion of differentiated THP-1 cells through a basement membrane matrix at all m-CSF concentrations examined (Fig. 11A). Invasion, normalized to nonspecific invasion in the absence of stimulus, was 31,779 ± 10,798 in the untreated group versus -5080 ± 6656 in the TTX group at 25 ng/ml m-CSF (n = 6; p = 0.019); 71,382 ± 12,209 versus 12,850 ± 12,406 at 100 ng/ml m-CSF (n = 6; p = 0.007); and 87,446 ± 15,216 versus 25,133 ± 17,752 (n = 6; p = 0.02).
In a similar fashion, TTX inhibited invasion of HTB-66 cells in response to 10% serum (Fig. 11B). In the untreated condition, invasion was 70,691 ± 14,700 RFU as compared with -10,244 ± 9302 (n = 5; p = 0.003).
In contrast, in a transwell assay, migration of THP-1 cells through a 5-μm pore in response to MCP-1 (monocyte chemotractant protein) (Fig. 11C) was not affected by TTX. These migration results suggest that TTX specifically inhibits actin-dependent cellular invasion as mediated by podosomes and invadopodia but not cortical actin-dependent amoeboid-type migration through a pore (26).
To confirm the TTX results, invasion assays with the shRNA-knockdown THP-1 clones were performed (Fig. 11D). In response to m-CSF (100 ng/ml), SCN8A knockdown clone 89 demonstrated near complete absence of invasion (3,126 ± 3,793 RFU, n = 6), whereas control clone 92 (65,716 ± 5,774 RFU, n = 6) and wild-type THP-1 cells (42,053 ± 1,761 RFU, n = 6) showed similar responses (p < 0.0001 for clone 89 as compared with either clone 92 or wild type).
DISCUSSION
We here demonstrate expression of a splice variant of SCN8A, the gene that encodes NaV1.6, in the human monocytic cell line THP-1. Prior studies of SCN8A variants demonstrated that fetal neurons and non-neuronal cells produce two variant transcripts, one containing the alternative exon 18N and one that skips exon 18 (19). Because 18N includes a stop codon, the prediction was that fetal neurons and non-neuronal cells would express a truncated, non-functional variant of NaV1.6. Our present data suggest that macrophages can express a full-length variant of NaV1.6 through deletion of exon 18N in SCN8A transcripts.
From a functional standpoint, our results also suggest that these NaV1.6 channels are required for macrophage podosome formation, melanoma invadopodia formation, and cellular invasion. Similar results were obtained with pharmacologic blockade with TTX, a naturally occurring mutant form of mouse SCN8A, which are NaV1.6 deficient (med mouse peritoneal macrophages), and shRNA gene knockdown. These results also suggest a potentially novel mechanism of intracellular signaling through the release of sodium from cationic intracellular stores, rapid uptake by anionic mitochondria, and subsequent calcium release from mitochondria. Prior studies in other cell types demonstrated a link between calcium signaling and elevated cytosolic sodium (2-5). However, in those experiments, sodium entered the cytosol from the extracellular space through plasma membrane channels. This study is the first to show intracellular sodium release coupled to calcium signaling and a specific physiologic effect, F-actin cytoskeletal remodeling.
We speculate that the interactions of vesicular NaV1.6, the mitochondrial Na/Ca exchanger, and plasma membrane calcium channels provide a molecular switch to permit rapid assembly and disassembly of podosomes and invadopodia. Elevation of cytosolic calcium would be most important for disassembly through activation of the calcium-dependent, actin severing protein gelsolin and potentially other calcium activated factors (10, 16). Subsequent assembly would then require buffering of cytosolic calcium, possibly through mitochondrial re-uptake to permit continuous cycling of the process. This intracellular signaling process mirrors that seen at the neuronal synapse, where tight coupling between sodium currents and calcium influx is responsible for vesicular transmitter release (27, 28). However, synaptic signaling is dependent primarily on entry of sodium and calcium ions from the extracellular space as opposed to macrophages where ionic fluxes may be primarily intracellular. This mechanism in macrophages may be crucial to their ability to function in a variety of extracellular environments to respond to injury and infection.
A prior study from this laboratory demonstrated that human macrophages also express the cardiac voltage-gated sodium channel NaV1.5 following priming of the cells with interferon-γ or lipopolysaccharide (6). Under the conditions examined in the present study (unprimed human cells or mouse macrophages), NaV1.5 is not expressed. When it is expressed, NaV1.5, which is TTX resistant, enhances lipopolysaccha-ride-mediated acidification of late endosomes through sodium efflux and proton influx into the endosome. NaV1.5 appears to be activated by lipopolysaccharide-mediated signaling. In contrast, NaV1.6 is inactivated by stimulation of the p38/MAPK (mitogen-activated protein kinase) signaling pathway when the channel is expressed in HEK-293 cells (29). These results suggest a reciprocal relationship between the two macrophage channels. In unprimed cells, where NaV1.5 is not expressed, activation of NaV1.6 by m-CSF or other podosome-inducing agents stimulates cellular invasion. Once the macrophage is at an inflammatory focus, NaV1.6 and additional cellular movement is turned off through the p38 or other signaling pathways, and NaV1.5 is synthesized and activated to enhance endosomal and phagosomal processing. However, it remains unclear how these intracellular sodium channels are gated, and future studies are required to characterize their biophysical properties.
In both the present and prior (6) study, we were able to inhibit intracellular voltage-gated sodium channel function with TTX, which is not membrane permeable. Using prolonged exposure (4 h) to 300 nm TTX in the present study, we observed an effect on podosome formation and cellular invasion. Because TTX does not readily cross membranes, it is likely that it was taken up by pinocytosis or by endocytosis/phagocytosis. Rapid distribution of TTX throughout the endosomal compartment is suggested by its ability to block NaV1.5 function when applied for 1 h in primed human cells during endocytosis or phagocytosis (6). In contrast, the present results suggest TTX does not appear to enter the membrane compartment where NaV1.6 resides unless there is more prolonged exposure, possibly because access to these compartments requires a combination of pinocytotic, endocytic, and phagocytic mechanisms. Even in that situation, it may only affect a subset of F-actin processes in the cell because the TTX may only reach NaV1.6 compartments that are important for podosome formation. This possibility is suggested by the greater overall effect on total cellular F-actin following shRNA knockdown (Fig. 10) as compared with TTX treatment (Fig. 4). It is possible that shRNA knockdown of NaV1.6 function can affect other F-actin-mediated processes in monocyte-macrophages such as cellular proliferation of monocyte precursors and phagocytosis in macrophages. Additional studies are required to address this issue. Irrespective of the full span of effects of TTX and shRNA knockdown of NaV1.6, however, it is notable that TTX and shRNA knockdown both have substantial inhibitory effects on podosome formation and cellular invasion.
Given the availability of orally active channel blockers, NaV1.6 and related channel proteins may provide unique targets for immune-mediated and neoplastic diseases. We propose that blockade of intracellular NaV1.6 channels to prevent macrophage podosome formation may be useful in autoimmune disorders such as multiple sclerosis where the amplification of inflammatory injury requires local invasion and peripheral recruitment of microglia, macrophages, and monocytes. Prior studies of sodium channel blockers such as phenytoin and carbamazepine in the rodent model of multiple sclerosis, experimental autoimmune encephalomyelitis, demonstrated efficacy in reduction of disease severity (30-32). Although these results were originally hypothesized to be due to a direct protective effect on neuronal axons, the present results combined with the rapid inflammatory rebound effect seen following withdrawal of these agents (8) suggest an anti-inflammatory effect in addition to a neuro-protective one (33). A similar mechanism of action of sodium channel blockade also may be relevant in tumor cell biology. In neoplastic cells that express intracellular sodium channels, particularly NaV1.6-positive melanomas, functional block of sodium channels might prevent metastasis through both a direct interaction with the tumor cell as well as effects on macrophages and endothelial cells (9, 34, 35). Additional studies are required to assess the therapeutic potential of sodium channel blockers in these disease states.
Acknowledgments
We thank the Yale University School of Medicine Electron Microscopy Facility in the Center for Cell and Molecular Imaging for the cryo-immuno-EM.
The nucleotide sequence(s) reported in this paper has been submitted to the Gen-Bank™/EBI Data Bank with accession number(s) FJ11941.
This work was supported, in whole or in part, by the National Institutes of Health. This work was also supported by the National Multiple Sclerosis Society, the Nancy Davis Foundation, and a Dana Foundation award in Clinical Hypotheses in Neuroimmunology (to M. D. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1 and S2.
Footnotes
The abbreviations used are: TTX, tetrodotoxin; m-CSF, macrophage colony-stimulating factor; MCP-1, monocyte chemotractant protein; NMDG, N-methyl-d-glucamine; SBFI, sodium binding benzofuran isopthalate; shRNA, small hairpin RNA; FBS, fetal bovine serum; PBS, phosphate-buffered saline; HBSS, Hanks' balanced salt solution; RFU, relative fluorescent unit.
References
- 1.Catterall, W., Goldin, A., and Waxman, S. (2005) Pharmacol. Rev. 57 397-409 [DOI] [PubMed] [Google Scholar]
- 2.Lowe, D., Richardson, B., Taylor, P., and Donatsch, P. (1976) Nature 260 337-338 [DOI] [PubMed] [Google Scholar]
- 3.Leblanc, N., and Hume, J. (1990) Science 248 372-376 [DOI] [PubMed] [Google Scholar]
- 4.Blumenstein, Y., Maximyuk, O., Lozovaya, N., Yatsenko, N., Kanevsky, N., Krishtal, O., and Dascal, N. (2004) J. Physiol. 556 121-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikolaeva, M., Mukherjee, B., and Stys, P. (2005) J. Neurosci. 25 9960-9967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrithers, M., Dib-Hajj, S., Carrithers, L., Tokmoulina, G., Pypaert, M., Jonas, E., and Waxman, S. (2007) J. Immunol. 178 7822-7832 [DOI] [PubMed] [Google Scholar]
- 7.Craner, M., Damarjian, T., Liu, S., Hains, B., Lo, A., Black, J., Newcombe, J., Cuzner, M., and Waxman, S. (2005) Glia 49 220-229 [DOI] [PubMed] [Google Scholar]
- 8.Black, J., Liu, S., Carrithers, M., Carrithers, L., and Waxman, S. (2007) Ann. Neurol. 62 21-33 [DOI] [PubMed] [Google Scholar]
- 9.Fraser, S., Salvador, V., Manning, E., Mizal, J., Altun, S., Raza, M., Berridge, R., and Djamgoz, M. (2003) J. Cell. Physiol. 195 479-487 [DOI] [PubMed] [Google Scholar]
- 10.Miyauchi, A., Hruska, K., Greenfield, E., Duncan, R., Alvarez, J., Barattolo, R., Colucci, S., Zambonin-Zallone, A., Teitelbaum, S., and Teti, A. (1990) J. Cell Biol. 111 2543-2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans, J., and Matsudaira, P. (2006) Eur. J. Cell Biol. 85 145-149 [DOI] [PubMed] [Google Scholar]
- 12.Calle, Y., Burns, S., Thrasher, A., and Jones, G. (2006) Eur. J. Cell Biol. 85 151-157 [DOI] [PubMed] [Google Scholar]
- 13.Tsuboi, S. (2007) J. Immunol. 178 2987-2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carman, C., Sage, P., Sciuto, T., de la Fuente, M., Geha, R., Ochs, H., Dvorak, H., Dvorak, A., and Springer, T. (2007) Immunity 26 784-797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans, J., Correia, I., Krasavina, O., Watson, N., and Matsudaira, P. (2003) J. Cell Biol. 161 697-705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chellaiah, M., Kizer, N., Silva, M., Alvarez, U., Kwiatkowski, D., and Hruska, K. (2000) J. Cell Biol. 148 665-678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarone, G., Cirillo, D., Giancotti, F., Comoglio, P., and Marchisio, P. (1985) Exp. Cell Res. 159 141-157 [DOI] [PubMed] [Google Scholar]
- 18.Chen, W. (1989) J. Exp. Zool. 251 167-185 [DOI] [PubMed] [Google Scholar]
- 19.Plummer, N. W., McBurney, M. W., and Meisler, M. H. (1997) J. Biol. Chem. 272 24008-24015 [DOI] [PubMed] [Google Scholar]
- 20.Oh, Y., and Waxman, S. G. (l998) NeuroReport 9 1261-1266 [Google Scholar]
- 21.McNiven, M., Baldassarre, M., and Buccione, R. (2004) Front. Biosci. 9 1944-1953 [DOI] [PubMed] [Google Scholar]
- 22.Bruzzaniti, A., Neff, L., Sanjay, A., Horne, W., De Camilli, P., and Baron, R. (2005) Mol. Biol. Cell 16 3301-3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavazzi, I., Nermut, M. V., and Marchisio, P. C. (1989) J. Cell Sci. 94 85-99 [DOI] [PubMed] [Google Scholar]
- 24.Ochoa, G. C., Slepnev, V. I., Neff, L., Ringstad, N., Takei, K., Daniell, L., Kim, W., Cao, H., McNiven, M., Baron, R., and De Camilli, P. (2000) J. Cell Biol. 150 377-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess, D. L., Kohrman, D. C., Gait, J., Plummer, N. W., Jones, J. M., Spear, B., and Meisler, M. H. (1995) Nat. Genet. 10 461-465 [DOI] [PubMed] [Google Scholar]
- 26.Wolf, K., Mazo, I., Leung, H., Engelke, K., vonAndrian, U. H., Deryugina, E. I., Strongin, A. Y., Brocker, E. B., and Friedl, P. (2003) J. Cell Biol. 160 267-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker, P., Hodgkin, A., and Ridgway, E. (1971) J. Physiol. 218 709-755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodgkin, A., and Katz, B. (1949) J. Physiol. 108 37-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittmack, E., Rush, A., Hudmon, A., Waxman, S., and Dib-Hajj, S. (2005) J. Neurosci. 25 6621-6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo, A., Saab, C., Black, J., and Waxman, S. (2003) J. Neurophysiol. 90 3566-3571 [DOI] [PubMed] [Google Scholar]
- 31.Bechtold, D., Kapoor, R., and Smith, K. (2004) Ann. Neurol. 55 607-616 [DOI] [PubMed] [Google Scholar]
- 32.Waxman, S. G. (2008) Nat. Clin. Neurol. 4 159-169 [DOI] [PubMed] [Google Scholar]
- 33.Smith, K. (2007) Brain Pathol. 17 230-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghersi, G., Zhao, Q., Salamone, M., Yeh, Y., Zucker, S., and Chen, W. (2006) Cancer Res. 66 4652-4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyckoff, J., Wang, Y., Lin, E., Li, J., Goswami, S., Stanley, E., Segall, J., Pollard, J., and Condeelis, J. (2007) Cancer Res. 67 2649-2656 [DOI] [PubMed] [Google Scholar]