Abstract
A 39‐year‐old Zimbabwean man presented with a 1 week history of fever, general malaise and acute‐onset chest pain. He had a urethral stricture, which had been managed with an indwelling supra‐pubic catheter. The electrocardiography on admission showed inferior ST‐T segments elevation. His chest pain and electrocardiography changes resolved subsequent to thrombolysis, and he remained haemodynamically stable. The 12‐h troponin I was increased at 10.5 μg/l (NR <0.04 μg/l). Echocardiography confirmed severe mitral regurgitation and a flail anterior mitral valve leaflet with an independently oscillating mobile vegetation. Enterococci faecalis were grown on blood cultures. A diagnosis of enterococci infective endocarditis with concomitant acute myocardial infarction due to possible septic emboli was made. Despite the successful outcome from thrombolysis in the setting of acute myocardial infarction with infective endocarditis, the case highlights the current lack of definitive data on the optimal acute management of such an unusual clinical scenario. Although there is serious concern that thrombolytic treatment for myocardial infarction in the setting of infective endocarditis may be associated with higher risk of cerebral haemorrhage, there is little documented evidence supporting the safety of primary percutaneous coronary intervention with these patients.
A 39‐year‐old Zimbabwean man presented with a 1‐week history of fever, general malaise and acute‐onset chest pain. He had a urethral stricture, which had been managed with an indwelling supra‐pubic catheter for the past 8 months. On examination, the patient was pyrexial, clubbed and had a clearly audible pan‐systolic murmur. The electrocardiography (ECG) on admission showed inferior ST‐T segments elevation and the patient was thrombolysed with tenectaplase. His chest pain and ECG changes resolved subsequently to thrombolysis, and he remained haemodynamically stable. The 12 h troponin I was increased at 10.5 μg/l (NR <0.04 μg/l).
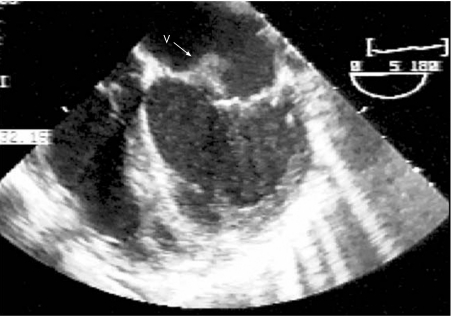
Transthoracic echocardiography showed a moderately enlarged, hyperdynamic left ventricle and a large eccentric jet of mitral regurgitation. Subsequent transoesophageal echocardiography confirmed severe mitral regurgitation and a flail anterior mitral valve leaflet with an independently oscillating mobile vegetation (fig 1). Enterococci faecalis were grown on blood cultures. Urine cultures showed a mixed organism growth.
Figure 1 Transoesophageal echocardiogram showing vegetation (V) on the anterior mitral valve leaflet.
The patient was started on a 4‐week course of intravenous antibiotics, with a good clinical response. He underwent insertion of a urethral stent, and the supra‐pubic catheter was removed. Coronary angiography 6 weeks after presentation showed unobstructed coronary arteries, and he proceeded to surgery to replace the mitral valve. The patient made an uneventful recovery and was discharged home 10 weeks after his initial presentation.
Infective endocarditis can be associated with embolic phenomena carrying a high risk of mortality and morbidity. Intracoronary septic embolisation resulting in acute myocardial infarction (AMI) is an uncommon complication of infective endocarditis. Pathological studies suggest that the involvement of coronary artery is more prevalent than clinical case reports would suggest.1 Although there was no angiographic evidence of coronary emboli in our case, it is likely that the initial thrombolytic treatment restored coronary flow with distal embolisation and degeneration of the embolic material. The probability of an AMI secondary to coronary embolisation from the valvular vegetation is supported by the clinical presentation of chest pain, ST‐T segment elevation on the ECG and a significant rise in troponin. However, one cannot definitively exclude the possibility of the concomitant occurrence of infective endocarditis as well as an acute thrombotic infarct in this case.
Enterococci are often encountered in urinary, biliary and gastrointestinal tract infections. At present, multiresistant enterococci is the third most common cause of infective endocarditis, after streptococci and Staphylococcus aureus. This patient had a poorly managed indwelling supra‐public catheter, which was likely to be the source of enterococcal septicaemia. There are no current guidelines suggesting the use of prophylactic antibiotic in patients with long‐term indwelling supra‐pubic or urethral catheter, despite the risk of chronic bacteriaemia.
The optimal acute treatment for AMI in the setting of infective endocarditis remains controversial. Current literature suggests that thrombolytic treatment in this setting is unfavourable, with concerns regarding a higher risk of cerebral vascular haemorrhage.2 This is because of a higher prevalence of central nervous system involvement such as microinfarction/haemorrhage and mycotic aneurysms in infective endocarditis. Risk of cerebral bleed may be further enhanced in the presence of bacteraemia as disturbance in haemostasis occurs as a result of activation of antithrombin and antiplatelet cascade activation in sepsis.
Coronary angiography seems to be safe in the presence of active infective endocarditis.3 However, the safety and efficacy of primary percutaneous coronary intervention (PCI) in the setting of septic embolic coronary occlusion is not established and should not be simply extrapolated from the success of the technique in treating acute atherothrombotic coronary disease. There is a theoretical concern that balloon/stent deployment in the setting of ongoing bacteraemia may result in localised septic mycotic aneurysm. Furthermore, septic emboli from vegetation may not be as amenable to PCI as atherosclerotic thrombotic lesions.4 Technologies such as mechanical rheolysis and distal protection devices may reduce the risk of distal embolisation, but their use has not been proved in the setting of infective endocarditis and septic occlusive coronary emboli.
In summary, this case of enterococcal endocarditis presenting as an AMI highlights the current lack of definitive data on the optimal management of such an unusual clinical scenario. Despite the concern that thrombolytic treatment for myocardial infarction in the setting of infective endocarditis may be associated with higher risk of cerebral haemorrhage, there is little documented evidence supporting the safety of primary PCI with these patients.
Abbreviations
AMI - acute myocardial infarction
ECG - electrocardiography
PCI - percutaneous coronary intervention
Footnotes
Competing interests: None declared.
Informed consent was obtained from the patient for publication of his details in this paper.
References
- 1.Brunson J G. Coronary embolism in bacterial endocarditis. Am J Pathol 195320689–701. [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter A J, Girard D E. Thrombolytics in infectious endocarditis associated myocardial infarction. J Emerg Med 20014401–406. [DOI] [PubMed] [Google Scholar]
- 3.Welton D E, Young J E, Raizner A E.et al Value and safety of cardiac catheterization during active infective endocarditis. Am J Cardiol 1979441306–1310. [DOI] [PubMed] [Google Scholar]
- 4.Glazier J J. Interventional treatment of septic coronary embolism: Sailing into uncharted and Dangerous Waters. J Intervent Cardiol 20024305–307. [DOI] [PubMed] [Google Scholar]