Abstract
Adipocyte-secreted proteins play important roles in metabolic regulation through autocrine, paracrine, and endocrine mechanisms. Using transcriptional profiling, we identified coiled-coil domain containing 80 (Ccdc80; also known as DRO1 and URB) as a novel secreted protein highly expressed in white adipose tissue. In 3T3-L1 cells Ccdc80 is expressed and secreted in a biphasic manner with high levels in postconfluent preadipocytes and terminally differentiated adipocytes. To determine whether Ccdc80 regulates adipocyte differentiation, Ccdc80 expression was manipulated using both knockdown and overexpression approaches. Small hairpin RNA-mediated silencing of Ccdc80 in 3T3-L1 cells inhibits adipocyte differentiation. This phenotype was partially reversed by treating the knockdown cells with Ccdc80-containing conditioned medium from differentiated 3T3-L1 cells. Molecular studies indicate that Ccdc80 is required for the full inhibition of T-cell factor-mediated transcriptional activity, down-regulation of Wnt/β-catenin target genes during clonal expansion, and the subsequent induction of C/EBPα and peroxisome proliferator-activated receptor γ. Surprisingly, overexpression of Ccdc80 in 3T3-L1 cells also inhibits adipocyte differentiation without affecting the repression of the Wnt/β-catenin signaling pathway. Taken together, these data suggest that Ccdc80 plays dual roles in adipogenesis by mechanisms that involve at least in part down-regulation of Wnt/β-catenin signaling and induction of C/EBPα and peroxisome proliferator-activated receptor γ.
The metabolic abnormalities associated with obesity underlie the development of insulin resistance and type 2 diabetes (1, 2). The emergence of white adipose tissue as an endocrine organ has refined our understanding of the mechanisms by which adipocytes can affect whole-body energy homeostasis (3). Adipose cells not only serve as lipid storage sites but can also secrete various autocrine, paracrine, and/or endocrine mediators referred to as adipocyte-secreted proteins or adipokines. These secreted proteins can have profound effects on energy balance and insulin sensitivity and are affected by hormonal changes, nutritional status, inflammatory mediators, and/or pharmacological treatments (4, 5). Understanding the full set of secreted proteins present in adipocytes and their regulation and activities might help reveal novel molecular links between obesity and metabolic disorders and potential therapeutic opportunities.
Commitment of mesenchymal progenitor cells to the adipocyte lineage involves a complex transcriptional cascade that culminates in the induction of the basic leucine-region zipper proteins C/EBPα,2 C/EBPβ, and C/EBPδ, the Krüppel-like transcription factor KLF5, and the nuclear receptor PPARγ (6-8). In the mouse 3T3-L1 cell line (9, 10) initiation of this cascade requires the stimulation of growth-arrested fibroblast-like cells with adipogenic inducers forcing the cells to re-enter the cell cycle allowing transcription of early adipogenic genes (11, 12). This clonal expansion phase is required for subsequent expression of master regulators of adipogenesis, C/EBPα and PPARγ, and the acquisition of the adipocyte phenotype (11, 12).
Adipocyte differentiation also involves extracellular-mediated signaling events by secreted factors such as the Wnt proteins. The canonical Wnt/β-catenin signaling pathway is a crucial regulator of cellular function (13, 14). In the unstimulated state, β-catenin is bound to the cytoplasmic destruction complex where it is phosphorylated by casein kinase-1 and glycogen synthase kinase-3, thereby promoting its ubiquitination and subsequent degradation by the proteasome. Activation of the pathway requires the binding of secreted Wnt to the Frizzled/low density lipoprotein receptor-related protein (LRP) co-receptor complex and the recruitment of Axin to phosphorylated LRP. The dissociation of Axin from the destruction complex stabilizes β-catenin, allowing its shuttling to the nucleus. Once in the nucleus, β-catenin binds to transcription factors of the TCF/LEF (lymphoid enhancer binding factor) family and promotes transcription of target genes, such as Axin-2, Frizzled, and cyclin D1. Activation or repression of canonical β-catenin signaling is believed to represent an important molecular switch in cell fate determination of mesenchymal stem cells (8, 15). C/EBPα and PPARγ repression by Wnt signaling drives osteoblastogenesis, whereas down-regulation of β-catenin-mediated transcriptional activity promotes adipogenesis via the induction of C/EBPα and PPARγ (15-20).
In this study we identified coiled-coil domain containing 80 (Ccdc80; also known as DRO1 and URB) as a novel adipocyte-secreted protein. Ccdc80 was previously identified as a gene regulated by estrogen in rat uterus and mammary gland (21). Ccdc80 has also been shown to be up-regulated in brown adipose tissue of mildly obese bombesin receptor subtype-3 (BRS-3-/-) mice (22) and to be down-regulated in cancer cell lines (23, 24) and during the osteoblastic differentiation of bone marrow stromal cells (25). Ectopic expression of Ccdc80 inhibits growth properties of cancer cells in vitro, suggesting that Ccdc80 might be a candidate tumor suppressor gene (23). Here, we show that Ccdc80 is highly expressed in white adipose tissue and that its expression is regulated during adipocyte differentiation in vitro. Using knockdown and overexpression studies, we found that Ccdc80 plays dual roles in adipogenesis by modulating C/EBPα and PPARγ expression. Molecular studies further indicate that Ccdc80 is necessary for the full repression of β-catenin signaling during differentiation. Our results establish Ccdc80 as a novel modulator of adipogenesis.
EXPERIMENTAL PROCEDURES
Gene Expression Profiling and Quantitative Reverse Transcription-PCR—RNA from undifferentiated and differentiated 3T3-L1 adipocytes and tissues from normal, 8-12-week-old male C57BL/6J mice as well as tissues from 10-week-old male ob/ob and age-matched wild-type control mice were obtained as described (26). For thiazolidinedione (TZD) treatment, 10-week-old male ob/ob mice were gavaged once per day with 15 mg/kg rosiglitazone or vehicle for 21 days. Total RNA was extracted using Trizol (Invitrogen) and purified using the RNeasy kit (Qiagen). Gene expression profiling was performed using the Mouse Genome 430 2.0 array (Affymetrix) as previously described (27). Taqman real-time quantitative PCR was performed on a 7900HT fast real-time PCR system (Applied Biosystems) according to the manufacturer's instructions using 18S as an endogenous control. Pre-designed gene-specific primers and probes were obtained from Applied Biosystems.
Secretion Experiments—HEK293T cells were seeded at a density of 2 × 106 cells in 10-cm Petri dishes. Cells were transfected with pSmed2 or pSmed2-Ccdc80-FLAG using FuGENE 6 (Roche Applied Science). Two days after transfection, cells were placed in serum-free DMEM, and medium was collected 24 h later. Endogenous secretion of Ccdc80 was evaluated in 3T3-L1 cells during differentiation. 3T3-L1 cells were rinsed twice with PBS and incubated in serum-free DMEM for 24 h before medium was collected. Conditioned media were analyzed by 4-10% SDS-PAGE followed by silver staining or immunological detection with anti-FLAG M2 (293T) or anti-Ccdc80 (3T3-L1) antibodies.
Antibody Production—Two peptides with 100% sequence homology with mouse and human Ccdc80 were synthesized: KNRVWVISAPHASEGYYR (corresponding to amino acid 148-165 in both mouse and human sequences) and KIDHFQLDNEKPMR (corresponding to amino acid 672-685 and 671-684 for human and mouse sequences, respectively). Peptides were conjugated to keyhole limpet hemocyanin and injected in a set of two rabbits for 90 days before serum collection (Open Biosystems). A separate antibody was produced by injecting a peptide corresponding to amino acid 841-855 and 840-854 for human and mouse sequences, respectively. Antibodies from terminal bleed were then purified on an affinity column against the immunizing peptide (New England Peptide). Endogenous Ccdc80 in 3T3-L1-conditioned medium was analyzed using the affinity-purified antibody (amino acids 840-854), except for Fig. 2D.
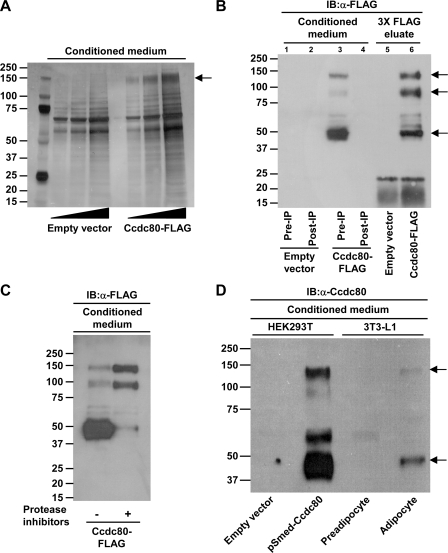
FIGURE 2.
Ccdc80 is a secreted protein. A, secretion of full-length Ccdc80 (∼140-kDa; denoted by an arrow). Conditioned medium from 293T cells transfected with a plasmid encoding Ccdc80-FLAG was analyzed by silver staining. Identity of Ccdc80 was confirmed by mass spectrometry analysis. B, secretion of full-length (∼140-kDa, upper arrow) and cleaved fragments (∼95 and ∼50 kDa, middle and lower arrow, respectively) of Ccdc80. Conditioned medium from 293T cells expressing a FLAG-tagged (C terminus) version of Ccdc80 (Ccdc80-FLAG) before (Pre-IP) and after (Post-IP) immunoprecipitation with an anti-FLAG M2 resin was analyzed by Western blotting (IB) using an anti-FLAG antibody. C, cleavage of Ccdc80 is partially prevented by the addition of protease inhibitors. 293T cells expressing Ccdc80-FLAG were incubated in the presence of a mixture of protease inhibitors for 48 h. Conditioned medium was analyzed by Western blotting using an anti-FLAG antibody. D, secretion of Ccdc80 by 3T3-L1 adipocytes. Conditioned medium from 293T cells expressing Ccdc80-FLAG or 3T3-L1 preadipocytes and adipocytes were analyzed by Western blotting using an antibody raised against amino acids 148-165 and 671-684 of Ccdc80. 3T3-L1 adipocytes secrete full-length (∼140 kDa) and a cleaved fragment (∼50 kDa) of Ccdc80 (indicated by arrows).
Immunofluorescence—3T3-L1 cells were grown on Permanox growth chamber slide (Nunc) until 2-day postconfluency or at the end of the differentiation protocol as described below. Cells were fixed in ice-cold methanol for 5 min, permeabilized in 0.1% PBS-buffered Triton X-100 for 15 min, and incubated in blocking buffer (PBS containing 0.1% Triton X-100 and 1% bovine serum albumin) for 30 min. Cells were then incubated overnight with anti-Ccdc80 (1:750 in blocking buffer) followed by Alexa fluor 594 goat anti-rabbit IgG (1:200 in blocking buffer) for 1 h. Slides were extensively washed with PBS containing Triton X-100 in between each step and rinsed with water before being mounted with Fluoro-gel II containing 4′,6-diamidino-2-phenylindole (EM Sciences). Slides were visualized with a Nikon Eclipse 80i microscope equipped with a digital imaging head using the appropriate filter.
Retroviral Vector and Infection—Retroviral vectors encoding non-silencing and mouse Ccdc80 shRNA were obtained from Open Biosystems. Hairpin sequences were as follows: non-silencing: ATCTCGCTTGGGCGAGAGTAAGTGCTGTTGACAGTGAGCGATCTCGCTTGGGCGAGAGTAAGTAGTGAAGCCACAGATGTACTTACTCTCGCCCAAGCGAGAGTGCCTACTGCCTCGGA and Ccdc80 (targeting position 2016-2037) and TGCTGTTGACAGTGAGCGCCCTGAGAAGGAGAAGAAGAAATAGTGAAGCCACAGATGTATTTCTTCTTCTCCTTCTCAGGTTGCCTACTGCCTCGGA. Viral packaging was achieved by transfecting 293-VSVG cells with plasmids using FuGENE 6. Viral supernatants supplemented with 10 μg/ml Polybrene were used to infect 3T3-L1 cells for 48 h followed by selection with 2 μg/ml puromycin. Additional cell lines expressing different shRNA constructs against Ccdc80 were created using pSIREN-RetroQ vectors (Clontech). Sequences for mouse Ccdc80 (targeting positions 229-247, 436-454, 2105-2123, and 2402-2420) and hairpin design were determined using the Clontech RNAi designer tool. Oligonucleotides were annealed and ligated into RNAi-ready pSIREN vector. Viral supernatants were obtained after transfection of RetroPack PT67 cells with pSIREN-RetroQ vectors and used to infect 3T3-L1 cells as described above.
Adenoviral Vector and Infection—Mouse Ccdc80 cDNA was generated by reverse transcription-PCR. Briefly, total RNA was isolated from mouse white adipose tissue using TRIZOL (Invitrogen). cDNAs were synthesized by reverse transcription using random decamers (Ambion). Full-length Ccdc80 was obtained by PCR and ligated into the SalI and XbaI sites of pSmed2. Ccdc80 cDNA was subcloned into pShuttle-CMV followed by linearization with PmeI and electroporation in Escherichia coli BJ5183 cells pre-transformed with the pAdEasy-1 plasmid. Recombinant adenovirus particles encoding mouse Ccdc80 or LacZ (control) were generated according to the manufacturer's instructions (Stratagene). Infection of 3T3-L1 with adenovirus was performed essentially as previously described (28). Briefly, cells were seeded at a density of 1.5 × 105 cells/well in 6-well plates and grown for 24 h. Adenovirus were incubated in serum-free DMEM containing 0.5 μg/ml poly-l-lysine (Sigma) for 100 min, and the mixture was layered onto PBS-washed cells for 90 min before the addition of DMEM containing 20% calf serum. Medium was removed 48 h later, and cells were differentiated as described below.
Adipocyte Differentiation—3T3-L1 cells were maintained in DMEM containing 20% calf serum in an atmosphere of 10% CO2 at 37 °C. Two days postconfluence (defined as Day 0), cells were induced to differentiate into adipocyte using DMEM containing 10% FBS supplemented with 500 μm 3-isobutyl-1-methylxanthine (IBMX), 1 μm dexamethasone, and 1.7 μm insulin for 4 days (Day 4) followed by DMEM containing 10% FBS and 0.85 μm insulin for 2 days then DMEM containing only 10% FBS for an additional 2-4 days (Day 8). Neutral lipid accumulation in formalin-fixed adipocytes was determined by oil red O staining.
Insulin Stimulation and Immunoblot Analysis—Differentiated 3T3-L1 adipocytes were deprived of serum for 2 h before stimulation with 10 nm insulin for 10 min. Cells were rinsed twice in ice-cold PBS and lysed as previously described (29). Equal amounts of proteins were separated on 4-12% SDS-PAGE and transferred to nitrocellulose membranes. Phosphorylation of Akt (Ser-473) and ERK1/2 (Thr-202/Tyr-204) was determined using phosphospecific antibodies (Cell Signaling Technologies).
Luciferase Reporter Assay—HepG2 cells were seeded at a density of 8 × 104 cells per well in 24-well plates and grown for 24 h in antibiotic-free DMEM containing 10% FBS. Canonical Wnt/β-catenin-mediated transcriptional activity was measured in cells transfected with 0.8 μg of TOPFLASH reporter plasmid that contains multiple TCF binding sites using Lipofectamine 2000. Transcriptional activity of nuclear receptors (ligand binding domain of human PPARα, PPARδ, PPARγ, or ERRα fused to GAL4 DNA binding domain) was measured in cells transfected with 0.8 μg of receptor plasmid and 0.8 μg of pFR-Luc that contains GAL4 upstream activating sequences. β-Galactosidase was used to normalize for transfection efficiency. 4 h after transfection, cells were rinsed with PBS and infected with adenovirus encoding either GFP or human Ccdc80 in Opti-MEM. Serum (final concentration, 10% FBS) was added to each well 2 h after infection, and cells were collected 24 h later. 3T3-L1 cells were seeded at a density of 2.5 × 104 cells per well in 24-well plates. Cells were transfected with 1 μg of TOPFLASH and 0.2 μg of β-galactosidase reporter plasmids using FuGENE 6 and grown in DMEM containing 20% calf serum until 2 days postconfluency. Cells were collected before and 24, 48, and 96 h after induction of differentiation with insulin, IBMX, and dexamethasone as described above. Luciferase and β-galactosidase activities were measured according to the manufacturer's instructions (Promega).
Statistical Analysis—Results are expressed as the mean ± S.E. Differences between groups were determined by using unpaired two-tailed Student's t-tests and were considered to be statistically significant at p < 0.05.
RESULTS
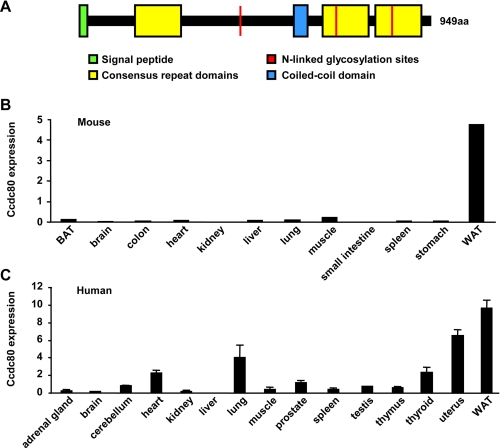
Identification of Ccdc80 as a Gene Encoding a Potential New Adipocyte-secreted Protein—In an attempt to identify new genes encoding adipocyte-secreted proteins, we analyzed changes in gene expression occurring in 3T3-L1 adipocytes and mouse white adipose tissue during metabolic paradigms. More specifically, we searched for genes encoding signal sequences that are regulated during 1) adipogenesis, 2) fasting, 3) obesity, and 4) insulin sensitization. This transcriptional profiling approach revealed Ccdc80 as a gene encoding a potential novel adipocyte-secreted protein. Mouse Ccdc80 (NM_026439) encodes a 949-amino acid protein of a predicted molecular mass of 108-kDa and is closely related to human Ccdc80 (NM_199511; 84% amino acid identity). The amino acid sequence of both human and mouse Ccdc80 show the presence of a putative cleavable signal peptide, three N-linked glycosylation sites, a coiled-coil domain, and three consensus repeat domains sharing homology (∼30%) with the fifth domain of sushi-repeat-containing protein, X-linked 1/2 (SRPX and SRPX2; schematically represented in Fig. 1A). Although Ccdc80 is highly conserved between species, no close homologs are found in either human, mouse, or rat genomic sequences (22, 23). Using real-time reverse transcription-PCR, we found that mouse Ccdc80 is highly expressed in white adipose tissue (WAT) with significantly lower mRNA levels in other tissues, including brown adipose tissue (BAT) (Fig. 1B). Similarly, human Ccdc80 mRNA was most abundant in white adipose tissue (Fig. 1C).
FIGURE 1.
Ccdc80 is highly expressed in white adipose tissue. A, schematic representation of mouse Ccdc80 protein. Ccdc80 expression in is shown in mouse tissues brown adipose tissue (BAT) and white adipose tissue (WAT) (B) and human tissues (C). Gene expression was measured by real-time PCR. n = 3-6 samples per tissue.
Ccdc80 Is a Secreted Protein—To confirm that Ccdc80 is a secreted protein, human Ccdc80 containing an in-frame C-terminal FLAG epitope was expressed in HEK293T cells. Analysis of conditioned medium revealed the presence of a prominent 140-kDa band readily detectable in medium from cells expressing Ccdc80 but not from those transfected with an empty vector (Fig. 2A). This band was excised from the gel, and mass spectrometry analysis confirmed that this protein was full-length Ccdc80 (63% amino acid coverage; data not shown). Examination of HEK293T supernatants by Western blotting using an anti-FLAG antibody showed that Ccdc80 is not only secreted in its full-length form (∼140 kDa) but also as cleaved fragments of ∼95 and ∼50 kDa (Fig. 2B). In our hands full-length (140-kDa) Ccdc80 transfers very poorly to both polyvinylidene fluoride and nitrocellulose blotting membranes, explaining the relatively lower abundance of full-length Ccdc80 compared with cleaved fragments during immunoblot analysis. As expected, full-length and cleaved fragments of Ccdc80 were depleted from the conditioned medium after immunoprecipitation with anti-FLAG resin (Fig. 2B, lane 3 versus lane 4) and recovered after elution with FLAG peptide (Fig. 2B, lane 3 versus lane 6). To determine whether processing of Ccdc80 involves an extracellular proteolytic event, HEK293T cells were incubated with a mixture of protease inhibitors. As shown in Fig. 2C, secretion of the 50-kDa fragment of Ccdc80 was almost totally abrogated by the addition of protease inhibitors with a concomitant increase in the presence of the full-length and 95-kDa cleaved form of Ccdc80, suggesting that high molecular mass forms (140- and 95-kDa) of Ccdc80 serve as substrates for a cell surface-anchored or extracellular protease.
To examine endogenous Ccdc80 secretion, we generated a polyclonal antibody using two peptides with 100% sequence homology between mouse and human Ccdc80 (see “Experimental Procedures”). Secretion of Ccdc80 was analyzed in conditioned medium obtained from 3T3-L1 preadipocytes and adipocytes and compared with that from HEK293T cells ectopically expressing human Ccdc80. Immunoblot analysis revealed two bands in conditioned medium from fully differentiated adipocytes, but not preconfluent preadipocytes, which corresponded in size to the full-length protein (140-kDa) and processed fragment (50-kDa) of Ccdc80 secreted from HEK293T cells (Fig. 2D). Both bands were significantly decreased in adipocytes in which Ccdc80 expression was reduced by RNA interference (see Fig. 4B and data not shown). These data demonstrate that differentiated adipocytes, but not dividing preadipocytes, secrete endogenously expressed Ccdc80 protein.
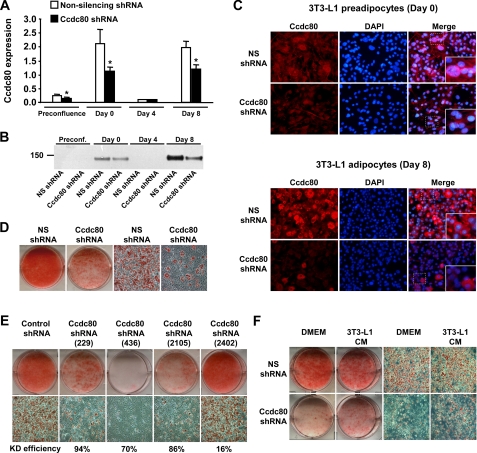
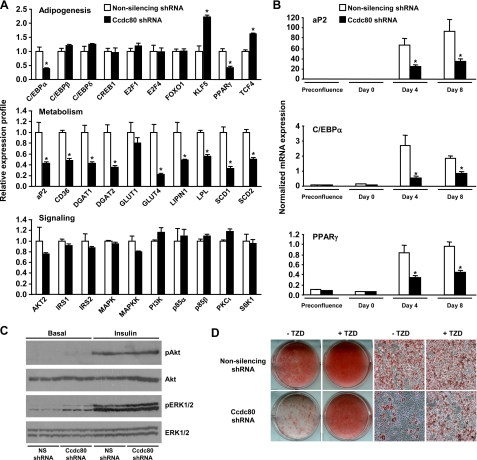
FIGURE 4.
Knockdown of Ccdc80 inhibits adipocyte differentiation. A-F, stable 3T3-L1 cell lines transduced with retrovirus encoding a non-silencing (NS) shRNA (white bars) or an shRNA against mouse Ccdc80 (black bars) were created. A, knockdown efficiency during adipocyte differentiation. Ccdc80 expression was determined by real-time PCR. *, p < 0.05 versus NS shRNA. B, secretion of full-length Ccdc80. Conditioned medium obtained at various time points during differentiation was analyzed by Western blotting using an antibody raised against amino acids 840-854 of Ccdc80. C, localization of Ccdc80 by immunofluorescence (200× magnification). Endogenous Ccdc80 was detected in postconfluent (Day 0) and differentiated (Day 8) 3T3-L1 cells using Alexa fluor 594 secondary antibody. 4′,6-diamidino-2-phenylindole (DAPI) was used to stain nucleus. D, lipid accumulation in 3T3-L1 cell lines expressing a NS shRNA or Ccdc80 shRNA. E, lipid accumulation in additional 3T3-L1 cell lines expressing a control shRNA or various shRNA against mouse Ccdc80 (targeting position 229-247, 436-454, 2105-2123, 2402-2420). Knockdown efficiency is shown below each micrograph. F, lipid accumulation in 3T3-L1 cell lines expressing a NS shRNA or Ccdc80 shRNA treated with DMEM or conditioned medium from terminally differentiated 3T3-L1 cells for 8 days. D-F, lipid accumulation was determined at the end of the adipocyte differentiation protocol by staining fixed cells with oil red O. Macroscopic and microscopic (100× magnification) images are shown. CM, conditioned medium.
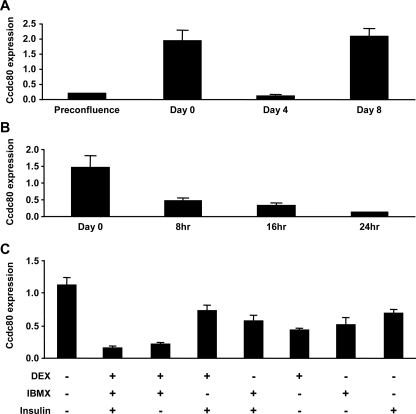
Ccdc80 Is Expressed in a Biphasic Manner during Adipogenesis—We next examined the temporal regulation of Ccdc80 gene expression during the differentiation of 3T3-L1 cells into adipocytes. Although classical adipokines such as leptin or adiponectin are up-regulated only during the terminal phase of adipocyte differentiation (30, 31), Ccdc80 was expressed in a biphasic manner (Fig. 3A). Ccdc80 mRNA was relatively low in proliferating 3T3-L1 preadipocytes but increased almost 10-fold when cells reached postconfluence before the initiation of differentiation (defined as Day 0, Fig. 3A). The addition of adipogenic inducers (dexamethasone, IBMX, and insulin) led to a dramatic reduction in Ccdc80 expression (Fig. 3A), an effect that was observed as early as 8 h after the addition of the mixture and was maximal after 24 h (Fig. 3B). Ccdc80 mRNA levels increased again during the late stage of adipocyte differentiation (Fig. 3A, Days 4-8).
FIGURE 3.
Ccdc80 is expressed in a biphasic manner in 3T3-L1 during differentiation. A, Ccdc80 expression during differentiation of 3T3-L1 cells into adipocytes. B, time-course of Ccdc80 repression during the early stage of differentiation. Ccdc80 expression was measured in postconfluent cells (Day 0) and upon induction of differentiation by the addition of adipogenic inducers (dexamethasone (DEX), IBMX, and insulin) for 8, 16, and 24 h. C, effect of adipogenic inducers on Ccdc80 expression. Postconfluent 3T3-L1 cells were left untreated or treated with one or more adipogenic inducers for 96 h. Ccdc80 gene expression was measured by real-time PCR. n = 3 per group.
To determine which component of the adipogenic mixture was able to induce down-regulation of Ccdc80, we assessed the individual and combined contribution of various adipogenic inducers during the early stages of differentiation (Day 0 to Day 4). Although each individual component of the mixture was able to significantly reduce Ccdc80 mRNA levels, a combination of both dexamethasone and IBMX was required to fully repress the expression of Ccdc80, and this was not further enhanced by the addition of insulin (Fig. 3C).
Silencing of Ccdc80 by RNAi Inhibits Adipocyte Differentiation—The biphasic expression of Ccdc80 during adipocyte differentiation raised the possibility that Ccdc80 regulates adipogenesis. To examine this hypothesis, we generated stable 3T3-L1 cell lines expressing either a control (non-silencing) or a Ccdc80 shRNA. Silencing of Ccdc80 by RNA interference reduced the levels of Ccdc80 mRNA by 40-50% (Fig. 4A). Analysis of Ccdc80 secretion revealed a similar pattern to that of Ccdc80 mRNA levels (see Figs. 3A and 4A), where Ccdc80 was mainly detected in conditioned medium from postconfluent preadipocytes (Day 0) and terminally differentiated adipocytes (Day 8) (Fig. 4B). As expected, secretion of full-length Ccdc80 by Ccdc80 KD cells was reduced when compared with control cells (Fig. 4B). Further experiments in intact cells showed that Ccdc80 was evenly distributed within the cytoplasm of both postconfluent preadipocytes (Day 0) and differentiated adipocytes (Day 8). Although Ccdc80 immunostaining was significantly reduced in Ccdc80 KD cells during both stages of adipocyte differentiation, the very few Ccdc80 KD cells that differentiate into adipocytes showed significant levels of Ccdc80 (Fig. 4C). Interestingly, we observed some overlapping between Ccdc80 and nuclear staining in some postconfluent preadipocytes (Day 0) but not in fully differentiated adipocytes (Fig. 4C).
The ability of Ccdc80 KD cells to differentiate into adipocytes was strongly inhibited as shown by reduced oil red O staining at the end of the differentiation protocol (Fig. 4D). To confirm this phenotype, four additional 3T3-L1 cell lines expressing different shRNA constructs directed against mouse Ccdc80 were created and induced to differentiate. Three of the four lines (229, 436, and 2105) showed significant reduction in Ccdc80 expression when compared with cells expressing a control non-targeting shRNA (Fig. 4E). Cells from the same three lines failed to accumulate lipids during differentiation, indicating defective adipogenesis (Fig. 4E). These data confirm that Ccdc80 is indeed required for adipocyte differentiation. We next determined whether the secreted form of Ccdc80 was sufficient to rescue the phenotype of Ccdc80 KD cells. Conditioned medium from fully differentiated 3T3-L1 cells previously shown to contain Ccdc80 (see Fig. 4B, Non-silencing shRNA at Day 8) was used to treat postconfluent control and Ccdc80 KD cells. As shown in Fig. 4F, the addition of Ccdc80-containing medium to Ccdc80 KD cells was able to partially restore the ability of knockdown cells to differentiate into adipocytes.
To explore the mechanisms by which Ccdc80 modulates adipocyte differentiation, we examined gene expression in control and Ccdc80 KD cells using transcriptional profiling (Fig. 5A) and Taqman real-time reverse transcription-PCR (Fig. 5B). Consistent with the decreased triglyceride accumulation in Ccdc80 KD cells (Fig. 4, D and E), silencing of Ccdc80 was accompanied by decreased expression of genes involved in fatty acid uptake (e.g. CD36 and lipoprotein lipase), triglyceride formation (e.g. diacylglycerol acyltransferase 1/2, lipin 1), and lipid metabolism (e.g. stearoyl-CoA desaturase 1/2 and aP2) on day 8 of differentiation (Fig. 5, A, middle panel, and B, upper panel). Expression of the insulin-sensitive glucose transporter GLUT4, a gene known to be up-regulated during adipocyte differentiation (32), was also decreased in Ccdc80 KD cells, whereas expression of the basal glucose transporter GLUT1 was unchanged after Ccdc80 gene silencing (Fig. 5A, middle panel). The decrease in metabolic genes in Ccdc80 KD cells can be explained by the decreased expression of C/EBPα and PPARγ, two master regulators of adipogenesis that are required for induction of metabolic marker genes (Fig. 5A, upper panel; Fig. 5B, middle and lower panel). Interestingly, expression of several transcription factors that function early in adipogenesis, such as C/EBPβ, C/EBPδ, and cAMP-responsive element-binding protein 1, was unchanged, and expression of KLF5, a positive regulator of PPARγ gene expression (33), was increased in Ccdc80 KD cells (Fig. 5A, upper panel). These data suggest that Ccdc80 acts downstream of C/EBPβ/δ and KLF5, but upstream of C/EBPα and PPARγ.
FIGURE 5.
Characterization of Ccdc80 KD cells. Stable 3T3-L1 cell lines transduced with retrovirus encoding a non-silencing (NS) shRNA (white bars) or an shRNA against mouse Ccdc80 (black bars) were created. A, expression profile of genes involved in adipogenesis, metabolism, and signaling. Samples were analyzed at the end of the differentiation protocol (Day 8) using a mouse genome microarray. *, p < 0.05 versus NS shRNA. B, induction of adipogenic markers during differentiation. Expression of aP2, C/EBPα, and PPARγ was determined by real-time PCR during differentiation. *, p < 0.05 versus NS shRNA. C, activation of Akt and ERK. Serum-deprived 3T3-L1 cells were left untreated or treated with insulin (10 nm) for 10 min. Cell lysates were analyzed by Western blotting. Phosphorylation of Akt at Ser-473 and ERK1/2 at Thr-202/Tyr-204 was determined using phosphospecific antibodies. Total levels of Akt and ERK1/2 are also shown. D, effect of TZD on adipogenesis. Postconfluent 3T3-L1 cells were differentiated with adipogenic inducers (dexamethasone, IBMX, and insulin) in the presence or absence of rosiglitazone (100 nm). Lipid accumulation was visualized by oil red O staining. Macroscopic and microscopic (100× magnification) images are shown.
We found no obvious differences in the expression of common mediators of the insulin signaling pathway (Fig. 5A, lower panel) and no defects in the activation of two mediators of the insulin signaling cascade, Akt and ERK, after insulin stimulation (Fig. 5C). Although Ccdc80 KD cells exhibited elevated basal phosphorylation of ERK1/2 at the end of the differentiation protocol (Fig. 5C), this increase was not seen in the earlier stages of adipocyte differentiation (data not shown). Treatment of cells during differentiation with the MEK (mitogen-activated protein/ERK kinase) inhibitor U0126, although abolishing ERK phosphorylation, did not increase adipogenesis (data not shown), demonstrating that altered ERK phosphorylation is unlikely to contribute to the lack of adipocyte differentiation in Ccdc80 KD cells. Phosphorylation of Akt after insulin stimulation was similar between control and Ccdc80 KD cells (Fig. 5C).
Given that TZDs are potent inducers of adipogenesis (34), we determined their effect in control and Ccdc80 KD cells. The addition of rosiglitazone (100 nm) to the adipogenic mixture increased differentiation in both control and Ccdc80 KD cells but did not restore differentiation of Ccdc80 KD cells to the same level as control cells (Fig. 5D).
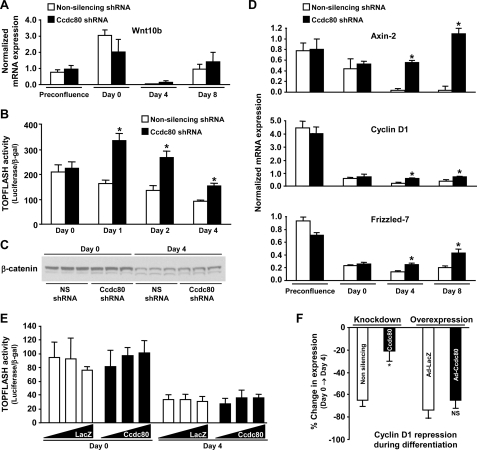
Silencing of Ccdc80 by RNAi Impairs Repression of TCF-mediated Transcriptional Activity and Expression of Wnt/β-Catenin Target Genes during Adipocyte Differentiation—Increased expression of C/EBPα and PPARγ during adipogenesis requires down-regulation of Wnt/β-catenin signaling (6, 8). In our initial experiments we noted that expression of the transcription factor TCF4, an important mediator of Wnt/β-catenin signaling in adipocytes (20), was elevated in Ccdc80 KD cells (Fig. 5A, upper panel). Furthermore, Wnt10b, a secreted protein that has been shown to negatively affect adipocyte differentiation (20, 35), is expressed in a biphasic manner similar to Ccdc80 during adipogenesis (compare Figs. 3A and Fig. 6A). To determine whether modulation of canonical Wnt signaling might be involved in the phenotype of Ccdc80 KD cells, we measured TOPFLASH activity as an index of β-catenin-mediated transcriptional activity via TCF. Upon reaching postconfluence (Day 0), cells expressing a non-silencing or Ccdc80 shRNA displayed similar TOPFLASH activity (Fig. 6B). After induction of differentiation, TOPFLASH activity in control cells declined slowly, reaching ∼ 50% of its initial activity after 4 days (Fig. 6B). In contrast, Ccdc80 KD cells showed a significant, ∼2-fold increase in TOPFLASH activity compared with control cells as early as 1 day after induction of differentiation and maintained this difference throughout the 4-day period examined (Fig. 6B). In agreement with these observations, we found that several target genes of the Wnt/β-catenin signaling pathway (Axin-2, cyclin D1, Frizzled-7) were not significantly decreased after induction of differentiation (i.e. Day 4 and Day 8) in Ccdc80 KD cells (Fig. 6D), suggesting a failure to down-regulate Wnt/β-catenin signaling in these cells upon induction of differentiation. The observed changes in TOP-FLASH activity and Wnt/β-catenin target genes expression occurred in the absence of changes in mRNA levels of Wnt10b (Fig. 6A) and in the presence of normal down-regulation of β-catenin protein levels (Fig. 6C), suggesting that Ccdc80 modulates the activity of the TCF transcriptional complex in a manner independent of Wnt10b and β-catenin.
FIGURE 6.
Silencing of Ccdc80 by RNAi impairs repression of TCF-mediated transcription and Wnt/β-catenin target genes expression during adipocyte differentiation. A-D, stable 3T3-L1 cell lines transduced with retrovirus encoding a non-silencing (NS) shRNA (white bars) or an shRNA against mouse Ccdc80 (black bars) were created. Expression of Wnt 10b (A) and Wnt/β-catenin targets (D) (Axin-2, cyclin D1 and Frizzled-7). Gene expression was determined by real-time PCR using a low density array. *, p < 0.05 versus NS shRNA. TOPFLASH activity (B) and β-catenin protein expression (C) were determined in postconfluent cells (Day 0) and after the addition of adipogenic inducers (dexamethasone, IBMX, and insulin) for 1, 2, and 4 days. *, p < 0.05 versus NS shRNA. E, 3T3-L1 cells were infected with adenovirus encoding either LacZ (Ad-LacZ, white bars) or mouse Ccdc80 (Ad-Ccdc80, black bar) at a m.o.i. of 500, 1000, and 2000. TOPFLASH activity was determined in postconfluent cells (Day 0) and after the addition of adipogenic inducers (dexamethasone, IBMX, and insulin) for 4 days. F, cyclin D1 repression during clonal expansion. Cyclin D1 expression was determined by real-time PCR in 3T3-L1 stably transduced with retrovirus encoding a NS shRNA (white bars) or an shRNA against mouse Ccdc80 (black bars) (knockdown; left portion of the graph) or in 3T3-L1 infected with adenovirus encoding either LacZ (Ad-LacZ, white bars) or mouse Ccdc80 (Ad-Ccdc80, black bar) at a m.o.i. of 2000 (overexpression; right portion of the graph). Data are presented as % change in cyclin D1 expression during differentiation (Day 0 to Day 4).
We next tested the hypothesis that increased expression of Ccdc80 in 3T3-L1 would potentiate repression of TCF-mediated transcription during adipogenesis. Overexpression of Ccdc80 in 3T3-L1 cells had no effect on TOPFLASH activity in unstimulated postconfluent cells (Day 0) when compared with cells expressing the control gene LacZ (Fig. 6E). As expected, LacZ-expressing 3T3-L1 cells showed repressed TOPFLASH activity upon induction of differentiation (Day 4). Overexpression of Ccdc80 did not further enhance this repression (Fig. 6E). Similarly, cyclin D1 levels were decreased to a similar extent in LacZ- and Ccdc80-overexpressing cells upon induction of differentiation (Fig. 6F). As expected, both the inhibition of TOPFLASH activity (Fig. 6B) and the repression of cyclin D1 expression (Fig. 6F) were attenuated in Ccdc80 KD cells during differentiation. These data suggest that endogenous Ccdc80 is both necessary and sufficient for repression of Wnt/β-catenin signaling during adipocyte differentiation.
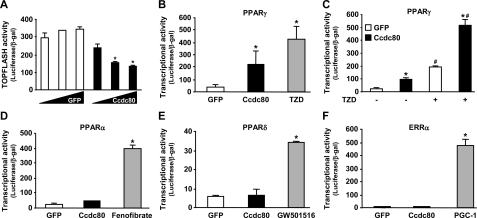
Ccdc80 Regulates TCF- and PPARγ-mediated Transcriptional Activity in HepG2 Cells—To investigate whether Ccdc80 can repress TCF-mediated transcription in a cell type that does not express appreciable amounts of endogenous Ccdc80, we overexpressed Ccdc80 in HepG2 cells. These cells express a stabilized and constitutively active form of β-catenin (36), eliminating potential effects of upstream modulators of the canonical Wnt/β-catenin signaling pathway. Adenovirus-mediated overexpression of Ccdc80 in HepG2 cells resulted in a dose-dependent inhibition of TOPFLASH reporter activity (Fig. 7A) without affecting β-catenin protein expression (data not shown), suggesting that Ccdc80 can modulate the transcriptional activity of the TCF transcriptional complex independently from changes in differentiation status. Because oncogenic activation of β-catenin can impair the expression of PPARγ target genes (18, 37), we tested the hypothesis that inhibition of Wnt signaling by Ccdc80 (Fig. 7A) would increase PPARγ transcriptional activity using a luciferase reporter assay. Indeed, in HepG2 cells overexpressing Ccdc80, PPARγ activity was increased by ∼5-fold (Fig. 7B). The addition of the TZD rosiglitazone, a potent PPARγ agonist, increased PPARγ activity by ∼10-fold in GFP-expressing cells (Fig. 7B) and potentiated the Ccdc80-mediated increase in PPARγ activity by 2-3-fold (Fig. 7C). Importantly, no changes in PPARα (Fig. 7D), PPARδ (Fig. 7E), and ERRα (Fig. 7F) activities were detected after the overexpression of Ccdc80 in HepG2 cells. This lack of effect of Ccdc80 was not because of a failure in the ability of these nuclear receptors to be activated, as each of them was induced by its specific ligand (fenofibrate for PPARα, GW501516 for PPARδ) or co-activator (PPARγ coactivator-1 (PGC-1) for ERRα) (Fig. 7, D-F).
FIGURE 7.
Ccdc80 regulates TCF- and PPARγ-mediated transcriptional activity. HepG2 cells were infected with adenovirus encoding either GFP (Ad-GFP, white bars) or human Ccdc80 (Ad-Ccdc80, black bar) at m.o.i. of 100, 250, and 500 were transfected with TOPFLASH reporter plasmid (A), PPARγ/pFR-Luc (B and C), PPARα/pFR-Luc (D), PPARδ/pFR-Luc (E), and ERRα/pFR-Luc (F) as described under “Experimental Procedures.” Luciferase and β-galactosidase activities were measured 24 h later. *, p < 0.05 versus Ad-GFP; #, p < 0.05 versus TZD-untreated cells. Positive controls for PPARγ (B and C), PPARα (D), PPARδ (E), and ERRα (F) transcriptional activities were TZD rosiglitazone (100 nm), fenofibrate (10 μm), GW501516 (100 nm), and ectopic expression of PPARγ coactivator-1 (PGC-1), respectively.
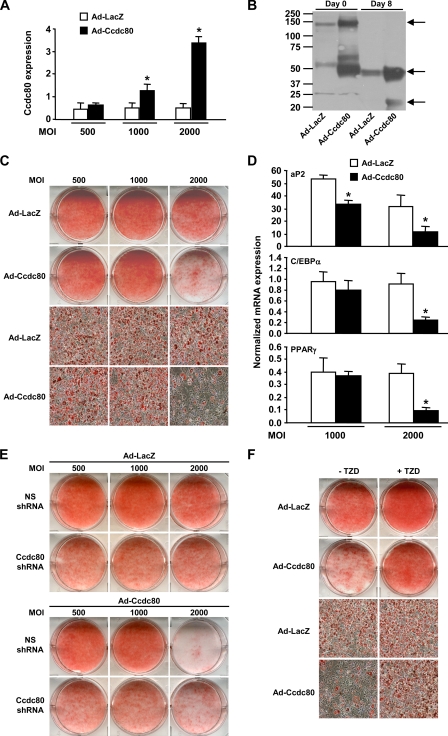
Adenovirus-mediated Overexpression of Ccdc80 Inhibits Adipogenesis—Although increasing Ccdc80 expression in 3T3-L1 cells had no effect on canonical Wnt signaling during adipogenesis (Fig. 6, E-F), it is possible that Ccdc80 controls additional functions that are important for adipocyte differentiation and might be revealed during overexpression experiments. To test this hypothesis, Ccdc80 levels were increased by infecting 3T3-L1 preadipocytes with adenovirus encoding mouse Ccdc80. We obtained cells that showed no overexpression (a multiplicity of infection (m.o.i.) 500), 2-fold (m.o.i. 1000) or 5-fold (m.o.i. 2000) overexpression of Ccdc80 mRNA (Fig. 8A). When cells overexpressing Ccdc80 5-fold above that of LacZ-infected cells were induced to differentiate, significantly higher amounts of Ccdc80 protein were detected in conditioned medium from both postconfluent (Day 0) and terminally differentiated cells (Day 8) (Fig. 8B). These cells showed impaired adipogenesis as reflected by decreased oil red O staining (Fig. 8C) and decreased expression of the adipocyte markers aP2, C/EBPα, and PPARγ (Fig. 8D). Cells overexpressing more modest amounts of Ccdc80 (m.o.i. 1000) did not show an overt decrease in oil red O staining, C/EBPα and PPARγ expression but showed a significant decrease in aP2 gene expression (Fig. 8D), indicating a more subtle differentiation defect. We next determined the effect of Ccdc80 overexpression in control and Ccdc80 KD cells. The defective lipid accumulation observed in Ccdc80 KD cells (see Fig. 4D) was still evident after LacZ overexpression (Fig. 8E, upper part) and was not prevented by Ccdc80 overexpression (Fig. 8E, lower part). Overexpression of Ccdc80 (m.o.i. 2000) in cells expressing a non-silencing shRNA impaired lipid accumulation as was previously observed in the parental 3T3-L1 cell line (compare Fig. 8, C and E). Although adenovirus-mediated overexpression of Ccdc80 had no significant effect on lipid accumulation in Ccdc80 KD cells (Fig. 8E, compare Ccdc80 shRNA-expressing cells infected with ad-LacZ versus ad-Ccdc80), the knockdown cells were largely protected against the deleterious effect of massive Ccdc80 overexpression (m.o.i. 2000) on adipogenesis (Fig. 8E, compare NS shRNA versus Ccdc80 shRNA-expressing cells infected with Ad-Ccdc80). Similar to Ccdc80 KD cells (Fig. 5D), treatment with TZD mostly, but not completely, reversed the impaired adipogenesis phenotype associated with Ccdc80 overexpression (Fig. 8F).
FIGURE 8.
Overexpression of Ccdc80 impairs adipogenesis. 3T3-L1 cells were infected with adenovirus encoding either LacZ (Ad-LacZ, white bars) or mouse Ccdc80 (Ad-Ccdc80, black bar) at various multiplicity of infection (m.o.i.). A, expression of Ccdc80 was determined by real-time PCR. B, secretion of Ccdc80. Conditioned medium from postconfluent (Day 0) and fully differentiated (Day 8) 3T3-L1 infected with adenovirus at a m.o.i. of 2000 was analyzed by Western blotting using an antibody raised against amino acids 840-854 of Ccdc80. Arrows indicate the full-length (∼140 kDa) and cleaved fragments (∼50 and ∼22 kDa) of Ccdc80 in conditioned medium from postconfluent (Day 0) and fully differentiated (Day 8) adipocytes. C, lipid accumulation. D, expression of adipogenic markers. Expression of aP2, C/EBPα, and PPARγ was determined by real-time PCR. E, lipid accumulation in 3T3-L1 cell lines expressing a NS shRNA or Ccdc80 shRNA infected with adenovirus encoding either LacZ or Ccdc80. F, effect of TZD on adipogenesis. Postconfluent (Day 0) 3T3-L1 cells infected with adenovirus at an m.o.i. of 2000 were differentiated with adipogenic inducers (dexamethasone, IBMX, and insulin) in the presence or absence of rosiglitazone (100 nm). C, E, and F, lipid accumulation was visualized by oil red O staining. Macroscopic and microscopic (100× magnification) images are shown. *, p < 0.05 versus Ad-LacZ.
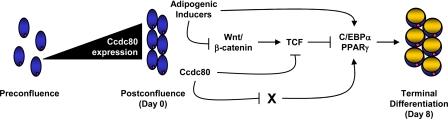
Bidirectional Modulation of Adipogenesis by Ccdc80—Taken together, our data suggest a model (schematically illustrated in Fig. 9) in which Ccdc80 positively modulates adipocyte differentiation by repressing the activity of the TCF transcriptional complex after induction of adipocyte differentiation. Ccdc80 also appears to play a negative role in adipogenesis by a mechanism that does not involve Wnt signaling repression. Achieving a balanced and timely expression of Ccdc80 throughout differentiation is essential for the induction and activation of C/EBPα and PPARγ and the acquisition of the adipocyte phenotype in 3T3-L1 cells.
FIGURE 9.
Proposed mechanism by which Ccdc80 bidirectionally regulates adipogenesis. 3T3-L1 preadipocytes express high levels of Ccdc80 upon reaching postconfluence, which are required for the efficient repression of TCF-mediated transcriptional activity after the addition of adipogenic inducers. Ccdc80 also plays a negative role in adipocyte differentiation by a mechanism distinct from inhibition of canonical Wnt signaling. Balanced levels of Ccdc80 are required for the induction of C/EBPα and PPARγ and the subsequent terminal differentiation into mature adipocytes.
DISCUSSION
The identification and characterization of secreted proteins from adipocytes is a critical step in our understanding of the tight links uniting obesity and metabolic disorders such as type 2 diabetes and cardiovascular diseases (4, 5). Here we report the functional characterization of the adipocyte-secreted protein Ccdc80 (also known as DRO1 and URB). Our initial observation that Ccdc80 is highly expressed in mouse and human white adipose tissue and is regulated during adipogenesis prompted us to further investigate its function.
A key finding of our study is that Ccdc80 is required for adipocyte differentiation. Ccdc80 is regulated in a biphasic manner during adipocyte differentiation. This pattern of expression is distinct from classical secreted adipokines, such as adiponectin (30) or leptin (31), but reminiscent of that of Wnt10b, a known adipocyte-secreted protein with an important role in adipogenesis (35). Reducing the expression of Ccdc80 in 3T3-L1 cells by RNAi provoked a marked impairment in the ability of these cells to differentiate into adipocytes and accumulate lipids. This phenotype appeared to be caused by a blunted induction of C/EBPα and PPARγ during differentiation. Surprisingly, the expression of upstream activators of C/EBPα and PPARγ, such as C/EBPβ, C/EBPδ, and KLF5, were not reduced, suggesting that Ccdc80 acts downstream and/or independently from these factors. Our data clearly show that the repression of Wnt/β-catenin target genes early during induction of differentiation is defective in cells depleted for Ccdc80 and that this depletion not only fails to repress TCF-mediated transcription but in fact increases signaling through this pathway.
Importantly, our study provides evidence for a causal role of Ccdc80 in inhibiting Wnt/β-catenin signaling. The ectopic expression of Ccdc80 in HepG2 cells was shown to decrease TOPFLASH reporter activity in the absence of any hormonal stimulation or treatment with chemical inducers. This cellular model also revealed that inhibition of canonical Wnt signaling by Ccdc80 positively regulates the activation of PPARγ but not that of other nuclear receptors involved in metabolic regulation. Because the effects of Ccdc80 are observed in the absence of any changes in β-catenin protein levels (in 3T3-L1 cells) as well as in cells expressing a constitutively active form of β-catenin (HepG2 cells), it is unlikely that Ccdc80 modulates TCF-mediated transcription by affecting upstream components of the Wnt/β-catenin signaling cascade, such as Wnt proteins, their receptors, or components of the β-catenin destruction complex. Rather, we propose that Ccdc80 regulates TCF-mediated transcription using a signaling pathway that is distinct from the upstream elements of the Wnt/β-catenin pathway. Together, our data define Ccdc80 as a new element involved in the switch between β-catenin signaling and PPARγ, a transition required for adipocyte differentiation (6, 8). It remains to be determined whether Ccdc80 directly attenuates TCF-mediated transcription or if Ccdc80-dependent changes in adipogenesis through another pathway(s) indirectly affects the activation of canonical Wnt signaling.
Surprisingly, overexpression of Ccdc80 did not promote adipocyte differentiation but, rather, inhibited this process. It is important to note that inhibition of adipocyte differentiation upon overexpression and knockdown of the same gene has been previously observed for modulators of adipogenesis. For instance, both depletion and overexpression of the Notch signaling target Hes-1 (hairy and enhancer of split-1) (38), the nuclear receptor Rev-erbα (39), or xanthine oxidoreductase (40) cause impaired adipogenesis. The kinase ERK also appears to play a dual role during adipocyte differentiation. On the one hand, phosphorylation of C/EBPβ by ERK is required for the induction of C/EBPα and PPARγ and the subsequent differentiation of preadipocytes into mature adipocytes (41-43). On the other hand, treatment with exogenous Pref-1 (preadipocyte factor-1) or ablation of Dok1 (downstream of tyrosine kinase 1) were both shown to prevent normal adipogenesis via elevated activation of ERK (44, 45). It is possible that the continued and unregulated expression of Ccdc80 throughout adipocyte differentiation activates signaling pathways at times or stages where it needs to be shut down (or vice versa), leading to inhibition of adipocyte differentiation. In contrast to Ccdc80 KD cells, the mechanism by which overexpression of Ccdc80 impairs adipogenesis is still unclear but does not seem to involve modulation of canonical Wnt signaling.
An important question that remains is how Ccdc80 can have both positive and negative effects on adipogenesis. The first clue probably came with our initial observation that Ccdc80 is expressed in a biphasic manner during adipocyte differentiation. Although not without precedent, most genes involved in adipocyte differentiation are regulated in a monophasic manner. Notable exceptions include Rev-erbα and Rev-erbβ, two regulators of circadian rhythm that are expressed in a biphasic fashion during differentiation of 3T3-L1 cells into adipocytes (46). Interestingly, Rev-erbα has been recently shown to play a dual role in adipogenesis (39). An early increase in Rev-erbα protein levels is required for mitotic clonal expansion, whereas down-regulation of the protein is necessary to relieve PPARγ2 repression during later stages of differentiation (39). Similar to Rev-erbα, it is possible that Ccdc80, although required for adipogenesis, also needs to be down-regulated for differentiation to occur in 3T3-L1 cells. As a consequence, disruption of that equilibrium by either reduced Ccdc80 peak expression by RNAi or deregulated overexpression of Ccdc80 prevents normal adipocyte differentiation.
Despite the strong prediction that Ccdc80 contains an N-terminal signal peptide sequence, published experiments had yielded contradictory results (23, 25, 47) prompting us to reexamine whether Ccdc80 is indeed a secreted protein. We found that human Ccdc80 ectopically expressed in HEK293T and, more importantly, endogenous Ccdc80 in 3T3-L1 adipocytes are secreted proteins. A recent report demonstrating secretion of Ccdc80 from human adipocytes further supports our conclusions (47). Importantly, we show in this study that secretion of Ccdc80 is required at least in part for normal adipocyte differentiation. It is interesting to note that multiple forms of human Ccdc80 are present in conditioned medium, and at least one of these forms (∼50-kDa fragment) is generated by an extracellular proteolytic event. A similar 50-kDa fragment is present in the supernatant from differentiated mouse adipocytes, suggesting that processing of this protein is not an overexpression artifact or specific to human Ccdc80. We currently do not know whether the processed forms of Ccdc80 are biologically active. Functionally relevant processing events of secreted proteins have been described. For instance, the preadipocyte-secreted protein Pref-1 (preadipocyte factor-1) can be proteolytically processed and secreted as a 25- and 50-kDa form, of which only the latter inhibits adipocyte differentiation (48, 49).
Although we have clearly shown that Ccdc80 can be secreted from postconfluent preadipocytes and mature adipocytes and that secreted Ccdc80 present in adipocyte-conditioned medium can rescue at least part of the Ccdc80 KD phenotype, we cannot exclude the possibility that additional intracellular forms of Ccdc80 exist that may contribute to the modulation of adipocyte differentiation. It is well known that secreted proteins can also have intracellular isoforms, generated by alternative mRNA transcription initiation, splicing, and/or translation initiation and that these intracellular isoforms often have distinct functions (for example, insulin-like growth factor-binding protein-3 or osteopontin; Refs. 50-52). We currently do not know whether intracellular isoforms that do not reside in the secretory pathway exist for Ccdc80 and, if so, what their origin(s) and specific roles are within adipose cells.
Selective modulation of adipocyte differentiation has recently emerged as a strategy for the prevention of both obesity and insulin resistance. Wnt signaling pathways, in particular, have been implicated in the regulation of adiposity and insulin sensitivity in both mice and humans. Although inhibition of Wnt signaling by decreased levels or activity of Wnt10b promotes obesity in mice and has been associated with obesity in humans, activation of Wnt signaling through transgenic overexpression of Wnt10b prevents obesity and improves insulin sensitivity in mice (35, 53, 54). Modulation of Wnt signaling by the small molecule harmine has also been shown to affect adipocyte differentiation in vitro and insulin sensitivity in vivo (55). Our results suggest that Ccdc80 plays a critical role in mediating the transition between preadipocyte and adipocyte, in part by controlling the switch between Wnt/β-catenin signaling and the induction of master regulators of adipogenesis C/EBPα and PPARγ. Thus, strategies to modulate Ccdc80 expression and/or secretion might lead to new approaches to prevent and/or treat obesity and its associated co-morbidities.
Acknowledgments
We thank Joe Wooters for mass spectrometry analysis of conditioned medium, and Dongmei Li for help with Taqman analysis as well as Gustave Hebert and Thadeus J. Unger for advice on immunofluorescence microscopy.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: C/EBPα/β/δ, CCAAT/enhancer binding protein α/β/δ; KLF5, Krüppel-like factor 5; PPARα/δ/γ, peroxisome proliferator-activated receptor α/δ/γ; TCF, T-cell factor; DRO1, down-regulated by oncogenes 1; URB, up-regulated in bombesin receptor subtype-3 deficient mice; ERK1/2, extracellular signal-regulated kinase 1/2; aP2, adipocyte protein 2; ERRα, estrogen-related receptor α; IGFBP-3; Ccdc80, coiled-coil domain containing 80; TZD, thiazolidinedione; DMEM, Dulbecco's modified Eagle's medium; PBS, phosphate-buffered saline; FBS, fetal bovine saline; GFP, green fluorescent protein; shRNA, short hairpin RNA; m.o.i., multiplicity of infection.
References
- 1.Kahn, B. B., and Flier, J. S. (2000) J. Clin. Investig. 106 473-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn, S. E., Hull, R. L., and Utzschneider, K. M. (2006) Nature 444 840-846 [DOI] [PubMed] [Google Scholar]
- 3.Scherer, P. E. (2006) Diabetes 55 1537-1545 [DOI] [PubMed] [Google Scholar]
- 4.Nawrocki, A. R., and Scherer, P. E. (2005) Drug Discov. Today 10 1219-1230 [DOI] [PubMed] [Google Scholar]
- 5.Trujillo, M. E., and Scherer, P. E. (2006) Endocr. Rev. 27 762-778 [DOI] [PubMed] [Google Scholar]
- 6.Farmer, S. R. (2006) Cell Metab. 4 263-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen, E. D., and Spiegelman, B. M. (2006) Nature 444 847-853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen, E. D., and MacDougald, O. A. (2006) Nat. Rev. Mol. Cell Biol. 7 885-896 [DOI] [PubMed] [Google Scholar]
- 9.Green, H., and Kehinde, O. (1975) Cell 5 19-27 [DOI] [PubMed] [Google Scholar]
- 10.Green, H., and Kehinde, O. (1976) Cell 7 105-113 [DOI] [PubMed] [Google Scholar]
- 11.Cornelius, P., MacDougald, O. A., and Lane, M. D. (1994) Annu. Rev. Nutr. 14 99-129 [DOI] [PubMed] [Google Scholar]
- 12.MacDougald, O. A., and Lane, M. D. (1995) Annu. Rev. Biochem. 64 345-373 [DOI] [PubMed] [Google Scholar]
- 13.Clevers, H. (2006) Cell 127 469-480 [DOI] [PubMed] [Google Scholar]
- 14.Moon, R. T., Kohn, A. D., De Ferrari, G. V., and Kaykas, A. (2004) Nat. Rev. Genet. 5 691-701 [DOI] [PubMed] [Google Scholar]
- 15.Krishnan, V., Bryant, H. U., and MacDougald, O. A. (2006) J. Clin. Investig. 116 1202-1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett, C. N., Ross, S. E., Longo, K. A., Bajnok, L., Hemati, N., Johnson, K. W., Harrison, S. D., and MacDougald, O. A. (2002) J. Biol. Chem. 277 30998-31004 [DOI] [PubMed] [Google Scholar]
- 17.Kang, S., Bennett, C. N., Gerin, I., Rapp, L. A., Hankenson, K. D., and MacDougald, O. A. (2007) J. Biol. Chem. 282 14515-14524 [DOI] [PubMed] [Google Scholar]
- 18.Liu, J., and Farmer, S. R. (2004) J. Biol. Chem. 279 45020-45027 [DOI] [PubMed] [Google Scholar]
- 19.Moldes, M., Zuo, Y., Morrison, R. F., Silva, D., Park, B. H., Liu, J., and Farmer, S. R. (2003) Biochem. J. 376 607-613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross, S. E., Hemati, N., Longo, K. A., Bennett, C. N., Lucas, P. C., Erickson, R. L., and MacDougald, O. A. (2000) Science 289 950-953 [DOI] [PubMed] [Google Scholar]
- 21.Marcantonio, D., Chalifour, L. E., Alaoui-Jamali, M. A., Alpert, L., and Huynh, H. T. (2001) Endocrinology 142 2409-2418 [DOI] [PubMed] [Google Scholar]
- 22.Aoki, K., Sun, Y. J., Aoki, S., Wada, K., and Wada, E. (2002) Biochem. Biophys. Res. Commun. 290 1282-1288 [DOI] [PubMed] [Google Scholar]
- 23.Bommer, G. T., Jager, C., Durr, E. M., Baehs, S., Eichhorst, S. T., Brabletz, T., Hu, G., Frohlich, T., Arnold, G., Kress, D. C., Goke, B., Fearon, E. R., and Kolligs, F. T. (2005) J. Biol. Chem. 280 7962-7975 [DOI] [PubMed] [Google Scholar]
- 24.Visconti, R., Schepis, F., Iuliano, R., Pierantoni, G. M., Zhang, L., Carlomagno, F., Battaglia, C., Martelli, M. L., Trapasso, F., Santoro, M., and Fusco, A. (2003) Oncogene 22 1087-1097 [DOI] [PubMed] [Google Scholar]
- 25.Liu, Y., Monticone, M., Tonachini, L., Mastrogiacomo, M., Marigo, V., Cancedda, R., and Castagnola, P. (2004) Biochem. Biophys. Res. Commun. 322 497-507 [DOI] [PubMed] [Google Scholar]
- 26.Lake, A. C., Sun, Y., Li, J. L., Kim, J. E., Johnson, J. W., Li, D., Revett, T., Shih, H. H., Liu, W., Paulsen, J. E., and Gimeno, R. E. (2005) J. Lipid Res. 46 2477-2487 [DOI] [PubMed] [Google Scholar]
- 27.Berasi, S. P., Huard, C., Li, D., Shih, H. H., Sun, Y., Zhong, W., Paulsen, J. E., Brown, E. L., Gimeno, R. E., and Martinez, R. V. (2006) J. Biol. Chem. 281 27167-27177 [DOI] [PubMed] [Google Scholar]
- 28.Orlicky, D. J., and Schaack, J. (2001) J. Lipid Res. 42 460-466 [PubMed] [Google Scholar]
- 29.Tremblay, F., and Marette, A. (2001) J. Biol. Chem. 276 38052-38060 [DOI] [PubMed] [Google Scholar]
- 30.Hu, E., Liang, P., and Spiegelman, B. M. (1996) J. Biol. Chem. 271 10697-10703 [DOI] [PubMed] [Google Scholar]
- 31.MacDougald, O. A., Hwang, C. S., Fan, H., and Lane, M. D. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 9034-9037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaestner, K. H., Christy, R. J., McLenithan, J. C., Braiterman, L. T., Cornelius, P., Pekala, P. H., and Lane, M. D. (1989) Proc. Natl. Acad. Sci. U. S. A. 86 3150-3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oishi, Y., Manabe, I., Tobe, K., Tsushima, K., Shindo, T., Fujiu, K., Nishimura, G., Maemura, K., Yamauchi, T., Kubota, N., Suzuki, R., Kitamura, T., Akira, S., Kadowaki, T., and Nagai, R. (2005) Cell Metab. 1 27-39 [DOI] [PubMed] [Google Scholar]
- 34.Tontonoz, P., Hu, E., and Spiegelman, B. M. (1994) Cell 79 1147-1156 [DOI] [PubMed] [Google Scholar]
- 35.Longo, K. A., Wright, W. S., Kang, S., Gerin, I., Chiang, S. H., Lucas, P. C., Opp, M. R., and MacDougald, O. A. (2004) J. Biol. Chem. 279 35503-35509 [DOI] [PubMed] [Google Scholar]
- 36.de La Coste, A., Romagnolo, B., Billuart, P., Renard, C. A., Buendia, M. A., Soubrane, O., Fabre, M., Chelly, J., Beldjord, C., Kahn, A., and Perret, C. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 8847-8851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, J., Wang, H., Zuo, Y., and Farmer, S. R. (2006) Mol. Cell. Biol. 26 5827-5837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross, D. A., Rao, P. K., and Kadesch, T. (2004) Mol. Cell. Biol. 24 3505-3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, J., Yin, L., and Lazar, M. A. (2006) J. Biol. Chem. 281 33842-33848 [DOI] [PubMed] [Google Scholar]
- 40.Cheung, K. J., Tzameli, I., Pissios, P., Rovira, I., Gavrilova, O., Ohtsubo, T., Chen, Z., Finkel, T., Flier, J. S., and Friedman, J. M. (2007) Cell Metab. 5 115-128 [DOI] [PubMed] [Google Scholar]
- 41.Bost, F., Aouadi, M., Caron, L., Even, P., Belmonte, N., Prot, M., Dani, C., Hofman, P., Pages, G., Pouyssegur, J., Marchand-Brustel, Y., and Binetruy, B. (2005) Diabetes 54 402-411 [DOI] [PubMed] [Google Scholar]
- 42.Prusty, D., Park, B. H., Davis, K. E., and Farmer, S. R. (2002) J. Biol. Chem. 277 46226-46232 [DOI] [PubMed] [Google Scholar]
- 43.Tang, Q. Q., Gronborg, M., Huang, H., Kim, J. W., Otto, T. C., Pandey, A., and Lane, M. D. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 9766-9771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosooka, T., Noguchi, T., Kotani, K., Nakamura, T., Sakaue, H., Inoue, H., Ogawa, W., Tobimatsu, K., Takazawa, K., Sakai, M., Matsuki, Y., Hiramatsu, R., Yasuda, T., Lazar, M. A., Yamanashi, Y., and Kasuga, M. (2008) Nat. Med. 14 188-193 [DOI] [PubMed] [Google Scholar]
- 45.Kim, K. A., Kim, J. H., Wang, Y., and Sul, H. S. (2007) Mol. Cell. Biol. 27 2294-2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu, M., Sun, T., Bookout, A. L., Downes, M., Yu, R. T., Evans, R. M., and Mangelsdorf, D. J. (2005) Mol. Endocrinol. 19 2437-2450 [DOI] [PubMed] [Google Scholar]
- 47.Okada, T., Nishizawa, H., Kurata, A., Tamba, S., Sonoda, M., Yasui, A., Kuroda, Y., Hibuse, T., Maeda, N., Kihara, S., Hadama, T., Tobita, K., Akamatsu, S., Maeda, K., Shimomura, I., and Funahashi, T. (2008) Biochem. Biophys. Res. Commun. 367 370-376 [DOI] [PubMed] [Google Scholar]
- 48.Mei, B., Zhao, L., Chen, L., and Sul, H. S. (2002) Biochem. J. 364 137-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, Y., and Sul, H. S. (2006) Mol. Cell. Biol. 26 5421-5435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Firth, S. M., and Baxter, R. C. (2002) Endocr. Rev. 23 824-854 [DOI] [PubMed] [Google Scholar]
- 51.Lee, K. W., and Cohen, P. (2002) J. Endocrinol. 175 33-40 [DOI] [PubMed] [Google Scholar]
- 52.Shinohara, M. L., Kim, H. J., Kim, J. H., Garcia, V. A., and Cantor, H. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 7235-7239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christodoulides, C., Scarda, A., Granzotto, M., Milan, G., Dalla, N. E., Keogh, J., De Pergola, G., Stirling, H., Pannacciulli, N., Sethi, J. K., Federspil, G., Vidal-Puig, A., Farooqi, I. S., O'Rahilly, S., and Vettor, R. (2006) Diabetologia 49 678-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright, W. S., Longo, K. A., Dolinsky, V. W., Gerin, I., Kang, S., Bennett, C. N., Chiang, S. H., Prestwich, T. C., Gress, C., Burant, C. F., Susulic, V. S., and MacDougald, O. A. (2007) Diabetes 56 295-303 [DOI] [PubMed] [Google Scholar]
- 55.Waki, H., Park, K. W., Mitro, N., Pei, L., Damoiseaux, R., Wilpitz, D. C., Reue, K., Saez, E., and Tontonoz, P. (2007) Cell Metab. 5 357-370 [DOI] [PubMed] [Google Scholar]