Abstract
The RAF–MEK–ERK pathway regulates both myoblast proliferation and differentiation; however, it is unclear how these events are coordinated. Here, we show that human phosphatidylethanolamine-binding protein 4 (PEBP4), a RAF kinase inhibitory protein (RKIP) family protein expressed preferentially in muscle, regulates the activity of the ERK pathway and myoblast differentiation by acting as a scaffold protein. In contrast to RKIP, which disrupts the RAF1–MEK interaction, PEBP4 forms ternary complexes with RAF1 and MEK, and can scaffold this interaction. PEBP4 expression is induced during the differentiation of primary human myoblasts. Consistent with the properties of a scaffold, PEBP4 enhances the RAF1–MEK interaction and the activation of MEK at low expression levels, whereas it inhibits these parameters at higher expression levels. Downregulation of PEBP4 by short hairpin RNA in human myoblasts increases MEK signalling and inhibits differentiation; by contrast, PEBP4 overexpression enhances differentiation. Thus, PEBP4 participates in the control of muscle cell differentiation by modulating the activity of MEK and ERK.
Keywords: human myoblast differentiation, PEBP4, RAF–MEK–ERK pathway, scaffold protein
Introduction
The RAF–MEK–ERK pathway participates in the regulation of many biological processes, including proliferation and differentiation. It has a crucial function in muscle cell differentiation, although both negative (Rommel et al, 1999; Yang et al, 2006; Yokoyama et al, 2007) and positive effects have been reported (Gredinger et al, 1998; Li & Johnson, 2006; Cho et al, 2007). These discrepant observations are not necessarily mutually exclusive as, in other cell systems, for example, cancer cell line PC12, the activation kinetics of the pathway is crucial, with transient activation mediating proliferation and sustained activation inducing neuronal differentiation (Marshall, 1995).
Increasing evidence shows that ERK signalling is modulated by proteins that regulate the interaction between pathway components (Kolch, 2005). Negative regulators include the RAF kinase inhibitory protein (RKIP; Yeung et al, 1999). RKIP inhibits the phosphorylation and activation of MEK by RAF1 by disrupting its interaction (Yeung et al, 1999). Recently, a new member of the RKIP family, human phosphatidylethanolamine-binding protein 4 (PEBP4), was described (Wang et al, 2004); PEBP4 expression was reported in human cancer cells. Tumour necrosis factor-α (TNFα) stimulated PEBP4 expression, its translocation from lysosomes to the cell membrane, and its association with RAF1 and MEK. Overexpression of PEBP4 in L929 fibroblasts inhibited TNFα-induced apoptosis, and the activation of ERK and JNK. Conversely, the downregulation of endogenous PEBP4 in human MCF7 breast cancer cells sensitized cells to TNFα-induced apoptosis and cell-cycle arrest (Wang et al, 2004, 2005). The reduction of endogenous PEBP4 expression in MCF7 cells augmented the levels of proapoptotic proteins such as p53, p21CIP/WAF and BAX (BCL2-associated X protein), while reducing the levels of protective proteins such as BCL2 (B-cell CLL/lymphoma 2) and BCLXL (Wang et al, 2005). Further investigations have shown that PEBP4 can protect CaoV-3 ovarian cancer cells against TRAIL (TNFα-related apoptosis inducing ligand)-induced apoptosis by limiting TRAIL-induced ERK and JNK activation (Li et al, 2006). Similarly, in prostate cancer cell lines, PEBP4 expression correlated with sensitivity to TRAIL-induced apoptosis. TRAIL-sensitive DU145 prostate cancer cells lacked PEBP4, whereas TRAIL-resistant LNCaP cells showed high expression (Li et al, 2007). Overexpressing PEBP4 in DU145 cells inhibited the activity of ERK but promoted AKT activation, which made the cells resistant to TRAIL-induced apoptosis. Downregulation of endogenous PEBP4 in LNCaP cells had the opposite effect. As PEBP4 expression was also elevated in advanced prostate cancer, PEBP4 might promote tumorigenesis by enhancing the survival capabilities of cancer cells. Recently, a mouse homologue of human PEBP4 was identified, which is specifically expressed in retinal ganglion cells and promotes cell migration and protects against drug-induced apoptosis (Zhang et al, 2007). The sequence identity of mouse PEBP4 with human PEBP4 is only 44.6% on the protein level and, owing to the different pattern of expression, it is unclear whether the mouse and human homologues have similar functions.
As PEBP4 shares homology to RKIP and binds to RAF1 and MEK, it was surmised to act in a similar manner to RKIP (Wang et al, 2004); however, there are clear functional differences. First, PEBP4 protects cancer cells against apoptosis, whereas RKIP overexpression is associated with the enhancement of apoptosis (Chatterjee et al, 2004; Al-Mulla et al, 2006) and good prognosis (Fu et al, 2003; Schuierer et al, 2004; Hagan et al, 2005; Al-Mulla et al, 2006). In addition, RKIP does not regulate the JNK pathway (Yeung et al, 1999). These differences show that RKIP and PEBP4 are not functionally equivalent. Importantly, the molecular mechanism of PEBP4 action is not characterized. Here, we show that PEBP4 uses a different molecular mechanism to regulate ERK by acting as a scaffold. We also show a hitherto unknown role for PEBP4 in myoblast differentiation.
Results And Discussion
PEBP4 forms ternary complexes with RAF1 and MEK
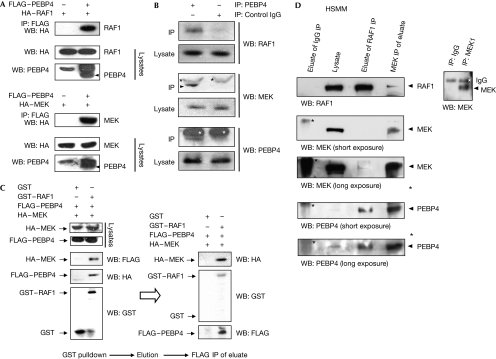
We isolated PEBP4 as RKIP-related protein (supplementary Fig S1 online). PEBP4 co-immunoprecipitated with RAF1 and MEK when exogenously expressed as tagged proteins (Fig 1A). PEBP4 is mainly expressed in muscle tissues (supplementary Fig S2 online). Thus, these interactions were verified by co-immunoprecipitation of endogenous proteins from primary SkMC human muscle cells. Endogenous PEBP4 co-immunoprecipitated with both endogenous RAF1 and MEK (Fig 1B). We examined whether PEBP4 can form ternary complexes with RAF1 and MEK by using a sequential immunoprecipitation procedure (Fig 1C). For this purpose, glutathione S-transferase (GST) or GST–RAF1 was transiently coexpressed with FLAG–PEBP4 and haemagglutinin (HA)–MEK. GST proteins were pulled down using glutathione sepharose beads and eluted with glutathione. Subsequently, the eluted proteins were immunoprecipitated with FLAG antibodies to isolate the proteins associated with FLAG–PEBP4. Both HA–MEK and GST–RAF1 were detected in the FLAG–PEBP4 precipitate, suggesting that PEBP4 can form ternary complexes with RAF1 and MEK, and might function as a scaffold protein for RAF1–MEK interactions. This sharply distinguishes it from RKIP, which disrupts the interaction between RAF1 and MEK (Yeung et al, 1999), acting as a competitive inhibitor of MEK binding (Yeung et al, 2000). Importantly, a ternary endogenous RAF1–MEK–PEBP4 complex also exists in muscle cells (Fig 1D) and MCF7 cells (supplementary Fig S3 online).
Figure 1.
PEBP4 associates with RAF1 and MEK. (A) Co-immunoprecipitation between HA-tagged RAF1 or HA–MEK with FLAG-tagged PEBP4 transiently expressed in COS1 cells. The PEBP4 antibody detects an unspecific band migrating just above FLAG–PEBP4. (B) Co-immunoprecipitations of endogenous PEBP4 with endogenous RAF1 or MEK from SkMC myotubes differentiated for 2 days. A rabbit pre-immune serum was used as a control (IgG); asterisks denote IgG heavy chains. (C) PEBP4 forms ternary complexes with RAF and MEK. FLAG–PEBP4 was coexpressed with the GST vector or with GST–RAF and HA–MEK in COS1 cells. GST proteins were purified by glutathione sepharose, eluted with glutathione and the eluate was immunoprecipitated with FLAG antibody. HA–MEK and GST–RAF were detected by immunoblotting with the indicated antibodies. (D) PEBP4 forms ternary complexes with RAF and MEK in myoblasts. Lysates from growing HSMM were immunoprecipitated with unrelated IgG or RAF1 C12 antibodies (directed against the carboxy-terminal 12 aa of RAF1) covalently crosslinked to beads. RAF1 protein was eluted from the beads by competition with the synthetic C12 peptide. The eluate was split into two aliquots that were immunoprecipitated with a polyclonal MEK1/2 antibody. The immunoprecipitates were blotted with antibodies against RAF1, MEK and PEBP4 as indicated. GST, glutathione S-transferase; HA, haemagglutinin; HSMM, human skeletal muscle myoblast; IgG, immunoglobulin G indicated with an asterisk; IP, immunoprecipitation; PEBP4, human phosphatidylethanolamine-binding protein 4; WB, Western blot.
PEBP4 scaffolds RAF1–MEK interactions
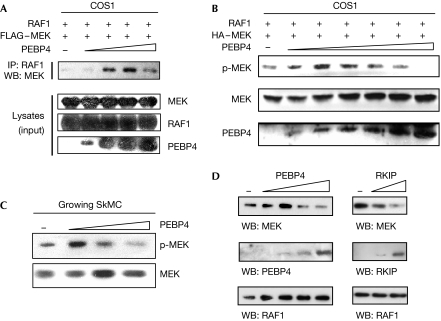
Increasing PEBP4 expression in COS1 cells initially enhanced the binding of MEK to RAF1, but dissipated the complex at higher concentrations (Fig 2A). Similarly, increasing PEBP4 expression first enhanced the activation of MEK, but became inhibitory at high levels (Fig 2B). Such behaviour is typical for scaffold proteins; they promote complex formation of client proteins until their expression levels exceed the concentrations of their clients resulting in sequestration, which functionally results in inhibition. Overexpression of PEBP4 in proliferating SkMC myoblasts, where PEBP4 levels are low (Fig 3A), resulted in an initial enhancement of MEK activation followed by suppression typical for a scaffold (Fig 2C). To test the scaffolding function of PEBP4 more directly, we incubated in vitro RAF1 immobilized on beads with soluble MEK and PEBP4 or RKIP (Fig 2D). Low concentrations of PEBP4 promoted the RAF1–MEK complex, formation, whereas higher concentrations interfered with it; by contrast, RKIP only inhibited it. These results confirm that RKIP acts as an inhibitor of the RAF1–MEK interaction, whereas PEBP4 can scaffold.
Figure 2.
PEBP4 regulates the RAF1–MEK association and activation of MEK. (A) COS1 cells were transiently transfected with RAF1 and FLAG–MEK and increasing amounts of PEBP4 (0.3–1.2 μg) expression plasmids as indicated. RAF1 immunoprecipitates (IP) were blotted for associated MEK. (B) Phosphorylation of MEK was determined in cells transfected as in (A) except that 0.2–2 μg PEBP4 was co-transfected. (C) Growing SkMC myoblasts were transfected with 0.5–3 μg PEBP4 expression vector and lysates were blotted for the phosphorylation of MEK. (D) FLAG–RAF1 beads (ca.100 ng RAF1) were incubated with 500 ng soluble GST–MEK and increasing amounts of PEBP4 (0, 50, 150, 500 and 1500 ng) or RKIP (0, 150 and 500 ng). Proteins bound to RAF1 beads were detected by Western blotting (WB). GST, glutathione S-transferase; PEBP4, human phosphatidylethanolamine-binding protein 4; RKIP, RAF kinase inhibitory protein.
Figure 3.
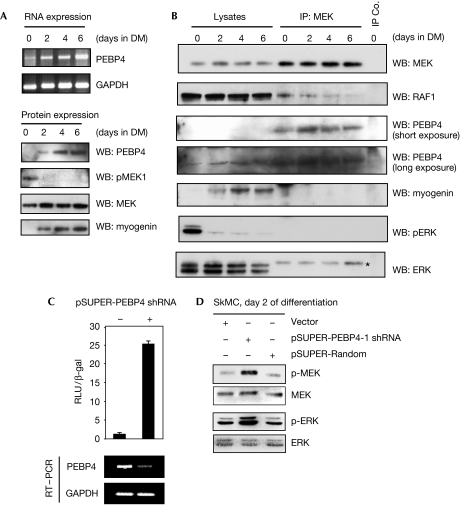
PEBP4 regulates MEK and ERK signalling in human muscle cells. (A) SkMCs were placed in a differentiation medium (DM) and examined for the expression of PEBP4 messenger RNA by RT–PCR (upper panel) and expression of PEBP4 protein, the myogenin differentiation marker and activation of MEK (lower panel). (B) Endogenous MEK was immunoprecipitated (IP) from differentiating SkMCs and blotted with the indicated antibodies. Rabbit IgG was used as a control; myogenin expression was assayed as a differentiation marker. The band marked by an asterisk in the ERK blot is a remnant of the MEK blot that was not removed by stripping. (C) Downregulation of endogenous PEBP4 in SkMC cells activates ELK-dependent transcription. SkMCs were co-transfected with pSUPER-PEBP4 shRNA or empty vector control, and Gal-luc plus Gal-ELK plasmids. Luciferase activity and PEBP4 mRNA expression (by RT–PCR) were assayed after 2 days of differentiation. (D) Phosphorylation of MEK and ERK was determined in SkMC cells transfected with the pSUPER-PEBP4 shRNA vector and cultured in differentiation medium for 2 days. β-gal, β-galactosidase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IgG, immunoglobulin G; PEBP4, human phosphatidylethanolamine-binding protein 4; RT–PCR, reverse transcription–PCR; shRNA, short hairpin RNA; WB, western blot.
PEBP4 regulates MEK–ERK signalling
Muscle differentiation is well studied in the mouse C2C12 myoblast cell line. There, the ERK pathway mediates not only myoblast proliferation and inhibits myoblast fusion (Rommel et al, 1999; Yang et al, 2006; Yokoyama et al, 2007), but also has a positive role in muscle differentiation (Gredinger et al, 1998; Li & Johnson, 2006; Cho et al, 2007). Therefore, we determined the expression of PEBP4 and activity of the ERK pathway during differentiation of primary human SkMC myoblasts. Differentiation induced PEBP4 expression both on the messenger RNA and protein levels, and a concomitant decrease in the activation of MEK (Fig 3A). Importantly, differentiation also induced an increase in PEBP4 association with MEK at the expense of RAF1 binding correlating with a decrease in ERK activation (Fig 3B). Although PEBP4 could scaffold the MEK–ERK interaction when overexpressed in COS1 cells (supplementary Fig S4 online), we could not consistently detect ERK co-precipitating with MEK and PEBP4 in primary muscle cells, presumably because the endogenous interaction is at the detection limit of our antibodies. In summary, these results suggest that PEBP4 regulates ERK pathway activation during differentiation by modulating protein interactions within the pathway.
To test this hypothesis, we downregulated the expression of endogenous PEBP4 in SkMC myoblasts by short hairpin RNA (shRNA). Owing to the difficulties of transfecting primary human cells and stably downregulating protein expression over a prolonged period of time, we assayed ERK pathway activation at day 2 of differentiation. Although the exact time course of differentiation is dependent on the batch of cells and passage number, the effects of PEBP4 on RAF1–MEK complexes and the activation of MEK were consistently observed at day 2. Reduction of PEBP4 mRNA by shRNA caused a robust activation of ELK-mediated transcription in a reporter gene assay (Fig 3C). ELK is a direct ERK substrate involved in the suppression of myogenesis (Wang et al, 2004). It is an appropriate indicator of ERK activity in SkMCs, as ELK activity is induced by both activated RAF1 and MEK, and inhibited by a selective MEK1/2 inhibitor (supplementary Fig S5 online). In addition, these assays confirmed that PEBP4 modulates the ERK pathway upstream from MEK, as activated RAF1 but not activated MEK was susceptible to the inhibition of PEBP4 (supplementary Fig S5 online). Downregulation of endogenous PEBP4 expression induced the activation of MEK and ERK on day 2 of differentiation (Fig 3D). These results indicate that PEBP4 modulates RAF1 activation of MEK and ERK, and hence might affect myoblast differentiation.
PEBP4 regulates human myoblast differentiation
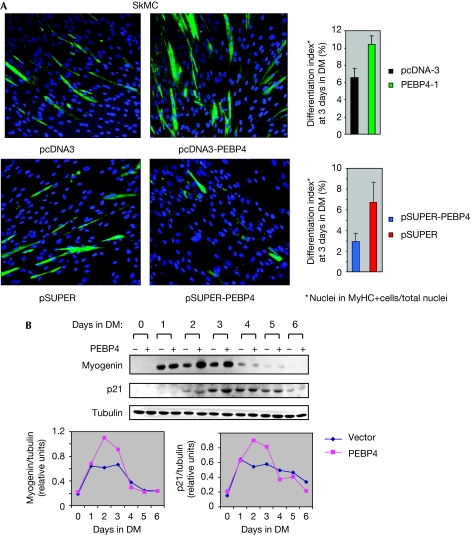
Therefore, we assessed the effects of PEBP4 overexpression or downregulation on SkMC differentiation. As the transfection efficiency of SkMC is poor, we co-transfected a low level of DsRed mito expression plasmid to allow enrichment of transfected cells by fluorescence-activated cell sorting (FACS). Sorted cells were induced to differentiate and were examined for the formation of multinucleated myotubes and the expression of myosin heavy chains (MyHCs) as morphological and biochemical markers for terminal differentiation, respectively. PEBP4 shRNA reduced myotube formation and the expression of MyHC, whereas transfection with a PEBP4 expression plasmid augmented both parameters (Fig 4A). PEBP4-transfected SkMC cells also increased the expression of the early muscle differentiation markers, myogenin and p21CIP/WAF (Fig 4B), supporting a role for PEBP4 in myoblast differentiation. These results were fully corroborated in human skeletal muscle myoblasts (HSMMs), another primary human myoblast cell strain using MyHC expression as a marker for terminal differentiation (supplementary Fig S6 online). PEBP4 overexpression also decreased DNA synthesis agreeing with a role in promoting differentiation (supplementary Fig S7 online).
Figure 4.
PEBP4 regulates myoblast differentiation. (A) SkMC myoblasts were co-transfected with DsRed mito plasmid and pcDNA3-PEBP4, pSUPER-PEBP4 shRNA or the respective empty vectors as indicated. FACS-sorted cells were placed in a differentiation medium (DM) and examined 3 days later for myosin heavy chain (MyHC) expression (green) and myotube formation as markers for terminal differentiation. Nuclei were stained with DAPI (blue). Results shown are representative of three independent experiments with ⩾4,000 nuclei counted per experiment. (B) SkMC myoblasts were co-transfected with vector or pcDNA3-PEBP4 and induced to differentiate. Lysates were blotted for the differentiation markers, myogenin and p21CIP/WAF. Blots were quantified by laser densitometry (lower panel). Results are representative of three independent experiments. DAPI, 4,6-diamidino-2-phenylindole; FACS, fluorescence-activated cell sorting; PEBP4, human phosphatidylethanolamine-binding protein 4; shRNA, short hairpin RNA.
PEBP does not mediate AKT inhibition of ERK signalling
AKT can inhibit the ERK pathway in differentiated mouse C2C12 muscle cells (Rommel et al, 1999), and the direct phosphorylation of RAF1 on the inhibitory Ser 259 site by AKT was suggested as a mechanism (Zimmermann & Moelling, 1999). As AKT could bind to RAF1 in differentiated C2C12 myotubes but not in myoblasts, it was plausible to suggest that myotubes express an adaptor protein that facilitates the interaction between AKT and RAF1 (Rommel et al, 1999). As PEBP4 co-immunoprecipitated with transfected AKT and endogenous AKT, and the interaction increased during myoblast differentiation (supplementary Fig S8A online), we investigated whether or not PEBP4 is this adaptor protein. PEBP4 could form a ternary complex with AKT and RAF1 (supplementary Fig S8B online). AKT could inhibit serum-stimulated RAF1 kinase activity in MCF-7 cells (supplementary Fig S8C online). Surprisingly, inhibitors of PI3 kinase (LY294002) or AKT (AKTIV) only partly relieved this inhibition. Co-transfection of increasing amounts of PEBP4 also partly rescued this inhibition, in contrast to what would be expected from the postulated adaptor protein. A RAF1 S259A mutant behaved similarly, except that LY294002 or AKTIV did not alleviate the repression of kinase activity caused by AKT. As expected, the phosphorylation of Ser 259 decreased not only on growth factor treatment (Dhillon et al, 2002), but surprisingly also when PEBP4 was co-transfected. Co-transfection of AKT had no effect on the phosphorylation of Ser 259, whereas treatment with LY294002 slightly increased it (supplementary Fig S8D online). Similarly, phosphorylation of Ser 259 did not change when SkMC cells were induced to differentiate despite a robust activation of AKT (supplementary Fig S8E online). In addition, PEBP4 did not affect AKT kinase activity (supplementary Fig S9 online), suggesting that the role of PEBP4 in myoblast differentiation is not exerted through the direct regulation of AKT.
These data suggest that (i) RAF1 inhibition by AKT is not mediated by the phosphorylation of Ser 259 and that (ii) PEBP4 is unlikely to represent the postulated adaptor protein that mediates RAF1 inhibition by AKT. As PEBP4 attenuates the inhibition of RAF1 by AKT, a function as a positive modulator of the RAF1–AKT cross-talk is more plausible. For example, PEBP4 could act as part of a control mechanism that fine-tunes RAF1 activity during myoblast differentiation and eventually facilitates recovery from AKT-mediated inhibition.
Methods
Plasmids. The complete open reading frame sequence of CORK1 was deduced from sequencing IMAGE clone 1840766 and deposited in GenBank, accession number AY730275. For consistency with existing nomenclature, the CORK1 constructs are called PEBP4 throughout.
Cell culture and transfections. SkMC and HSMM primary human myoblasts were propagated as specified by the supplier (Clonetics, Lonza, Wokingham, UK). To induce differentiation, confluent cells were switched to DMEM+2% horse serum. COS1, MCF-7 and HEK293 cells were maintained in DMEM+10% FCS. Subconfluent cells were transfected by lipofection, and HSMM by nucleofection (Amaxa, Walkersville, MD, USA).
Reporter gene assays. SkMCs were transfected with Gal-ELK plasmids, which contain a Gal4 DNA-binding domain fused to an ELK transactivation domain that is activated by ERK (Kortenjann et al, 1994). The readout was performed by assessing the activity of a Gal4 promoter-driven luciferase reporter plasmid using the luciferase assay system I (Promega, Southampton, UK).
Immunoprecipitations, immunoblotting and RAF kinase assays. Immunoprecipitations, immunoblotting and RAF kinase assays were performed as described previously (Dhillon et al, 2002) except that cells were lysed in immunoprecipitation buffer (50 mM Tris–HCl pH 7.5, 15 mM EGTA, 150 mM NaCl, 0.1% (w/v) Triton X-100, 1 mM phenylmethyl sulphonyl fluoride, 1 mM dithiothreitol, 10 μg/ml leupeptin and 10 μg/ml aprotinin).
In vitro binding assays. FLAG–RAF1 was expressed in HEK293 and immobilized on anti-FLAG M2 agarose beads (Sigma, Dorset, UK). GST–MEK was prepared from Sf9 cells as described previously (Dhillon et al, 2002). PEBP4 and RKIP were expressed as GST fusion products in Escherichia coli and purified using glutathione sepharose (GE Healthcare) and released from GST by thrombin cleavage. FLAG–RAF1 beads were incubated simultaneously with soluble GST–MEK and PEBP4 or RKIP for 1 h at 4°C in immunoprecipitation buffer.
Fluorescence-activated cell sorting and immunocytochemistry. Myoblasts were co-transfected with the plasmids of interest and DsRed mito (Clontech). DsRed-positive cells were isolated by using an FACS Vantage SE cell sorter, seeded on eight-chamber glass slides at high density overnight and switched to a differentiation medium. Cells were fixed with 2% formaldehyde, permeabilized with 0.1% Triton X-100 and stained with the indicated antibodies.
RNA interference. The target region of shRNA corresponding to codons 27–34 of the PEBP4 sequence was cloned into pSUPER as described previously (Brummelkamp et al, 2002).
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
supplementary Information
Acknowledgments
We thank Peter Shaw for the Gal-ELK plasmids. This study was supported by Cancer Research UK.
Footnotes
The authors declare that they have no conflict of interest.
References
- Al-Mulla F et al. (2006) Raf kinase inhibitor protein expression in a survival analysis of colorectal cancer patients. J Clin Oncol 24: 5672–5679 [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 [DOI] [PubMed] [Google Scholar]
- Chatterjee D et al. (2004) RKIP sensitizes prostate and breast cancer cells to drug-induced apoptosis. J Biol Chem 279: 17515–17523 [DOI] [PubMed] [Google Scholar]
- Cho YY et al. (2007) RSK2 mediates muscle cell differentiation through regulation of NFAT3. J Biol Chem 282: 8380–8392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon AS, Meikle S, Yazici Z, Eulitz M, Kolch W (2002) Regulation of Raf-1 activation and signalling by dephosphorylation. EMBO J 21: 64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z, Keller ET (2003) Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J Natl Cancer Inst 95: 878–889 [DOI] [PubMed] [Google Scholar]
- Gredinger E, Gerber AN, Tamir Y, Tapscott SJ, Bengal E (1998) Mitogen-activated protein kinase pathway is involved in the differentiation of muscle cells. J Biol Chem 273: 10436–10444 [DOI] [PubMed] [Google Scholar]
- Hagan S, Al Mulla F, Mallon E, Oien K, Ferrier R, Gusterson B, Curto Garcia JJ, Kolch W (2005) Reduction of raf-1 kinase inhibitor protein expression correlates with breast cancer metastasis. Clin Cancer Res 11: 7392–7397 [DOI] [PubMed] [Google Scholar]
- Kolch W (2005) Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol 6: 827–837 [DOI] [PubMed] [Google Scholar]
- Kortenjann M, Thomae O, Shaw PE (1994) Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol Cell Biol 14: 4815–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang X, Li N, Qiu J, Zhang Y, Cao X (2007) hPEBP4 resists TRAIL-induced apoptosis of human prostate cancer cells by activating Akt and deactivating ERK1/2 pathways. J Biol Chem 282: 4943–4950 [DOI] [PubMed] [Google Scholar]
- Li J, Johnson SE (2006) ERK2 is required for efficient terminal differentiation of skeletal myoblasts. Biochem Biophys Res Commun 345: 1425–1433 [DOI] [PubMed] [Google Scholar]
- Li P, Wang X, Li N, Kong H, Guo Z, Liu S, Cao X (2006) Anti-apoptotic hPEBP4 silencing promotes TRAIL-induced apoptosis of human ovarian cancer cells by activating ERK and JNK pathways. Int J Mol Med 18: 505–510 [PubMed] [Google Scholar]
- Marshall CJ (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80: 179–185 [DOI] [PubMed] [Google Scholar]
- Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ (1999) Differentiation stage-specific inhibition of the raf–MEK–ERK pathway by Akt. Science 286: 1738–1741 [DOI] [PubMed] [Google Scholar]
- Schuierer MM, Bataille F, Hagan S, Kolch W, Bosserhoff AK (2004) Reduction in Raf kinase inhibitor protein expression is associated with increased Ras-extracellular signal-regulated kinase signaling in melanoma cell lines. Cancer Res 64: 5186–5192 [DOI] [PubMed] [Google Scholar]
- Wang X, Li N, Liu B, Sun H, Chen T, Li H, Qiu J, Zhang L, Wan T, Cao X (2004) A novel human phosphatidylethanolamine-binding protein resists tumor necrosis factor α-induced apoptosis by inhibiting mitogen-activated protein kinase pathway activation and phosphatidylethanolamine externalization. J Biol Chem 279: 45855–45864 [DOI] [PubMed] [Google Scholar]
- Wang X, Li N, Li H, Liu B, Qiu J, Chen T, Cao X (2005) Silencing of human phosphatidylethanolamine-binding protein 4 sensitizes breast cancer cells to tumor necrosis factor-α-induced apoptosis and cell growth arrest. Clin Cancer Res 11: 7545–7553 [DOI] [PubMed] [Google Scholar]
- Yang W, Chen Y, Zhang Y, Wang X, Yang N, Zhu D (2006) Extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase pathway is involved in myostatin-regulated differentiation repression. Cancer Res 66: 1320–1326 [DOI] [PubMed] [Google Scholar]
- Yeung K et al. (1999) Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 401: 173–177 [DOI] [PubMed] [Google Scholar]
- Yeung K, Janosch P, McFerran B, Rose DW, Mischak H, Sedivy JM, Kolch W (2000) Mechanism of suppression of the Raf/MEK/extracellular signal-regulated kinase pathway by the raf kinase inhibitor protein. Mol Cell Biol 20: 3079–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama T, Takano K, Yoshida A, Katada F, Sun P, Takenawa T, Andoh T, Endo T (2007) DA-Raf1, a competent intrinsic dominant-negative antagonist of the Ras–ERK pathway, is required for myogenic differentiation. J Cell Biol 177: 781–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang X, Xiang Z, Li H, Qiu J, Sun Q, Wan T, Li N, Cao X, Wang J (2007) Promotion of cellular migration and apoptosis resistance by a mouse eye-specific phosphatidylethanolamine-binding protein. Int J Mol Med 19: 55–63 [PubMed] [Google Scholar]
- Zimmermann S, Moelling K (1999) Phosphorylation and regulation of raf by akt (protein kinase B). Science 286: 1741–1744 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Information