Abstract
Use of aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs) may reduce the risk of gastric or oesophageal adenocarcinomas. We examined the association between self-reported use of aspirin or non-aspirin NSAIDs in the earlier 12 months and gastric non-cardia (N=182), gastric cardia (N=178), and oesophageal adenocarcinomas (N=228) in a prospective cohort (N=311 115) followed for 7 years. Hazard ratios (HRs) and 95% confidence intervals (CIs) come from Cox models adjusted for potential confounders. Use of any aspirin (HR, 95% CI: 0.64, 0.47–0.86) or other NSAIDs (0.68, 0.51–0.92) was associated with a significantly lower risk of gastric non-cardia adenocarcinoma. Neither aspirin (0.86, 0.61–1.20) nor other NSAIDs (0.91, 0.67–1.22) had a significant association with gastric cardia cancer. We found no significant association between using aspirin (1.00, 0.73–1.37) or other NSAIDs (0.90, 69–1.17) and oesophageal adenocarcinoma. We also performed a meta-analysis of the association between the use of NSAIDs and risk of gastric and oesophageal adenocarcinoma. In this analysis, aspirin use was inversely associated with both gastric and oesophageal adenocarcinomas, with summary odds ratios (95% CI) for non-cardia, cardia, and oesophageal adenocarcinomas of 0.64 (0.52–0.80), 0.82 (0.65–1.04), and 0.64 (0.52–0.79), respectively. The corresponding numbers for other NSAIDs were 0.68 (0.57–0.81), 0.80 (0.67–0.95), and 0.65 (0.50–0.85), respectively.
Keywords: aspirin, NSAIDs, oesophageal cancer, gastric cancer, cohort, meta-analysis
Aspirin may prevent heart disease (Hennekens and Schneider, 2008) and colon cancer (Dubé et al, 2007), but the US Preventive Services Task Force does not endorse an aspirin regimen for primary chemoprevention of colon cancer (US Preventive Services Task Force, 2007). Daily aspirin use carries the risk of gastrointestinal bleeding and haemorrhagic stroke and the expected benefits do not outweigh the risks, at least in individuals at average risk for colorectal cancer. If additional chemopreventive benefits at sites other than the colon could be included in the risk benefit analysis, this calculation may change.
Worldwide, gastric cancer remains the second leading cause of death due to cancer, with over 900 000 incident cases and about 700 000 deaths (Parkin et al, 2002). Although gastric cancer incidence rates are decreasing in the United States, about 21 000 incident cases occur each year (Ries and Melbert, 2007). In contrast, oesophageal adenocarcinoma rates have increased dramatically over the last 30 years in many Western countries, and there are about 9000 incident cases in the United States each year (Ries and Melbert, 2007). Both stomach and oesophageal cancers have high fatality rates, only 24 and 16% 5-year survival respectively, so preventive strategies are particularly important for these cancers. Earlier studies suggest that the incidence of adenocarcinomas of the oesophagus and stomach may be reduced by the use of aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs) (Corley et al, 2003; Gonzalez-Perez et al, 2003; Wang et al, 2003), but few studies have been prospective, used data collected directly from subjects, and controlled for the many potential confounders.
We aimed to examine the association between aspirin and non-aspirin NSAID use and risk of oesophageal, gastric cardia, and gastric non-cardia adenocarcinoma in the NIH-AARP Diet and Health Study cohort, a large prospective study conducted in the United States.
Methods
The NIH-AARP Diet and Health Study is a large prospective cohort study designed to investigate the association between diet and other factors and risk of cancer and has been described in detail previously (Schatzkin et al, 2001). Between 1995 and 1996, a questionnaire was mailed to 3.5 million AARP members (aged 50–71 years) in eight US states (California, Florida, Georgia, Louisiana, Michigan, New Jersey, North Carolina, and Pennsylvania). In total, 617 119 individuals returned the questionnaire. A second mailed questionnaire (1996–1997) collected additional information including NSAID use and 334 908 individuals were available for analysis. We excluded subjects for whom either the baseline (N=6959) or the follow-up questionnaire (N=3424) was completed by proxies, those with prevalent cancer at the second questionnaire baseline (N=4543), those with incomplete information about NSAID use (N=6353), those with total energy intake more than twice the interquartile range from the median (N=2506), and those who exited on the first day of follow-up (N=8). The resulting cohort included 311 115 participants including 180 337 men and 130 778 women. The NIH-AARP Diet and Health Study was approved by the Special Studies Institutional Review Board of the US National Cancer Institute.
As described earlier (Michaud et al, 2005), addresses for members of the NIH-AARP cohort were updated annually by matching the cohort database to that of the National Change of Address database maintained by the US Postal Service. We ascertained vital status by annual linkage of the cohort to the Social Security Administration Death Master File, cancer registry linkage, questionnaire responses, and responses to other mailings. Incident cases of cancer through the year 2003 were identified by probabilistic linkage between the NIH-AARP cohort membership and the cancer registry databases of the states of residence at the time of mailing the questionnaire with the addition of Arizona, Nevada, and Texas, each of which has been certified by the North American Association of Central Cancer Registries for meeting the highest standards of data quality. For matching purposes, first and last name, address history, gender, date of birth, and Social Security number (available for 85% of our participants) were used. We estimate the sensitivity of case identification to be about 90%. Cancer sites were identified by anatomic site and histologic code as detailed earlier (Freedman et al, 2007), using the International Classification of Disease for Oncology, third edition. We classified tumours with site codes C15.0–C15.9 as oesophageal cancers, site code C16.0 as gastric cardia tumours, and site codes C16.1–C16.9 as non-cardia tumours. All included cancers were classified as adenocarcinomas.
Our questionnaire assessed aspirin (generic aspirin and trade names) use and non-aspirin NSAID use separately and the latter named 19 non-aspirin NSAIDs (e.g., ibuprofen, sulindac etc., using both generic and trade names) and specifically excluded Tylenol, acetaminophen, and other pain relievers. Both questions asked about any use in the past 12 months and asked users to mark how frequently they took them: less than two times per month, two to three times per month, one to two times per week, three to four times per week, five to six times per week, one time per day, or two or more times per day. Owing to small numbers in some of the categories, we collapsed these into monthly, weekly, or daily use.
The baseline questionnaire contained questions about demographic information, cigarette use, alcohol consumption, education, and a food frequency questionnaire of 124 items. Potential confounders were categorised as described earlier (Abnet et al, 2008).
Statistics
We computed two-sided tests and considered P-values <0.05 or estimates with confidence intervals (CIs) that excluded 1.0 as statistically significant. To assess the potential for confounding, we tabulated and compared known or potential risk factors by NSAID use status. Hazard ratios (HRs) and 95% CIs were calculated using Cox proportional hazards regression with follow-up time as the underlying time metric using SAS 9.1 (Cary, NC, USA). All presented estimates come from models adjusted for age at cohort entry, sex, cigarette smoking status and intensity, alcohol use, education, fruit intake, vegetable intake, BMI, total energy intake, and both vigorous physical activity and usual physical activity throughout the day. A small number of subjects were missing values for some adjusting covariates and these subjects were retained using dummy variables in the models. We modelled the HR (95% CI) for any use of aspirin and of non-aspirin NSAIDs in a single model (i.e., mutually adjusted models) and for frequency of use with adjustment for the other category of NSAIDs. We tested the proportional hazards assumption using cross-product terms for interaction between follow-up time and any use of NSAIDs and found no significant deviations from proportionality. Furthermore, we dropped 1, 2, or 3 years of initial follow-up and refitted the models to assess lag effects and found none. Age-standardised incidence rates were calculated as in Breslow and Day (1987) with 5-year age bands and age- and sex-specific rates standardised to the entire NIH-AARP Diet and Health Study population.
Meta-analysis
To conduct the meta-analysis, we searched PubMed on 20 November 2008, with the following terms: (aspirin OR nsaid OR nsaids OR non-steroidal) AND (gastric cancer OR oesophageal cancer) AND (case-control OR cohort OR epidemiologic). We reviewed the 128 retrieved abstracts to find relevant papers, reading those in full in which the abstracts were not entirely informative. We also reviewed earlier meta-analyses on aspirin and NSAIDS in relation to oesophageal and gastric cancers (Corley et al, 2003; Gonzalez-Perez et al, 2003; Wang et al, 2003) and other review articles (Baron, 2003; Bosetti et al, 2003). We limited our analysis to papers reporting case–control or cohort studies of the association between the use of either aspirin or non-aspirin NSAIDS and the risk of oesophageal or gastric adenocarcinomas. We included the following studies for the oesophagus (Farrow et al, 1998; Cheng et al, 2000; Lindblad et al, 2005; Anderson et al, 2006; Jayaprakash et al, 2006; Ranka et al, 2006; Fortuny et al, 2007; Duan et al, 2008; Sadeghi et al, 2008), cardia (Farrow et al, 1998; Zaridze et al, 1999; Akre et al, 2001; Fortuny et al, 2007; Duan et al, 2008; Sadeghi et al, 2008), non-cardia (Farrow et al, 1998; Zaridze et al, 1999; Akre et al, 2001; Fortuny et al, 2007; Duan et al, 2008), and gastric NOS (Thun et al, 1993; Schreinemachers and Everson, 1994; Coogan et al, 2000; Langman et al, 2000; Akre et al, 2001; Sorensen et al, 2003; Ratnasinghe et al, 2004; Lindblad et al, 2005) and excluded a few studies from certain sections that did not specify the agent (Garidou et al, 1996; Suleiman et al, 2000) or the histology of the oesophageal tumours (Funkhouser and Sharp, 1995; Langman et al, 2000). Two other studies, one prospective and one retrospective, reported on the association between aspirin and oesophageal adenocarcinoma, but included only subjects with Barrett's oesophagus in the reference group (Tsibouris et al, 2004; Vaughan et al, 2005) and these papers are discussed separately. From each selected paper, the effect measures (odds ratio (OR) or HR) and 95% CIs were tabulated by two investigators. In each case, we selected the most expansive measure of NSAID use and the maximally adjusted model that did not include adjustment for reflux symptoms (where possible). In some cases, we collapsed multiple exposure groups into a single measure of association using fixed effects models, and these are indicated by asterisks in the figure. We used Stata/SE version 8.0 (Stat Corp., College Station, TX, USA) and the meta and metabias commands to complete the analyses. We report the results from random effects models, but the results for fixed effects are similar in each case. Plots were created using SigmaPlot 8.0 (Systat Corp., Chicago, IL, USA).
Results
In the 12 months before baseline, 73% of the cohort had used aspirin and 56% had used non-aspirin NSAIDs (Table 1), 25% reported daily aspirin use and 10% reported daily non-aspirin NSAID use. Aspirin users and non-aspirin NSAID users appeared similar to each other and to the cohort as a whole in their age, smoking histories, alcohol-drinking habits, education, diet, BMI, and amount of physical activity.
Table 1. Distribution of covariates in NSAID users and non-users in NIH-AARP Diet and Health Study.
| Variable | Cohort | Aspirin users in the past 12 months | Non-aspirin NSAID users in the past 12 months |
|---|---|---|---|
| Number | 311 115 (100%) | 227 198 (73%) | 175 591 (56%) |
| Age, years, mean (s.d.) | 62.3 (5.3) | 62.3 (5.3) | 61.7 (5.4) |
| Sex, male, N (%) | 180 337 (58%) | 141 387 (62%) | 97 001 (55%) |
| Tobacco smoking | |||
| Never | 111 128 (37%) | 79 079 (36%) | 61 947 (36%) |
| Former<20 cigarettes per day, N (%) | 84 125 (28%) | 61 840 (28%) | 48 356 (28%) |
| Former⩾20 cigarettes per day, N (%) | 66 306 (22%) | 50 395 (23%) | 37 966 (22%) |
| Current<20 cigarettes per day, N (%) | 25 506 (8%) | 18 088 (8%) | 14 150 (8%) |
| Current⩾20 cigarettes per day, N (%) | 13 924 (5%) | 10 323 (5%) | 7505 (4%) |
| Education | |||
| High school or less, N (%) | 72 276 (24%) | 49 679 (22%) | 38 088 (22%) |
| Post-high school training, N (%) | 103 305 (34%) | 75 067 (34%) | 58 775 (34%) |
| College graduate, N (%) | 61 520 (20%) | 46 410 (21%) | 35 516 (21%) |
| Post-graduate education, N (%) | 66 493 (22%) | 50 702 (23%) | 39 087 (23%) |
| Alcohol, drinks per day, mean (s.d.) | 0.9 (2.3) | 1.0 (2.3) | 0.9 (2.1) |
| Fruit intake, servings per day, mean (s.d.) | 3.0 (2.4) | 2.9 (2.3) | 2.9 (2.3) |
| Vegetable intake, servings per day, mean (s.d.) | 3.9 (2.4) | 3.9 (2.4) | 3.9 (2.4) |
| Body mass index, kg m−2, mean (s.d.) | 26.9 (5.0) | 26.9 (4.8) | 27.2 (5.1) |
| Vigorous physical activity | |||
| Never, N (%) | 12 309 (4%) | 7619 (3%) | 6154 (4%) |
| Rarely, N (%) | 40 323 (13%) | 27 952 (12%) | 22 580 (13%) |
| 1–3 times per month, N (%) | 41 327 (13%) | 30 307 (13%) | 24 164 (14%) |
| 1–2 times per week, N (%) | 66 624 (22%) | 49 501 (22%) | 38 486 (22%) |
| 3–4 times per week, N (%) | 85 859 (28%) | 64 141 (28%) | 49 257 (28%) |
| ⩾5 times per week, N (%) | 62 226 (20%) | 46 028 (20%) | 33 710 (20%) |
| Activity throughout the day | |||
| Sit during day, not much walking, N (%) | 23 614 (8%) | 17 063 (8%) | 13 887 (8%) |
| Sit much of the day, walk a fair amount, N (%) | 74 037 (33%) | 74 037 (33%) | 58 204 (34%) |
| Stand/walk a lot, no lifting, N (%) | 118 404 (39%) | 86 292 (39%) | 65 899 (38%) |
| Lift carry light loads, N (%) | 54 906 (18%) | 40 220 (18%) | 30 199 (18%) |
| Heavy work, N (%) | 7874 (3%) | 5670 (3%) | 4331 (3%) |
Abbreviation: NSAID=non-steroidal anti-inflammatory drug.
The cohort members had an average of 6.7 years of follow-up and we collected 2 078 248 person years of follow-up in total. The HRs and 95% CIs for both any use and the frequency of aspirin or non-aspirin NSAID use are given in Table 2. Models adjusted for only age and sex produced similar results to these fully adjusted models. For gastric non-cardia cancer, we found a strong dose-dependent protective association for aspirin. Any aspirin use had an HR (95% CI) of 0.64 (0.47–0.86). The HRs decreased from 0.74 for monthly use to 0.57 for weekly or daily use and the test for trend across categories was significant (P=0.0032). For non-aspirin NSAIDs, any reported use showed a significantly decreased risk of non-cardia gastric cancer, 0.68 (0.51–0.92). But there was no clear trend; the HRs were 0.71, 0.48, and 0.82, for monthly, weekly, and daily use, respectively, and the test for trend across categories was borderline non-significant (P=0.050). We saw no significant associations between any use of aspirin (1.00, 0.73–1.37) or non-aspirin NSAIDs (0.90, 0.69–1.17) and the risk of oesophageal adenocarcinoma.
Table 2. Hazard ratios (95% CI)a for the association between NSAID use and the risk of cancer in the NIH-AARP Diet and Health Study.
|
Any aspirin use in the past 12 months
|
Frequency of aspirin use in the past 12 months
|
|||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | None | Monthly | Weekly | Daily | |||
| Cohort, N (%) | 83 917 (27%) | 227 198 (73%) | 83 917 (27%) | 96 863 (31%) | 52 096 (17%) | 78 239 (25%) | ||
| HR (95% CI) | Reference | HR (95% CI) | HR (95% CI) | HR (95% CI) | P trend b | |||
| Oesophageal adenocarcinoma, N (%) | 52 (23%) | 176 (77%) | 1.00 (0.73–1.37) | 1.0 | 0.95 (0.65–1.37) | 0.91 (0.59–1.40) | 1.11 (0.78–1.57) | 0.52 |
| Gastric cardia adenocarcinoma, N (%) | 48 (27%) | 130 (73%) | 0.86 (0.61–1.20) | 1.0 | 0.80 (0.53–1.20) | 0.71 (0.43–1.18) | 0.99 (0.67–1.45) | 0.96 |
| Gastric non-cardia adenocarcinoma, N (%) | 67 (37%) | 115 (63%) | 0.64 (0.47–0.86) | 1.0 | 0.74 (0.51–1.07) | 0.57 (0.35–0.92) | 0.57 (0.39–0.85) | 0.0032 |
|
Any non-aspirin NSAID use in the past 12 months
|
Frequency of non-aspirin NSAID use in the past 12 months
|
|||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | None | Monthly | Weekly | Daily | |||
| Cohort, N (%) | 135 524 (44%) | 175 591 (56%) | 135 524 (44%) | 102 165 (33%) | 41 568 (13%) | 31 858 (10%) | ||
| HR (95% CI) | Reference | HR (95% CI) | HR (95% CI) | HR (95% CI) | P trend | |||
| Oesophageal adenocarcinoma, N (%) | 113 (50%) | 115 (50%) | 0.90 (0.69–1.17) | 1.0 | 0.87 (0.64–1.19) | 0.99 (0.65–1.51) | 0.89 (0.55–1.43) | 0.62 |
| Gastric cardia adenocarcinoma, N (%) | 88 (49%) | 90 (51%) | 0.91 (0.67–1.22) | 1.0 | 0.83 (0.59–1.19) | 1.13 (0.72–1.78) | 0.87 (0.51–1.48) | 0.80 |
| Gastric non-cardia adenocarcinoma, N (%) | 106 (58%) | 76 (42%) | 0.68 (0.51–0.92) | 1.0 | 0.71 (0.50–1.02) | 0.48 (0.26–0.87) | 0.82 (0.50–1.34) | 0.050 |
Abbreviations: BMI=body mass index; CI=confidence interval; HR=hazard ratio; NSAID=non-steroidal anti-inflammatory drug.
HRs and 95% CIs come from models adjusted for age at cohort entry, sex, cigarette smoking status, alcohol, education, fruit intake, vegetable intake, BMI, total energy intake, and both vigorous physical activity and usual physical activity throughout the day. Estimates for any aspirin and any non-aspirin NSAID are from a single model (i.e., mutually adjusted) and those for frequency of use were adjusted for any use of the other class of NSAID.
Trend tests used the category of intake as an ordinal variable (0–3).
We combined aspirin and non-aspirin NSAIDs into a single category and found statistically significantly reduced risk of non-cardia gastric cancer, 0.64 (0.44–0.91), compared with never using either agent, but no significant associations with risk of cancer at the other two sites. As adenocarcinomas of the oesophagus and cardia can be difficult to separate, we combined these two sites into a single outcome, but still found no significant associations with either aspirin or non-aspirin NSAID use. We adjusted for and also stratified by reported use of antacids, a proxy marker for reflux disease or heartburn, and found no differences compared with the presented results (data not shown). We tested for and found no evidence of a significant interaction between sex and NSAID use. Finally, we deleted the first 1, 2, and 3 years of follow-up and found similar results to those for the full follow-up period.
We calculated age-standardised incidence rates for non-cardia gastric cancer in aspirin users and non-users. The rates (95% CI) per 100 000 person years dropped from 11.0 (8.4–13.6) in non-aspirin users to 7.0 (5.7–8.3) in users. We also calculated rates in men and women separately because of the underlying difference in risk for this cancer. The number of non-cardia gastric cases in women in our cohort was small (N=53), and among aspirin non-users and users we found rates of 6.4 (3.7–9.1) and 5.1 (3.3–6.9), respectively. In men (N=129), we found rates of 16.4 (11.6–21.1) and 8.2 (6.4–9.9), respectively.
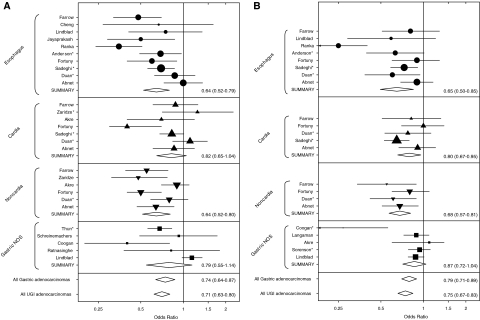
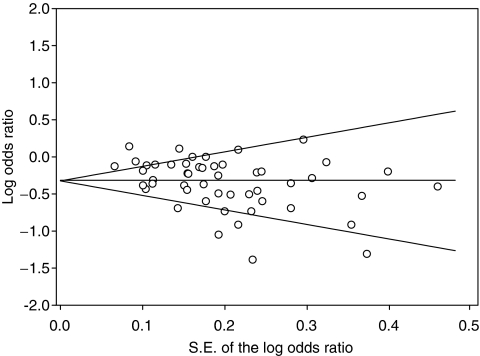
To put our results in a larger perspective, we completed a meta-analysis of 49 risk estimates from 17 published studies reporting the association between either aspirin (Figure 1A) or non-aspirin NSAID (Figure 1B) use and the risk of oesophageal or gastric adenocarcinoma. In the meta-analysis, aspirin use was inversely associated with both gastric and oesophageal adenocarcinomas, with summary ORs (95% CI) for non-cardia, cardia, and oesophageal adenocarcinomas of 0.64 (0.52–0.80), 0.82 (0.65–1.04), and 0.64 (0.52–0.79), respectively. The corresponding numbers for other NSAIDs were 0.68 (0.57–0.81), 0.80 (0.67–0.95), and 0.65 (0.50–0.85), respectively. Figure 2 shows a Begg funnel plot for detecting publication bias in all the NSAID and upper GI adenocarcinoma literature combined. The summary OR (95% CI) for all estimates included in Figure 1A and B was 0.72 (0.67–0.79). Using either the Begg test (P=0.019) or the Egger test (P=0.002), we found evidence of publication bias and the figure suggests that the current literature may overestimate the beneficial effects of NSAIDs. But, when we excluded the smaller studies (those with standard error greater than 0.2; N=20) or those that showed strong protection (log OR less than −0.50; N=14), we found that the association was essentially unchanged.
Figure 1.
Forest plots for the association between any aspirin (A) or non-aspirin NSAID (B) use and risk of oesophageal, gastric cardia, or gastric non-cardia cancer. Summary estimates and study weights (proportional to symbol size) come from random effects models. Studies are listed by the last name of the first author and Abnet refers to this study. We used the broadest measure of NSAID exposure and multivariable-adjusted estimates whenever possible. To make the exposure measures more comparable, we generated new combined estimates of effect when the published estimates were stratified on dose or duration, and this is indicated by an asterisk after the first author's name. Gastric NOS means that the location of the tumours within the stomach was not specified. The summary estimate for all studies included in this figure was 0.72 (0.67–0.79).
Figure 2.
Begg funnel plot with pseudo 95% confidence intervals for all estimates included in the meta-analysis of NSAID use and oesophageal or stomach adenocarcinoma. Both the Begg test (P=0.010) and the Egger test (P=0.001) suggested publication bias, but, dropping the studies with a standard error greater than 0.2 (N=20) or a log OR less than −0.50 (N=14) left the association essentially unchanged. Therefore, publication bias probably had little effect on the summary estimates.
Discussion
We found that reported use of aspirin or non-aspirin NSAIDs was associated with a significant 36% reduction in the risk of non-cardia gastric cancer consistent with the earlier studies. For ever use of aspirin in the previous 12 months, age-adjusted rates of gastric non-cardia cancer dropped from 11.0 in non-users to 7.0/100 000 person years in users. Although we did not find a significant association between use of aspirin or other NSAIDs with cardia cancer, the point estimate in our study was very close to the summary estimate from the meta-analysis, which showed a protective effect. Unlike most earlier observational studies, we found no such association with oesophageal adenocarcinoma.
Our finding in the NIH-AARP cohort study that aspirin or other NSAIDs had a protective association with gastric non-cardia adenocarcinoma is consistent with the literature published earlier, which is summarised in Figure 1. It appears that aspirin and non-aspirin NSAIDs have similar effects, which may have implications for cancer prevention. Eradication of Helicobacter pylori, the strongest risk factor for gastric non-cardia adenocarcinoma, may reduce its incidence (Talley, 2008). However, recent studies suggest that H. pylori may have health benefits, such as preventing asthma (Blaser et al, 2008) or oesophageal adenocarcinoma (Islami and Kamangar, 2008). Beyond the direct benefits and risks of eradication to the individual, the methods and consequences of attempted widespread eradication, such as increasing antibiotic resistance, must also be considered (Graham and Shiotani, 2008). A single trial has tested the effect of the NSAID rofecoxib on subjects with gastric pre-neoplastic lesions over 24 months and found no evidence of benefit, but this study was small and did not use cancer as an end point (Leung et al, 2006). The remarkably consistent observational results showing that NSAID use is associated with a reduced risk of gastric cancer may warrant a randomised trial in a suitable population at high risk for the disease in which side effects can be monitored closely.
The epidemiology of gastric cardia tumours in the United States is similar to that of oesophageal adenocarcinoma. The incidence of this tumour may have increased in recent years, but this change may have occurred because of changing patterns of diagnosis or because of the difficulty of separating adenocarcinomas in the gastric cardia from those in the oesophagus (Kubo and Corley, 2002). We found no significant association between use of either aspirin or non-aspirin NSAIDs and risk of gastric cardia adenocarcinoma, but our point estimates are similar to the summary estimates from our meta-analysis, which suggests a significant protective effect.
We found no evidence that ever or daily aspirin use lowered the risk of oesophageal adenocarcinoma, for which, as shown in Figure 1, our results are discordant with many earlier studies. Most of these showed some evidence, albeit not always significantly, that use of aspirin or non-aspirin NSAIDs was associated with reduced risk.
The reasons for these differences are not clear. Most earlier studies had retrospective designs and may be prone to reverse causality for NSAIDs, as subjects with reflux symptoms, and therefore at higher risk of oesophageal adenocarcinoma, may avoid using NSAIDs producing the appearance of protection among users. But at least one earlier prospective study found that NSAID use reduced risk of progression to oesophageal adenocarcinoma among subjects with Barrett's oesophagus (Vaughan et al, 2005). Recently, in the same cohort, the association is found strongest among subjects with multiple molecular abnormalities that confer the greatest risk of progressing to cancer, but absent in those at the lowest risk (Galipeau et al, 2007). One study using subjects with Barrett's oesophagus as controls found that oesophageal adenocarcinoma cases and subjects with Barrett's used aspirin and NSAIDs at similar rates, but this differed with long-term reflux symptoms (Tsibouris et al, 2004). One small, short-term trial tested the effect of twice-daily treatment with 200 mg of celecoxib for 48 weeks on the proportion of dysplastic biopsies in subjects with Barrett's oesophagus and did not find any benefit (Heath et al, 2007). A large trial of proton pump inhibitors with or without aspirin for the chemoprevention of oesophageal adenocarcinoma in men with Barrett's oesophagus is underway (Jankowski and Moayyedi, 2004).
Our study has several strengths, being based on a large prospective cohort that provided adequate power and minimised recall bias, which may alter the associations found in case–control studies. We used subject-completed questionnaires that captured both over-the-counter and pharmacy-provided NSAIDs and information on many potentially confounding exposures, many of which (e.g., age, tobacco smoking, and physical activity) were similar among NSAID users and non-users. On the other hand, we captured only NSAID use over the previous 12 months and did not collect the duration of use, which could have caused misclassification of subjects who, for example, recently ceased NSAID use due to upper gastrointestinal symptoms. But, we did adjust for and stratify by antacid use without change in our risk estimates. We could not assess infection with H. pylori and infected subjects may have different patterns of NSAID use, which would lead to different confounding effects in the stomach and oesophagus. Finally, this study, being observational, is susceptible to confounding by other unmeasured or poorly measured confounders, supporting the need for randomised controlled trials.
In this large prospective cohort study, we found further evidence that regular use of aspirin or non-aspirin NSAIDs may reduce the risk of non-cardia gastric cancer; in contrast, this was not associated with reduced risk of oesophageal adenocarcinoma, thereby differing from most earlier studies.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry; from Georgia by the Georgia Center for Cancer Statistics; from California by the California Department of Health Services, Cancer Surveillance Section; from Michigan by the Michigan Cancer Surveillance Program; from Florida by the Florida Cancer Data System under contract to the Department of Health (DOH); from Louisiana by the Louisiana Tumor Registry; from Nevada by the Nevada Central Cancer registry; from New Jersey by the New Jersey State Cancer Registry; from North Carolina by the North Carolina Central Cancer Registry; from Pennsylvania by the Division of Health Statistics and Research, Pennsylvania Department of Health; and from Texas by the Texas Cancer Registry. The views expressed herein are solely those of the authors and do not necessarily reflect those of the cancer registries or contractors. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions. We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation.
CCA had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of data analysis. All authors have given full approval to the final paper. CCA, NDF, FK, MFL, and AS were involved in the study design, analysis, and interpretation of the data. CCA, NDF, FK, MFL, ARH, and AS were all involved in the critical revision of the paper.
Footnotes
Competing interests
The authors declare that no competing interests exist.
References
- Abnet CC, Freedman ND, Hollenbeck AR, Fraumeni Jr JF, Leitzmann M, Schatzkin A (2008) A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 44: 465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akre K, Ekstrom AM, Signorello LB, Hansson LE, Nyren O (2001) Aspirin and risk for gastric cancer: a population-based case–control study in Sweden. Br J Cancer 84: 965–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LA, Johnston BT, Watson RGP, Murphy SJ, Ferguson HR, Comber H, McGuigan J, Reynolds JV, Murray LJ (2006) Nonsteroidal anti-inflammatory drugs and the esophageal inflammation-metaplasia-adenocarcinoma sequence. Cancer Res 66: 4975–4982 [DOI] [PubMed] [Google Scholar]
- Baron JA (2003) Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog Exp Tumor Res 37: 1–24 [DOI] [PubMed] [Google Scholar]
- Blaser MJ, Chen Y, Reibman J (2008) Does Helicobacter pylori protect against asthma and allergy? Gut 57: 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosetti C, Talamini R, Franceschi S, Negri E, Garavello W, La Vecchia C (2003) Aspirin use and cancers of the upper aerodigestive tract. Br J Cancer 88: 672–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow NE, Day NE (1987) Statistical methods in cancer research. Vol. 2, The design and analysis of cohort studies. IARC Scientific Publication No. 82, Lyon: IARC [PubMed] [Google Scholar]
- Cheng KK, Sharp L, McKinney PA, Logan RF, Chilvers CE, Cook-Mozaffari P, Ahmed A, Day NE (2000) A case–control study of oesophageal adenocarcinoma in women: a preventable disease. Br J Cancer 83: 127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan PF, Rosenberg L, Palmer JR, Strom BL, Zauber AG, Stolley PD, Shapiro S (2000) Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol Biomarkers Prev 9: 119–123 [PubMed] [Google Scholar]
- Corley DA, Kerlikowske K, Verma R, Buffler P (2003) Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 124: 47–56 [DOI] [PubMed] [Google Scholar]
- Duan L, Wu AH, Sullivan-Halley J, Bernstein L (2008) Nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric adenocarcinomas in Los Angeles County. Cancer Epidemiol Biomarkers Prev 17: 126–134 [DOI] [PubMed] [Google Scholar]
- Dubé C, Rostom A, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D (2007) The use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med 146: 365–375 [DOI] [PubMed] [Google Scholar]
- Farrow DC, Vaughan TL, Hansten PD, Stanford JL, Risch HA, Gammon MD, Chow WH, Dubrow R, Ahsan H, Mayne ST, Schoenberg JB, West AB, Rotterdam H, Fraumeni Jr JF, Blot WJ (1998) Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev 7: 97–102 [PubMed] [Google Scholar]
- Fortuny J, Johnson CC, Bohlke K, Chow WH, Hart G, Kucera G, Mujumdar U, Ownby D, Wells K, Yood MU, Engel LS (2007) Use of anti-inflammatory drugs and lower esophageal sphincter-relaxing drugs and risk of esophageal and gastric cancers. Clin Gastroenterol Hepatol 5: 1154–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman ND, Abnet CC, Leitzmann MF, Mouw T, Subar AF, Hollenbeck AR, Schatzkin A (2007) A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 165: 1424–1433 [DOI] [PubMed] [Google Scholar]
- Funkhouser EM, Sharp GB (1995) Aspirin and reduced risk of esophageal carcinoma. Cancer 76: 1116–1119 [DOI] [PubMed] [Google Scholar]
- Galipeau PC, Li X, Blount PL, Maley CC, Sanchez CA, Odze RD, Ayub K, Rabinovitch PS, Vaughan TL, Reid BJ (2007) NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med 4: e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garidou A, Tzonou A, Lipworth L, Signorello LB, Kalapothaki V, Trichopoulos D (1996) Life-style factors and medical conditions in relation to esophageal cancer by histologic type in a low-risk population. Int J Cancer 68: 295–299 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez A, Garcia Rodriguez LA, Lopez-Ridaura R (2003) Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC cancer 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DY, Shiotani A (2008) New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol 5: 321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath EI, Canto MI, Piantadosi S, Montgomery E, Weinstein WM, Herman JG, Dannenberg AJ, Yang VW, Shar AO, Hawk E, Forastiere AA (2007) Secondary chemoprevention of Barrett's esophagus with celecoxib: results of a randomized trial. J Natl Cancer Inst 99: 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekens CH, Schneider WR (2008) The need for wider and appropriate utilization of aspirin and statins in the treatment and prevention of cardiovascular disease. Expert Rev Cardiovasc Ther 6: 95–107 [DOI] [PubMed] [Google Scholar]
- Islami F, Kamangar F (2008) Helicobacter pylori and Esophageal Cancer Risk: A Meta-analysis. Cancer Prev Res 1: 329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski J, Moayyedi P (2004) Re: Cost-effectiveness of aspirin chemoprevention for Barrett's esophagus. J Natl Cancer Inst 96: 885–887 [DOI] [PubMed] [Google Scholar]
- Jayaprakash V, Menezes RJ, Javle MM, McCann SE, Baker JA, Reid ME, Natarajan N, Moysich KB (2006) Regular aspirin use and esophageal cancer risk. Int J Cancer 119: 202–207 [DOI] [PubMed] [Google Scholar]
- Kubo A, Corley DA (2002) Marked regional variation in adenocarcinomas of the esophagus and the gastric cardia in the United States. Cancer 95: 2096–2102 [DOI] [PubMed] [Google Scholar]
- Langman MJ, Cheng KK, Gilman EA, Lancashire RJ (2000) Effect of anti-inflammatory drugs on overall risk of common cancer: case–control study in general practice research database. BMJ 320: 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung WK, Ng EKW, Chan FKL, Chan WY, Chan KF, Auyeung ACM, Lam CCH, Lau JYW, Sung JJY (2006) Effects of long-term rofecoxib on gastric intestinal metaplasia: results of a randomized controlled trial. Clin Cancer Res 12: 4766–4772 [DOI] [PubMed] [Google Scholar]
- Lindblad M, Lagergren J, Garcia Rodriguez LA (2005) Nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev 14: 444–450 [DOI] [PubMed] [Google Scholar]
- Michaud DS, Midthune D, Hermansen S, Leitzmann M, Harlan LC, Kipnis V, Schatzkin A (2005) Comparison of Cancer Registry Case Ascertainment with SEER Estimates and Self-reporting in a Subset of the NIH-AARP Diet and Health Study. Journal of Registry Management 32: 70–75 [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P (2002) Global cancer statistics 2002. CA Cancer J Clin 55: 74–108 [DOI] [PubMed] [Google Scholar]
- Ranka S, Gee JM, Johnson IT, Skinner J, Hart AR, Rhodes M (2006) Non-steroidal anti-inflammatory drugs, lower oesophageal sphincter-relaxing drugs and oesophageal cancer. A case–control study. Digestion 74: 109–115 [DOI] [PubMed] [Google Scholar]
- Ratnasinghe LD, Graubard BI, Kahle L, Tangrea JA, Taylor PR, Hawk E (2004) Aspirin use and mortality from cancer in a prospective cohort study. Anticancer Res 24: 3177–3184 [PubMed] [Google Scholar]
- Ries LAG, Melbert D (2007) SEER Cancer Statistics Review, 1975–2004. National Cancer Institute: Bethesda, MD, USA [Google Scholar]
- Sadeghi S, Bain CJ, Pandeya N, Webb PM, Green AC, Whiteman DC (2008) Aspirin, nonsteroidal anti-inflammatory drugs, and the risks of cancers of the esophagus. Cancer Epidemiol Biomarkers Prev 17: 1169–1178 [DOI] [PubMed] [Google Scholar]
- Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS, Freedman LS, Brown CC, Midthune D, Kipnis V (2001) Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 154: 1119–1125 [DOI] [PubMed] [Google Scholar]
- Schreinemachers DM, Everson RB (1994) Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology 5: 138–146 [DOI] [PubMed] [Google Scholar]
- Sorensen HT, Friis S, Norgard B, Mellemkjaer L, Blot WJ, McLaughlin JK, Ekbom A, Baron JA (2003) Risk of cancer in a large cohort of nonaspirin NSAID users: a population-based study. Br J Cancer 88: 1687–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleiman UL, Harrison M, Britton A, McPherson K, Bates T (2000) H2-receptor antagonists may increase the risk of cardio-oesophageal adenocarcinoma: a case–control study. Eur J Cancer Prev 9: 185–191 [PubMed] [Google Scholar]
- Talley NJ (2008) Is it time to screen and treat H pylori to prevent gastric cancer? The Lancet 372: 350–352 [DOI] [PubMed] [Google Scholar]
- Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW (1993) Aspirin use and risk of fatal cancer. Cancer Res 53: 1322–1327 [PubMed] [Google Scholar]
- Tsibouris P, Hendrickse MT, Isaacs PET (2004) Daily use of non-steroidal anti-inflammatory drugs is less frequent in patients with Barrett's oesophagus who develop an oesophageal adenocarcinoma. Aliment Pharmacol Ther 20: 645–655 [DOI] [PubMed] [Google Scholar]
- US Preventive Services Task Force (2007) Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 146: 361–364 [PubMed] [Google Scholar]
- Vaughan TL, Dong LM, Blount PL, Ayub K, Odze RD, Sanchez CA, Rabinovitch PS, Reid BJ (2005) Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett's oesophagus: a prospective study. Lancet Oncol 6: 945–952 [DOI] [PubMed] [Google Scholar]
- Wang W-H, Huang J-Q, Zheng G-F, Lam S-K, Karlberg J, Wong BC-Y (2003) Non-steroidal anti-inflammatory drug use and the risk of gastric cancer: a systematic review and meta-analysis. J Natl Cancer Inst 95: 1784–1791 [DOI] [PubMed] [Google Scholar]
- Zaridze D, Borisova E, Maximovitch D, Chkhikvadze V (1999) Aspirin protects against gastric cancer: results of a case–control study from Moscow, Russia. Int J Cancer 82: 473–476 [DOI] [PubMed] [Google Scholar]