Abstract
Arsenic is a well-known human skin carcinogen but the underlying mechanisms of carcinogenesis are unclear. Transcription factor Nrf2-mediated antioxidant response represents a critical cellular defense mechanism, and emerging data suggest that constitutive activation of Nrf2 contributes to malignant phenotype. In the present study when an immortalized, non-tumorigenic human keratinocyte cell line (HaCaT) was continuously exposed to environmentally relevant level of inorganic arsenite (100 nM) for 28 weeks, malignant transformation occurred as evidenced by the formation of highly aggressive squamous cell carcinoma after inoculation into nude mice. To investigate the mechanisms involved, a broad array of biomarkers for transformation were assessed in these arsenic-transformed cells (termed As-TM). In addition to increased secretion of matrix metalloproteinase-9 (MMP-9), a set of markers for squamous differentiation and skin keratinization, including keratin-1, keratin-10, involucrin, and loricrin, were significantly elevated in As-TM cells. Furthermore, As-TM cells showed increased intracellular glutathione, elevated expression of Nrf2 and its target genes, as well as generalized apoptotic resistance. In contrast to increased basal Nrf2 activity in As-TM cells, a diminished Nrf2-mediated antioxidant response induced by acute exposure to high dose of arsenite or tert-butyl hydroxyquinone occurred. The findings that multiple biomarkers for malignant transformation observed in As-TM cells, including MMP-9 and cytokeratins, are potentially regulated by Nrf2 suggest constitutive Nrf2 activation may be involved in arsenic carcinogenesis of skin. The weakened Nrf2 activation in response to oxidative stressors observed in As-TM cells, coupled with acquired apoptotic resistance, would potentially have increased the likelihood of transmittable oxidative DNA damage and fixation of mutational/DNA damage events.
Keywords: Arsenic, Carcinogenesis, Nrf2, Oxidative stress, CK2
Introduction
Inorganic arsenic is a well-recognized, multi-site human carcinogen and exposure is associated with an increased risk for dermal malignancies [1]. Arsenic is also carcinogenic in rodent models, producing liver, lung, ovary and adrenal tumors after transplacental exposure [2] and skin tumors in combination with ultraviolet (UV) irradiation or phorbol esters in mice [3, 4]. However, arsenic alone does not appear to induce skin cancer in these mouse models [2–4], suggesting events associated with arsenic-induced dermal carcinogenesis may be distinct from other target tissues. Accumulating evidence suggests that oxidative stress occurs in response to arsenic exposure [5, 6] and may be one factor in dermal arsenic carcinogenesis. Indeed, evidence of arsenic-induced oxidative DNA damage has been observed in cells [7–12], in rodents [13] and in humans [14].
Transcription factor NF-E2-related factor 2 (Nrf2), a Cap‘n’ collar basic leucine zipper protein, regulates critically important cellular defense transcriptional programs that maintain cellular redox homeostasis and serve to limit oxidative damage and inflammation [15]. Nrf2 controls expression of a variety of genes encoding for antioxidative and phase 2 drug-metabolizing enzymes through antioxidant response elements (ARE) [15]. Nrf2-controlled genes encode for various enzymes, including detoxification enzymes like glutathione S-transferases (GST), NAD(P)H: quinone oxidoreductase 1 (NQO1), and heme oxygenase 1 (HO-1) and antioxidant enzymes like the γ-glutamate cysteine ligase catalytic subunit (GCLC) and regulatory subunit (GCLM). Nrf2 also controls expression of several transporters including the multidrug resistance protein-1 and cysteine-glutamate-exchange transporter, among many other proteins. Supporting the importance of Nrf2 in cellular defense is the finding that Nrf2-deficient mice show a deficiency in this coordinated gene regulatory program and have a higher susceptibility to both oxidative damage and chemical carcinogenesis [16, 17]. In apparent contrast to extensive data showing that low Nrf2 activity predisposes cells to chemical carcinogenesis, emerging evidence suggests that constitutive activation of Nrf2 may contribute to a malignant phenotype [18]. Indeed, increased expression and activity of Nrf2 has been observed in various tumor cells [19, 20]. These inconsistencies suggest that Nrf2 may play paradoxical roles in different stages of tumorigenesis.
Given the potential importance of oxidative DNA damage in dermal carcinogenesis, as well as the critical roles of Nrf2 in the defense against oxidative damage, here we study whether Nrf2 and its target genes are involved in arsenic-induced skin carcinogenesis using a human dermal cell model system in which we find arsenic induces malignant transformation of human keratinocytes.
Materials and Methods
Cell culture
The HaCaT cell is a spontaneously immortalized human epithelial cell line developed by Boukamp et al. [21]. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 U penicillin/ml, and 100 μg streptomycin/ml. Cultures were maintained at 37°C in a humidified 5% CO2 atmosphere with an oxygen concentration of 19.9%. For chronic arsenic exposure, cells were maintained continuously in medium containing 100 nM of sodium arsenite (NaAsO2, Sigma, St. Louis, MO) for 28 weeks as described previously [10]. All the materials for cell culture were purchased from Invitrogen (Carlsbad, CA)
Zymographic analysis of metalloproteinase-9 (MMP-9) activity
Cells at 70–80% confluence were washed twice with phosphate-buffered saline (PBS), and the medium was changed to serum-free DMEM. After 48 hrs, the conditioned medium was collected and centrifuged for 5 min at 600 × g. A 500-μl aliquot was concentrated to < 100 μl in a Microcon concentrator at 14,000 × g at 4°C. Protein concentration was determined using a commercial assay (Bio-Rad, Hercules, CA), and 1 μg of total protein (6–10 μl) from each sample was electrophoresed on a 10% zymography gel containing 0.1% gelatin (Novex, San Diego, CA). MMP-9 activity was detected by incubating the gel in 1× zymogram renaturing buffer for 30 min at room temperature and then in 1× zymogram developing buffer (Novex) overnight at 37 °C, followed by staining with GelCode Blue (Pierce Corp., Rockford, IL). After staining, the bands were quantified using Bio-Rad Gel Doc 2000™ Systems with Bio-Rad TDS Quantity One software.
Establishing malignant transformation
To test their ability to form tumors, the chronic arsenic-treated and passage-matched control HaCaT cells were inoculated bilaterally into the renal capsules (2 million cells/kidney capsule/animal) of male nude mice (NCr-nu, n = 11). Mice were obtained at 8 weeks of age from the NCI-Frederick Animal Production Area and were housed in an American Association for Accreditation of Laboratory Animal Care (AAALAC) accredited facility under conditions that met or exceeded recommendations outlined in the Guide for Care and Use of Laboratory Animals (National Institutes of Health Publication No. 86–23, 2985). The study proposal was approved by the Institutional Animal Care and Use Committee. Tumor formation was assessed biweekly. The first tumor was detected 10 weeks after injection of arsenic-treated cells and the average sacrifice time for the mice in this group was 150 days. Controls were sacrificed when the last mouse inoculated with arsenic-treated cells was sacrificed. Kidneys and any resulting tumors were embedded in paraffin, sectioned, stained with H&E, and analyzed by light microscopy.
Acute cytotoxicity assay
A minimum of 5 replicates of 10,000 cells per well were plated in 96-well plates and allowed to adhere to the plate for 24 hrs, at which time the media was removed and replaced with fresh serum-free media containing arsenic compounds. Cells were then incubated for an additional 24 hrs and cell viability and cytotoxicity was determined using Non-Radioactive Cell-Proliferation Assay Kit (Promega, Madison, WI) and CytoTox96 Non-Radioactive Cytotoxicity Assay Kit (Promega), respectively.
Determination of apoptosis by flow cytometry
Cells were seeded in 6-wells plate and grown to ~80% confluence. 18 hrs post UVB exposure the cells, including floating and attached cells, were harvested for apoptosis analysis. Detection of phosphatidylserine on the outer leaflet of apoptotic cells was performed using TACS Annexin V-FITC Apoptosis Detection Kit (Trevigen, Gaithersburg, MD) detailed previously [10]. For each sample, 10,000 cells were examined by flow cytometry using a Becton Dickinson FACSort (Becton Dickinson, San Jose, CA). The percent of apoptotic cells was determined by statistical analysis of the various dot plots using CellQuest software.
Preparation of protein extracts and western blotting
The whole cell extracts were obtained by using Cell Lysis Buffer (Cell Signaling Technology, Inc., Beverly, MA) with 0.5% of Protease Inhibitor Cocktail (Sigma) and 1% of Phosphatase Inhibitor Cocktail I (Sigma). Nuclear and cytosolic fractions were separated by TransFactor Extraction Kit (BD Biosciences Clontech, Palo Alto, CA). All the protein fractions were stored at −70 °C until use. Proteins were separated by Novex 4–12% or 4–20% Tris-Glycine Gel (Invitrogen) and transferred onto nitrocellulose membranes. The blots were probed with the primary antibodies followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Antibody incubations were performed in Blocker™ BLOTTO in TBS (Pierce, Rockford, IL). Immunoreactive proteins were detected by chemiluminescence using ECL reagent (Amersham Pharmacia, Piscataway, NJ) and subsequent autoradiography. Antibodies for Nrf2 (sc-13032), keratin-10 (sc-6258), involucrin (sc-15223), HO-1 (sc-1797), NQO1 (sc-16464), CK2α (sc-9030), CK2α′ (sc-6481), and CK2β (sc-12739) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies for caspase-3 (#9661), caspase-7 (#9491); PARP (#9541) and β-actin (#4967) were from Cell Signaling.
Measurement of CK2 activity
The activity of CK2 in nuclear fractions was determined using Casein Kinase 2 Assay Kit (Upstate, Lake Placid, NY). Briefly, nuclear fraction (30 μg protein) was incubated with CK2 substrate peptide (200 μM) in CK2 buffer (pH 7.2) containing 20 mM MOPS, 25 mM β-glycerol phosphate, 5 mM EGTA, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 25 mM magnesium chloride, 100 μM ATP, 0.2 mM substrate peptide, 0.4 μM PKA inhibitor cocktail, and 100 μCi of [γ-32P]ATP (PerkinElmer Life Sciences, Inc., Boston, MA) for 30 min at 37 °C. After the reaction was stopped by adding 20 μl of 40% trichloroacetic acid, a 25 μl aliquot was transferred to a P81 paper square, and the radiolabeled substrate was allowed to bind to the paper for 30 s. The paper squares were rinsed three times with 30 ml of 0.75% phosphoric acid each and followed by a 5-min washing with 20 ml of acetone. Dried squares were transferred to scintillation vial filled with 5 ml of scintillation cocktail (ICN Biomedicals Inc., Costa Mesa, CA) and read in LS 6500 Multi-Purpose Scintillation Counter (Beckman Coulter, Fullerton, CA).
Quantitative real-time RT–PCR analysis
Total RNA was isolated by using TRIzol (Invitrogen), and then subjected to DNase I digestion using RNase-Free DNase Set (Qiagen, Valencia, CA), followed by cleanup with RNeasy Mini kit (Qiagen). RNA from each sample was reverse transcribed with MuLV reverse transcriptase (Applied Biosystems, Foster City, CA) and Oligo d(T) primers. The SYBR Green PCR Kit (Applied Biosystems) was used for quantitative real-time RT-PCR analysis. The primers (Table S1, Online materials) were designed using Primer Express software (Applied Biosystems). Relative differences in gene expression between groups were expressed using cycle time values; these values were first normalized with that of β-actin in the same sample, and the expression in the experimental group was expressed as folds of controls. Real time fluorescence detection was carried out using a MyiQ™ SingleColor Real-Time PCR Detection System (Bio-Rad, Hercules, CA).
Statistics
All statistical analyses were performed using Graphpad Prism 4 (GraphPad Software, San Diego, CA), with p < 0.05 taken as significant. For comparisons between As-TM and control cells, Students’ t test were performed. Statistical analyses to evaluate the acute effect of arsenite on Nrf2 protein levels as well as the time- and dose-dependent effects of arsenite or tBHQ exposure on gene expression between As-TM and control cells were performed using two-way ANOVA with Bonferroni post hoc testing. A Fischer’s exact test was used to examine differences in tumor incidence after inoculation of control or As-TM cells into nude mice.
RESULTS
Low level, chronic arsenic treatment of HaCaT cells induces malignant transformation
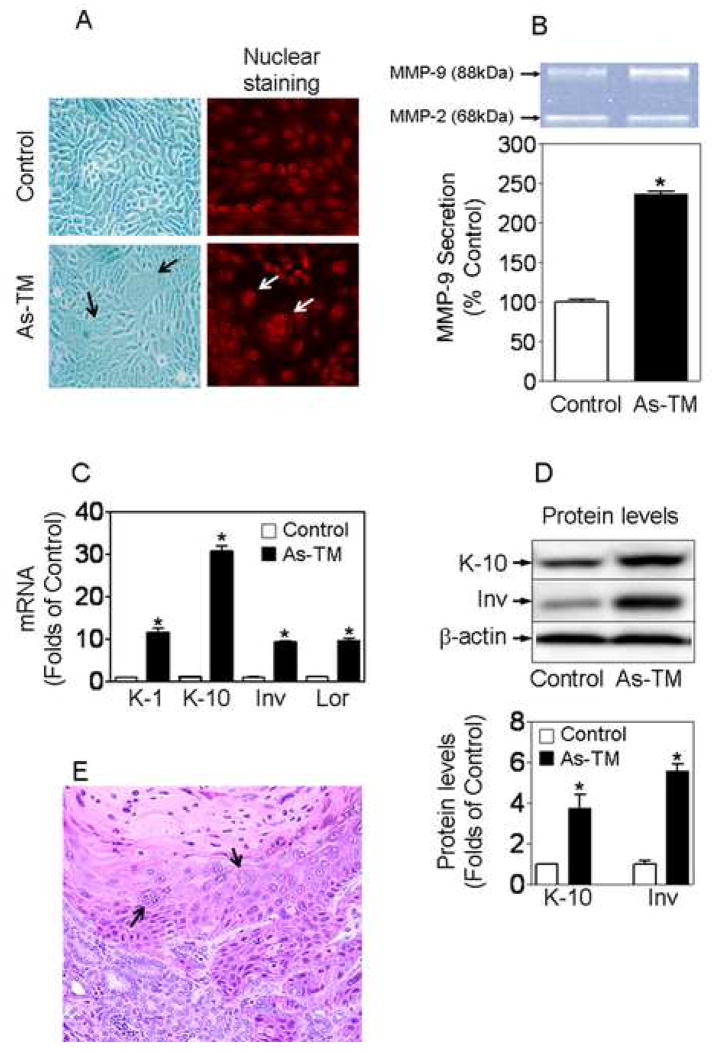
To achieve oncogenic transformation, HaCaT cells were continuously exposed to a low level (100 nM) of inorganic arsenite. The arsenic concentration used in this study is comparable to human blood arsenic levels found in chronic arsenosis patients in Inner Mongolia, China, where arsenic-induced skin lesions and cancers are common [22]. After 28 weeks of continuous arsenic exposure, the arsenic-treated cells exhibited unique morphological alterations with the frequent occurrence of giant multinuclear cells (Fig. 1A), which are common during malignant transformation and in tumors [23]. In addition, a marked increase in secretion of active MMP-9, but not MMP-2, from arsenic-treated cells occurred (Fig. 1B). Extracellular MMP-9 activity was 2.3-fold higher in arsenic-treated cells compared to control. Furthermore, As-TM cells expressed dramatically higher expression levels of keratin-1, keratin-10, involucrin and loricrin, markers of keratinocyte differentiation and skin keratinization, compared to control cells (Fig. 1C and 1D). Malignant transformation was confirmed when the arsenic-treated cells produced aggressive cancers after inoculation into nude mice, and these cells are henceforth termed As-TM to designate the acquisition. Within 20 weeks of inoculation, 5/11 mice inoculated with the As-TM cells developed tumors (P = 0.035) compared to 0/11 mice inoculated with the control HaCaT cells. Histologic examination revealed the tumors to be highly aggressive squamous cell carcinoma (SCC), showing clear evidence of invasion into the renal parenchyma (Fig. 1E). Thus, based on variety of criteria, it is clear that the As-TM cells have acquired a highly malignant phenotype that duplicates a common form of skin cancer in arsenic-exposed humans [1].
Figure 1.
Low-level, chronic arsenic exposure induces malignant transformation in HaCaT cells. (A) Morphological alterations in arsenic-exposed cells. Images were taken by a microscope with a Polarold camera (200 × magnification) (left) and a laser scanning confocal microscope (LSM 510 NLO) after the cells were stained with propidium iodide (right). Arrows indicate giant multinuclear cells. (B) Analysis of MMP-9 secretion from As-TM and passage-matched control cells. Changes in MMP-9 activity were quantified by densitometric analysis of the bands. n = 3. *, p < 0.05 vs. control cells. (C–D) Increased expression of keratin-1, keratin-10, involucrin and loricrin in As-TM cells. Gene expression and protein levels were determined using real-time RT-PCR (C) and Western blot (D), respectively. Lower panel of (D) is the quantified results of Western blots. n = 3 – 4. *, p < 0.05 vs. control cells. (E) Histologic examination of tumors formed by As-TM cells after inoculation into the renal capsules of male nude mice. 20 weeks after inoculation, the tumors were removed, fixed in formalin, embedded in paraffin, sectioned, and stained with H&E. The staining pattern revealed the tumors to be aggressive squamous cell carcinoma. Arrows indicate giant multinuclear cells.
As-TM cells resistant to arsenic cytotoxicity and UVB-induced apoptosis
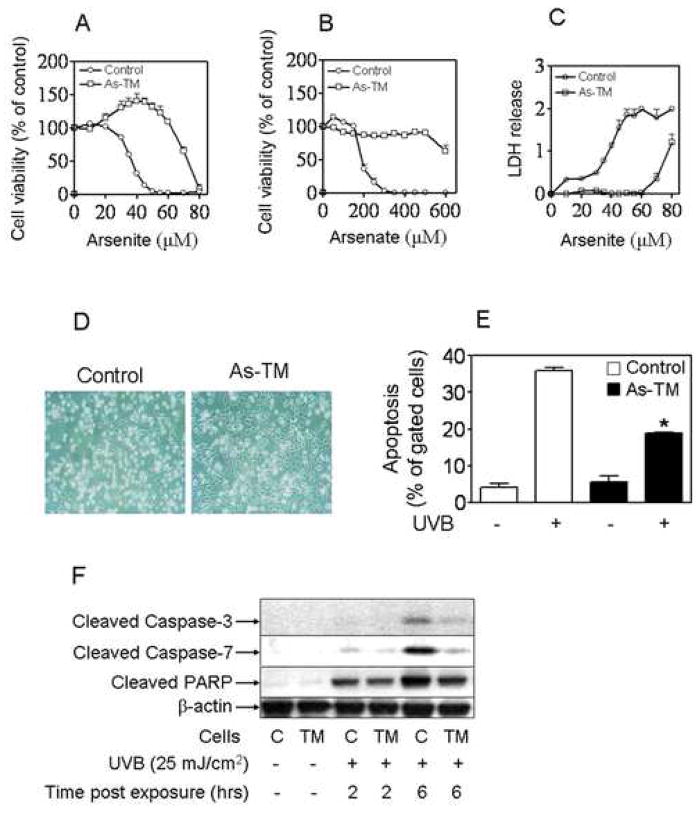
Our previous data [10] indicated that HaCaT cells chronically treated with arsenic show resistance to apoptosis induced by high concentrations of arsenite or UVA. Here, a generalized resistance to arsenic cytotoxicity is also observed in As-TM cells. When As-TM and control cells were exposed to different concentrations of arsenite (Fig. 2A and 2C) or inorganic arsenate (Fig. 2B) for 24 hrs, assays of metabolic integrity (Fig. 2A and 2B) as well as lactate dehydrogenase (LDH) release (Fig. 2C) revealed a dramatic difference in cytotoxicity between the two cell lines. Since methylation is a primary metabolic process of arsenic in vivo and also is involved in arsenic toxicity and carcinogenetic effect, the methylation activity was evaluated in As-TM and control cells. Surprisingly, no arsenic methylation activity was detected in both As-TM and control cells (Online material Fig. S1). Thus, whatever the basis of toxicity in this case the agent is likely not a methylated arsenical.
Figure 2.
As-TM cells acquired generalized apoptotic resistance. (A–C) As-TM cells resistant to cytotoxicity induced by inorganic arsenicals. After one-week arsenic-free cultivation, cells were seeded into 96-well plates (10000 cells/well) and grown to 80% confluence at which time the indicated concentrations of arsenicals including arsenite (A and C) and arsenate (B) were added. Cytotoxicity was assessed 24 hrs later by metabolic integrity MTS assay (A–B) and LDH release (absorbance at 490 nm) (C). Results are presented as the mean ± SEM, n = 6. Results of MTS assay expressed as percent of untreated control of appropriate cells, which are set at 100%. (D–F) As-TM cells resistant to apoptosis induced by UVB. (D) 18 hr after cells were exposed to UVB (25 mJ/cm2), images were taken by a microscope with a Polarold camera. (E) Percentage of total apoptosis calculated based on the measurements of flow cytometry. n = 4. *, p < 0.05 vs. control cells with UVB. (F) Immunoblotting of cleaved caspase-3 and 7 and PARP in As-TM and control cells. C, control; TM, As-TM cells.
To examine whether the As-TM cells acquired apoptotic resistance to UVB, a potent human dermal carcinogen, As-TM and control cells were exposed to environmental exposure-relevant doses of UVB radiation (25 mJ/cm2). At 18 hr after cells were exposed to UVB, general cell damage and apoptosis was determined by microscopy examination and flow cytometry, respectively. As shown in Fig. 2D, a significantly lower portion of As-TM cells (32%) vs. control cells (81%) showed morphological changes including shrinkage or floating. Consistent with this microscopy observation, 35.4% of the control cells underwent apoptosis after UVB exposure, whereas only 18.8% of the As-TM cells were apoptotic (Fig. 2E). These results were supported by caspase-3, caspase-7 and PARP analysis. Western blot analysis indicated that the As-TM cells exhibited much less activated caspase-3, caspase-7 and PARP than control cells after high UVB exposure (Fig. 2F). These data, together with our previous findings [10], indicate As-TM cells acquired generalized apoptotic resistance.
As-TM cells show increased CK2 expression and nuclear activity
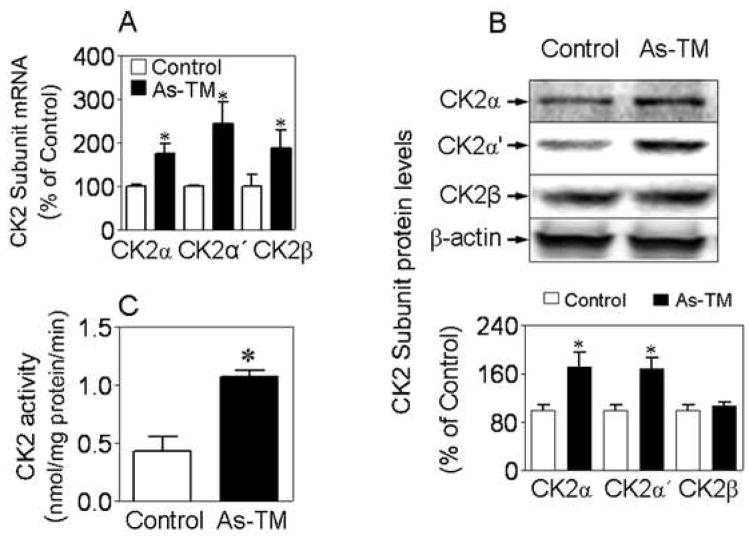
Our recent results [24] provided evidence that endogenous human Nrf2 is constitutively phosphorylated and that casein kinase-2 (CK2) is the major kinase responsible for the phosphorylation, which is critical in regulating the Nrf2-mediated cellular antioxidant response. CK2 is composed of two catalytic subunits (α and α′ assembled as αα, α′ α′ or αα′), and a dimer of two regulatory β subunits [25]. As shown in Fig. 3A, the As-TM cells showed enhanced CK2 expression, including α, α′, and β subunits when compared to control. Furthermore, the nuclear protein levels of CK2 catalytic subunits α and α′ (Fig. 3B), as well as CK2 specific activity in nuclear fractions (Fig. 3C) were significantly higher in As-TM cells than in control cells.
Figure 3.
As-TM cells exhibit increased CK2 expression and activity. (A) Gene expression of CK2 subunits in As-TM and controls. (B) Protein levels of CK2 subunits in nuclear fractions extracted from As-TM and controls. Lower panel, quantified results of the Western blot results. (C) CK2 activity in nuclear fractions extracted from As-TM and control cells. *, p < 0.05 vs. control cells.
As-TM cells exhibit enhanced antioxidant levels and weakened Nrf2-mediated antioxidant response
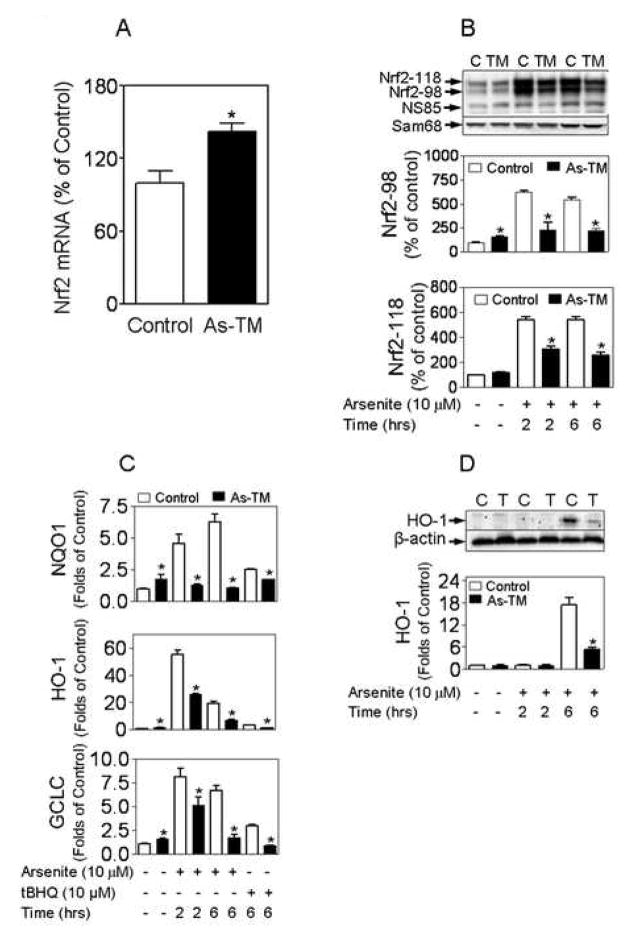
Since oxidative damage has been recognized as a possible mode of action of arsenic carcinogenesis [9, 26], levels of intracellular GSH and its oxidized form (GSSG) were measured in As-TM and control cells. GSH, synthesized by GCL and glutathione synthetase, is the most important, as well as abundant, redox buffer in cells [27]. In its reaction to scavenge peroxides, GSH is converted by oxidation to GSSG, which can subsequently be reduced back to GSH by glutathione reductase. The balance between GSH and GSSG has long been recognized as an important indicator of oxidative stress [27]. As shown in Fig. S2A (Online materials), intracellular GSH levels of As-TM cells are slightly, but statistically higher than that of control cells. Along with this, Fig. S2B illustrates the dramatic increase in GSSG levels in the As-TM cells, suggesting a chronic oxidative stress occurred in these cells. Consistent with the results that As-TM cells contain elevated levels of GSH and GSSG, the gene expression of GCLC and its key regulatory nuclear factor Nrf2 is significantly increased in the cells (Fig. 4 A and 4C). In addition, without acute stress challenge the mRNA levels of NQO1 and HO-1, both Nrf2 targets, are also significantly higher in As-TM cells than controls (Fig. 4 C).
Figure 4.
As-TM cells show enhanced basal Nrf2 activity and weakened Nrf2-mediated antioxidant response. (A) Gene expression of Nrf2 in As-TM cells. n = 3–6. *, p < 0.05 vs. control. (B) Representative images of Nrf2 immunoblots with nuclear fractions (upper) and quantitative results (middle and lower). *, p < 0.05 vs. exposure- and time-matched control cells. C, control; TM, As-TM cells. (C) Real-time RT-PCR analysis of expression of Nrf2-target genes. Data are shown as folds of the control cells without acute treatment. *, p < 0.05 vs. exposure- and time-matched control cells. (D) Protein levels of HO-1 in As-TM and control cells acutely exposed to arsenite. C, control cells; TM, As-TM cells. *, p < 0.05 vs. exposure-and time-matched control cells.
Our previous data indicated that two phosphorylated forms of endogenous Nrf2 accumulate in the nucleus after chemically-induced oxidative stress including acute arsenite exposure [24]. CK2-mediated sequential phosphorylation, resulting in Nrf2-98 with transcriptional activity and Nrf2-118 causing Nrf2 degradation, plays a critical role in Nrf2 activation and degradation [24]. Consistent with the findings that As-TM cells acquired increased nuclear CK2 activity (Fig. 3C) and the regulatory roles of CK2 on Nrf2 phosphorylation [24], As-TM cells showed a higher ratio of the form Nrf2-118 to Nrf2-98 after the cells were acutely exposed to arsenite at a dose (10 μM, 2–6 hrs) which can induce Nrf2 activation (Fig. 4B). In particular, the Nrf2-98, with transcriptional activity, was much lower in As-TM than in control cells under the same acute treatment conditions (Fig. 4B). Importantly, in As-TM cells the increases in the Nrf2-target genes, including NQO1, HO-1 and GCLC, induced by acute arsenite or tert-butylhydroquinone (tBHQ) treatment were significantly lower than control cells (Fig. 4B). Furthermore, compared to control cells a dramatic decrease in protein levels of HO-1 induced by acute arsenite challenge was observed in As-TM cells (Fig. 4D).
Discussion
Low-level, chronic arsenic exposure can induce malignant transformation in various human and rodent cells [28–32]. In the present study when HaCaT cells were continuously exposed to environmentally relevant levels of arsenic for a protracted period, malignant transformation occurred as evidenced by the formation of highly aggressive SCC, a common form of skin cancer in arsenic-exposed humans [1], after inoculation of nude mice. Importantly, by studying this cell model system several important biomarkers for arsenic-induced malignant transformation, including increased secretion of MMP-9, expression profile of Nrf2-target genes and cytokeratins, acquired apoptotic resistance and decreased Nrf2-mediated antioxidant response, were indentified.
MMPs are a family of matrix-degrading enzymes that play a critical role in invasion and metastasis of malignant tumors and hypersecretion of MMPs is common in highly aggressive tumors [33]. MMP-9 specifically targets type IV collagen, a major component of basement membrane, and appears to play a crucial role in tumor invasion across the basement membrane [34]. Elevated expression levels of MMP-9 are strongly correlated with malignant phenotype in SCC [34] and are characteristic of malignant transformation of cells [29]. In human keratinocyes constitutive production and secretion of MMP-9 correlate with the degree of tumorigenicity in malignantly transformed clones [33]. Thus, the dramatically increased secretion of MMP-9 observed in As-TM cells might be an important biomarker for arsenic-induced malignant transformation in human keratinocytes. It is noteworthy that the promoter region of MMP-9 gene contains multiple highly conserved AREs [35], suggesting Nrf2 may act as a direct transcriptional regulator of MMP-9.
Skin hyperkeratosis is common in chronic arsenosis [22]. Importantly, SCC can arise from arsenic-induced hyperkeratotic lesions [36], suggesting dysfunctional keratinization may be critical in arsenic-induced skin carcinogenesis. In the current study, As-TM cells were observed to exhibit dramatically increased expression levels of keratin-1, keratin-10, involucrin, and loricrin, major markers of squamous differentiation that play important roles in skin keratinization [37]. Since aberrant over-expressions of these markers have been observed in skin hyperkeratosis [38, 39], skin SCC [40], bladder SCC [41] and lung cancer [42], the enhanced expressions observed in As-TM cells may represent additional indicators for arsenic-induced malignant transformation. Interestingly, emerging data suggests that Nrf2 may act as a direct transcriptional regulator of certain squamous differentiation genes through their respective AREs [43]. Thus, the enhanced expression of these genes and marked increase in secretion of active MMP-9 observed in As-TM cells might be a consequence of chronic Nrf2 activation. It should be noted that the expression of keratin-10 in As-TM cells is different from what was observed in higher concentrations of arsenite-induced malignant transformation of keratinocyte [30].
CK2 is a ubiquitous and highly conserved pleiotropic serine/threonine protein kinase, and it appears to function predominantly in regulating the activities of nuclear proteins, including the regulation of cell growth and proliferation, as well as cell survival [25, 44]. Dysregulation of CK2 expression imparts a potent oncogenic potential to cells and overexpression of CK2 is often a key factor in carcinogenic transformation [45]. A consistent elevation of CK2 has been observed in rapidly proliferating tissues and a wide variety of tumors [46]. In the present study, increased expression and activity of CK2 was observed in As-TM cells, suggesting increase in CK2 might be a critical event during the process of arsenic-induced malignant transformation of human keratinocytes.
Apoptosis normally functions to control the integrity of cell populations by eliminating aberrant clones, whereas failure of apoptosis likely is a key contributor to tumor initiation and progression as well as drug resistance in skin cancer and cancer in general [47, 48]. Thus, the acquired, generalized apoptotic resistance in As-TM cells may be an important event in the process of arsenic-induced malignant transformation. Our previous studies have indicated that arsenic-induced apoptotic resistance is potentially associated with PI3K/PKB-mediated cellular survival signaling pathways [10]. In the present study, increased levels of CK2, which participates in inhibition of apoptosis and negatively regulates caspase activity in tumor cells [49], was observed. This finding suggests the enhanced CK2 expression of As-TM cells might be involved in their acquired apoptotic resistance.
In contrast to increased expression of Nrf2 and its target genes, including GCLC as well as elevated levels of intracellular GSH, a weakened Nrf2-mediated antioxidant response was observed in As-TM cells. The decrease of Nrf2 activation in response to high levels of oxidative stressors observed in As-TM cells, coupled with their acquired apoptotic resistance might increase the likelihood to acquire heritable oxidative DNA damage [7, 9]. This may help explain the remarkable co-carcinogenic effects of arsenic and UV irradiation observed in mouse models of dermal carcinogenesis but the absence of activity for arsenic alone [3, 4]. Our previous study indicated that exogenous GSH might markedly suppress hypochlorous acid-induced Nrf2 activation in mouse macrophages [50], suggesting the enhanced GSH levels in As-TM cells might be a critical factor for the weakened Nrf2 activation. In addition, increased activities of Nrf2 downstream phase II enzymes in As-TM cells might detoxify both arsenic and tBHQ, and thus results in decreased Nrf2 activation. Furthermore, our recent studies revealed that CK2 is an important protein kinase for Nrf2 phosphorylation, which is involved in Nrf2 activation and degradation [24]. The enhanced activity of CK2 and subsequent Nrf2 phosphorylation of As-TM cells might also contribute to their decreased Nrf2-medicated antioxidant response.
In summary, long-term arsenic exposure at levels in the range of human exposure causes malignant transformation of human keratinocytes in vitro. This occurs concurrently with acquired apoptotic resistance, increased expression of CK2, elevated basal Nrf2 activity, and decreased Nrf2-mediated antioxidant response. Arsenic-induced apoptotic resistance and weakened antioxidant response may be critical steps in development of dermal cancer after exposure to the metalloid.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This research was also funded in part with Federal Funds from the National Cancer Institute under contract #NO1-CO-124000. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the United States Government. We thank Drs. E. Tokar, C. Kojima, C.W. Woods, and L.K. Keefer for their critical reviews of this manuscript.
Abbreviations
- ARE
antioxidant response element
- As-TM
arsenic-transformed cells
- CK2
casein kinase 2
- DMAs
dimethylated arsenic
- DMEM
Dulbecco’s modified Eagle’s Medium
- GCL
γ-glutamate cysteine ligase
- GCLC
γ-glutamate cysteine ligase catalytic subunit
- GCLM
γ-glutamate cysteine ligase regulatory subunit
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- GST
glutathione S-transferase
- HO-1
heme oxygenase 1
- iAs
inorganic arsenic
- Inv
involucrin
- K-1
keratin-1
- K-10
keratin-10
- Lor
Loricrin
- MMAs
monomethylated arsenic
- MMP-2
matrix metalloproteinase-2
- MMP-9
matrix metalloproteinase-9
- NQO1, NAD(P)H
quinone oxidoreductase 1
- Nrf2
nuclear factor E2-related factor 2
- PBS
phosphate buffered saline
- PKB
protein kinase B
- ROS
reactive oxygen species
- SCC
squamous cell carcinoma
- tBHQ
tert-butyl hydroxyquinone
- UVA
ultraviolet A
- UVB
ultraviolet B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.IARC. IARC monograph on the evaluation of the carcinogenic risk to human-overall evaluations of carcinogenicity: an update of IARC monographs 1 to 42. IARC; Lyon (France): 1987. Arsenic and arsenic compounds. [Google Scholar]
- 2.Waalkes MP, Ward JM, Diwan BA. Induction of tumors of the liver, lung, ovary and adrenal in adult mice after brief maternal gestational exposure to inorganic arsenic: promotional effects of postnatal phorbol ester exposure on hepatic and pulmonary, but not dermal cancers. Carcinogenesis. 2004;25:133–41. doi: 10.1093/carcin/bgg181. [DOI] [PubMed] [Google Scholar]
- 3.Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: an animal model for arsenic carcinogenesis. Toxicol Appl Pharmacol. 2001;176:64–71. doi: 10.1006/taap.2001.9277. [DOI] [PubMed] [Google Scholar]
- 4.Germolec DR, Spalding J, Yu HS, Chen GS, Simeonova PP, Humble MC, Bruccoleri A, Boorman GA, Foley JF, Yoshida T, Luster MI. Arsenic enhancement of skin neoplasia by chronic stimulation of growth factors. Am J Pathol. 1998;153:1775–85. doi: 10.1016/S0002-9440(10)65692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pi J, Yamauchi H, Kumagai Y, Sun G, Yoshida T, Aikawa H, Hopenhayn-Rich C, Shimojo N. Evidence for induction of oxidative stress caused by chronic exposure of Chinese residents to arsenic contained in drinking water. Environ Health Perspect. 2002;110:331–6. doi: 10.1289/ehp.02110331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pi J, Qu W, Reece JM, Kumagai Y, Waalkes MP. Transcription factor Nrf2 activation by inorganic arsenic in cultured keratinocytes: involvement of hydrogen peroxide. Exp Cell Res. 2003;290:234–45. doi: 10.1016/s0014-4827(03)00341-0. [DOI] [PubMed] [Google Scholar]
- 7.Kessel M, Liu SX, Xu A, Santella R, Hei TK. Arsenic induces oxidative DNA damage in mammalian cells. Mol Cell Biochem. 2002:234–235. 8. [PubMed] [Google Scholar]
- 8.Shi H, Hudson LG, Ding W, Wang S, Cooper KL, Liu S, Chen Y, Shi X, Liu KJ. Arsenite causes DNA damage in keratinocytes via generation of hydroxyl radicals. Chem Res Toxicol. 2004;17:871–8. doi: 10.1021/tx049939e. [DOI] [PubMed] [Google Scholar]
- 9.Hei TK, Filipic M. Role of oxidative damage in the genotoxicity of arsenic. Free Radic Biol Med. 2004;37:574–81. doi: 10.1016/j.freeradbiomed.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Pi J, He Y, Bortner C, Huang J, Liu J, Zhou T, Qu W, North SL, Kasprzak KS, Diwan BA, Chignell CF, Waalkes MP. Low level, long-term inorganic arsenite exposure causes generalized resistance to apoptosis in cultured human keratinocytes: Potential role in skin co-carcinogenesis. Int J Cancer. 2005;116:20–6. doi: 10.1002/ijc.20990. [DOI] [PubMed] [Google Scholar]
- 11.Ding W, Hudson LG, Liu KJ. Inorganic arsenic compounds cause oxidative damage to DNA and protein by inducing ROS and RNS generation in human keratinocytes. Mol Cell Biochem. 2005;279:105–12. doi: 10.1007/s11010-005-8227-y. [DOI] [PubMed] [Google Scholar]
- 12.Wang TC, Jan KY, Wang AS, Gurr JR. Trivalent arsenicals induce lipid peroxidation, protein carbonylation, and oxidative DNA damage in human urothelial cells. Mutat Res. 2007;615:75–86. doi: 10.1016/j.mrfmmm.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Piao F, Ma N, Hiraku Y, Murata M, Oikawa S, Cheng F, Zhong L, Yamauchi T, Kawanishi S, Yokoyama K. Oxidative DNA damage in relation to neurotoxicity in the brain of mice exposed to arsenic at environmentally relevant levels. J Occup Health. 2005;47:445–9. doi: 10.1539/joh.47.445. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi H, Aminaka Y, Yoshida K, Sun G, Pi J, Waalkes MP. Evaluation of DNA damage in patients with arsenic poisoning: urinary 8-hydroxydeoxyguanine. Toxicol Appl Pharmacol. 2004;198:291–6. doi: 10.1016/j.taap.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–40. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410–5. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci U S A. 2001;98:4611–6. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stacy DR, Ely K, Massion PP, Yarbrough WG, Hallahan DE, Sekhar KR, Freeman ML. Increased expression of nuclear factor E2 p45-related factor 2 (NRF2) in head and neck squamous cell carcinomas. Head Neck. 2006;28:813–8. doi: 10.1002/hed.20430. [DOI] [PubMed] [Google Scholar]
- 21.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pi J, Kumagai Y, Sun G, Yamauchi H, Yoshida T, Iso H, Endo A, Yu L, Yuki K, Miyauchi T, Shimojo N. Decreased serum concentrations of nitric oxide metabolites among Chinese in an endemic area of chronic arsenic poisoning in inner Mongolia. Free Radic Biol Med. 2000;28:1137–42. doi: 10.1016/s0891-5849(00)00209-4. [DOI] [PubMed] [Google Scholar]
- 23.Bershadsky AD, Gelfand VI, Vasiliev JM. Multinucleation of transformed cells normalizes their spreading on the substratum and their cytoskeleton structure. Cell Biol Int Rep. 1981;5:143–50. doi: 10.1016/0309-1651(81)90022-9. [DOI] [PubMed] [Google Scholar]
- 24.Pi J, Bai Y, Reece JM, Williams J, Liu D, Freeman ML, Fahl WE, Shugar D, Liu J, Qu W, Collins S, Waalkes MP. Molecular mechanism of human Nrf2 activation and degradation: Role of sequential phosphorylation by protein kinase CK2. Free Radic Biol Med. 2007;42:1797–806. doi: 10.1016/j.freeradbiomed.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinna LA. Protein kinase CK2: a challenge to canons. J Cell Sci. 2002;115:3873–8. doi: 10.1242/jcs.00074. [DOI] [PubMed] [Google Scholar]
- 26.Shi H, Hudson LG, Liu KJ. Oxidative stress and apoptosis in metal ion-induced carcinogenesis. Free Radic Biol Med. 2004;37:582–93. doi: 10.1016/j.freeradbiomed.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 28.Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad Sci U S A. 1997;94:10907–12. doi: 10.1073/pnas.94.20.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J Natl Cancer Inst. 2002;94:1888–91. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- 30.Chien CW, Chiang MC, Ho IC, Lee TC. Association of chromosomal alterations with arsenite-induced tumorigenicity of human HaCaT keratinocytes in nude mice. Environ Health Perspect. 2004;112:1704–10. doi: 10.1289/ehp.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sens DA, Park S, Gurel V, Sens MA, Garrett SH, Somji S. Inorganic cadmium- and arsenite-induced malignant transformation of human bladder urothelial cells. Toxicol Sci. 2004;79:56–63. doi: 10.1093/toxsci/kfh086. [DOI] [PubMed] [Google Scholar]
- 32.Wen G, Calaf GM, Partridge MA, Echiburu-Chau C, Zhao Y, Huang S, Chai Y, Li B, Hu B, Hei TK. Malignant (Neoplastic) Transformation of Human Small Airway Epithelial Cells Induced by Arsenic. Mol Med. 2007 doi: 10.2119/2007-00090.Wen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachmeier BE, Boukamp P, Lichtinghagen R, Fusenig NE, Fink E. Matrix metalloproteinases-2,-3,-7,-9 and-10, but not MMP-11, are differentially expressed in normal, benign tumorigenic and malignant human keratinocyte cell lines. Biol Chem. 2000;381:497–507. doi: 10.1515/BC.2000.064. [DOI] [PubMed] [Google Scholar]
- 34.Bernhard EJ, Gruber SB, Muschel RJ. Direct evidence linking expression of matrix metalloproteinase 9 (92-kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proc Natl Acad Sci U S A. 1994;91:4293–7. doi: 10.1073/pnas.91.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Tomso DJ, Chorley BN, Cho HY, Cheung VG, Kleeberger SR, Bell DA. Identification of polymorphic antioxidant response elements in the human genome. Hum Mol Genet. 2007;16:1188–200. doi: 10.1093/hmg/ddm066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong SS, Tan KC, Goh CL. Cutaneous manifestations of chronic arsenicism: review of seventeen cases. J Am Acad Dermatol. 1998;38:179–85. doi: 10.1016/s0190-9622(98)70596-1. [DOI] [PubMed] [Google Scholar]
- 37.Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol Rev. 1997;77:397–424. doi: 10.1152/physrev.1997.77.2.397. [DOI] [PubMed] [Google Scholar]
- 38.Sumitomo S, Kumasa S, Iwai Y, Mori M. Involucrin expression in epithelial tumors of oral and pharyngeal mucosa and skin. Oral Surg Oral Med Oral Pathol. 1986;62:155–63. doi: 10.1016/0030-4220(86)90038-1. [DOI] [PubMed] [Google Scholar]
- 39.Shen CS, Tabata K, Matsuki M, Goto T, Yokochi T, Yamanishi K. Premature apoptosis of keratinocytes and the dysregulation of keratinization in porokeratosis. Br J Dermatol. 2002;147:498–502. doi: 10.1046/j.1365-2133.2002.04853.x. [DOI] [PubMed] [Google Scholar]
- 40.Said JW, Sassoon AF, Shintaku IP, Banks-Schlegel S. Involucrin in squamous and basal cell carcinomas of the skin: an immunohistochemical study. J Invest Dermatol. 1984;82:449–52. doi: 10.1111/1523-1747.ep12260937. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Carbayo M, Socci ND, Charytonowicz E, Lu M, Prystowsky M, Childs G, Cordon-Cardo C. Molecular profiling of bladder cancer using cDNA microarrays: defining histogenesis and biological phenotypes. Cancer Res. 2002;62:6973–80. [PubMed] [Google Scholar]
- 42.Zhang H, Yousem SA, Franklin WA, Elder E, Landreneau R, Ferson P, Keenan R, Whiteside T, Levitt ML. Differentiation and programmed cell death-related intermediate biomarkers for the development of non-small cell lung cancer: a pilot study. Hum Pathol. 1998;29:965–71. doi: 10.1016/s0046-8177(98)90202-7. [DOI] [PubMed] [Google Scholar]
- 43.Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–45. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed K, Gerber DA, Cochet C. Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol. 2002;12:226–30. doi: 10.1016/s0962-8924(02)02279-1. [DOI] [PubMed] [Google Scholar]
- 45.Seldin DC, Leder P. Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science. 1995;267:894–7. doi: 10.1126/science.7846532. [DOI] [PubMed] [Google Scholar]
- 46.Munstermann U, Fritz G, Seitz G, Lu YP, Schneider HR, Issinger OG. Casein kinase II is elevated in solid human tumours and rapidly proliferating non-neoplastic tissue. Eur J Biochem. 1990;189:251–7. doi: 10.1111/j.1432-1033.1990.tb15484.x. [DOI] [PubMed] [Google Scholar]
- 47.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 48.Guzman E, Langowski JL, Owen-Schaub L. Mad dogs, Englishmen and apoptosis: the role of cell death in UV-induced skin cancer. Apoptosis. 2003;8:315–25. doi: 10.1023/a:1024112231953. [DOI] [PubMed] [Google Scholar]
- 49.Yamane K, Kinsella TJ. CK2 inhibits apoptosis and changes its cellular localization following ionizing radiation. Cancer Res. 2005;65:4362–7. doi: 10.1158/0008-5472.CAN-04-3941. [DOI] [PubMed] [Google Scholar]
- 50.Pi J, Zhang Q, Woods CG, Wong V, Collins S, Andersen ME. Activation of Nrf2-mediated oxidative stress response in macrophages by hypochlorous acid. Toxicol Appl Pharmacol. 2008;226:236–43. doi: 10.1016/j.taap.2007.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.