Abstract
Background
Hyperglycemia after traumatic brain injury (TBI) is associated with poor outcome. This study examined the incidence and risk factors for perioperative hyperglycemia in children with TBI.
Methods
A retrospective cohort study of children ≤ 13 years who underwent urgent or emergent craniotomy for TBI at Harborview Medical Center (level I Adult and Pediatric Trauma Center) between 1994 and 2004 was performed. Preoperative (Emergency department to general anesthesia start), intraoperative (during general anesthesia), and immediate postoperative (first 24 hours after surgery) glucose values for each patient were retrieved. The incidence of hyperglycemia (glucose ≥ 200 mg/dL) and hypoglycemia (glucose < 60 mg/dL) was determined. Persistent hyperglycemia was defined as hyperglycemia during any 2/3 (preoperative, intraoperative and immediate postoperative) study periods whereas transient hyperglycemia was defined as hyperglycemia during any one study period. Multivariate logistic regression analysis was used to determine the independent predictors of perioperative hyperglycemia. Data are presented as AOR (95% CI) and p < 0.05 reflects significance.
Results
At-least one serum glucose value was recorded during each study period: preoperative (86 [82%]), intraoperative (94 [89%]), and postoperative (101 [97%]. Sixty four percent of children had less than one glucose recorded per anesthetic hour. Forty-seven (45%) children had hyperglycemia during at least one study period. Transient hyperglycemia occurred in 29 (28%) and persistent hyperglycemia occurred in 18 (17%) of children. Independent predictors of perioperative hyperglycemia were age < 4 years (AOR [95% CI]; 3.5 [1.2–10.6]), GCS ≤ 8 (AOR 95% CI; 7.2 (2.4–21.5)) and the presence of multiple lesions including SDH (AOR 95% CI; 34.7 [2.3– 525.5]). Six children were treated with insulin, and 2 children had hypoglycemia, unrelated to insulin treatment.
Conclusions
Perioperative hyperglycemia was common and intraoperative hypoglycemia was not rare but more frequent intraoperative glucose sampling may be needed to better determine the incidence of hypo and hyperglycemia during the perioperative period. Age < 4 years, severe TBI and the presence of multiple lesions including SDH were risk factors for perioperative hyperglycemia.
Keywords: hyperglycemia, children, traumatic brain injury
Introduction
Severe pediatric traumatic brain injury (TBI) is associated with high morbidity and mortality.1–5 While brain damage due to trauma accounts for the majority of early mortality following TBI, secondary injury to the brain can occur as a result of hypotension, hypoxia, increased intracranial pressure and hyperglycemia; thereby leading to poor outcome.1–3, 5–7 The prevention and rapid correction of these secondary insults can result in improved outcome after TBI.
Previous studies have shown that pediatric patients with TBI have higher serum glucose levels than those suffering from trauma without TBI, and that hyperglycemia occurs more frequently in children with severe TBI than in those with mild and moderate TBI.2,4 Additionally, admission hypergylcemia after TBI is associated with poor outcome, especially when it persists beyond the first 24 hours.2 High Pediatric Intensive Care Unit (PICU) admission glucose is associated with longer PICU length of stay and higher in-hospital mortality8 and lower admission glucose in severely head injured infants has been reported to be associated with good Glasgow Outcome Score (GOS) at one year.5 Although severe TBI indicated by lower Glasgow Coma Scale (GCS) score and head computed tomography (CT) findings have been reported to correlate with admission hyperglycemia in pediatric patients,1,2 perioperative hyperglycemia has never been considered. Despite the fact that the intraoperative period is physiologically very stressful and may be associated with secondary insults, the incidence of perioperative hyperglycemia including the intraoperative period is not known. In this study, we aimed to examine the incidence and risk factors for perioperative hyperglycemia in children with TBI and hypothesized that the incidence of perioperative hyperglycemia, which includes the intraoperative period, in children with TBI was high and was associated with young age and severe TBI.
Methods
Study design
A retrospective cohort study of children ≤ 13 years who underwent urgent or emergent craniotomy for TBI at Harborview Medical Center (HMC: level I Adult and Pediatric Trauma Center) was performed after approval by the University of Washington’s Human Subjects Institutional Review Board.
Subjects and setting
Children ≤ 13 years who underwent urgent or emergent craniotomy for TBI over a 10 year period between 1994 and 2004 at HMC were included. Anesthetic records with CPT codes reflecting evacuation of subdural hematoma (SDH), epidural hematoma (EDH), intracerebral hemorrhage (ICH), decompressive craniotomy or craniotomy were retrieved to generate a complete list of eligible children. Children with history of diabetes mellitus and those returning to operating room for repeat intracranial surgery were excluded. Medical and anesthetic records were reviewed for demographics, GCS upon arrival to the emergency department (ED), radiographic findings corresponding to the time immediately preceding surgery, insulin administration and in-hospital mortality. Preoperative (the period from admission to the ED to the commencement of general anesthesia in the operating room), intraoperative and immediate postoperative (first 24 hours after surgery) glucose values for each patient were retrieved from the HMC Laboratory Information Systems (LIS) database. When intraoperative glucose values were not available through LIS, they were abstracted from the anesthetic records.
Outcomes
The main outcome was the incidence of hyperglycemia, defined as serum glucose ≥ 200mg/dL at any point of time during the preoperative, intraoperative and immediate postoperative (first 24 hour) period. Secondary outcomes were: (1) insulin treatment of hyperglycemia, (2) incidence of hypoglycemia (glucose < 60mg/dL) and (3) incidence of persistent hyperglycemia. Persistent hyperglycemia was defined as hyperglycemia during any two of the three (preoperative, intraoperative and immediate postoperative) study periods and transient hyperglycemia was defined as any episode of hyperglycemia during any one study period. We also examined mortality.
Statistical Analysis
Serum glucose values were categorized into bins as follows: < 60 mg/dL, 60–110 mg/dL, 111–149 mg/dL, 150–199 mg/dL, 200–299 mg/dL, 300–399 mg/dL and ≥ 400 mg/dL. Descriptive statistics were used to examine clinical characteristics, perioperative (preoperative, intraoperative and immediate postoperative) glucose data, insulin treatment, and incidence of hyperglycemia and hypoglycemia. The relationship between 1) preoperative and intraoperative glucose, and 2) intraoperative and postoperative glucose was examined using Spearman’s rank correlation. Student’s T test was used to examine age differences, perioperative fluids, and duration of anesthesia in children with and without perioperative hyperglycemia. The univariate association between age < 4 years, severe TBI (ED GCS ≤ 8), TBI lesion type (isolated EDH / isolated SDH / isolated ICH / any SDH / multiple lesions), extracranial injuries, mannitol administration, ICP > 20 mmHg, fever (temperature ≥ 38.5°C), hypotension (systolic blood pressure less than fifth percentile for age and gender), and hyperglycemia was examined using Fisher’s Exact and Chi-square test. We also examined the relationship between hyperglycemia and death. Significant univariate factors (p ≤ 0.05) were then entered in a multivariate logistic regression model to determine the independent predictors of perioperative hyperglycemia (SPSS 11.0, Tx). Data are presented as mean ± SD (range), median (range), number (percent), or adjusted odds ratios (95% CI) as appropriate. p < 0.05 was considered significant.
Results
Insitutional Management of pediatric TBI
Currently, the management of children with TBI at HMC aims to achieve the goals described in the 2003 pediatric guidelines.9 Prior to publication of the guidelines, the clinical management of these children was more variable. However, during the study period, none of the patients received perioperative steroids and all were administered dextrose free intravenous fluids during the pre and intraoperative period. All patients received either D5NS or D ½ NS postoperatively in the PICU as maintenance fluids. During the study period, while there was no fixed anesthetic protocol and the anesthetic regime was determined by attending anesthesiologist, all patients received inhalational anesthesia with < 1 MAC isoflurane or sevoflurane without nitrous oxide and intermittent boluses of fentanyl / morphine to provide analgesia with the overall goals of maintaining cerebral blood flow. Mild hyperventilation and mannitol were employed to facilitate brain relaxation during surgery, if required, as they are in children with severe TBI. While ICP monitoring is used at our institution, most of these patients came to the operating room directly from the ED, and did not have ICP monitoring. In this study, 11 patients had ICP monitors in place prior to surgery and general anesthesia. Insulin treatment for control of blood glucose was at the discretion of attending anesthesiologist, and glucose treatment thresholds may have changed over time.
Patients
One hundred and twelve children underwent urgent or emergent craniotomy for TBI during the 10 year study period. One anesthetic record was missing and 6 cases were misclassified for procedures (non urgent craniotomy or repeat surgery). After excluding these 7 children, data from the remaining 105 children were included in the final analysis. The perioperative clinical characteristics of these children are described in Table 1.
Table 1.
Perioperative clinical characteristics of 105 children with TBI. Data expressed as mean ± SD (range), median (range) or number (%). GCS: Glasgow Coma Scale score, Hypotension = Systolic BP < 5th percentile, Fever = Temperature > 38.5°C.
| Age in years | 5.3 ± 3.7 (range 0–12) |
| Male gender | 65 (62%) |
| Type of injury | |
| Isolated TBI | 94 (89.5%) |
| TBI with extracranial injury | 11 (10.5%) |
| Type of TBI | |
| Subdural Hematoma (SDH) | 53 (51%) |
| Extradural Hematoma (EDH) | 23 (22%) |
| Intracerebral Hematoma (ICH) | 11 (11%) |
| Multiple lesions | 10 (10%) |
| GCS | 8.8 ± 5.6 (range 3–15) |
| Duration of preoperative period (minutes) | 85.5 (range 12–405) |
| Duration of anesthesia (minutes) | 180 (range 60–420) |
| Patients receiving mannitol | 55 (52%) |
| Patients with hypotension | 56 (53%) |
| Patients with fever | 16 (15%) |
| Patients with ICP monitoring | 11 (11%) |
| In-hospital mortality | 15 (14.3%) |
Incidence of perioperative hyperglycemia
In all, 47 (45%) children had hyperglycemia during at least one study period (preoperative, intraoperative or postoperative within 24 hours of surgery).
Preoperative glucose (Table 2)
Table 2.
Perioperative glucose data in 105 children with traumatic brain injury. Hyperglycemia = glucose ≥ 200 mg/dL, hypoglycemia = < 60 mg/dL. Preoperative period = period from emergency department admission to arrival in operating room and immediate postoperative period = the first 24 hours after surgery. Data expressed as median (range) or number (percent).
| Preoperative (86 patients with 95 values) | Intraoperative (94 patients with 244 values) | Immediate Postoperative (101 patients with 292 values) | |
|---|---|---|---|
| Average First glucose (mg/dL) | 156 (69 –379) | 165 (24 – 492) | 150 (72 – 418) |
| Median glucose (mg/dL) | 158.5 (69 – 379) | 149.5 (78 – 418) | 146.0 (75 – 355) |
| Patients with hyperglycemia | 22 (26%) | 30 (32%) | 24 (24%) |
| Patients with hypoglycemia | 0 | 2 (2%) | 1 (1%) |
| Hypoglycemic episodes | 0 | 2 (1%) | 1 (0.3%) |
| Patients treated with insulin | 0 | 6 (9%) | 0 |
At-least one preoperative glucose [median 158.5 (range 69 – 379) mg/dL] was recorded in 86 (82%) children. Hyperglycemia occurred in 22 (26%) children and none had hypoglycemia. None of the children with preoperative hyperglycemia was treated with insulin prior to surgery.
Intraoperative glucose (Table 2; Figure 1 Figure 2, Figure 3)
Figure 1.
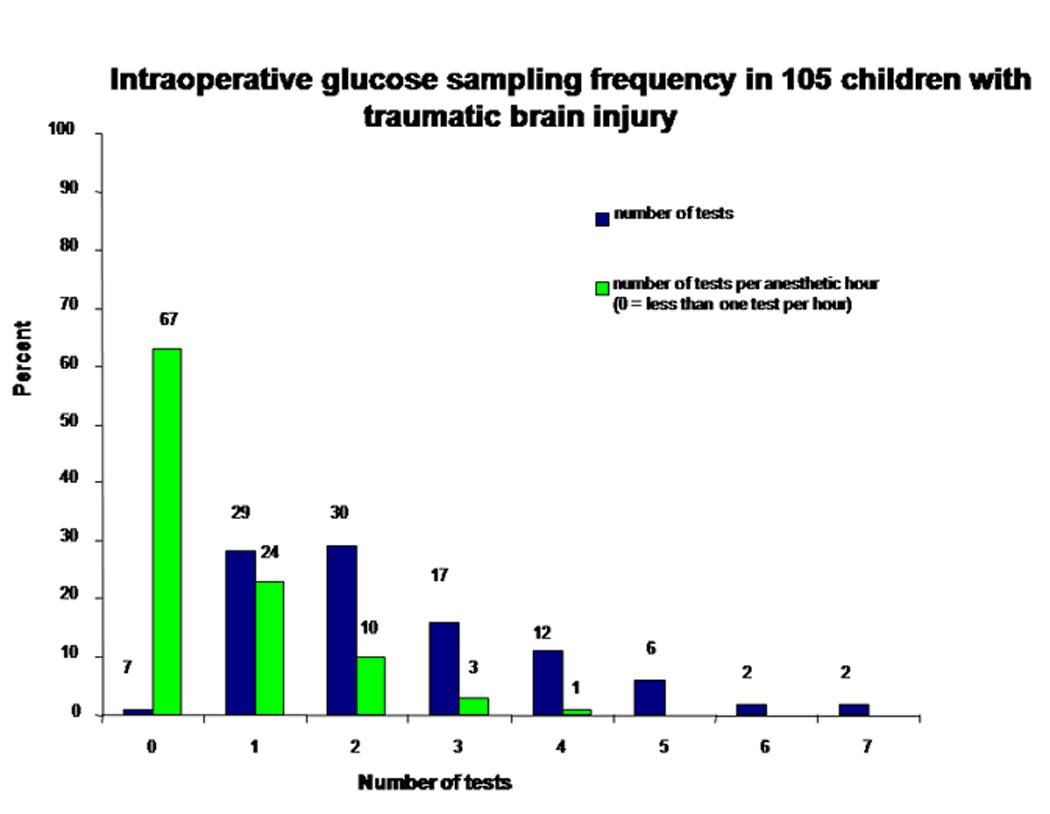
Intraoperative glucose sampling frequency in 105 children with traumatic brain injury. The number above each bar represents the number of children.
Figure 2.
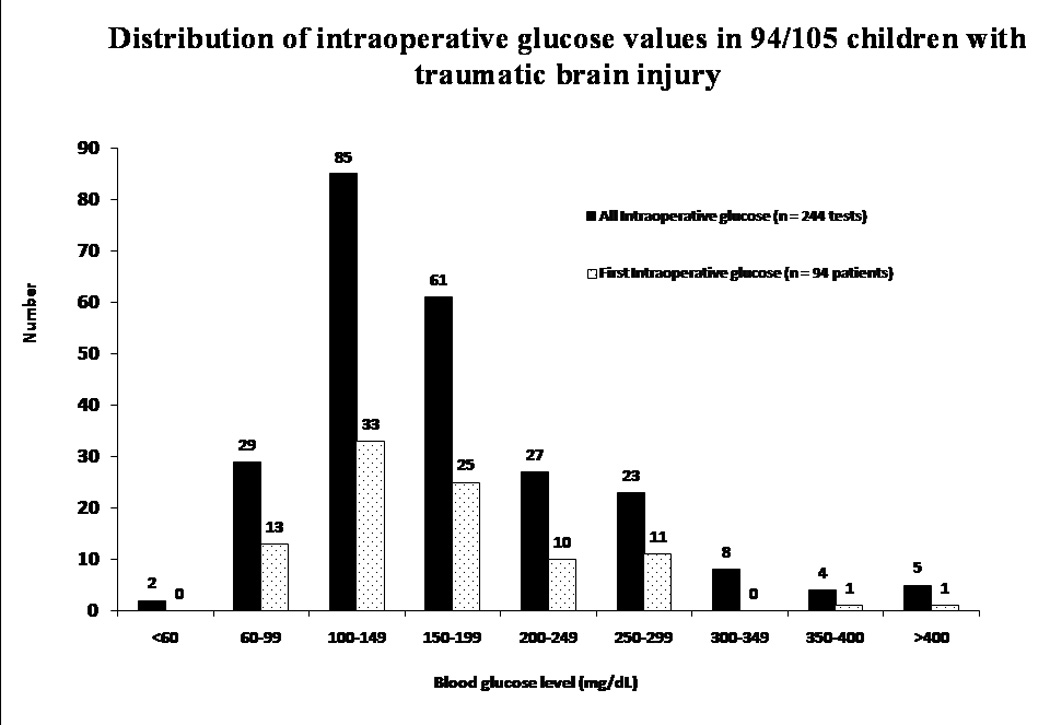
Distribution of intraoperative glucose values in 94/105 children with traumatic brain injury. Thirty (32%) children had hyperglycemia with 67 (27.5%) glucose values ≥ 200 mg/dL.
Figure 3.
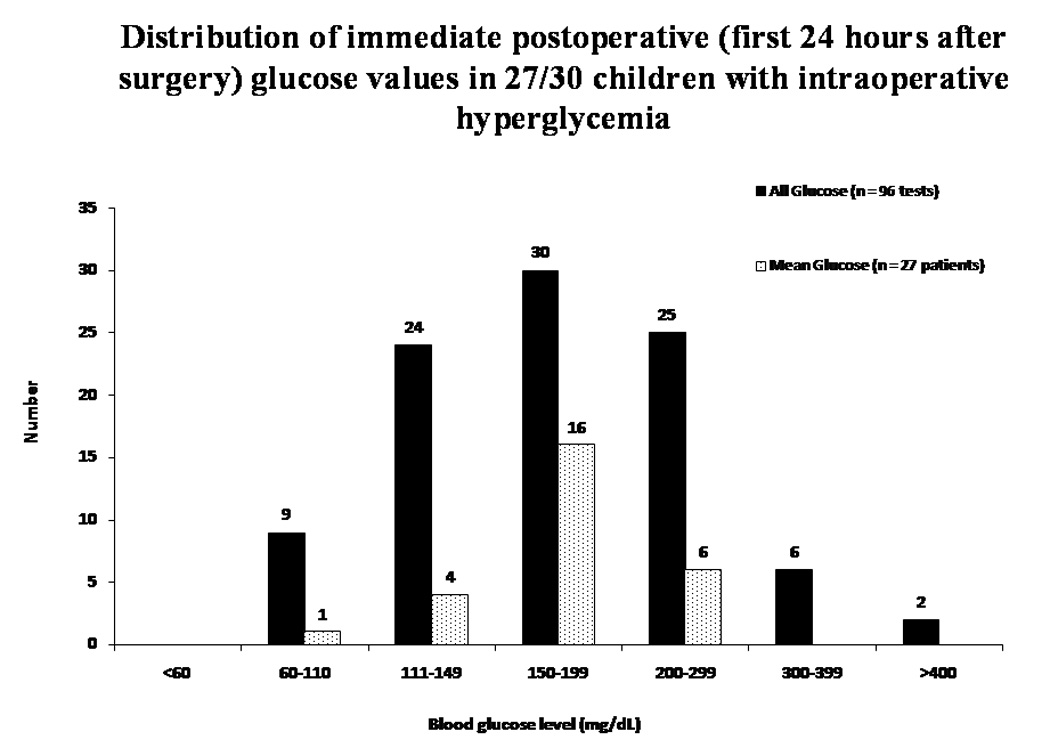
Distribution of immediate postoperative (first 24 hours after surgery) glucose values in 27/30 children with intraoperative hyperglycemia. Hyperglycemia defined as blood glucose ≥ 200 mg/dL. Ninety-six postoperative glucose values were recorded.
At-least one intraoperative glucose [median 149.5 (range 78 – 418) mg/dL] was recorded in 94 (89%) children. The median anesthetic time was 180 (range 60–420) minutes. On average, glucose was sampled once every 75 minutes during general anesthesia, but it was recorded less than once per anesthetic hour in most (64%) children.
Thirty (32%) children had intraoperative hyperglycemia. There was no association between preoperative and intraoperative glucose (R2= 0.25). Most (90%) children who had initial hyperglycemia had glucose checked subsequently during general anesthesia.
Six children received intravenous insulin for median glucose of 299 (range 193– 420)mg/dL. Intravenous insulin boluses ranged from 0.07–0.8 units/kg. None of the children treated with insulin had subsequent hypoglycemia.
Two children had hypoglycemia (glucose < 60 mg/dL), unrelated to insulin treatment. Each child had one hypoglycemic episode (24 and 52 mg/dL, respectively). The 5 month old child with glucose of 24mg/dL was treated with dextrose (D25solution; 2mL/kg), with a subsequent increase in glucose to 145mg/dL.
Postoperative glucose (Table 2; Figure 3)
One hundred and one (97%) children had at least one glucose [median (range) 146.0 (75 – 355) mg/dL] recorded during the first 24 hours after surgery. Twenty-four (24%) children had postoperative hyperglycemia, and none received insulin. There was no correlation between intraoperative and immediate postoperative glucose (R2= 0.28). One child, without prior hypoglycemia had a single episode of immediate postoperative hypoglycemia (glucose = 45mg/dL) which resolved without dextrose administration.
Transient vs. persistent hyperglycemia
Transient hyperglycemia occurred in 29 (28%) children and persistent hyperglycemia occurred in 18 (17%) children. Eleven (50%) children with preoperative hyperglycemia had intraoperative hyperglycemia and 14 (47%) children with intraoperative hyperglycemia had postoperative hyperglycemia. Hyperglycemia occurred in all three study periods in 8 (7.6%) children,
Factors associated with perioperative hyperglycemia (Table 5, Table 6)
Table 5.
Univariate factors associated with any perioperative hyperglycemia (preoperative, intraoperative or immediate postoperative period) in 105 children with traumatic brain injury. ED = Emergency Department, GCS = Glasgow Coma Scale Score. SDH = Subdural hematoma, EDH = Epidural hematoma, ICH = Intracerebral hemorrhage, Fever = Temperature ≥ 38.5°C, Hypotension = systolic blood pressure < 5th percentile for age/gender. n = number of patients. p < 0.05 = significant
| Variable | Any Hyperglycemia (Any glucose ≥ 200 mg/dL) (n = 47) |
No Hyperglycemia (All glucose < 200 mg/dL) (n = 58) |
p-value |
|---|---|---|---|
| Age (mean ± SD) (range) | 4.4 ± 3.9 (0.3–11) | 6.1 ± 3.6 (0.3–12) | 0.03 |
| Male gender (n=64) | 31 | 33 | 0.34 |
| Age < 4 years (n=43) | 27 | 16 | 0.004 |
| ED GCS ≤ 8 (n = 53) | 36 | 17 | <0.0001 |
| Isolated SDH (n=23) | 15 | 08 | 0.02 |
| Any SDH (n = 33) | 24 | 09 | <0.0001 |
| Isolated EDH (n = 53) | 14 | 39 | <0.0001 |
| Isolated ICH (n=11) | 05 | 06 | 1.0 |
| Multiple lesions (n=10) | 09 | 01 | 0.005 |
| In- hospital mortality (n = 15) | 12 | 3 | 0.006 |
| Extracranial injuries (n=11) | 5 | 6 | 0.96 |
| Receiving manitol (n=55) | 32 | 23 | 0.002 |
| Perioperative fluid (n=76) (Mean ± SD) (range) | 229.6 ± 111.9 (101–568) ml/kg | 141 ± 57.6 (30–308) ml/kg | < 0.001 |
| Perioperative ICP > 20 mmHg (n=6/11 with ICP monitoring) | 4 | 2 | 0.24 |
| Perioperative fever (n=16) | 9 | 7 | 0.31 |
| Perioperative hypotension (n=56) | 30 | 26 | 0.05 |
| Duration of anesthesia median (range) | 180 (60–420) minutes | 180 (120–360) minutes | 0.49 |
Table 6.
Independent predictors of perioperative hyperglycemia. Data from 99/105 children (missing data for 6 patients). Data are adjusted for age and gender. ED = Emergency Department, GCS = Glasgow Coma Scale Score, SDH = Subdural Hematoma
| Risk Factors | Adjusted Odds Ratio (95% CI) |
|---|---|
| Age < 4 years | 3.5 (1.2–10.6) |
| GCS ≤ 8 | 7.2 (2.4–21.5) |
| Multiple lesions including SDH | 34.7 (2.3– 525.5) |
Univariate risk factors for perioperative hyperglycemia were age < 4 years, severe (GCS ≤ 8) TBI, isolated SDH, any SDH, multiple lesions on head CT, mannitol administration and volume of perioperative fluid administered. In-hospital mortality was higher in children with perioperative hyperglycemia than those without hyperglycemia (p=0.006). Twelve of the 15 children who died had perioperative hyperglycemia and 7 (39%) children with persistent hyperglycemia died. Independent predictors of perioperative hyperglycemia were age < 4 years, severe TBI, and the presence of multiple lesions including SDH on head CT.
Discussion
Hyperglycemia is a negative prognostic indicator in adult and pediatric TBI1–3, 5, 6, 10, 11 and this study provides the first estimate of the incidence and risk factors for perioperative hyperglycemia in children with TBI who underwent urgent/emergent craniotomy at our institution. The main findings of this study are that in children with TBI: 1) perioperative hyperglycemia was common, 2) although most patients had at-least one glucose checked during general anesthesia, the sampling frequency for the majority of children was less than one serum glucose per anesthetic hour, 3) intraoperative hyperglycemia was common but few patients were treated with insulin, 5) Age < 4 years, severe TBI and presence of multiple lesions that include SDH on preoperative CT head were independent predictors of perioperative hyperglycemia, and 6) intraoperative hypoglycemia occurred independent of insulin treatment and was not rare.
In this study, we used a somewhat “arbitrary” definition of hyperglycemia (> 200 mg/dl). Previous studies have used different glucose values to define hyperglycemia in the context of pediatric TBI. These values vary and range from 150 mg/dl2 to as high as 270 mg/dl.4 However, the treatment threshold for hyperglycemia in both adults and children is controversial and some clinicians may not administer insulin for a glucose of 150 mg/dl for fear of hypoglycemia, and others may consider a glucose of 270 mg/dl to be too high a treatment threshold. In a randomized, controlled study involving more than 1500 critically ill adults, Van den Berghe et al. recommended intensive insulin therapy to maintain blood glucose ≤ 110 mg/dL to reduce morbidity and mortality.12 In a subsequent study analyzing of 63 of these patients with isolated TBI, they observed significant reduction in mean and maximum intracranial pressure, incidence of seizures and diabetes insipidus, and suggested that tight glucose control with insulin protects the central and peripheral nervous system and shortens the intensive care dependency, with improved long term rehabilitation.13 Despite the lack of consensus regarding the hyperglycemia definition threshold in TBI, we used a value of 200 mg/dl because in current clinical practice, this is a commonly used treatment threshold. Our data show that glucose > 200mg/dL is common during the perioperative period. In this study, we based our estimates of the incidence of hyperglycemia on available data, which was almost always obtained during each of the 3 study periods but only intermittently obtained within each study period. Therefore, the actual prevalence of intraoperative or perioperative hyperglycemia may be higher than currently estimated. In support of this idea is a recent study which reported that hyperglycemia was frequently not detected with intermittent laboratory glucose measurements in critically ill children.14 A continuous glucose monitoring system with real time read-outs might be one solution to decrease sampling bias and valuable for the real time detection and treatment of hyper and hypoglycemia during the perioperative period.14
Following initial trauma, TBI evolves and different periods of injury may be particularly stressful. This may be one explanation for the lack of correlation between glucose values that we observed during different study periods. Our data show that it may not be possible to predict intraoperative glucose on the basis of preoperative glucose or predict postoperative glucose on the basis of intraoperative glucose levels. Frequent sampling of glucose before, during and after surgery is therefore important.
In this study, risk factors for perioperative hyperglycemia were age < 4 years and severe TBI. We dichotomized age into a young vs. older age group because children less than 4 years are known to have the highest rate for TBI-related deaths, hospitalizations, and emergency department visits15 and have worse outcome than older chidren.16–18 The relationship between hyperglycemia and young age in this study suggests that hyperglycemia may be one mechanism that explains the observation that young children have poor outcomes after TBI. Our finding that children with severe TBI have more perioperative (including the intraoperative period) hyperglycemia is new and adds to the information on hyperglycemia in children with TBI, previously provided by Michaud et al.1 and Chiaretti et al.2 Unlike Michaud et al.,1 however, we found an association between hyperglycemia and presence of multiple lesions including SDH on head CT. This difference could be due to the fact that we included surgical patients whereas Michaud et al.1 did not consider the intraoperative period. Similarly, the lack of association between head CT lesion type and hyperglycemia in Parish and Webb’s study4 may be due to operational differences in hyperglycemia definition (glucose > 270 mg/dL). Regardless of these differences, however, our finding suggests that severe TBI, whether it is clinically scored or radiographically assessed, predicts perioperative hyperglycemia in children undergoing urgent/emergent craniotomy.
Hyperglycemia may reflect TBI severity 2, 9, 11, 19 as it often occurs in children as a normal response to stress, secondary to an increase in the concentrations of stress hormones resulting in stimulation of gluconeogenesis and glycogenolysis.20, 21 However, we did not have hormone levels to directly assess stress. We did examine surrogates of stress and potential confounding factors for hyperglycemia such as extracranial injuries, perioperative fluid administration, use of mannitol, duration of anesthesia, hypotension, and fever were not found to affect hyperglycemia in our study (Table 5,Table 6). We did not enter perioperative fluid administered and presence of any SDH into the final multivariate analysis model because there were large numbers of missing data (29 missing data) for perioperative fluid, and every patient who had multiple lesions had SDH. On the other hand, hyperglycemia may exacerbate the impact of ischemia and hypoxia and lead to poor outcome22, 23 by potentiating brain cellular and tissue lactic acidosis.6, 7, 24–26
Therefore, there is no consensus as to whether transient hyperglycemia after TBI should be treated and Parish and Webb have suggested that insulin should not be used to treat hyperglycemia during first 40 hours after pediatric TBI4. However, when hyperglycemia is persistent poor outcomes may ensue. 2, 4, 5 Twelve of the 15 children with in-hospital mortality in our series had perioperative hyperglycemia and 7 (39%) of the 18 children with persistent hyperglycemia died. Although the aim of this study was to examine the risk factors for hyperglycemia and we did not study mortality as an outcome measure (and thus did not enter mortality into multivariate analysis model), we observed higher in-hospital mortality in children with perioperative hyperglycemia than those without hyperglycemia (Table 5), a finding which has previously been reported.2,3,5
Approximately 2% children had perioperative hypoglycemia and this can be equally detrimental to outcome of and the real incidence of hypoglycemia may also be higher with more frequent sampling. Vespa et al. have demonstrated that intensive insulin therapy results in a net reduction in cerebral microdialysis glucose, but elevates lactate/pyruvate ratio and global oxygen extraction fraction, with no functional advantage.27 While the observations of elevated lactate/pyruvate ratio in the context of reduction of blood glucose from insulin remain unexplained, the occurrence of increased number of hypoglycemic events during the intensive insulin treatment raises concern regarding its routine use in TBI.28–31 The occurrence of 2 episodes of hypoglycemia in 2 patients in our study, independent of insulin, suggests that the risk of hypoglycemia is not theoretical. We speculate that the concern for hypoglycemia was primarily responsible for the infrequent use of intraoperative insulin during general anesthesia, even when glucose values exceeded 200g/dL. However, currently there are insufficient data to support insulin treatment and our study does not address the issue whether or not glucose above 200 mg/dL should be treated. Insulin administration in such circumstances may be dangerous and should be initiated with caution.
The major limitations of this study are the retrospective design of the study and the lack of long term outcome data. This study represents data from one institution and other biological markers of stress injury following TBI were not available. We could not determine the effect of multiple lesions without SDH on perioperative hyperglycemia because all these patients had SDH in addition to other lesions on preoperative head CT. In this study, perioperative hyperglycemia was associated with in-hospital mortality, but the number of deaths was small and we were not able to determine if perioperative hyperglycemia was an independent predictor of death. Despite these limitations, these data provide new information regarding the incidence and risk factors for perioperative hyperglycemia, and of the incidence of hypoglycemia in children during general anesthesia and the perioperative period.
This study demonstrates that perioperative hyperglycemia was common and that intraoperative hypoglycemia was not rare in children with TBI requiring urgent/emergent craniotomy. We have also shown that perioperative hyperglycemia can be predicted by young age, severe TBI and multiple TBI lesions that include SDH. Since intermittent intraoperative sampling may have underestimated the actual frequency of both hyper-and hypoglycemia, more frequent if not continuous perioperative glucose monitoring in children with TBI may be needed.
Table 3.
Intraoperative insulin treatment in 6 children with traumatic brain injury. SDH = Subdural Hematoma, ICH = Intracerebral Hematoma, N/A = Not Available
| Age (years) | Gender | Intracranial Injury | Preoperative glucose (mg/dL) | Number of glucose tests (number per anesthetic hour | Glucose range (mg/dL) | First glucose (mg/dL) | Last glucose (mg/dL) | Insulin units/ kg (Triggering glucose) |
|---|---|---|---|---|---|---|---|---|
| 2 | M | SDH | N/A | 7 (3) | 125–273 | 273 | 125 | 0.4(273),0.8(245),0.8(229),0.8(186),0.8(200) |
| 2 | F | SDH | 201 | 3 (1) | 420–471 | 420 | 471 | 0.3(420),0.5(463) |
| 8 | M | ICH | 316 | 5 (1) | 237–329 | 320 | 261 | 0.25(320),0.4(329) |
| 9 | M | SDH | 132 | 2 (1) | 252–278 | 278 | 252 | 0.1(278),0.1(252) |
| 9 | M | SDH | N/A | 6 (2) | 130–379 | 379 | 130 | 0.03(362),0.07(257) |
| 12 | M | SDH | 190 | 4 (1) | 93–193 | 193 | 97 | 0.1(193) |
Table 4.
Clinical characteristics of children with traumatic brain injury with intraoperative hypoglycemia. EDH = Extradural Hematoma.
| Age | Gender | Intracranial Lesion | Preoperative glucose (mg/dL) | Number of Glucose tests number per anesthetic hour) | Glucose range (mg/dL) | First glucose (mg/dL) | Last glucose (mg/dL) | Lowest glucose mg/dL) | Dextrose dose* | Post Dextrose Glucose (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 months | M | EDH | 217 | 5(1) | 24 | 24–145 | 116 | 24 | 14mL | 145 |
| 6 years | F | EDH | 69 | 3(1) | 52 | 69–143 | 89 | 52 | - | - |
Dextrose was administered as D25 injection.
Acknowledgments
Funding was provided by: NIH/1K23HDO44632-05 (MSV), HARC (OC) and Departmental resources (DS, JJ, RC)
Footnotes
Implications Statement: Hyperglycemia after traumatic brain injury (TBI) is associated with poor outcome. In this study of children with TBI undergoing urgent/emergent craniotomy, perioperative hyperglycemia was common and hypoglycemia was not rare. Perioperative hyperglycemia was predicted by age < 4 years, severe TBI and the presence of multiple lesions that include SDH.
Conflict of Interest Statement: The authors have no conflicts of interest.
References
- 1.Michaud CJ, Riavara FP, Longstreth WT, Jr, Grady S. Elevated initial blood glucose levels and poor outcome following severe brain injuries in children. J Trauma. 1991;31:1356–1362. doi: 10.1097/00005373-199110000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Chiaretti A, Benedictis RD, Langer A, Di Rocco C, Bizzarri C, Iannelli A, Polidori G. Prognostic implications of hyperglycemia in paediatric head injury. Childs Nerv Syst. 1998;14:455–459. doi: 10.1007/s003810050259. [DOI] [PubMed] [Google Scholar]
- 3.Cochran A, Scaife ER, Hansen KW, Downey EC. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma. 2003;55:1035–1038. doi: 10.1097/01.TA.0000031175.96507.48. [DOI] [PubMed] [Google Scholar]
- 4.Parish RA, Webb KS. Hyperglycemia is not a poor prognostic sign in head-injured children. J Trauma. 1988;28:517–519. doi: 10.1097/00005373-198804000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Marton E, Mazzucco M, Nascimben E, Martinuzzi A, Longatti P. Severe head injury in early infancy: analysis of causes and possible predictive factors for outcome. Childs Nerv Syst. 2007;23:873–880. doi: 10.1007/s00381-007-0314-9. [DOI] [PubMed] [Google Scholar]
- 6.Jeremitsky E, Omert L, Dunham C, Protetch J, Rodriguez A. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia and hypoperfusion. J Trauma. 2003;54:312–319. doi: 10.1097/01.TA.0000037876.37236.D6. [DOI] [PubMed] [Google Scholar]
- 7.Rosner MJ, Newsome HH, Becker DP. Mechanical brain injury: the sympathoadrenal response. J Neurosurg. 1984;61:76–86. doi: 10.3171/jns.1984.61.1.0076. [DOI] [PubMed] [Google Scholar]
- 8.Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118:173–179. doi: 10.1542/peds.2005-1819. [DOI] [PubMed] [Google Scholar]
- 9.American Association for the Surgery of Trauma; Child Neurology Society; International Society for Pediatric Neurosurgery; International Trauma Anesthesia and Critical Care Society; Society of Critical Care Medicine; World Federation of Pediatric Intensive and Critical Care Societies; National Center for Medical Rehabilitation Research; National Institute of Child Health and Human Development; National Institute of Neurological Disorders and Stroke; Synthes USA; International Brain Injury Association. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. J Trauma. 2003;54(6 Suppl):S235–S310. doi: 10.1007/s00068-003-1288-2. [DOI] [PubMed] [Google Scholar]
- 10.Lam AM, Winn HR, Cullen BF, Sundling N. Hyperglycemia and neurologic outcome in patients with head injury. J Neurosurgery. 1991;75:545–551. doi: 10.3171/jns.1991.75.4.0545. [DOI] [PubMed] [Google Scholar]
- 11.Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000;46:335–342. doi: 10.1097/00006123-200002000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 13.Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64:1348–1353. doi: 10.1212/01.WNL.0000158442.08857.FC. [DOI] [PubMed] [Google Scholar]
- 14.Allen HF, Rake A, Roy M, Brenner D, McKiernan CA. Prospective detection of hyperglycemia in critically ill children using continuous glucose monitoring. Pediatr Crit Care Med. 2008;9:153–158. doi: 10.1097/PCC.0b013e3181668b33. [DOI] [PubMed] [Google Scholar]
- 15.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21:544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Freeman SS, Udomphorn Y, Armstead WM, Fisk DM, Vavilala MS. Young age as a risk factor for impaired cerebral autoregulation after moderate to severe pediatric traumatic brain injury. Anesthesiology. 2008;108:588–595. doi: 10.1097/ALN.0b013e31816725d7. [DOI] [PubMed] [Google Scholar]
- 17.Duhaime AC, Christian CW, Moss E, Seidl T. Long-term outcome in infants with the shaking impact syndrome. Pediatr Neurosurg. 1996;24:292–298. doi: 10.1159/000121058. [DOI] [PubMed] [Google Scholar]
- 18.Ewing-Cobbs L, Kramer L, Prasad M, et al. Neuroimaging, physical, and developmental findings after inflicted and non-inflicted traumatic brain injury in young children. Pediatrics. 1998;102:300–337. doi: 10.1542/peds.102.2.300. [DOI] [PubMed] [Google Scholar]
- 19.Young B, Ott L, Dempsey R, Haack D, Tibbs P. Relationship between admission hyperglycemia and neurologic outcome of severely brain injured patients. Ann Surg. 1989;210:466–472. doi: 10.1097/00000658-198910000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolih CA, Ober KP. The endocrine response to critical illness. Med Clin North Am. 1995;79:211–224. doi: 10.1016/s0025-7125(16)30093-1. [DOI] [PubMed] [Google Scholar]
- 21.De Salles AA, Muizelaar JP, Young HF. Hyperglycemia, cerebrospinal fluid lactic acidosis and cerebral blood flow in severely head-injured patients. Neurosurgery. 1987;21:45–50. doi: 10.1227/00006123-198707000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Pentelenyi T, Kammerer L, Stutzel M, Balazsi I. Alterations of the basal serum insulin and blood glucose in brain-injured patients. Injury. 1979;10:201–208. doi: 10.1016/0020-1383(79)90009-3. [DOI] [PubMed] [Google Scholar]
- 23.Longstreth J, Inui T. High blood glucose level on hospital admission and poor neurological recovery after cardiac arrest. Ann Neurol. 1984;15:59–63. doi: 10.1002/ana.410150111. [DOI] [PubMed] [Google Scholar]
- 24.Rehncrona S, Rosen I, Seisjo BK. Excessive cellular acidosis: an important mechanism of neuronal damage in the brain? Acta Physiol Scand. 1980;110:435–437. doi: 10.1111/j.1748-1716.1980.tb06692.x. [DOI] [PubMed] [Google Scholar]
- 25.Zygun DA, Steiner LA, Johnston AJ, Hutchinson PJ, Al-Rawi PG, Chatfield D, Kirkpatrik PJ, Menon DK, Gupta AK. Hyperglycemia and brain tissue pH after traumatic brain injury. Neurosurgery. 2004;55:877–881. doi: 10.1227/01.neu.0000137658.14906.e4. [DOI] [PubMed] [Google Scholar]
- 26.Sood SC, Gulati SC, Kumar M, Kak VK. Cerebral metabolism following brain injury II Lactic acid changes. Acta Neurochir. 1980;53:47–51. doi: 10.1007/BF02074520. [DOI] [PubMed] [Google Scholar]
- 27.Vespa P, Boonyaputthikul R, McArthur DL, Miller C, Etchepare M, Bergsneider M, Glenn T, Martin N, Hovda D. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;34:850–856. doi: 10.1097/01.CCM.0000201875.12245.6F. [DOI] [PubMed] [Google Scholar]
- 28.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, O’Brien PC, Johnson MG, Williams AR, Cutshall SM, Mundy LM, Rizza RA, McMahon MM. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146:233–243. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- 29.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. German Competence Network Sepsis (SepNet). Intensive Insulin therapy and Pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 30.Bilotta F, Caramia R, Cernak I, Paoloni FP, Doronzio A, Cuzzone V, Santoro A, Rosa G. Intensive insulin -therapy after severe traumatic brain injury: A Randomized Clinical Trial. Neurocrit Care. 2008 Mar 29; doi: 10.1007/s12028-008-9084-9. [DOI] [PubMed] [Google Scholar]
- 31.Treggiari MM, Karir V, Yanez ND, Weiss NS, Daniel S, Deem SA. Intensive insulin therapy and mortality in critically ill patients. Crit Care. 2008 Feb 29;12(1):R29. doi: 10.1186/cc6807. [DOI] [PMC free article] [PubMed] [Google Scholar]


