Abstract
Objectives
To test the analgesic efficacy of topical morphine on superficial burns within the emergency department by comparing pain scores, comfort ratings and analgesia taken by participants.
Method
A placebo‐controlled three‐treatment randomised controlled trial was undertaken. 59 participants were randomly allocated to receive a dressing containing Intrasite gel and morphine sulphate, Intrasite gel and water or the conventional Jelonet dressing. The study design enabled double‐blinding between the two Intrasite gel treatments.
Results
49 participants were included in the final analysis as 10 were lost to follow‐up. No significant differences were observed between the pain scores or comfort ratings of the three treatments. Participants receiving Jelonet and the placebo reduced their pain scores by the greatest amount overall. However, participants receiving morphine were the only group to reduce pain scores by >20 mm on two consecutive time intervals (2 and 6 h). At 12 h the morphine group reported the highest pain scores. Only 4/15 participants receiving topical morphine administered additional analgesia compared with 12/17 receiving the Jelonet dressing and 6/17 receiving Intrasite and water (p = 0.055). However, when all analgesia was taken into account, the morphine group was administered the greatest amount. Overall, the placebo group reported their dressings to be the most comfortable and took the least amount of analgesia. Minor adverse reactions included itching, burning and a rash. No serious adverse reactions were reported.
Conclusions
Topical morphine sulphate does not seem to be as effective when used for the pain associated with superficial burns as when used for the pain associated with chronic inflammatory wounds. (The European Clinical Trials Database number for this study is 2005‐003285‐42.)
Burns (and scalds) account for 175 000 attendances at emergency departments (ED) in the UK every year, where there are inconsistencies with wound care and pain management.1 National guidelines recommend the administration of simple analgesia (paracetamol and ibuprofen) and the application of a moist wound dressing.2 Locally, patients receive the application of the first‐aid dressing Burnshield to the injured site, which cools the area before the application of a Jelonet dressing. However, Burnshield is expensive and not always readily available, and some patients become distressed when it is removed to apply the Jelonet dressing. This is because of the mechanical stimulation of the nociceptors and the possible anticipation of further pain and discomfort.3,4
Evidence on the use of local anaesthetic and topical anti‐inflammatory preparations on burns is limited, with mixed results being reported.5,6 However, topical opioids are reported to significantly reduce the pain associated with leg ulcers and chronic inflammatory skin conditions.7,8,9,10,11,12,13,14,15,16,17,18 This is thought to be because of their action on peripheral opioid receptors that “sprout” within minutes of inflammation.19 As superficial burns prompt an intense inflammatory response, they would seem to be ideal wounds for assessing the use of topical opioids for acute pain.
The aim of this study was to test whether topically applied opioids have an analgesic effect when applied to patients attending the ED with superficial burns. The objective was to compare pain scores recorded at 2, 6 and 12 h after dressing, comfort ratings and analgesia taken by participants who receive a conventional Jelonet dressing (Smith and Nephew, Yorkshire, UK), or a dressing containing Intrasite gel (Smith and Nephew) and morphine, or a placebo of Intrasite gel and water.
Ethical approval was obtained from the Southampton and South West Hampshire Research Ethics Committee, and the study was jointly sponsored by the University of Portsmouth and the Portsmouth Hospitals National Health Service (NHS) Trust.
Method
This single‐centred randomised, placebo‐controlled trial was undertaken in the ED of the Portsmouth Hospitals NHS Trust. The target population was the cohort of patients presenting to the ED during 2006 with minor superficial burns that were <5% total body surface area. Participants were identified by a checklist on arrival to the ED. Table 1 shows the inclusion and exclusion criteria.
Table 1 Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| ⩾16 years | Burns to the neck, face, head and genitalia |
| Weight >50 kg | Electrical and chemical burns |
| Burn caused by hot liquid, touching a hot surface or sun burn | Patients taking codeine‐based drugs |
| Burn <8 h since injury | Presence of other injuries |
| No signs of infection | Presence of any pathophysiology affecting perception to pain |
| Patients under the influence of alcohol or drugs |
Paracetamol and/or ibuprofen was administered orally and Burnshield applied before patients were approached about consenting to participate in the study. After recruitment, participants were randomised to receive one of the following interventions:
six‐layer Jelonet dressing, covered with gauze and bandage (control);
15 g Intrasite gel mixed with 1 ml 10 mg/ml morphine sulphate, applied evenly over the burn using a 20 ml syringe, covered with a six‐layer Jelonet dressing, gauze and bandage; or
placebo—15 g Intrasite gel mixed with 1 ml sterile water, applied evenly over the burn using a 20 ml syringe and covered with a six‐layer Jelonet dressing, gauze and bandage.
Intrasite gel was selected as the medium to mix with the morphine to aid application to the burn. As Intrasite gel is primarily composed of water, it has a cooling effect, is non‐adherent and has a neutral pH that does not irritate the skin. It maintains a moist environment and helps keep exposed nerve endings bathed in fluid. Moreover, it contains the two mediums (water and propylene glycol) that have been shown to aid the absorption of drugs from the surface of a burn.20,21 Morphine sulphate was the chosen opioid as it is cheap, available in the ED and has recently been shown to remain stable when mixed with Intrasite gel.22
Randomisation was performed by a receptionist at the satellite minor injuries unit by using a table of random digits obtained from the Research randomizer website (http://www.randomizer.org).
Blinding
The treatments were sealed in envelopes, which were numbered in the order in which they were to be opened. To ensure double‐blinding of the two Intrasite gel treatments, the envelopes were stored in the paediatric area, which is separated from the main ED. The treatments were prepared by a registered children's nurse once a participant had been recruited. After preparation, the treatments were given to the research team to apply. At no point did the paediatric nurses have direct contact with the participants. Owing to the double‐blinding between the two Intrasite gel treatments, all participants receiving an Intrasite gel dressings were observed in the ED for 30 min after application.
Pain scores were recorded in the ED before any treatment had been received, and by the participants on discharge 2, 6 and 12 h after treatment in a pain diary. The 0–100 mm Visual Analogue Scale (VAS) was chosen as it is a reliable and valid univariate measure of pain assessment when used with patients in a variety of clinical settings23 and variations have been successfully used in similar studies.8,9,11,12,13,14,15,17
Assuming a between‐subject standard deviation in VAS pain scores of 20 mm and applying a Bonferroni adjustment to a conventional 5% significance level, we calculated the need for 21 participants in each arm of the study to detect a 20 mm difference in mean pain scores between the test treatment (Intrasite and morphine) and each of the two control treatments, with a power of 80% (B Higgins, personal communication, 10 May 2005). A reduction of 20 mm was chosen as it is within the upper limit that has been identified as a meaningful reduction in pain, by patients, following administration of analgesia in the ED.24
Sample size calculations were based on the assumption that the VAS would be analysed using analysis of variance. However, the data obtained from the VAS failed to satisfy the parametric assumptions and a non‐parametric alternative (the Kuskal–Wallis one‐way analysis of variance) was used to analyse the pain scores at the allotted time intervals. Baseline demographic data were recorded for each patient.
All participants were reviewed the following day and an overall comfort rating, using a 5‐point Likert scale (1, very comfortable; 5, very uncomfortable), was recorded. Analgesia administered was recorded by the participants in a pain diary. The researcher then converted the amount of analgesia taken into units 1 unit = 1 g paracetamol or 400 mg ibuprofen; ½ unit = 500 mg paracetamol or 200 mg ibuprofen.
Comfort ratings and analgesic units were also analysed using the Kruskal–Willis one‐way analysis of variance test. In addition, the research team recorded whether participants experienced any side effects after discharge by completion of a checklist at the time of follow‐up to identify any relationship between the treatment used and the presence of adverse reactions.
Calculations were performed using the statistical software package SPSS V.13.0.
Results
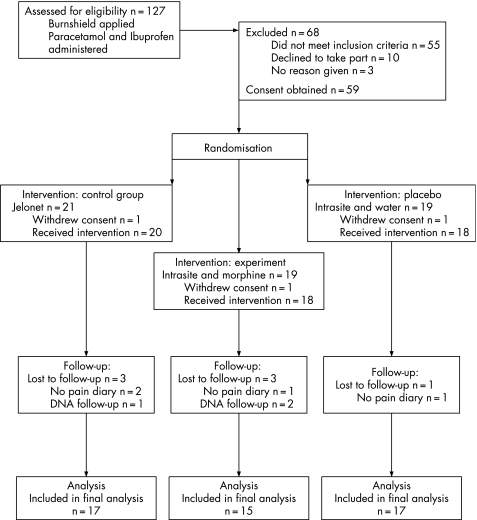
A total of 127 patients were approached about the study during the study period (3 February to 31 December 2006). Of these, 68 patients declined to participate or failed to meet the strict inclusion criteria, and 59 patients were recruited. Of these, 10 patients were lost to follow‐up, leaving a total of 49 patients included in the final analysis (fig 1).
Figure 1 CONSORT flow diagram of patients assessed for eligibility. DNA = did not attend follow‐up appointment.
Table 2 defines the baseline demographics of participants included in the final analysis.
Table 2 Demographics and baseline data of participants included in the final analysis.
| Baseline data | Jelonet (n = 17) | Water (n = 17) | Morphine (n = 15) |
|---|---|---|---|
| Gender, n | |||
| Male | 8 | 4 | 10 |
| Female | 9 | 13 | 5 |
| Mean (SD) age (years) | 34.71 (15.63) | 32.00 (13.23) | 32.20 (11.62) |
| Mean (SD) weight (kg) | 78.03 (17.13) | 73.32 (21.70) | 77.19 (17.93) |
| Mean (SD) size (cm2) | 78.88 (127.44) | 19.70 (18.89) | 26.93 (34.22) |
| Site | |||
| Upper limb | 11 | 14 | 11 |
| Lower limb | 3 | 3 | 1 |
| Torso/chest | 3 | 0 | 3 |
| Cause | |||
| Hot liquid | 15 | 12 | 13 |
| Hot surface | 2 | 5 | 2 |
| ED pain scores (mm) | |||
| Median | 69.5 | 57.5 | 65.0 |
| Range | 43–96.5 | 28–87 | 10–78.5 |
ED, emergency department.
Despite randomisation, burns in the Jelonet group were considerably larger, there were four burns which were >100 cm2 compared with only one burn in the morphine group. The largest burn in the placebo group was 63 cm2. Median pain scores recorded before receiving any treatment were similar in the Jelonet and morphine groups but slightly lower in the placebo group; however, this difference was not significant (p = 0.271).
The three treatment groups were first compared with regard to pain assessed 2, 6 and 12 h after dressing. Table 3 presents the median pain scores and p values, after performing a Kruskal–Wallis one‐way analysis of variance test on the scores.
Table 3 Comparison of median pain scores recorded in the emergency department and at 2, 6 and 12 h after dressing.
| Variable | Jelonet (n = 17) | Water (n = 17) | Morphine (n = 15) | p Value |
|---|---|---|---|---|
| ED pain scores (mm) | 69.5 (43–96.5) | 57.5 (28–87) | 65.0 (10–78.5) | 0.271 |
| Pain scores at 2 h (mm) | 40.0 (0–90) | 30.0 (0–88) | 41.0 (0–60) | 0.640 |
| Pain scores at 6 h (mm) | 30.0 (0–96) | 23.0 (0–75) | 19.75 (0–43.5) | 0.818 |
| Pain scores at 12 h (mm) | 10.0 (0–86) | 10.8 (0–44.5) | 18.0 (0–97) | 0.857 |
| Total pain reduction, median (%) | 59.5 (85%) | 46.7 (81%) | 47.0 (72%) |
ED, emergency department.
Values are median (range) unless specified otherwise.
The three groups recorded a similar reduction in median pain scores at 2 h when compared with pain scores recorded in the ED, with the Jelonet group recording a loss of 29.5 mm, the placebo group 27.5 mm and the morphine group 24.0 mm. All three groups therefore reached the level of pain relief reported to be considered a significant reduction after analgesia in the ED.24 Those in the morphine group reduced their median pain scores by the greatest amount when recorded at 6 h (21.25 mm) compared with 10 and 7 mm by those receiving Jelonet and placebo. At 12 h the morphine group recorded the highest pain scores, almost twice that of the other treatments. However, the difference in pain scores at all the allotted time intervals was not significant at the 5% significance level (table 3).
Alternative outcome measures were the comfort rating of the dressings and additional analgesia participants administered after discharge from the ED. Table 4 provides a summary of these results.
Table 4 Median comfort ratings and total analgesic units administered by each group.
| Jelonet | Water | Morphine | p Value at 5% significance level | |
|---|---|---|---|---|
| Comfort rating (1–5 Likert scale*), median (range) | 2.0 (1–5) | 1.5 (1–5) | 3.0 (1–5) | 0.390 |
| Analgesia units taken after discharge | ||||
| Participants administering additional analgesia, n | 12/17 | 6/17 | 4/15 | 0.055 |
| Total units administered | 24.5 | 12.0 | 10.0 | |
| Mean (SD) | 1.44 (1.44) | 0.75 (1.24) | 0.67 (1.24) |
*1, very comfortable; 5, very uncomfortable.
In all, 27/49 (55%) participants took no further analgesia after leaving the ED. To obtain a true comparison of the total analgesia taken by each group, the units of paracetamol, ibuprofen and morphine administered in the ED were added to the additional units administered after discharge (table 5).
Table 5 Total analgesic units for each group.
| Jelonet | Water | Morphine | p Value at 5% significance level | |
|---|---|---|---|---|
| Oral analgesia administered in ED | 34.5 | 35.0 | 35.0 | |
| Morphine applied topically | — | — | 15 | |
| Additional analgesia administered after discharge | 24.5 | 12.0 | 10.0 | 0.055 |
| Total units per group | 59.0 | 47.0 | 60.0 | 0.089 |
ED, emergency deparment.
The morphine group actually received the most analgesia over the study period. The placebo group received the least amount of analgesia, and still rated their dressings the most comfortable. Performing a Spearman r correlation on these two variables confirmed this relationship (r = 0.441; p = 0.002).
A total of 15 participants reported 20 minor symptoms that were recorded as possible adverse reactions to the dressing received (table 6).
Table 6 Adverse reactions reported by participants in each group.
| Jelonet | Water | Morphine | |
|---|---|---|---|
| Number reporting symptoms | 6/17 | 3/17 | 6/15 |
| Burning | 4 | 1 | 1 |
| Itching | 0 | 1 | 3 |
| Localised adhesive rash | 1 | 1 | 1 |
| Headache | 0 | 1 | 2 |
| Nausea | 2 | 1 | 0 |
| Drowsiness | 0 | 1 | 0 |
No participant in the morphine group reported symptoms that could have indicated opioid toxicity, with nausea and drowsiness being reported only by participants receiving Jelonet or the placebo. At the follow‐up appointment, no irritation was noted to any wound bed, there was no macerated skin and no dressings were reported to have adhered to the wound. No participant had to return to the ED early as a result of an adverse reaction, no serious unsuspected or suspected adverse reactions were reported and no general practitioner reported any problems with infection or wound healing after the participants were discharged from the ED.
Discussion and conclusion
Pain management of burns is particularly difficult owing to the many factors affecting pain, including sensory, mechanical and anticipatory attributes.3,4 However, several studies and case reports have reported that topically applied opioids provide a quick‐acting and long‐lasting analgesic effect in a variety of chronic inflammatory skin conditions and partial‐and full‐thickness burns.7,8,9,10,11,12,13,14,15,16,17,18 This study is the first to test whether opioids could also provide a satisfactory analgesic effect on the acute pain associated with superficial burns.
Unfortunately, fewer participants were recruited than intended, reducing the power of the study, and the strict inclusion criteria of the study has ensured that the population to which the study can be generalised is limited to those >16 years of age, who are normally fit and well, with no underlying medical condition and not prescribed additional codeine‐based drugs.
This study identified that there was no statistical significance at the 5% significance level between the median pain scores recorded by the three groups at the allotted time intervals and that the morphine group recorded the highest median pain scores at 2 and 12 h. Those receiving the Jelonet and placebo reduced their pain by the greatest amount (85% and 81%, respectively), in contrast with the results of studies on chronic pain. Participants in this study were not administered codeine/morphine‐based drugs by other routes and may support the claim that peripheral opioid receptors only sprout in the presence of the administration of opioids given by other methods.25
Despite reducing their pain scores by the least amount, the morphine group was the only group to record a reduction in pain of >20 mm at two consecutive time intervals (2 and 6 h) despite taking considerably less analgesia after discharge from the ED (p = 0.055), reflecting results of studies on chronic pain. However, the morphine group actually received more analgesia than the other treatments when all analgesic units (including the morphine) were added together. The group receiving the placebo, actually administered the least amount of analgesia (47 units), rated their dressings the most comfortable (median 1.5) and reduced their pain scores by 46.7 mm (81%).
The adverse reactions reported in this study do not indicate that any participant experienced systemic opioid toxicity, reflecting the results of studies on chronic pain.9,14,15,16,18 Morphine increases histamine release, which in turn results in itching, and although this was the main symptom reported by the morphine group, it could not be solely linked to the application of morphine as it also occurred in those receiving placebo.
A limitation of the study is that it lacks pharmacokinetic information that could assess the extent of systemic absorption of morphine when applied topically, and no assumption can be made on whether topical morphine has a true peripheral effect. This study seems to have shown that morphine applied topically is not as effective for the acute pain associated with minor superficial burns as it is for chronic pain.
Up‐to‐date evidence for best practice on the management of pain associated with burns is limited. Clinical guidelines to advise ED staff on the optimal method of management of burn injuries across the NHS are being developed.1 Until then, it is recommended that systemic analgesia for patients presenting with superficial burns should consist of paracetamol, ibuprofen and codeine. The dressing chosen should be easy to apply, be non‐adherent and maintain a moist wound environment. Further research into the use of alternative topical preparations are recommended in order to improve the management of pain associated with superficial burns.
Acknowledgements
I thank Alan Charters (Consultant Nurse, Portsmouth Hospitals NHS Trust), Joyce Wise (Portsmouth University), Martine Cross, Kate Greenwood, Bernie Higgins and Diane Gal (R&D Unit, Portsmouth University), Kerrie Drake and Linda Barton (ENPs) and all the paediatric nurses in the ED Portsmouth Hospitals Trust for their support throughout this study.
Abbreviations
ED - emergency department
NHS - National Health Service
VAS - Visual Analogue Scale
Footnotes
Funding: None.
Competing interests: None.
References
- 1.British Burn Association National Burn Care Review committee report—standards and strategy for burn care: a review of burn care in the British Isles. 2001. http://www.bapras.org.uk (accessed 2 Apr 2007)
- 2.Department of Health Prodigy guidance: burns and scalds. 2004. http://www.prodigy.nhs.uk/guidance (Accessed 2 Apr 2007)
- 3.Ptacek J T, Patterson D R, Doctor J. Describing and predicting the nature of procedural pain after thermal injuries: implications for research. J Burn Care Rehabil 200021318–326. [DOI] [PubMed] [Google Scholar]
- 4.Geisser M E, Bingham H G, Robinson M E. Pain and anxiety during burn dressing changes: concordance between patients' and nurses' ratings and relation to medication administration and patient variables. J Burn Care Rehabil 199516165–171. [DOI] [PubMed] [Google Scholar]
- 5.Alvi R, Jones S, Burrows D.et al The safety of topical anaesthetic and analgesic agents in a gel when used to provide pain relief at split skin donor sites. Burns 19982454–57. [DOI] [PubMed] [Google Scholar]
- 6.Brofeldt B T, Cornwell P, Doherty D.et al Topical lidocaine in the treatment of partial‐thickness burns. J Burn Care Rehabil 19891063–68. [DOI] [PubMed] [Google Scholar]
- 7.Westerling P, Hoglund P, Lundin S.et al Transdermal administration of morphine to healthy subjects. Br J Clin Pharmacol 199437571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Twillman R K, Long T D, Cathers T A.et al Treatment of painful skin ulcers with topical opioids. J Pain Symptom Manage 199917288–292. [DOI] [PubMed] [Google Scholar]
- 9.Krajnik M, Zylicz Z, Finlay I.et al Potential uses of topical opioids in palliative care—report of 6 cases. Pain 199980121–125. [DOI] [PubMed] [Google Scholar]
- 10.Flock P, Gibbs L, Sykes N. Diamorphine‐metronidazole gel effective for treatment of painful infected leg ulcers. J Pain Symptom Manage 200020396–397. [DOI] [PubMed] [Google Scholar]
- 11.Long T D, Cathers T A, Twillman R.et al Morphine‐infused silver sulfadiazine (MISS) cream for burn analgesia: a pilot study. J Burn Care Rehabil 200122118–123. [DOI] [PubMed] [Google Scholar]
- 12.Ballas S K. Treatment of painful sickle cell leg ulcers with topical opioids. Blood 2002991096. [DOI] [PubMed] [Google Scholar]
- 13.Flock P. Pilot study to determine the effectiveness of diamorphine gel to control pressure ulcer pain. J Pain Symptom Manage 200325547–554. [DOI] [PubMed] [Google Scholar]
- 14.Zeppetella G, Paul J, Ribeiro M D C. Analgesic efficacy of morphine applied topically to painful ulcers. J Pain Symptom Manage 200325555–558. [DOI] [PubMed] [Google Scholar]
- 15.Watterson G, Howard R, Goldman A. Peripheral opioids in inflammatory pain. Arch Dis Child 200489679–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribeiro M D, Joel S P, Zeppetella G. The bioavailability of morphine applied topically to cutaneous ulcers. J Pain Symptom Manage 200427434–439. [DOI] [PubMed] [Google Scholar]
- 17.Abbas S Q. Diamorphine‐Intrasite dressings for painful pressure ulcers. J Pain Symptom Manage 200428532–533. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher R E, Arndt D R, Hunt K L. Analgesic effects of topical methadone: a report of four cases. Clin J Pain 200521190–192. [DOI] [PubMed] [Google Scholar]
- 19.Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med 19853321685–1690. [DOI] [PubMed] [Google Scholar]
- 20.Aoyama H, Nishizaki A, Aoki Y.et al The effect of some ointment bases on the systematic absorption of tobramycin from various wound surfaces of burned patients. Burns 198410290–299. [DOI] [PubMed] [Google Scholar]
- 21.Stone H H, Kolb L D, Pettitt J.et al The systemic absorption of an antibiotic from the burn wound surface. Am Surg 196834639–643. [PubMed] [Google Scholar]
- 22.Zeppetella G, Joel S P, Ribeiro M D C. Stability of morphine sulphate and diamorphine hydrochloride in Intrasite gel™. Palliat Med 200519131–136. [DOI] [PubMed] [Google Scholar]
- 23.Byers J F, Bridges S, Kijek J.et al Burn patients' pain and anxiety experiences. J Burn Care Rehabil 200122144–149. [DOI] [PubMed] [Google Scholar]
- 24.Kelly A M. The minimum clinically significant difference in visual analogue scale pain scores does not differ with severity of pain. Emerg Med J 20011893205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vernassiere C, Cornet C, Trechot P.et al Study to determine the efficacy of topical morphine on painful chronic skin ulcers. J Wound Care 200514289–293. [DOI] [PubMed] [Google Scholar]