Abstract
Aim
To evaluate the efficacy and tolerability of formoterol delivered by Aerolizer in the emergency department.
Methods
A single‐centre, double‐blind, randomised, placebo‐controlled, parallel group study was conducted in patients seeking emergent care for an acute exacerbation of asthma. Patients were randomly assigned to one of two groups: group 1 (salbutamol), receiving a total dose of 600 μg salbutamol (200+200+200) delivered by a meter‐dose inhaler into a spacer device as two puffs at 20 min intervals; and group 2 (formoterol), receiving formoterol 24 μg (12+12) as two dry powder capsules each containing 12 μg of formoterol via Aerolizer at 20 min intervals. The peak expiratory flow rate (PEFR) was measured at baseline and 5 min after the second and third doses.
Results
60 subjects receiving salbutamol (n = 28) or formoterol (n = 32) completed the study. Age, gender, baseline PEFR, duration of asthma and previous medication were balanced between the two groups. Mean PEFR increased significantly over baseline values in both the salbutamol and formoterol groups (63% in the salbutamol group, p = 0.001, and 55% in the formoterol group, p = 0.001). No significant difference was observed in the increase in PEFR between the groups (p = 0.99, 95% CI −29.62 to 29.59). The proportion of patients reporting adverse events was similar in the two groups.
Conclusion
Formoterol was found to be well tolerated and as effective as salbutamol in the management of acute asthma. Further studies are needed to follow the patients after discharge from the emergency room to compare the long‐term effect of formoterol on patients' stability.
Despite the increasing use of long‐acting β2‐agonists in clinical practice, relatively few studies have addressed their role in acute asthma.1 β2‐agonists with long‐acting properties, formoterol and salmeterol, are recommended only as maintenance therapy in patients with moderate to severe asthma that is poorly controlled on inhaled corticosteroids.1,2 In addition to its use in maintenance therapy, formoterol is approved in Europe as a reliever medication. The National Heart, Lung, and Blood Institute, Maryland, USA emphasises that long‐acting β2‐agonists should not be used to treat acute symptoms or exacerbations.2
Currently, the repetitive administration of short‐acting inhaled β2‐agonists (2–4 puffs every 20 min for the first hour) is considered the preferred initial treatment for acute asthma.3 Thereafter, the dose of β2‐agonist required will depend on the severity of exacerbation, and varies from 2 puffs every 3 h to 10 puffs at internals of less than an hour.3
The rationale for using long‐acting β2‐agonists in the emergency department (ED) as a substitute to short‐acting β2‐agonists relates to their duration of action and potential for reducing the need of repeated administration of bronchodilator therapy.1
Formoterol, a long‐acting β2‐agonist with unique pharmacological properties as well as a favourable safety profile, seems ideal for the management of acute asthma. It has a fast onset of action, similar to that of short‐acting β2‐agonists such as salbutamol. In all, 80–90% of maximum bronchodilation occurs within 5–10 min of inhalation.4,5 An improvement in the mean forced expiratory volume in one second is sustained over 24 h after dosing, although the clinical duration of action is reported to be 12 h.6,7 In addition to rapid bronchodilation, the safety and tolerability high‐dose formoterol Turbuhaler relative to high‐dose terbutaline Turbuhaler and salbutamol meter‐dose inhaler (MDI) in the treatment of asthma exacerbation has been confirmed by previous comparative and non‐comparative studies.8,9
Formoterol is equally effective when given either by Aerolizer or by Turbuhaler.10,11 However, handling the Aerolizer is easier than handling the Turbuhaler, and patients make less critical errors while using an Aerolizer than while using a Turbuhaler.12,13 This feature could be especially valuable in choosing an alternative treatment for asthma exacerbation.
A recent study shows that rapid onset of bronchodilation can be achieved by administering formoterol via Turbuhaler (Oxis, Astrazeneca, Sweden) in patients with asthma exacerbation, which is associated with greater maximal efficacy during the third and fourth hour after dosing compared with salbutamol administered via MDI. In addition, it provides a safety profile at least as good as that of salbutamol when used up to 54 μg.9
Although the efficacy and safety of high‐dose formoterol delivered by Turbuhaler in asthma exacerbation has been demonstrated, that of low‐dose formoterol delivered by Aerolizer has not yet been studied.
The present study compares the efficacy and tolerability of equipotent doses of formoterol delivered by Aerolizer with that of salbutamol delivered by MDI.
Methods
Study population
We recruited adult patients with acute asthma over a 3‐month period. These patients had approached the ED of “Shaheed Labafinejad” hospital, Tehran, Iran. Approximately 22 000 patients visit this department annually, with nearly 1400 adult patients presenting with asthma exacerbation.
To be included in the study, patients had to fulfill the following criteria:
Age >18 years
American Thoracic Society's definition of asthma14
No history of hypersensitivity to β2‐agonists, thyrotoxicosis, ischaemic heart disease; severe tachyarrhythmia, heart failure, uncontrolled hypertension, pregnancy or breastfeeding
Able to perform a forced expiratory manoeuvre.
All patients with severe life‐threatening acute asthma who required admission to the intensive care unit were excluded from the study.
The study was approved by the medical ethics committee of the Shaheed Beheshti University of Medical Sciences, and Health Services and informed consent was obtained from all patients.
Study design
The study had a single‐centre, double‐blind, randomised, parallel‐group design. Demographic data, duration of asthma and treatment given at arrival to the ED were recorded.
The eligible patients were assigned by random number allocation to one of the two treatment groups—that is, either salbutamol or formoterol. Randomisation was carried out using sequential opaque sealed envelopes in which treatment allocation had been predetermined using blocked randomisation by a statistician not related to the study. Both treatments were started as soon as possible after the patient's arrival at the hospital.
In the salbutamol group, a total dose of 600 μg salbutamol (200+200+200) delivered by MDI with a spacer device (Jahan‐Behbood, Tehran, Iran) was given as two puffs (100 μg/puff) at 20‐min intervals. Patients in the second group were given formoterol 24 μg (12+12) as two dry powder capsules each containing 12 μg of formoterol via Aerolizer (Novartis Pharma AG, Basel, Switzerland) at 20 min intervals. The drugs were administered in a double‐blind manner. As placebo, the patients in the salbutamol group received two dry powder capsules, containing lactose, at the first and second doses in addition to salbutamol. The safety of lactose has been proven by previous studies.15
Patients in the formoterol group received two puffs from an identical MDI placebo (Jahan‐Behbood) at 20 min intervals concurrently. All treatments were administered by ED nurses who were trained to use the inhaler and were unaware of the treatment groups.
To omit the effect of other drugs on our outcome, systemic corticosteroids were added only to the treatment of those patients who did not respond to the third dose of the drug under study.
The decision to discharge or admit a patient was made by the ED attendant physician, who did not have any knowledge of the patient group allocation. Patients were discharged from the ED according to the British Thoracic Society guidelines16 and if they were improving clinically. All discharged patients were given prednisolone 60 mg daily for at least 5 days.
Measurements
The primary efficacy parameter was PEFR. It was measured with a peak flow meter (Jaeger, Germany) immediately before starting the treatment and 5 min after the second and third doses. At each assessment, the highest PEFR value was recorded from three manoeuvres. As self‐recording of PEFR by the patients themselves might be inaccurate, four ED nurses who were trained to use a peak flow meter performed all the measurements. After completing the full dose, patients were asked to indicate the presence or absence of each of the following symptoms: mouth dryness, dizziness, headache, nausea, palpitation, tremor and cramps. Secondary efficacy parameters included: proportion of patients who required hospitalisation, and proportion of patients who needed additional medication after completing the full dose of β2‐agonists.
Statistical methods
Differences in mean PEFR between the two groups were analysed by applying the Student's t test. Paired t test was used to compare PEFR before and after the second and third administrations to determine whether there were significant improvements in either group.
A previous trial has reported that the mean PEFR in persons with asthma exacerbation treated by salbutamol was 227.1 l/min, with a SD of 71.7 l/min.17 Estimations from power calculations predicted that 29 patients in each group (salbutamol and formoterol) would be required to detect a difference of 55 l/min in mean PEFR between the two treatment groups, at α (two‐sided) = 0.05 and power = 80%.
The proportion of patients who required hospitalisation or additional medication after the full dose of β2‐agonists was compared using the x2 test.
Results
Demographics and baseline characteristics
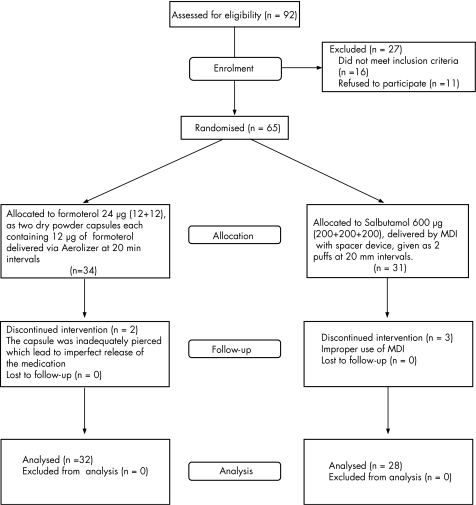
From a total of 92 patients enrolled in the study, 65 patients were randomised to treatment with either salbutamol or formoterol: 34 in the formoterol group and 31 in the salbutamol group. Figure 1 shows the progression of patients through the study phases. A total of five patients discontinued the study: 2 (6.25%) patients from the formoterol group (due to inadequate piercing of the capsule, which led to imperfect release of the medication) and 3 (9.6%) patients from the salbutamol group (due to improper use of MDI).
Figure 1 Flow chart describing the progress of patients through randomised trial.
Table 1 shows the demographic characteristics of the 60 patients who completed the study, and their baseline PEFR. The two treatment groups were well matched with regard to demographic characteristics. The mean PEFR value at baseline in the formoterol group (119.3 l/min) was higher than that in the salbutamol group (100.8 l/min). However, the difference was not statistically significant. A similar number of patients in each group had taken asthma medication. Of the 60 patients, 58 (96.7%) used inhaled β2‐agonists (salbutamol), 23 (38.3%) used inhaled glucocorticosteroids, 43 (71.7%) used theophylline, 30 (50%) used systemic glucocorticosteroids and 4 (6.7%) used cromolyn as previous medication.
Table 1 Baseline characteristics of the two treatment groups.
| Variables | Salbutamol (n = 28) | Formoterol (n = 32) |
|---|---|---|
| Sex (M/F) | 14/18 | 13/15 |
| Age (years), mean (SD) | 53.2 (15.0) | 56.4 (14.2) |
| Duration of asthma (years), mean (SD) | 12.4 (8.4) | 10.4 (8.1) |
| PEFR at baseline (l/min) | 100.8 | 119.3 |
F, female; M, male; PEFR, peak expiratory flow rate.
Efficacy results
In both groups, PEFR increased significantly over baseline values. Overall, there was no significant difference in mean PEFR changes after the third dose of treatment between the two groups (65.9 l/min in the salbutamol group vs 65.9 l/min in the formoterol group, p = 0.99, 95% CI, −29.62 to 29.59; table 2).
Table 2 Improvement in peak expiratory flow rate (l/min) after the second and third doses compared with baseline.
| PEFR | Salbutamol (n = 28) | Formoterol (n = 32) | p Value |
|---|---|---|---|
| Baseline–second dose | 40.5 (22.5 to 58.5) | 47.3 (32.1 to 62.5) | 0.55 |
| Baseline–third dose | 65.9 (41.2 to 90.5) | 65.9 (47.4 to 84.4) | 0.99 |
| Second–third dose | 25.3 (14.9 to 35.8) | 18.5 (7.7 to 29.4) | 0.36 |
PEFR, peak expiratory flow rate.
All p values are for two‐tailed t tests .
Values in parentheses are 95% CI.
Among the treatment groups, there were no statistically significant differences in the need for additional drugs or hospitalisation.
In all, 9 (28.1%) patients of the formoterol group and 9 (32.1%) patients of the salbutamol group needed an additional drug such as corticosteroids for the control of their exacerbation. In addition, 4 (12.5%) in the formoterol group and 5 (17.8%) in the salbutamol group required hospitalisation and were admitted.
Adverse events
Both treatments were well tolerated, and no unusual or unexpected adverse events (AEs) were reported. Overall, 34 AEs were reported. Typical β2‐receptor‐mediated subjective symptoms (reported as AEs) occurred during treatment with formoterol in 12 (43%) patients and during treatment with salbutamol in 11 (42.9%) patients. The most common AE was mouth dryness in 22 (36.7%) patients, which occurred with similar frequency in both groups. There were no differences in the formoterol and salbutamol groups with regard to reporting AEs (table 3).
Table 3 All important adverse events or side effects in each intervention group.
| Symptoms | Formoterol group n (%) | Salbutamol group n (%) | p Value |
|---|---|---|---|
| Mouth dryness | 12 (37.5) | 10 (35.7) | NS |
| Dizziness | 5 (15.6) | 2 (7.1) | NS |
| Headache | 3 (9.4) | 2 (7.1) | NS |
Discussion
We compared the efficacy and tolerability of the two treatment regimens (formoterol 24 μg as two dry powder capsules each containing 12 μg of formoterol via Aerolizer and salbutamol 600 μg via MDI with spacer in three equal doses) in providing relief of acute asthma exacerbation in the ED setting.
The results from this study demonstrated that formoterol delivered by Aerolizer was at least as effective as salbutamol delivered by MDI, based on improvement in PEFR at 5 min after the second and third doses in patients with acute asthma exacerbation.
Both formoterol Aerolizer and salbutamol MDI improved PEFR, and the difference between treatment groups for change in PEFR value from baseline to post second and third doses was not significant.
Furthermore, the rapid onset of action of formoterol Aerolizer in asthma exacerbation was in accordance with previous studies.6,7
The rapid onset of action of formoterol and salbutamol is explained by the ability of these drugs to reach the β2‐adrenoceptor from the aqueous phase, but, unlike salbutamol, formoterol is moderately lipophilic, enabling a considerable amount of drug, when inhaled, to diffuse into the lipid bilayer and to produce a long duration of action.18,19
This is the first study to compare formoterol Aerolizer and salbutamol MDI in asthma exacerbation. Boonsawat et al9 compared the efficacy and safety of high‐dose formoterol delivered by Turbuhaler with that of salbutamol delivered by MDI. Considering the fact that formoterol induces bronchodilation in a dose‐dependent manner,20,21 there would be a concern that a lower dose of formoterol could not be used in the management of acute asthma. Our results further extend the findings of this study to the effectiveness of a lower dose of formoterol in a population with asthma having more severe obstruction of airways.
Previous studies suggest that formoterol 12 μg Aerolizer is an equivalent bronchodilation dose to salbutamol 200 μg MDI.6 On the basis of the Global Initiative for Asthma guideline for the treatment of asthma exacerbation, which recommends two to four puffs of short‐acting β2‐agonist every 20 min for the first hour,3 we decided to compare formoterol Aerolizer with the lowest recommended dose of salbutamol.
In addition, patients with forced expiratory volume in one second <30% of predicted normal value were excluded in Boonsawat et al's9 study. Therefore, the study population did not represent the severe end of the spectrum of asthma exacerbation. However, in our study, any patient with asthma exacerbation who was able to generate a PEFR value was enrolled.
Unlike previous trials, the mean baseline PEFR value obtained in this study was very low in both groups (119.3 and 100.8 l/min), which was probably due to the age differences observed in these studies. The majority of our patients in both groups were aged >50 years, and this could have contributed to the lower mean PEFR value. In addition, our sample was drawn from a hospital which is a referral centre for patients with respiratory disorder with more severe forms of asthma exacerbation.
Given that our goal was to assess the role of formoterol in the management of asthma exacerbation, this low mean PEFR value did not seem to affect our results.
To manage asthma exacerbation in hospital, it is recommended that rapid‐acting inhaled β2‐agonists be administered via either MDI or a nebuliser. Current guidelines for the management of acute asthma, including The British Thoracic Society guidelines, have not mentioned that a nebuliser is superior to the MDI and spacer in treating mild and moderate exacerbations of asthma in children ⩾2 years old and in adults.22,23
Owing to the improper usage of the inhaler devices by most patients in Iran, β2‐agonists will be administered via an MDI by an appropriate volume spacer in hospital management of asthma exacerbation without life‐threatening features. Our chronic asthma treatment is based exactly on the Global strategy for asthma management and prevention.3
In our study, 2 (6.3%) patients in the formoterol group and 3 (10.7%) patients in the salbutamol group experienced a decline or no change in PEFR after administration of the sprays. All of these patients except one patient in the salbutamol group who was hospitalised, had an improvement in their symptoms.
One possible explanation could be that the improvement in the patient's well‐being has a mechanism different from the bronchodilatory and bronchoprotective effects of the β2‐agonists.
Another explanation could be related to the fact that each patient with asthma has a distinct number of β‐receptors, which respond to individualised doses of β2‐agonists.24
Therefore, the dose used in our study might not have been sufficient enough for them.
The safety profile of formoterol is well established.25 Several studies26,27,28 have shown that, despite having a prolonged bronchodilator effect in the airways, formoterol has a short duration of systemic effects. The proportions of patients reporting AE in this study were similar in the two groups, but more than those observed in previous studies. Mouth dryness and dizziness were the most frequent AEs. Neither tremor nor palpitation developed in any of our patients in the salbutamol group. There were no serious AEs and no discontinuations due to AEs. Formoterol was well tolerated, with no unusual or unexpected AEs. Patients in this trial received a maximum of 24 µg of formoterol via dry‐powder inhaler. We did not evaluate the cardiovascular and metabolic effects of this dose of formoterol in our patients. This dose was well within the tolerability range of formoterol, as evidence suggests that formoterol up to a daily dose of 120 μg delivered through Aerolizer has a safety profile comparable to that of short‐acting β2‐agonists.29
Our results support prior studies in suggesting formoterol as a very useful bronchodilator and as a potential substitute for controlling acute asthma attack. Our study was limited by several factors.
First, we did not follow patients after being discharged from the ED. The long‐term efficacy and safety of formoterol for management of asthma exacerbation needs to be assessed in future studies. We focused only on PEFR as the primary efficacy parameter in designing and implementing the study. The other data required for assessing the severity of asthma, such as respiratory rate, pulse or arterial blood gas results, were not included in this study, which could be another limitation. However, initial assessments (history, physical examination and tests) were performed for all patients according to the Global strategy for asthma management and prevention. Finally, the numbers enrolled were small, thus limiting our ability to detect smaller but potential clinically significant differences.
Abbreviations
AE - adverse event
ED - emergency department
MDI - meter‐dose inhaler
PEFR - peak expiratory flow rate
Footnotes
Competing interests: None declared.
References
- 1.Hospenthal M A, Peters J I. Long‐acting β2‐agonists in the management of asthma exacerbations. Curr Opin Pulm Med 20051169–73. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigo G J, Rodrigo C, Hall J B. Acute asthma in adults, a review. Chest 20041251081–1102. [DOI] [PubMed] [Google Scholar]
- 3.Global strategy for asthma management and prevention. NIH Publication 02–3659, 2004. http://www.ginasthma.org (accessed 8 March 2007)
- 4.van der Woude H J, Postma D S, Politiek M J.et al Relief of dyspnoea by beta2‐agonists after methacholine‐induced bronchoconstriction. Respir Med 200498816–820. [DOI] [PubMed] [Google Scholar]
- 5.Schermer T R, Hoff W J, Greefhorst A P.et al Profiles of measured and perceived bronchodilation. A placebo‐controlled cross‐over trial comparing formoterol and salmeterol in moderate persistent asthma. Pulm Pharmacol Ther 200417205–212. [DOI] [PubMed] [Google Scholar]
- 6.Bronsky E A, Yegen U, Yeh C M.et al Formoterol provides long‐lasting protection against exercise‐induced bronchospasm. Ann Allergy Asthma Immunol 200289407–412. [DOI] [PubMed] [Google Scholar]
- 7.Bensch G, Lapidus R J, Levine B E.et al A randomized, 12‐week, double‐blind, placebo‐controlled study comparing formoterol dry powder inhaler with albuterol metered‐dose inhaler. Ann Allergy Asthma Immunol 20018619–27. [DOI] [PubMed] [Google Scholar]
- 8.Malolepszy J, Boszormenyi Nagy G, Selroos O.et al Safety of formoterol turbuhaler at cumulative dose of 90 mg in patients with acute bronchial obstruction. Eur Respir J 200118928–934. [DOI] [PubMed] [Google Scholar]
- 9.Boonsawat W, Charoenratanakul S, Pothirat C.et al Formoterol (OXISs) Turbuhalers as a rescue therapy compared with salbutamol MDI plus spacer in patients with acute severe asthma. Respir Med 2003971067–1074. [DOI] [PubMed] [Google Scholar]
- 10.Eliraz A, Ramirez‐Rivera A, Ferranti P.et al Similar efficacy following four weeks treatment of asthmatics with formoterol 12 micrograms b.d. delivered by two different dry powder inhalers: differences in inhaler handling, Int J Clin Pract 200155164–170. [PubMed] [Google Scholar]
- 11.Lotvall J, Mellen A, Arvidsson P.et al Similar bronchodilation with formoterol delivered by aerolizer or turbuhaler. Can Respir J 19996412–416. [DOI] [PubMed] [Google Scholar]
- 12.Bronsky E A, Grossman J, Henis M J.et al Inspiratory flow rates and volumes with the Aerolizer dry powder inhaler in asthmatic children and adults. Curr Med Res Opin 200420131–137. [DOI] [PubMed] [Google Scholar]
- 13.Molimard M. How to achieve good compliance and adherence with inhalation therapy. Curr Med Res Opin 200521(Suppl 4)S33–S37. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society Standard for the diagnosis and care of patients with chronic obstructive pulmonary diseases (COPD) and asthma. Am Rev Resp Dis 1987136225–244. [DOI] [PubMed] [Google Scholar]
- 15.Maesen F P, Costongs R, Smeets S J.et al Formoterol as dry powder inhalation, a dose finding study in comparison with formoterol metered dose inhaler and placebo. Chest 19921011376–1381. [DOI] [PubMed] [Google Scholar]
- 16.British Thoracic Society, Scottish Intercollegiate Guidelines Network British guideline on the management of asthma. Thorax 200358(Suppl I)i1–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigo G J, Rodrigo C. First‐line therapy for adult patients with acute asthma receiving a multiple‐dose protocol of ipratropium bromide plus albuterol in the emergency department. Am J Respir Crit Care Med 20001611862–1868. [DOI] [PubMed] [Google Scholar]
- 18.Johnson M. Effects of β2‐agonists on resident and infiltrating inflammatory cells. J Allergy Clin Immunol 2002110S282–S290. [DOI] [PubMed] [Google Scholar]
- 19.Ringdal N, Derom E, Wahlin‐Boll E.et al Onset and duration of action of single doses of formoterol inhaled via Turbuhaler. Respir Med 1998921017–1021. [DOI] [PubMed] [Google Scholar]
- 20.Cazzola M, D'Amato M, Califano C.et al Formoterol as dry powder oral inhalation compared with salbutamol metered‐dose inhaler in acute exacerbations of chronic obstructive pulmonary disease. Clin Ther 200224595–604. [DOI] [PubMed] [Google Scholar]
- 21.Ketchell R I, Jensen M W, Spina D.et al Dose‐related effects of formoterol on airway responsiveness to adenosine 5'‐monophosphate and histamine. Eur Respir J 200219611–616. [DOI] [PubMed] [Google Scholar]
- 22.Idris A H, McDermott M F, Raucci J C.et al Emergency department treatment of severe asthma. Metered‐dose inhaler plus holding chamber is equivalent in effectiveness to nebulizer. Chest 1993103665–672. [DOI] [PubMed] [Google Scholar]
- 23.Newman K B, Milne S, Hamilton C.et al A comparison of albuterol administered by metered‐dose inhaler and spacer with albuterol by nebulizer in adults presenting to an urban emergency department with acute asthma. Chest 20021211036–1041. [DOI] [PubMed] [Google Scholar]
- 24.Maesen F P, Costongs R, Smeets J J.et al The effect of maximal doses of formoterol and salbutamol from a meter dose inhaler on pulse rates, ECG, and serum potassium concentrations. Chest 1991991367–1373. [DOI] [PubMed] [Google Scholar]
- 25.Rosenhall L, Elvstrand A, Tilling B.et al One‐year safety and efficacy of budesonide/formoterol in a single inhaler (Symbicort Turbuhaler) for the treatment of asthma. Respir Med 200397702–708. [DOI] [PubMed] [Google Scholar]
- 26.Tötterman K J, Huhti L, Sutinen E.et al Tolerability to high doses of formoterol and terbutaline via Turbuhaler for 3 days in stable asthmatic patients. Eur Respir J 199812573–579. [DOI] [PubMed] [Google Scholar]
- 27.Guhan A R, Cooper S, Oborne J.et al Systemic effects of formoterol and salmeterol: a dose‐response comparison in healthy subjects. Thorax 200055650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenborg J, Larsson R, Rott Z.et al Relative therapeutic index between inhaled formoterol and salbutamon in asthma patients. Respir Med 200296412–417. [DOI] [PubMed] [Google Scholar]
- 29.Lecaillon J B, Kaiser G, Palmisano M.et al Pharmacokinetics and tolerability of formoterol in healthy volunteers after a single high dose of Foradil dry powder inhalation via Aerolizer. Eur J Clin Pharmacol 199955131–138. [DOI] [PubMed] [Google Scholar]