Abstract
Background
Septic arthritis remains a challenging diagnosis in which the doctor often relies on laboratory tests.
Objective
To examine the diagnostic utility of three ancillary tests—namely, white blood cells (WBC), erythrocyte sedimentation rate (ESR) and the WBC in the joint fluid (jWBC)—using likelihood ratios (LRs) and receiver operating characteristic (ROC) curves.
Methods
This was a retrospective cohort study at the Jacobi Medical Center. Medical charts of patients who had undergone arthrocentesis were included. Patients who had “dry taps” were excluded from the study. Patients were considered to have septic arthritis if they had a positive arthrocentesis culture or operative findings. The primary outcomes of this study were the sensitivities, specificities, LR(+) and LR(−) values of the laboratory tests for septic arthritis. The performance characteristics of the laboratory tests were analysed using ROC curves.
Results
156 patients were enrolled, 16 (10%) had septic arthritis. The sensitivities for WBC, ESR and jWBC were 0.75, 0.75 and 0.50, and the specificities were 0.55, 0.11 and 0.88, respectively. The LR(+) values were 1.7, 0.84 and 4.0, and the LR(−) values were 0.46, 2.4 and 0.57, respectively. In ROC curve analysis, jWBC was the best test (area under the curve (AUC) 0.75, 95% confidence interval (CI) 0.58 to 0.92), followed by WBC (AUC 0.69, 95% CI 0.57 to 0.80) and ESR (AUC 0.55, 95% CI 0.37 to 0.74). A cut‐off of jWBC = 17 500 maximised sensitivity and specificity on the ROC curve.
Conclusions
jWBC was the best diagnostic test for septic arthritis, WBC and ESR were poor tests. However, no test was diagnostic, and the clinician must be careful with patients with a potential septic joint.
Septic arthritis is a disease associated with serious morbidity to the patient and a diagnostic challenge to the doctor. The causes of monoarticular arthritis are numerous, with septic arthritis being the most important entity. The diagnosis is rarely established by the history and physical examination, and the clinician is led to rely on ancillary tests, specifically the white blood cell (WBC) count from peripheral blood, the erythrocyte sedimentation rate (ESR) and the WBC count of the joint fluid (jWBC). Although septic arthritis is known to be associated with elevations in these ancillary tests, previous studies have found that they are insufficiently sensitive to rule out septic arthritis.1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29 Unfortunately, most of these studies are limited in their nature as case‐series studies,1,2,3,4,5,6,7,8,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28 and are unable to comment beyond sensitivity. In fact, there are few studies which have examined the diagnostic utility of these ancillary tests in septic arthritis using positive and negative likelihood ratios (LR(+) and LR(−)) or receiver operating characteristic (ROC) curves.29,30,31 The purpose of our study was to examine the diagnostic utility of these three tests in patients with septic arthritis, using LRs and ROC curves.
Methods
Study design, setting and population
This was a retrospective cohort study at the Jacobi Medical Center. We searched the hospital medical database for adult and paediatric patients who had undergone arthrocentesis from January 1998 to October 2004. The study was approved by the Committee on Clinical Investigations, Albert Einstein College of Medicine.
Study protocol and measurements
The medical charts were reviewed for demographic (age, sex), clinical (operative findings, diagnoses) and laboratory data (WBC, ESR, jWBC, culture results). Patients who had “dry taps” were excluded from the study. In our laboratory, WBC >11×103 cells/mm3 and ESR >20 mm/h were considered high. For the purposes of this study, jWBC >50 000 cells/mm3 was considered high. Patients were considered to have septic arthritis if they had a positive arthrocentesis culture or operative findings consistent with septic arthritis (ie, frank pus). Chart reviewers underwent a training session before data collection. In all, 40 (26%) charts were cross‐reviewed to determine inter‐rater reliability.
Data analysis
The primary outcomes of this study were the sensitivities, specificities, LR(+) and LR(−) values of the laboratory tests for septic arthritis. The performance characteristics of the laboratory tests were analysed using ROC curves. Inter‐rater reliability was determined using simple agreement. Data were analysed using Microsoft Excel X for Mac (Redmond, Washington, USA) and SPSS for Windows 11.5.0 (Chicago, Illinois, USA).
Results
The database identified 188 patients who had undergone arthrocentesis during the study period. We excluded 32 patients because they had dry taps, leaving 156 patients in the final analysis. The mean age was 53 (range 1–97) years, with 13% paediatric patients and 56% males. In all 16 (10%) patients had septic arthritis confirmed by arthrocentesis culture or operative findings. The remaining patients had a myriad of diagnoses, the most common being gout (33%), osteoarthritis (9%), traumatic effusion (6%) and pseudogout (5%). No specific diagnosis was given to 36 (23%) patients. Inter‐rater reliability was excellent (simple agreement 96%, 95% confidence interval (CI) 94% to 98%).
All patients had data on WBC count, 107 patients had data on ESR and 127 patients on jWBC. The mean (standard deviation (SD)) of the WBC count was 12 (7.2)×103 cells/mm3; ESR was 72 (39) mm/h and jWBC was 26 000 (38 000) cells/mm3. In all, 75 (48%) patients had a raised WBC count, 94 (60%) had a raised ESR, 20 (13%) had a raised jWBC count, and 14 (9%) patients had a positive joint fluid culture.
Table 1 shows the sensitivities, specificities and LRs of elevations in WBC, ESR and jWBC levels for patients with septic arthritis. The sensitivities of WBC, ESR and jWBC were 0.75, 0.75 and 0.50; the specificities were 0.55, 0.11 and 0.88; the LR(+) values were 1.7, 0.84 and 4.0, and the LR(−) values were 0.46, 2.4 and 0.57, respectively. All patients with septic arthritis had at least one abnormality in WBC, ESR or jWBC; thus the combined sensitivity of all three tests was 100%.
Table 1 Diagnostic utility of laboratory tests for septic arthritis.
| n | Sensitivity | Specificity | LR(+) | LR(−) | |
|---|---|---|---|---|---|
| WBC | 156 | 0.75 (0.48 to 0.93)* | 0.55 (0.46 to 0.63) | 1.7 (1.2 to 2.3) | 0.46 (0.19 to 1.1) |
| ESR | 107 | 0.75 (0.43 to 0.95) | 0.11 (0.05 to 0.19) | 0.84 (0.60 to 1.2) | 2.4 (0.76 to 7.4) |
| jWBC† | 127 | 0.50 (0.21 to 0.79) | 0.88 (0.80 to 0.93) | 4.0 (1.9 to 8.6) | 0.57 (0.32 to 1.0) |
| All tests‡ | 156 | 1.0 (0.79 to 1.0) | 0.24 (0.17 to 0.31) | 1.3 (1.2 to 1.4) | 0 (0 to 13) |
| jWBC§ | 127 | 0.83 (0.52 to 0.98) | 0.67 (0.57 to 0.75) | 2.5 (1.8 to 3.6) | .25 (0.07 to 0.89) |
ESR, erythrocyte sedimentation rate; jWBC, white blood cells in the joint; LR, likelihood ratio; WBC, white blood cells.
*95% confidence interval.
†using jWBC ⩾50 000.
‡if any of the three test counts are raised.
§using jWBC ⩾17 500; cut‐off which maximised sensitivity and specificity, retrospective analysis.
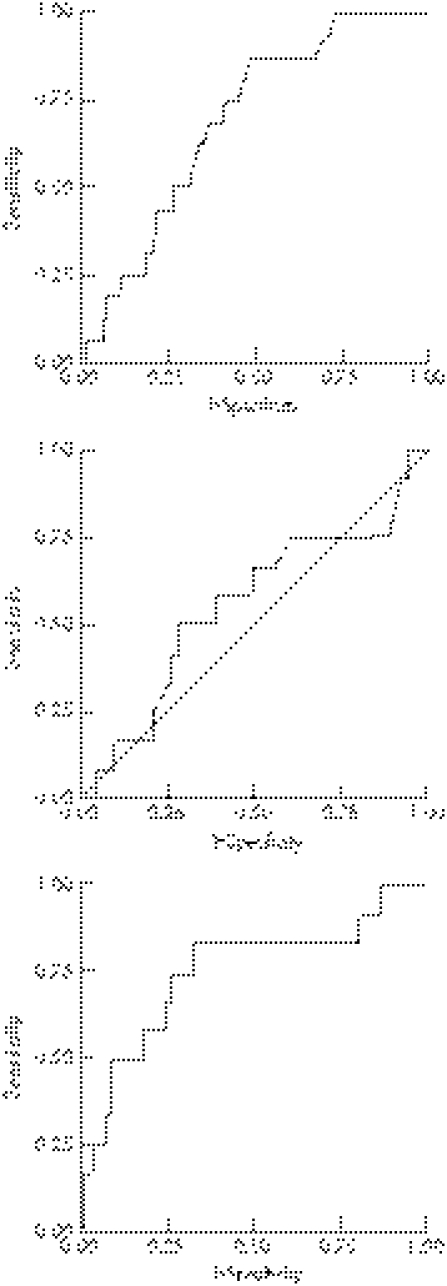
Figure 1 shows the performance characteristics of WBC, ESR and jWBC, as summarised by ROC curves. The area under the curve (AUC) values for WBC, ESR and jWBC were 0.69 (95% CI 0.57 to 0.80), 0.55 (95% CI 0.37 to 0.74) and 0.75 (95% CI 0.58 to 0.92), respectively. For jWBC, the diagnostic cut‐off that maximised the combination of sensitivity and specificity was a jWBC count of 17 500 cells/mm3, with a sensitivity of 83% (95% CI 0.52 to 0.98), a specificity of 67% (95% CI 0.57 to 0.75), an LR(+) value of 2.5 (95% CI 1.8 to 3.6) and an LR(−) value of 0.25 (95%CI 0.07 to 0.89).
Figure 1 Receiver operating characteristic curves for white blood cells, erythrocyte sedimentation rate and WBC in the joint fluid for septic arthritis.
Discussion
The WBC, ESR and jWBC values showed considerable variation in diagnostic utility for septic arthritis. The sensitivities of the three ancillary tests were fair at best, with a range from 0.50 to 0.75. The specificities were variable: the specificity of ESR was extremely poor at 0.11, whereas that of jWBC was rather good at 0.88. The LR(+) values of WBC and ESR were poor (1.7 and 0.84, respectively), but the LR(+) value of jWBC approached respectability at 4.0, with a 95% CI upper limit of 8.3. The LR(−) values of all three tests were poor, with a range from 0.46 to 2.4. If we were to use a reference standard of 10 as a “good” LR(+) and 0.10 as a “good” LR(−), then none of the three tests had “good” LR(+) or LR(−) values in this study, inclusive of the 95% CIs.
The combined sensitivity of all three tests was 100%, but the combined specificity was low (0.24), and coupled with the sample size of this study, the combined LR(−) value was insufficiently powerful to effectively rule out a septic joint, as indicated by the upper limit of the 95% CI, which was greater than 1.0 (95% CI 0 to 13).
The best diagnostic test, based on the results of ROC curves, was the jWBC, followed by WBC and ESR. The AUC for the jWBC ROC curve was 0.75, indicating a fair to good diagnostic test. The upper limit of the AUC was 0.92, indicating that it may be an excellent test. ESR was a poor diagnostic test with an AUC of 0.55, and WBC was a fair diagnostic test with an AUC of 0.69.
The upper limit cut‐off for jWBC count in the diagnosis of septic arthritis has never been clearly established. Medical folklore and emergency medicine texts have suggested cut‐offs of 2000, 10 000 or 50 000.32,33,34 In our ROC analysis, the jWBC curve makes a turn at jWBC counts of 15 000–20 000 cells/mm3, with 83% sensitivity and 60–67% specificity. The diagnostic cut‐off that maximised the combination of sensitivity and specificity was a jWBC count of 17 500 cells/mm3, with a sensitivity of 83% and a specificity of 67%. In comparison with a jWBC cut‐off of 50 000 cells/mm3, a jWBC cut‐off of 17 500 cells/mm3 improves on sensitivity by sacrificing some specificity. Consequently, a jWBC cut‐off of 17 500 cells/mm3 may allow the clinician to rule out a septic joint (LR(−) 0.25, 95% CI 0.07 to 0.89), which is the principal concern of clinicians.
The results of our ROC curve analysis must be interpreted with caution. Although our findings regarding sensitivity, specificity and LRs were consistent with many earlier studies,1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31 our findings based on ROC curve analysis may be unique and need to be validated. In our search of the literature, our study may be the first to examine these diagnostic tests in septic arthritis with ROC curve analysis. Thus, our ROC curve results may not be generaliseable, given the variations in laboratories and patient populations.
Limitations
A portion of our sample did not have data on all diagnostic tests. For instance, nearly one third of the sample did not have an ESR data.
There may be sampling bias in our study as we selected patients using reported arthrocentesis results. Presumably, patients were chosen to undergo arthrocentesis or operative intervention based on clinical suspicion.
We did not collect data on comorbid illnesses or antibiotic usage, which may be significant variables in the demographics and outcome of our patient population.
The sample size of the study was relatively small, and it limited our ability to comment on some of our findings with statistical confidence, such as the potential ability of the combined tests to rule out septic arthritis, as evidenced by the wide 95% CI associated with the LR(−) (table 1).
We used a stringent definition of septic arthritis in our study, and some patients with a possible septic joint may be excluded by our case definition (eg, a patient with positive blood cultures and a hot joint, but negative joint fluid culture).
Conclusion
As adjuncts in the diagnosis of septic arthritis, WBC and ESR are poor tests, whereas the jWBC count is a fairly good test. However, none of these tests are perfect, and the clinician should be conservative in approaching the patient with a hot joint.
Abbreviations
AUC - area under the curve
ESR - erythrocyte sedimentation rate
jWBC - WBC in the joint fluid
ROC - receiver operating characteristic
WBC - white blood cells
S F Li - C Cassidy, C Chang, S Gharib, J Torres
Footnotes
Competing interests: None declared.
References
- 1.Kunnamo I, Pelkonen P. Routine analysis of synovial fluid cells is of value in the differential diagnosis of arthritis in children. J Rheumatol 1986131076–1080. [PubMed] [Google Scholar]
- 2.Unkila‐Kallio L, Kallio M J T, Peltola H. The usefulness of C‐reactive protein levels in the identification of concurrent septic arthritis in children who have acute hematogenous osteomyelitis. J Bone Joint Surg Am 199476848–853. [DOI] [PubMed] [Google Scholar]
- 3.Culp R W, Eichenfield A H, Davidson R S. Lyme arthritis in children. J Bone Joint Surg Am 19876996–99. [PubMed] [Google Scholar]
- 4.Morrey B F, Bianco A J, Rhodes K H. Septic arthritis in children. Orthop Clin North Am 19756923–934. [PubMed] [Google Scholar]
- 5.Rotbart H A, Glode M P. Haemophilus influenza type b septic arthritis in children: report of 23 cases. Pediatrics 198575254–259. [PubMed] [Google Scholar]
- 6.Kallio M J T, Unkila‐Kallio L, Aalto K. Serum C‐reactive protein, erythrocyte sedimentation rate and white blood cell count in septic arthritis of children. Pediatr Infect Dis J 199716411–413. [DOI] [PubMed] [Google Scholar]
- 7.Kunnamo I, Kallio P, Pelkonen P. Clinical signs and laboratory tests in the differential diagnosis of arthritis in children. Am J Dis Child 198714134–40. [DOI] [PubMed] [Google Scholar]
- 8.DelBaccaro M A, Champoux A N, Bockers T. Septic arthritis versus transient synovitis of the hip: the value of screening laboratory tests. Ann Emerg Med 1992211418–1422. [DOI] [PubMed] [Google Scholar]
- 9.Eich G F, Superti‐Furga A, Umbricht F S. The painful hip: evaluation of criteria for clinical decision‐making. Eur J Pediatr 1999158923–928. [DOI] [PubMed] [Google Scholar]
- 10.Molteni R A. The differential diagnosis of benign and septic joint disease in children. Clinical, radiologic, laboratory, and joint fluid analysis, based on 37 children with septic arthritis and 97 with benign aseptic arthritis. Clin Pediatr 19781719–23. [DOI] [PubMed] [Google Scholar]
- 11.Klein D M, Barbera C, Gray S T. Sensitivity of objective parameters in the diagnosis of pediatric septic hips. Clin Orthop 1997338153–159. [DOI] [PubMed] [Google Scholar]
- 12.Kelly P J. Bacterial arthritis in the adult. Orthop Clin North Am 19756973–981. [PubMed] [Google Scholar]
- 13.Kortekangas P, Aro H T, Tuominen J. Synovial fluid leukocytosis in bacterial arthritis vs. reactive arthritis and rheumatoid arthritis in the adult knee. Scand J Rheumatol 199221283–288. [DOI] [PubMed] [Google Scholar]
- 14.Soderquist B, Jones I, Fredlund H. Bacterial or crystal‐associated arthritis? Discriminating ability of serum inflammatory markers. Scand J Infect Dis 199830591–596. [DOI] [PubMed] [Google Scholar]
- 15.Borenstein D G, Simon G L. Hemophilus influenza septic arthritis in adults. A report of four cases and a review of the literature. Medicine 198665191–201. [DOI] [PubMed] [Google Scholar]
- 16.Knight D J, Gilbert F J, Hutchison J D. Lesson of the week: septic arthritis in osteoarthritic hips. BMJ 199631340–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCutchan H J, Fisher R C. Synovial leukocytosis in infectious arthritis. Clin Orthop 1990257226–230. [PubMed] [Google Scholar]
- 18.Goldenberg D L, Brandt K D, Cathcart E S. Acute arthritis caused by gram‐negative bacilli: a clinical characterization. Medicine 197453197–208. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong R W, Bolding F, Joseph R. Septic arthritis following arthroscopy: clinical syndromes and analysis of risk factors. Arthroscopy 19928213–223. [DOI] [PubMed] [Google Scholar]
- 20.Brandt K D, Cathcart E S, Cohen A S. Gonococcal arthritis. Clinical features correlated with blood, synovial fluid and genitourinary cultures. Arthritis Rheum 197417503–510. [DOI] [PubMed] [Google Scholar]
- 21.Sharp J T, Lidsky M D, Duffy J. Infectious arthritis. Arch Intern Med 19791391125–1130. [PubMed] [Google Scholar]
- 22.Krey P R, Bailen D A. Synovial fluid leukocytosis. A study of extremes. Am J Med 197967436–442. [DOI] [PubMed] [Google Scholar]
- 23.Weitoft T, Makitalo S. Bacterial arthritis in a Swedish health district. Scand J Infect Dis 199931559–561. [DOI] [PubMed] [Google Scholar]
- 24.Tan R K, Miller D G. ESR in gonococcal arthritis. BMJ 19791621–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winblad S. Arthritis associated with Yersinia enterocolitica infections. Scand J Infect Dis 19757191–195. [DOI] [PubMed] [Google Scholar]
- 26.Seifert M H, Warin A P, Miller A. Articular and cutaneous manifestations of gonorrhoea. Ann Rheum Dis 197433140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldenberg D L, Cohen A S. Acute infectious arthritis. A review of patients with nongonococcal joint infections (with emphasis on therapy and prognosis). Am J Med 197660369–377. [DOI] [PubMed] [Google Scholar]
- 28.Li S F, Henderson J, Dickman E.et al Laboratory tests in adults with septic arthritis: can they rule out a septic joint? Acad Emerg Med 200411276–280. [DOI] [PubMed] [Google Scholar]
- 29.Shmerling R H, Delbanco T L, Tosteson A N A. Synovial fluid tests. What should be ordered? JAMA 19902641009–1014. [PubMed] [Google Scholar]
- 30.Kocher M S, Zurakowski D, Kasser J R. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence‐based clinical prediction algorithm. J Bone Joint Surg Am 1999811662–1670. [DOI] [PubMed] [Google Scholar]
- 31.Kocher M S, Mandiga R, Zurakowski D. Validation of a clinical prediction rule for the differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am 2004861629–1635. [DOI] [PubMed] [Google Scholar]
- 32.Tintinalli J E, Ruiz E, Krone R L.Emergency medicine: a comprehensive study guide, 4th edn. New York: McGraw‐Hill 1996315–316.
- 33.Rosen P, Barkin R.Emergency medicine: concepts and clinical practice. 4th edn. New York: Mosby, 19982663–2664.
- 34.Roberts J R, Hedges J R.Clinical procedures in emergency medicine. 3rd edn. Philadelphia: WB Saunders, 1998927