Abstract
Purpose of review
Recent work on the role of medial frontal cortex in cognition and its involvement in neurological disorders is critically reviewed.
Recent findings
The highly influential notion of conflict monitoring by the anterior cingulate has been called into question by monkey single-cell neurophysiology and lesion studies in monkeys and humans. An alternative role for this region in adapting behaviour in response to changing demands over time is gaining support. By contrast, the more dorsally placed pre-supplementary motor area and supplementary eye field have been implicated in direct executive control in situations of response conflict. Although more rostral medial areas have been linked to complex cognitive operations involving references to the self, conceptual obstacles make the evidence difficult to interpret. The role of orbitofrontal cortex in guiding action based on value has been reinforced.
Summary
This area continues to generate both interest and controversy. A few striking discrepancies between data from functional imaging and interventional techniques illustrate the hazards of drawing strong conclusions from merely correlative evidence. More broadly, a case can be made for tempering the empirical enthusiasm here with a little more theoretical restraint.
Keywords: medial frontal cortex, cingulate cortex, pre-supplementary motor area, supplementary eye field, orbitofrontal cortex, ventromedial cortex
Introduction
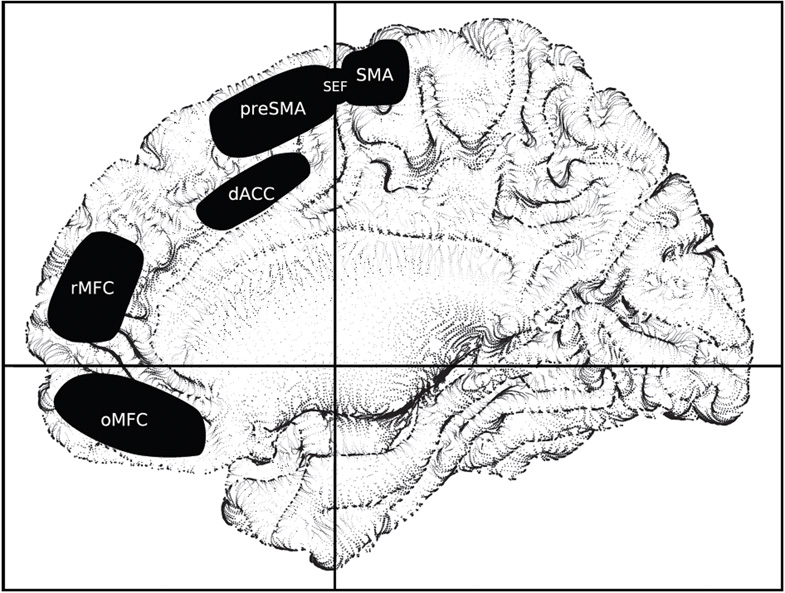
Despite intense interest over the past few decades, the role of the medial frontal cortex in cognition remains obscure. Since activity in this regions is known to be modulated by a wide range of behaviours - and impaired in common neurological conditions -there are good grounds for the belief that this role may be fundamental. Dysfunction within dorsomedial regions (see Figure) is well documented in Parkinson’s disease, and reverses in tandem with the improvements in motor function seen following deep brain stimulation(1). Focal damage to this region, such as may occur following vascular damage in the territory of the anterior cerebral artery or surgical resection is associated with deficits in voluntary movements(2). These observations implicate dorsomedial areas in the control of voluntary action(3).
Figure.
Sketch of the approximate locations of medial frontal areas referred to in the text, superimposed on a single subject template brain normalised into standard stereotactic space (colin brain, http://brainmap.wustl.edu/resources/caretnew.html) and rendered using non-photorealistic techniques (http://www.opennpar.org).
By contrast, dysfunction of ventromedial areas is associated with complex disturbances of goal-oriented decision-making consistent with impairments either in the evaluation of the relative values of goals, or the optimal means of attaining them(4). Intriguingly, these areas have also been implicated in disorders characterised by deficits in the ability to take another person’s perspective on events - an ability often referred to as mentalizing or having a “theory of mind”(5). Autism, and its purer variant Asperger’s syndrome, are perhaps defining of this type of disorder and have been linked to medial frontal abnormalities(6, 7). These findings, in conjunction with suggestive functional imaging, have lead to the notion that ventromedial regions are specialised for the cognitive operations involved in predicting and explaining other people’s behaviour by attributing to them independent mental states such as desires and beliefs(8).
Nonetheless, the relative rarity of focal medial frontal damage in humans makes it difficult to determine what precise processes these areas are actually necessary for. It may therefore be inevitable that, as outlined below, recent work in this field should have brought at least as many reversals as advances.
Dorsomedial cortex
Interest here has focussed on two regions in the vicinity of the VCA line (a line running through the anterior commissures perpendicular to the plane of the anterior and posterior commissures): the supplementary motor complex (comprising the pre-SMA, SEF, and SMA proper) and the dorsal cingulate cortex (see Figure). In both these regions, the parts anterior to the VCA line: the pre-SMA and the dorsal anterior cingulate (dACC), which both have extensive pre-frontal connectivity but relatively few motor projections, have received the most attention. It should be noted(3) that such is their proximity that activity in one may often be erroneously assigned to the other.
Conflict monitoring
A remarkable feature of dACC activity is its consistent modulation by task difficulty in the absence of marked specificity for any one cognitive task. This finding, derived almost exclusively from functional imaging in humans, has been taken as implying a fundamental role in cognition(9). According to the so-called conflict-monitoring hypothesis, there must be general mechanism in the brain whereby conflicting neural representations - whether sensory or motor - are first detected so that executive control systems can resolve the competition between them. A classic example of such a situation is the Stroop task where subjects are asked to respond to colour words printed in different ink with either the colour of the ink or the words themselves. It is suggested that the detection part of this mechanism is subserved by a macroanatomically circumscribed region, and that this region is the dACC.
Conceptually, this hypothesis has the considerable merit of confronting the implicit Cartesianism of much of the literature in the field: the view of parts of the brain as instruments of some “higher” area that somehow knows how and when to use them. But the idea of a general conflict monitor is open to the same criticisms: one has to explain the detection of conflict between conflict-detecting cells, inevitably leading to an infinite regress. Aside from theoretical considerations, recent studies have notably failed to show unitary cingulate activation under both stimulus and response conflict(10, 11), suggesting that the dACC may be confined to the more limited function of detecting conflict between sensorimotor representations. However, if conflict-related activity in the cingulate is specific to action it seems perverse to assume that this area is merely detecting conflict and not resolving it, particularly since it has the necessary connectivity to motor regions. The association with action also raises the possibility that heightened activity here may be reflect the co-activation of distinct neural subpopulations coding for different - and competing - actions; for example “respond to colour” vs “respond to word” in the Stroop task. If so, this activity would result from the conflict that is meant to be detected and not the detection itself. This question - which inevitably requires single cell recordings - was recently comprehensively explored in the SEF and the dACC using two well-designed tasks behaviourally proven to cause conflict. Neither area showed any pure conflict related activity, and the dACC did not show any conflict activity at all(12).
It must also be borne in mind that imaging evidence, being merely correlational, can only be suggestive. To establish necessity requires studies that examine loss of function. Importantly, since the cingulate is so ubiquitously activated even a small number of studies showing an absence of any functional deficits following damage to this area would be considered strong evidence against the fundamental role that has been proposed for it. Thus it is highly significant that two recent human studies have failed to show any evidence of abnormalities in executive control(13, 14). Similarly, recent work involving experimental lesions has failed to reveal analogous deficits in monkeys(15).
Adaptive behaviour
What, then, is the dACC for, and why is its activity apparently so indiscriminate? One attractive possibility is that it is driving the autonomic responses that accompany behaviourally challenging situations. Using both functional imaging and human lesion data, a recent study has comprehensively addressed this question(14). Not only was comparable dACC activation found during a difficult cognitive task and isometric exercise, but in both circumstances autonomic changes indexed by heart rate variation better accounted for signal changes in this region. More importantly, patients with lesions involving the dACC had impaired autonomic responses to stress but unremarkable performance on a range of tasks thought to recruit executive control.
Activity in the dACC seems to reflect the autonomic response appropriate to expected increases in the demand for action. The purpose of these autonomic changes is widely presumed to be the preparation of the body for the physical demands of the forthcoming action. The dACC may therefore be part of a more general system for adaptation in the face of changing requirements for the actions required to secure future goals. The consistent finding of dACC activity following errors or rewards(16, 17), would certainly fit with a process of evaluating the outcome of action after an event, thereby allowing for an appropriate adjustment in preparation for subsequent events. Indeed, activity specific to action-reward associations has recently been demonstrated in monkey dACC(18), and lesioning the dACC impairs reward-based selection in the monkey(19). A critical question is how this activity impacts on subsequent behaviour. To this end, Rushworth and colleagues recently examined the consequences of experimental dACC lesions in monkeys performing a series tasks requiring flexible adjustments in behaviour(15). The lesioned monkeys did not show impairments in adjusting behaviour immediately after errors - as conflict detection or simple error monitoring would predict - but rather were impaired in integrating reinforcement information over time to guide voluntary behaviour optimally. Similar inferences can be made from a recent neurophysiological study of adaptation to uncertain rewards(20). Rather than simply detecting conflict, it seems the dACC has a much more complex role in long term adjustments to goal-oriented behaviour.
Conflict resolution
A role in conflict between responses has also been ascribed to the more dorsally placed supplementary motor complex, particularly the pre-SMA. By contrast with the dACC, in addition to suggestive functional imaging(21-24), there is good evidence that damage or disruption of this area impairs behaviour in situations of response conflict(22, 25). Moreover, preliminary reports have shown single-cell activity specifically preceding switches between response rules in the pre-SMA, and improved switching behaviour following microstimulation of the same region. By varying the timing of the stimulation, these authors showed that behavioural effects were obtained with a delay estimated at 28 ms. Apart from the a priori considerations already referred to, this makes it much more likely that the pre-SMA is resolving conflict rather than just detecting it(26). This is also consistent with the medial frontal hypofunction(27) and selective degeneration of pre-SMA pyramidal neurones(28) in patients with Parkinson’s Disease, a condition widely conceptualised as involving a failure of inhibition of competing motor programs(29).
A shift away from a purely evaluative role is also evident in the SEF. Although this area has direct projections to saccadic centres in the brainstem it has traditionally been thought to monitor action rather than modulate it online(30). This view was challenged recently by the finding of a strikingly specific oculomotor deficit in a patient with a microlesion of the left SEF(31). This patient was shown to have a profound deficit when being forced to discard an ongoing voluntary saccadic plan in favour of one in the opposite direction. The direct executive role that this implies has now received support from monkey neurophysiology. Using a related paradigm - saccadic countermanding - in which the subject is forced to discard a reflexive saccadic plan in favour of continued fixation, Stuphorn & Schall(32) found that subthreshold microstimulation of the SEF improved countermanding performance by lengthening saccadic reaction times. Importantly, microstimulation in the same areas shortened purely reflexive saccades, demonstrating that the effect had the contextual specificity one would expect of executive control. The SEF therefore does not simply monitor action but exerts executive control in situations of response conflict.
Volition
Activity in the supplementary motor complex has also been traditionally associated with internally rather than externally guided movements, and much has been made of this distinction. Since a conventional criterion of voluntary action is that one has to be able to choose whether on not to perform it regardless of the external circumstances, “internal” has been taken as being paradigmatic of “voluntary” or “volitional”.
This idea may be misguided for two reasons. First, the processes involved in the performance of internally guided action are impossible to match to those involved in externally guided action. One can change the parameters of an external stimulus and observe the resulting modulation in the subject’s response to it, but one cannot know what is being changed when the impetus for an action is internal and therefore hidden from view. Second, conceptually, it is not clear what this internal quality really consists in or why it should be defining. Whereas reflexive acts are easy to distinguish on the basis that one may suppress them but cannot choose to perform them, it is hard to call one action more voluntary than another purely on the basis that it is less potently specified by external events. Whether apparently guided by external events or not we can choose whether to do something or not and our action is therefore volitional in both cases.
Following an influential theory of action, it has been suggested that the presence of a conscious urge to act is a criterion for voluntary action. If this is true then examining the processes underlying this urge might tell us something about how voluntary action arises. A recent imaging study purports to do just that, by asking subjects to respond freely and attend either to the timing of their urge to act or the timing of the act itself. The contrast between the two conditions revealed activation in the pre-SMA, which - it is argued - represented intention in the brain. The problem is that feeling an urge to move is neither a necessary nor a sufficient criterion for voluntary action(33). Indeed, so conceived, voluntary action would be like sneezing: the consequence of an urge that may be suppressed but never really chosen.
Aside from these theoretical difficulties, there may be many much simpler reasons why the pre-SMA should be more active when subjects attend more carefully to their movements, as they did in the critical condition here. Internally guided action is likely to be associated with greater co-activation of competing potential motor plans, and so may simply reflect their automatic and wholly non-volitional suppression so as to prevent conflict between competing plans. If so, then this process may be revealed in other circumstances involving conflict. This question has been explored by manipulating freedom of response selection and conflict in the same imaging paradigm that generated conflict between ongoing movement plans(24). Pre-SMA activation was shown in both cases, although in dissociable areas and without an interaction between them so a unitary mechanism was not demonstrated. Another study with similar aims found on the medial surface dACC activation only for conflict, and pre-SMA for free selection; however, this study is difficult to interpret owing to potential differences in arousal between the two critical conditions(34). This issue, therefore, remains open.
Ventromedial cortex
The functional topography of this region is unsettled. The orbitofrontal parts have been extensively studied and are heavily implicated in encoding the value associations of sensory percepts. Although the parts close to the frontal pole - here referred to as rostral ventromedial cortex - appear to be recruited by complex cognitive operations generally, interest here has focussed on their role in tasks involving thoughts about the self and others: what might be termed social cognition(8), particularly with reference to conditions associated with impairments in “theory of mind”.
Rostral ventromedial cortex
Perhaps the most poorly understood region of the prefrontal cortex is its most rostral part. Functional imaging has implicated this region in situations where subjects have to refer to their own mental states or those of others - past, present or future - particularly those charged with emotional significance. It has therefore been widely assumed that such “mentalizing” is its critical function(8), and recent research has sought to fractionate the anatomical substrates of such functions more finely, for example, by exploring the difference between thinking about the thoughts of others who are similar or dissimilar in outlook to oneself(35) or thinking emotionally charged or neutral thoughts(36). However, this begs the question of whether this region is specialised for such “mentalizing” or incidentally more strongly recruited in situations involving thoughts about people - self and other. Indeed, it is not difficult to argue that no cognitive state can match such states for both complexity and subject expertise. The activation that these experiments reveal may therefore simply reflect subjects’ expert exploration of a problem with a very large number of variables. Similarly overenthusiastic assumptions about the specificity for faces of the fusiform face area in extrastriate cortex have recently had to be revised in the light of evidence that it is merely concerned with general visual expertise(37, 38). Moreover, although rostromedial damage can lead to impaired performance at tasks requiring an understanding of the mental states of others, it is not clear that this deficit is specifically related to mentalizing(39).
In support of a less domain-specific function, a recent unconstrained volumetric brain morphometry study found that fluid intelligence correlated with grey matter volume most strongly in rostral ventromedial cortex(40). The authors of this study attempted - heroically - to reconcile this finding with the mentalizing account by suggesting that subjects with large medial prefrontal cortices might perform better because they make a rapid impression of the correct solution: a process requiring self-referencing because “an impression involves deciding what feels right to you”(my emphasis)(40). Clearly, a much simpler explanation is that it is merely the combination of complexity and expertise involved in mentalizing that is recruiting these regions, which in reality have a much broader role in situations where multiple cognitive processes are co-activated(41).
Orbitofrontal ventromedial cortex
The orbitofrontal cortex - perhaps unique amongst cortical structures in receiving the widest range of sensory inputs of any cortical area - is functionally characterised by strong modulation by rewards(42). Although initially shown in association with so called primary rewards - such as sweet tasting food - activity in this area is evoked by the associations between sensory stimuli and behaviourally relevant outcomes generally, whether of positive or negative valence. Anatomically, there is a anterior-posterior subdivision, with primary rewards being represented more posteriorly than conditioned rewards, and medial lateral subdivision with the outcomes of negative behavioural valence being represented more laterally than rewards(43). Critically, activity in this region conforms to the rules of temporal difference learning, being modulated by the discrepancy between expected outcomes and reality. The ventral striatum also shows such activity(44), which appears to be driven by a phasic signal arising from brainstem dopaminergic projections(45). It has recently been shown that this area and the dACC - which is also responsive to outcomes - can be dissociated on the basis of whether the outcome is determined by the subjects own actions (dACC) or external circumstances (oMFC). Such a role in establishing the value of things is consistent with the failure of patients with orbitofrontal damage to make advantageous choices when confronted with items whose different values they need to establish by trial and error, over time(4). It is also intriguing that patients with focal damage to this region are more likely to exhibit abnormal collecting behaviour - repetitively and indiscriminately acquiring useless objects - presumably as a result of abnormal neural representations of object value(46).
A notable aspect of recent studies is an increasing emphasis on exploring aspects of behaviour in situations of varying uncertainty(47-49). Not only is this ecologically more valid, but the response to uncertainty is of great interest in itself. A recent neurophysiological study of the reward-related signal carried by brainstem dopaminergic neurones has revealed tonic activity that is maximal when the monkey’s reward is most uncertain(50). What purpose could such “gambling” neurones serve? Perhaps the clue is that the monkeys in this experiment did not know they were gambling - that there was nothing in the external environment that could allow them to predict the rewards better. Thus the uncertainty signal may be modulating areas that would make the monkey attend to or explore the environment so as to attempt to minimise this uncertainty. This may well be why the human counterpart of this study has failed to show widespread modulation in cortical areas - humans can understand that no information is available(51). However, there are good indications that dorsomedial frontal areas might be activated in these circumstances - neuronal activity reflecting decision-making under obscure reward contingencies has been shown to occur very early in SEF, certainly much earlier than the frontal eye field or lateral intraparietal area(52). This region may therefore represent a key node in the link between uncertainty and action.
Conclusion
As self-consciousness is arguably a defining feature of humans (or possibly higher primates, e.g. (53)), brain areas most strongly activated during tasks defined with reference to the self will inevitably attract a great deal of interest. It is perhaps equally understandable that it should be so difficult to restrain the rather exuberant theorizing functional imaging seems to encourage here. However, there is an increasing realization of the need to test predictions from functional imagining rigorously using techniques that disrupt the function of the putatively critical area. It seems that the notion of conflict monitoring in the dACC will not survive such tests, and an alternative role in optimising behaviour in response to outcomes over time is suggested instead. By contrast, the pre-SMA and SEF are increasingly being implicated in immediate executive control. The role of orbitofrontal cortex in guiding action on the basis of value is now firmly established, but the tantalizing association of the neighbouring rostral ventromedial cortex with conceptions of the self needs closer scrutiny. If the self is to be grounded in a neural substrate, greater theoretical clarity is needed before the right questions can be posed, let alone answered.
Acknowledgements
The author is supported by the Wellcome Trust as part of a programme grant to Professors Christopher Kennard and Masud Husain.
References
- 1.Sestini S, Ramat S, Formiconi AR, Ammannati F, Sorbi S, Pupi A. Brain networks underlying the clinical effects of long-term subthalamic stimulation for Parkinson’s disease: a 4-year follow-up study with rCBF SPECT. J Nucl Med. 2005 Sep;46(9):1444–54. [PubMed] [Google Scholar]
- 2.Krainik A, Lehericy S, Duffau H, Vlaicu M, Poupon F, Capelle L, et al. Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology. 2001 Sep 11;57(5):871–8. doi: 10.1212/wnl.57.5.871. [DOI] [PubMed] [Google Scholar]
- 3.Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004 Sep;8(9):410–7. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994 Apr-Jun;50(1-3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 5.Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. Journal of Cognitive Neuroscience. 1998;10(5):640–56. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- 6.Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, et al. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999 Jun 3;10(8):1647–51. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- 7.Happe F, Ehlers S, Fletcher P, Frith U, Johansson M, Gillberg C, et al. ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport. 1996 Dec 20;8(1):197–201. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- 8.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006 Apr;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 9.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001 Jul;108(3):624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- *10.van Veen V, Carter CS. Separating semantic conflict and response conflict in the Stroop task: a functional MRI study. Neuroimage. 2005 Sep;27(3):497–504. doi: 10.1016/j.neuroimage.2005.04.042. [Evidence - in disagreement with the conflict monitoring hypothesis - that response conflict is dissociable from another kind of conflict.] [DOI] [PubMed] [Google Scholar]
- *11.Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006 May 18;50(4):643–53. doi: 10.1016/j.neuron.2006.04.015. [Evidence that different kinds of conflict do not engage the cingulate in the same way. The authors do not discard the concept of an anatomically discrete conflict detector but extend it to parietal cortex.] [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Roesch MR, Olson CR. Neuronal Activity in Macaque SEF and ACC During Performance of Tasks Involving Conflict. J Neurophysiol. 2004 Aug 4; doi: 10.1152/jn.00305.2004. [DOI] [PubMed] [Google Scholar]
- *13.Fellows LK, Farah MJ. Is anterior cingulate cortex necessary for cognitive control? Brain. 2005 Apr;128(Pt 4):788–96. doi: 10.1093/brain/awh405. [A direct test of the conflict detection hypothesis in patients with medial frontal damage fails to find any evidence of the predicted deficit.] [DOI] [PubMed] [Google Scholar]
- 14.Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003 Oct;126(Pt 10):2139–52. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- *15.Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006 Jul;9(7):940–7. doi: 10.1038/nn1724. [Monkey lesion study implicating the anterior cingulate cortex in flexibly adapting behaviour over time.] [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003 Oct 3;302(5642):120–2. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- 17.Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998 Nov 13;282(5392):1335–8. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto K, Suzuki W, Tanaka K. Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science. 2003 Jul 11;301(5630):229–32. doi: 10.1126/science.1084204. [DOI] [PubMed] [Google Scholar]
- 19.Hadland KA, Rushworth MF, Gaffan D, Passingham RE. The anterior cingulate and reward-guided selection of actions. J Neurophysiol. 2003 Feb;89(2):1161–4. doi: 10.1152/jn.00634.2002. [DOI] [PubMed] [Google Scholar]
- 20.Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex. 2006 Jul;16(7):1040–55. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001 Dec;14(6):1387–401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- 22.Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002 May;87(5):2577–92. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- 23.Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003 Oct;20(2):1132–9. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- 24.Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Curr Biol. 2005 Jan 26;15(2):122–8. doi: 10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura K, Sakai K, Hikosaka O. Effects of local inactivation of monkey medial frontal cortex in learning of sequential procedures. J Neurophysiol. 1999 Aug;82(2):1063–8. doi: 10.1152/jn.1999.82.2.1063. [DOI] [PubMed] [Google Scholar]
- **26.Isoda M, Hikosaka O. Switching from automatic to controlled behaviour. I. Role for the pre-SMA in overcoming automatic action. Society for Neuroscience Abstracts. 2005 [Sophisticated monkey neurophysiology - available only in abstract form - supporting the role of the pre-SMA in resolving conflict between responses.] [Google Scholar]
- 27.Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Ann Neurol. 1992 Aug;32(2):151–61. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald V, Halliday GM. Selective loss of pyramidal neurons in the pre-supplementary motor cortex in Parkinson’s disease. Mov Disord. 2002 Nov;17(6):1166–73. doi: 10.1002/mds.10258. [DOI] [PubMed] [Google Scholar]
- 29.Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996 Nov;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 30.Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000 Dec 14;408(6814):857–60. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- 31.Husain M, Parton A, Hodgson TL, Mort D, Rees G. Self-control during response conflict by human supplementary eye field. Nat Neurosci. 2003 Feb;6(2):117–8. doi: 10.1038/nn1005. [DOI] [PubMed] [Google Scholar]
- *32.Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006 Jul;9(7):925–31. doi: 10.1038/nn1714. [Confirmatory evidence of direct executive control by the supplementary eye field.] [DOI] [PubMed] [Google Scholar]
- 33.Bennett MR, Hacker PMS. Philosophical Foundations of Neuroscience. Oxford: Blackwell Publishing; 2003. [Google Scholar]
- 34.Lau H, Rogers RD, Passingham RE. Dissociating response selection and conflict in the medial frontal surface. Neuroimage. 2006 Jan 15;29(2):446–51. doi: 10.1016/j.neuroimage.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006 May 18;50(4):655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 36.Hynes CA, Baird AA, Grafton ST. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia. 2006;44(3):374–83. doi: 10.1016/j.neuropsychologia.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci. 2000 Feb;3(2):191–7. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y. Revisiting the role of the fusiform face area in visual expertise. Cereb Cortex. 2005 Aug;15(8):1234–42. doi: 10.1093/cercor/bhi006. [DOI] [PubMed] [Google Scholar]
- 39.Bird CM, Castelli F, Malik O, Frith U, Husain M. The impact of extensive medial frontal lobe damage on ‘Theory of Mind’ and cognition. Brain. 2004 Apr;127(Pt 4):914–28. doi: 10.1093/brain/awh108. [DOI] [PubMed] [Google Scholar]
- *40.Gong QY, Sluming V, Mayes A, Keller S, Barrick T, Cezayirli E, et al. Voxel-based morphometry and stereology provide convergent evidence of the importance of medial prefrontal cortex for fluid intelligence in healthy adults. Neuroimage. 2005 May 1;25(4):1175–86. doi: 10.1016/j.neuroimage.2004.12.044. [VBM study showing the rostral ventromedial frontal cortex grey matter as the only region significantly correlated with fluid intelligence.] [DOI] [PubMed] [Google Scholar]
- 41.Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004 Mar;5(3):184–94. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 42.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000 Mar;10(3):284–94. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 43.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004 Apr;72(5):341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 44.McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003 Apr 24;38(2):339–46. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 45.Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998 Aug;1(4):304–9. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- 46.Anderson SW, Damasio H, Damasio AR. A neural basis for collecting behaviour in humans. Brain. 2005 Jan;128(Pt 1):201–12. doi: 10.1093/brain/awh329. [DOI] [PubMed] [Google Scholar]
- 47.Sugrue LP, Corrado GS, Newsome WT. Choosing the greater of two goods: neural currencies for valuation and decision making. Nat Rev Neurosci. 2005 May;6(5):363–75. doi: 10.1038/nrn1666. [DOI] [PubMed] [Google Scholar]
- 48.Glimcher PW, Rustichini A. Neuroeconomics: the consilience of brain and decision. Science. 2004 Oct 15;306(5695):447–52. doi: 10.1126/science.1102566. [DOI] [PubMed] [Google Scholar]
- 49.Daw NDND, O’Doherty JPJP, Dayan PP, Seymour BB, Dolan RJRJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441(7095):876–9. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003 Mar 21;299(5614):1898–902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 51.Dreher JC, Kohn P, Berman KF. Neural coding of distinct statistical properties of reward information in humans. Cereb Cortex. 2006 Apr;16(4):561–73. doi: 10.1093/cercor/bhj004. [DOI] [PubMed] [Google Scholar]
- 52.Coe B, Tomihara K, Matsuzawa M, Hikosaka O. Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision-making task. J Neurosci. 2002 Jun 15;22(12):5081–90. doi: 10.1523/JNEUROSCI.22-12-05081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brosnan SF, De Waal FB. Monkeys reject unequal pay. Nature. 2003 Sep 18;425(6955):297–9. doi: 10.1038/nature01963. [DOI] [PubMed] [Google Scholar]