Abstract
The question of whether RNA interference (RNAi) acts as an antiviral mechanism in mammalian cells remains controversial. The antiviral interferon (IFN) response cannot easily be distinguished from a possible antiviral RNAi pathway owing to the involvement of double-stranded RNA (dsRNA) as a common inducer molecule. The non-structural protein 3 (NS3) protein of rice hoja blanca virus (RHBV) is an RNA silencing suppressor (RSS) that exclusively binds to small dsRNA molecules. Here, we show that this plant viral RSS lacks IFN antagonistic activity, yet it is able to substitute the RSS function of the Tat protein of human immunodeficiency virus type 1. An NS3 mutant that is deficient in RNA binding and its associated RSS activity is inactive in this complementation assay. This cross-kingdom suppression of RNAi in mammalian cells by a plant viral RSS indicates the significance of the antiviral RNAi response in mammalian cells and the usefulness of well-defined RSS proteins.
Keywords: RNAi suppression, complementation assay, HIV-1 Tat, RHBV NS3
Introduction
RNA interference (RNAi) acts as an antiviral response in plants, insects and invertebrates (Voinnet, 2001; Galiana-Arnoux et al, 2006; van Rij et al, 2006; Wang XH et al, 2006; Ding & Voinnet, 2007). RNAi is induced by double-stranded RNA (dsRNA) viral replication intermediates and extended secondary structures in viral RNA (Voinnet, 2005). These dsRNA molecules are recognized by Dicer proteins and processed into small interfering RNAs (siRNAs), which guide the RNA-induced silencing complex (RISC) to inactivate a target RNA in a sequence-specific manner (Sontheimer, 2005). To counteract this antiviral response, plant and insect viruses encode RNA silencing suppressor (RSS) proteins (Voinnet, 2005). Most plant viral RSS proteins have dsRNA-binding domains for short siRNAs (Voinnet, 2005; Lakatos et al, 2006; Merai et al, 2006) or longer dsRNAs (Merai et al, 2005; Deleris et al, 2006). Other plant viral RSS proteins interfere with protein components of the RNAi machinery (Deleris et al, 2006; Levy et al, 2008).
Mammalian cells have a functional RNAi pathway that can be instructed to become antiviral on transfection with siRNAs or constructs that express short hairpin RNAs against viral sequences (reviewed by Haasnoot et al, 2007; Marques & Carthew, 2007). However, the potential role of RNAi as a natural antiviral defence mechanism in mammalian cells remains controversial. The characteristic of antiviral RNAi—that is, accumulation of virus-derived siRNAs—could not be identified in infected cells (Pfeffer et al, 2004); however, such molecules have been described more recently for several endogenous and exogenous viruses, including human immunodeficiency virus type 1 (HIV-1; Bennasser et al, 2005; Soifer et al, 2005; Yang & Kazazian, 2006; Parameswaran et al, 2008), and yet the significance of these findings is still being debated (Lin & Cullen, 2007). There is accumulating evidence that mammalian cells use microRNAs (miRNAs) to control viruses (Berkhout & Jeang, 2007). HIV-1 is inhibited by miR-17 and miR-20a owing to the downregulation of histone acetylase p300/CBP-associated factor (PCAF), a cofactor of the transactivator of transcription (Tat) protein (Triboulet et al, 2007). miRNAs might also regulate components of the antiviral interferon (IFN) pathway, and thus provide a possible link between the RNAi and IFN pathways (reviewed by Sonkoly et al, 2008). These combined findings support the idea that RNAi, either siRNA- or miRNA- based, is part of the innate immune system in mammals.
Consistent with this idea, an increasing number of mammalian viruses have been shown to encode an RSS protein, for example, the hepatitis C virus (HCV) core and envelope protein 2 (Wang Y et al, 2006; Ji et al, 2008), vaccinia virus E3L (Li et al, 2004), Ebola virus VP35 (de Vries & Berkhout, 2008), primate foamy virus Tas (Lecellier et al, 2005), influenza A virus NS1 (Bucher et al, 2004; Li et al, 2004; Haasnoot et al, 2007) and HIV-1 Tat (Bennasser et al, 2005). These RSS proteins suppress RNAi-mediated downregulation of a reporter gene construct. NS1 and VP35 can also trans-complement the production of a Tat-negative HIV-1 variant (Haasnoot et al, 2007). HIV-1 Tat and HCV core were proposed to block the activity of Dicer (Bennasser et al, 2005; Chen et al, 2008), whereas NS1, E3L and VP35 probably act by sequestering dsRNA (Bucher et al, 2004; Li et al, 2004; Haasnoot et al, 2007). In addition, we showed that stable expression of mammalian RSS proteins increases virus replication (de Vries et al, 2008).
Intriguingly, most identified RSS proteins of mammalian viruses also have IFN or protein kinase R (PKR) antagonistic properties, and these activities usually map to the RNA-binding domain, which is also implicated in RNAi function (Wang et al, 1999, 2000; Bucher et al, 2004). To distinguish between the IFN and RNAi pathways, we used the NS3 protein of rice hoja blanca virus (RHBV) that binds exclusively to small dsRNAs and shows RSS activity in mammalian cells (Hemmes et al, 2007; Schnettler et al, 2008). Consistent with this property, we confirm that this plant virus protein lacks IFN antagonistic activity and yet is able to rescue a Tat-negative HIV-1 variant.
Results And Discussion
The plant virus NS3 protein complements Tat
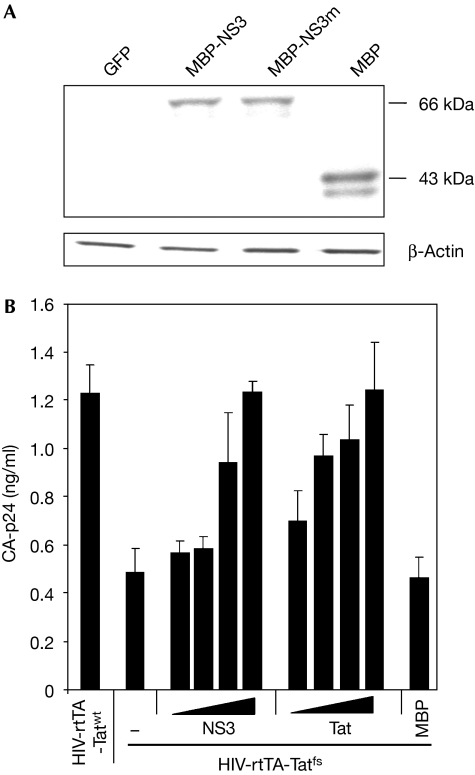
NS3 expression vectors were made that use the constitutive elongation factor (EF)-1α promoter. First, we tested protein expression in human embryonic kidney (HEK) 293T cells. NS3 is expressed as a fusion protein of 66 kDa with maltose-binding protein (MBP). The MBP domain is synthesized as a separate 43-kDa domain (Fig 1A). Functional complementation with a well-defined RSS protein is an effective strategy to identify new RSS functions (Li et al, 2004). For example, the Ebola virus VP35 protein can complement a Tat-negative HIV-1 variant in which the Tet system for doxycycline (dox)-inducible gene expression replaces the transcriptional function of Tat (Haasnoot et al, 2007). Here, we used this system to test whether the function of Tat RSS can be complemented by the RHBV NS3 protein, which exclusively binds to short dsRNA molecules (Hemmes et al, 2007) and is therefore not expected to modulate the IFN pathway. An increasing amount of NS3 expression plasmid was co-transfected with a Tat negative HIV-1 variant (HIV-rtTA-Tatfs) construct in HEK293T cells and the production of HIV-1 was monitored. Markedly, the RHBV NS3 protein was able to rescue virus production in trans to approximately the same extent as HIV-1 Tat (Fig 1B). The reason that more NS3 than Tat vector is needed could be due to differences in the RSS mechanism—siRNA binding versus Dicer blocking—or the protein's intracellular localization/stability/concentration, but this was not investigated further. Given the established role of NS3 in counteracting antiviral RNAi (Bucher et al, 2003; Hemmes et al, 2007), this result indicates that the production of HIV-1 is restricted by the RNAi mechanism.
Figure 1.
Plant viral RSS protein NS3 complements HIV-1 Tat. (A) Lysates of human embryonic kidney (HEK) 293T cells, transfected with expression plasmids for GFP, MBP-NS3, MBP-NS3m or MBP (900 ng), were analysed for protein expression by Western blot analysis, using a rabbit polyclonal antiserum against MBP. Immunological detection of β-actin acted as a loading control. (B) HEK293T cells were co-transfected with HIV-rtTA-Tatwt and HIV-rtTA-Tatfs (100 ng) in combination with increasing amounts (10, 100, 600 and 900 ng) of NS3 or Tat expression plasmids. The vector expressing MBP (900 ng) was used as a negative control. The production of HIV-1 was determined 2 days post-transfection by detecting CA-p24 in the supernatant using ELISA. The mean of at least three independent experiments is shown with standard error. ELISA, enzyme-linked immunosorbent assay; GFP, green fluorescence protein; HIV-1, human immunodeficiency virus type 1; MBP, maltose-binding protein; NS3, non-structural protein 3; NS3m, mutant protein; RSS, RNA silencing suppressor; Tat, transactivator of transcription.
NS3 shows no IFN antagonistic activity
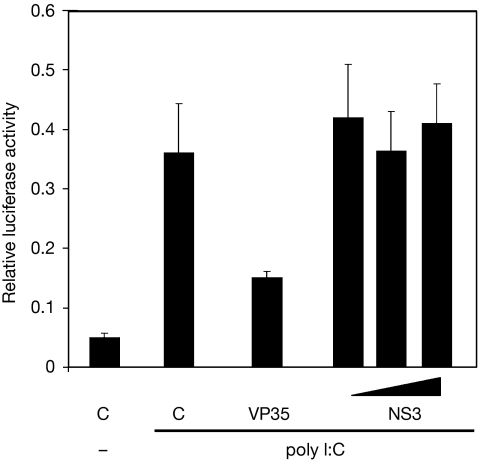
To rule out that the Tat-complementing property of NS3 was based on the modulation of the IFN pathway, this effect was probed in mammalian cells (HEK293T) using a firefly luciferase reporter construct under the control of an IFN-β inducible promoter and Renilla luciferase as an internal control (Fig 2). As a positive control, firefly luciferase expression—as a measure of IFN production—was induced by poly I:C, and this stimulatory effect was significantly reduced in the presence of the IFN-antagonistic VP35 protein of Ebola virus (Cardenas et al, 2006). As expected, the RHBV NS3 protein showed no IFN antagonistic activity, yielding the same firefly luciferase expression as the negative control (empty vector). We also tested the Tat wild-type HIV-1 variant (HIV-rtTA-Tatwt) and the Tat negative HIV-1 variant (HIV-rtTA-Tatfs) constructs for their ability to induce IFN in this assay; no such activity was measured (data not shown). Next, the possible involvement of the PKR component of the IFN pathway was tested by performing NS3 complementation in the presence of 2-aminopurine, a specific PKR inhibitor (Lu & Cullen, 2004). NS3 maintained Tat-complementation activity with 2-aminopurine (supplementary Fig S1 online), confirming that PKR is not involved. Thus, Tat inactivation and NS3 complementation do not have an impact on the IFN pathway.
Figure 2.
NS3 has no interferon and PKR antagonistic properties. HEK293T cells were co-transfected with expression vectors encoding firefly luciferase under the control of an IFN-β-inducible promoter, Renilla luciferase, and VP35, NS3 (10, 100 and 400 ng) or pBluescript (C), either in the presence (+) or absence (−) of poly I:C. Luciferase expression was measured 3 days post-transfection. A relative luciferase expression corrected for the internal Renilla control (firefly/Renilla) is shown. The mean of at least three independent experiments is shown with standard error. HEK, human embryonic kidney; IFN, interferon; NS3, non-structural protein 3; PKR, protein kinase R.
NS3 RSS activity requires dsRNA-binding
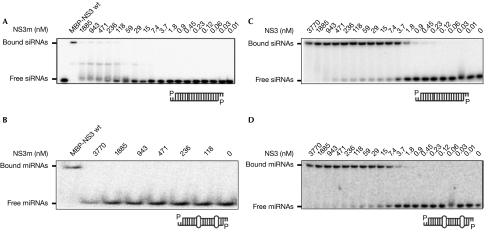
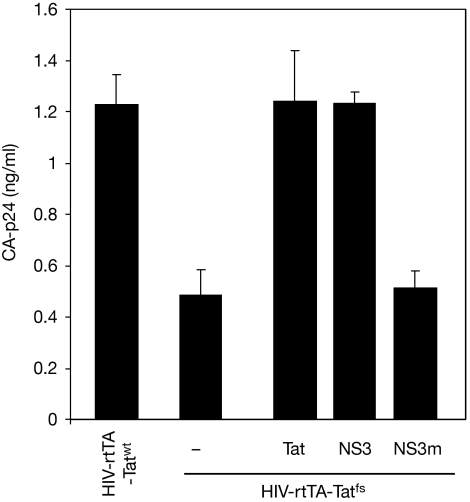
Next, we tested whether NS3 requires its RNA-binding domain for HIV-1 trans-complementation. By substituting a triple lysine motif (positions 173–175) with alanines, an NS3 mutant (NS3m) was obtained that is defective in siRNA binding (Fig 3A) and in suppressing antiviral RNAi in plants (data not shown). As the anti-HIV effect of the RNAi pathway might use miRNAs (Triboulet et al, 2007), the NS3m was tested for its ability to bind to miRNAs using an electrophoretic mobility shift assay (Fig 3B). In comparison to wild-type NS3, which is known to bind to miRNA and siRNA duplexes with high affinity (Fig 3C,D; Hemmes et al, 2007), this NS3m was unable to interact with miRNA molecules, even at the highest NS3 protein concentration (Fig 3B). The NS3m was subsequently tested in the HIV-1 trans-complementation assay (Fig 4), after confirming that the NS3m is expressed at a comparable level to the wild-type NS3, in the transfected cells (Fig 1A). Unlike wild-type NS3, NS3m was not able to restore the virus production defect of HIV-rtTA-Tatfs at any of the concentrations tested.
Figure 3.
NS3 mutant fails to bind to siRNA and miRNA. Various concentrations (0–3770 nM) of bacterially purified NS3 protein and wild-type or mutant (NS3m) protein were incubated for 20 min at 25°C with 100 pM of 32P-labelled RNA molecules, either (A,C) siRNA or (B,D) Arabidopsis thaliana miR171a. The RNA–protein complexes formed were separated on a native 5% polyacrylamide gel. A representative picture is shown from three independent experiments. MBP, maltose-binding protein; miRNA, microRNA; NS3, non-structural protein 3; siRNA, small interfering RNA; wt, wild type.
Figure 4.
Double-stranded RNA binding of NS3 is required for HIV-1 Tat complementation. HEK293T cells were transfected with a Tat wild-type HIV-1 variant (HIV-rtTA-Tatwt) or a Tat negative HIV-1 variant (HIV-rtTA-Tatfs) in combination with Tat, NS3, NS3 mutant (NS3m) or pBluescript (−; 900 ng). CA-p24 in the culture supernatant was measured at 2 days post-transfection. The mean of at least three independent experiments is shown with standard error. dsRNA, double-stranded RNA; HEK, human embryonic kidney; HIV-1, human immunodeficiency virus type 1; NS3, non-structural protein 3; Tat, transactivator of transcription.
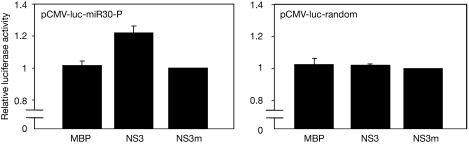
Whether NS3 is able to block the miRNA pathway in human cells was determined by using a firefly luciferase reporter containing multiple miR-30 target sites for either the sense (pCMV-luc-miR30-P) or antisense (pCMV-luc-miR30-AP) strand. This reporter is normally tested in co-transfection with an excess of miR-30 expression vector (Zeng et al, 2003), which might mask a subsequent RNAi suppression effect. HEK293T cells that express endogenous miR-30, where both strands can act as guide strands (Zeng et al, 2003), were co-transfected with NS3 and either pCMV-luc-miR30-P (Fig 5, left panel) or pCMV-luc-miR30-AP (supplementary Fig S2 online). Several controls were included: first, we used a control luciferase reporter with randomized miRNA target sites (pCMV-luc-random; Fig 5, right panel); second, NS3m and MBP were co-transfected as controls for NS3 RSS activity. Luciferase expression was measured 2 days post-transfection. We observed a modest stimulatory effect of NS3 on pCMV-luc-miR30-P (Fig 5, left panel) and aminopurine (supplementary Fig S2 online), but not on the pCMV-luc-random control (Fig 5, right panel), suggesting that NS3 is able to inhibit endogenous miRNA action in mammalian cells.
Figure 5.
NS3 inhibits endogenous miRNA action. HEK293T cells were co-transfected with expression vectors encoding pCMV-luc-miR30-P (left panel) or pCMV-luc-random (right panel), in combination with MBP, MBP-NS3 or MBP-NS3m (600 ng). Luciferase expression was measured 2 days post-transfection and relative luciferase expression (firefly/Renilla) was determined. The luciferase level measured with NS3m was set at 1.0. HEK, human embryonic kidney; MBP, maltose-binding protein; miRNA, microRNA; NS3, non-structural protein 3; NS3m, mutant protein.
Here, we have shown that a plant viral RSS protein that lacks IFN antagonistic properties can functionally replace the HIV-1 Tat RSS function and that this complementation is based on the sequestration of small dsRNA. These results corroborate further the RSS function of HIV-1 Tat (Bennasser et al, 2005; Haasnoot et al, 2007), which has been questioned by others (Lin & Cullen, 2007). Although cross-kingdom suppression of RNA silencing has been reported for several viral RSS proteins (Dunoyer et al, 2004; Schnettler et al, 2008), this is the first report, to our knowledge, of cross-kingdom RSS activity in a mammalian viral complementation assay. These results are in line with the observation that knockdown of the RNAi pathway by Drosha or Dicer silencing enhances HIV-1 replication (Triboulet et al, 2007). The ongoing debate about the physiological relevance of RNAi as an antiviral mechanism is spurred, in part owing to the presence of the antiviral IFN pathway (reviewed by Gantier & Williams, 2007). The results show that a plant virus-encoded RSS protein with well-defined biochemical activity can be used as a powerful tool to analyse the contribution of the antiviral RNAi pathway in mammalian systems in the presence of the IFN pathway. It is noted that other eukaryotes encode an alternative innate immune response next to the RNAi pathway (Dangl & Jones, 2001; Arbouzova & Zeidler, 2006). Taken together, we have shown that the production of HIV-1 is limited by endogenous small RNAs and that viral RSS function can counteract this restriction.
Methods
Plasmid constructs. Expression plasmids for MBP-NS3, MBP-NS3m, VP35, Tat, HIV-rtTA-Tatwt and HIV-rtTA-Tatfs were described previously (Haasnoot et al, 2007; Schnettler et al, 2008). miRNA-based firefly luciferase sensor constructs have also been described previously (Zeng et al, 2003).
Cell culture and transfection. HEK293T cells were grown as a monolayer in DMEM (Gibco BRL, Breda, the Netherlands) supplemented with 10% FCS (Hyclone, Etten-Leur, the Netherlands), streptomycin (100 μg/ml) and penicillin (100 U/ml) at 37°C and 5% CO2. To reach a confluence of 60–70% at the time of transfection, cells were trypsinated 24 h pre-transfection and seeded in a 24-well plate at a concentration of 1.5 × 105 cells per well. The transfection was performed using Lipofectamine 2000 (Invitrogen, Breda, the Netherlands) according to the manufacturer's instructions.
For the IFN assay, cells were co-transfected with 500 ng of a firefly luciferase expression plasmid under the control of an IFN β-inducible promoter, IFNβ-luc, 2 ng of a Renilla luciferase expression plasmid, 100 ng of poly I:C and 400 ng of pBluescript (Stratagene, Huissen, the Netherlands) or plasmids encoding MBP-NS3, MBP-NS3 mutant or VP35. Cells were lysed 3 days post-transfection and luciferase expression was determined using the dual luciferase reporter assay (Promega, Leiden, the Netherlands), according to the manufacturer's protocol.
For the miRNA sensor construct assay, cells were co-transfected with 25 ng firefly luciferase expression plasmid containing target sites for sense or antisense of human miRNA-30 (pCMV-luc-miR30-P or pCMV-luc-miR30-AP), or random miRNA target sites (pCMV-luc-random; Zeng et al, 2003), 0.5 ng of a Renilla luciferase expression plasmid and constructs encoding MBP-NS3, MBP-NS3m or MBP. At 48 h post-transfection, cells were lysed and assayed for luciferase expression by the dual luciferase assay (Promega).
The HIV Tat complementation assay was performed as described previously (Haasnoot et al, 2007).
Recombinant protein expression and electrophoretic mobility shift assay (EMSA). The wild-type and mutant MBP-NS3 proteins were expressed in BL21 DE3 cells and purified as described by Hemmes et al (2007). EMSA, either with radioactively labelled siRNA or miRNA molecules, was performed in triplicate as described previously (Lakatos et al, 2006), visualized by overnight exposure to a phosphor screen, scanned (Molecular Dynamics Typhoon Phosphor imager; Amersham Biosciences, Den Bosch, the Netherlands) and a representative picture was shown.
Protein expression in transfected HEK293T cells was analysed by Western blot analysis using a rabbit polyclonal antiserum specific for MBP (BioLabs, Leusden, the Netherlands). As a loading control, β-actin was detected with a mouse monoclonal antibody after stripping of the blot. For visualization, goat alkaline phosphatase-conjugated secondary antibodies (Dako/Sigma, Heverlee, Belgium) and NBT-BCIP substrate (Roche, Almere, the Netherlands) were used according to the manufacturer's recommendations.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Figures
Acknowledgments
We thank Alex Harwig for his assistance, Dr van Kuppeveld (Radboud University Nijmegen) for the IFN-inducible luciferase construct and Dr Cullen (Duke University Medical Center) for the miRNA-based luciferase sensor constructs. This study was financially supported by The Netherlands Organization for Scientific Research (NWO, sections Earth and Life Sciences (ALW, grant to R.G.) and Chemical Sciences (CW, TOP grant to B.B.).
Footnotes
The authors declare that they have no conflict of interest.
References
- Arbouzova NI, Zeidler MP (2006) JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development 133: 2605–2616 [DOI] [PubMed] [Google Scholar]
- Bennasser Y, Le SY, Benkirane M, Jeang KT (2005) Evidence that HIV-1 encodes an siRNA and a suppressor of RNA silencing. Immunity 22: 607–619 [DOI] [PubMed] [Google Scholar]
- Berkhout B, Jeang KT (2007) RISCy business: microRNAs, pathogenesis, and viruses. J Biol Chem 282: 26641–26645 [DOI] [PubMed] [Google Scholar]
- Bucher E, Sijen T, De Haan P, Goldbach R, Prins M (2003) Negative-strand tospoviruses and tenuiviruses carry a gene for a suppressor of gene silencing at analogous genomic positions. J Virol 77: 1329–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher E, Hemmes H, de Haan P, Goldbach R, Prins M (2004) The influenza A virus NS1 protein binds small interfering RNAs and suppresses RNA silencing in plants. J Gen Virol 85: 983–991 [DOI] [PubMed] [Google Scholar]
- Cardenas WB, Loo YM, Gale M Jr, Hartman AL, Kimberlin CR, Martinez-Sobrido L, Saphire EO, Basler CF (2006) Ebola virus VP35 protein binds double-stranded RNA and inhibits α/β interferon production induced by RIG-I signaling. J Virol 80: 5168–5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhang Z, Chen J, Zhang J, Zhang J, Wu Y, Huang Y, Cai X, Huang A (2008) HCV core protein interacts with Dicer to antagonize RNA silencing. Virus Res 133: 250–258 [DOI] [PubMed] [Google Scholar]
- Dangl JL, Jones JD (2001) Plant pathogens and integrated defence responses to infection. Nature 411: 826–833 [DOI] [PubMed] [Google Scholar]
- Deleris A, Gallego-Bartolome J, Bao J, Kasschau KD, Carrington JC, Voinnet O (2006) Hierarchical action and inhibition of plant Dicer-like proteins in antiviral defense. Science 313: 68–71 [DOI] [PubMed] [Google Scholar]
- de Vries W, Berkhout B (2008) RNAi suppressors encoded by pathogenic human viruses. Int J Biochem Cell Biol 40: 2007–2012 [DOI] [PubMed] [Google Scholar]
- de Vries W, Haasnoot J, van der Velden J, van Montfort T, Zorgdrager F, Paxton W, Cornelissen M, van Kuppeveld F, de Haan P, Berkhout B (2008) Increased virus replication in mammalian cells by blocking intracellular innate defense responses. Gene Therapy 15: 545–552 [DOI] [PubMed] [Google Scholar]
- Ding SW, Voinnet O (2007) Antiviral immunity directed by small RNAs. Cell 130: 413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunoyer P, Lecellier CH, Parizotto EA, Himber C, Voinnet O (2004) Probing the microRNA and small interfering RNA pathways with virus-encoded suppressors of RNA silencing. Plant Cell 16: 1235–1250 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, Imler JL (2006) Essential function in vivo for Dicer-2 in host defense against RNA viruses in Drosophila. Nat Immunol 7: 590–597 [DOI] [PubMed] [Google Scholar]
- Gantier MP, Williams BR (2007) The response of mammalian cells to double-stranded RNA. Cytokine Growth Factor Rev 18: 363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasnoot J, de Vries W, Geutjes EJ, Prins M, de Haan P, Berkhout B (2007) The Ebola virus VP35 protein is a suppressor of RNA silencing. PLoS Pathog 3: e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmes H, Lakatos L, Goldbach R, Burgyan J, Prins M (2007) The NS3 protein of rice hoja blanca tenuivirus suppresses RNA silencing in plant and insect hosts by efficiently binding both siRNAs and miRNAs. RNA 13: 1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Glaser A, Wernli M, Berke JM, Moradpour D, Erb P (2008) Suppression of short interfering RNA-mediated gene silencing by the structural proteins of hepatitis C virus. J Gen Virol 89: 2761–2766 [DOI] [PubMed] [Google Scholar]
- Lakatos L, Csorba T, Pantaleo V, Chapman EJ, Carrington JC, Liu YP, Dolja VV, Calvino LF, Lopez-Moya JJ, Burgyan J (2006) Small RNA binding is a common strategy to suppress RNA silencing by several viral suppressors. EMBO J 25: 2768–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecellier CH, Dunoyer P, Arar K, Lehmann-Che J, Eyquem S, Himber C, Saib A, Voinnet O (2005) A cellular microRNA mediates antiviral defense in human cells. Science 308: 557–560 [DOI] [PubMed] [Google Scholar]
- Levy A, Dafny-Yelin M, Tzfira T (2008) Attacking the defenders: plant viruses fight back. Trends Microbiol 16: 194–197 [DOI] [PubMed] [Google Scholar]
- Li WX et al. (2004) Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing. Proc Natl Acad Sci USA 101: 1350–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Cullen BR (2007) Analysis of the interaction of primate retroviruses with the human RNA interference machinery. J Virol 81: 12218–12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Cullen BR (2004) Adenovirus VA1 noncoding RNA can inhibit small interfering RNA and microRNA biogenesis. J Virol 78: 12868–12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JT, Carthew RW (2007) A call to arms: coevolution of animal viruses and host innate immune responses. Trends Genet 23: 359–364 [DOI] [PubMed] [Google Scholar]
- Merai Z, Kerenyi Z, Molnar A, Barta E, Valoczi A, Bisztray G, Havelda Z, Burgyan J, Silhavy D (2005) Aureusvirus P14 is an efficient RNA silencing suppressor that binds double-stranded RNAs without size specificity. J Virol 79: 7217–7226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merai Z, Kerenyi Z, Kertesz S, Magna M, Lakatos L, Silhavy D (2006) Double-stranded RNA binding may be a general plant RNA viral strategy to suppress RNA silencing. J Virol 80: 5747–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran P, Fire A, Jalili R, Gharizadeh B, Ronaghi M, Lu R et al. (2008) Investigating the interplay between viral replication and the RNAi machinery in vertebrates and invertebrates. In Proceedings of the Keystone Symposia, p. 127 (poster abstract number 258); Vancouver, Canada, 25–30 March 2008
- Pfeffer S et al. (2004) Identification of virus-encoded microRNAs. Science 304: 734–736 [DOI] [PubMed] [Google Scholar]
- Schnettler E, Hemmes H, Goldbach R, Prins M (2008) The NS3 protein of rice hoja blanca virus suppresses RNA silencing in mammalian cells. J Gen Virol 89: 336–340 [DOI] [PubMed] [Google Scholar]
- Soifer HS, Zaragoza A, Peyvan M, Behlke MA, Rossi JJ (2005) A potential role for RNA interference in controlling the activity of the human LINE-1 retrotransposon. Nucleic Acids Res 33: 846–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkoly E, Stahle M, Pivarcsi A (2008) MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol 18: 131–140 [DOI] [PubMed] [Google Scholar]
- Sontheimer EJ (2005) Assembly and function of RNA silencing complexes. Nat Rev Mol Cell Biol 6: 127–138 [DOI] [PubMed] [Google Scholar]
- Triboulet R et al. (2007) Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science 315: 1579–1582 [DOI] [PubMed] [Google Scholar]
- van Rij RP, Saleh MC, Berry B, Foo C, Houk A, Antoniewski C, Andino R (2006) The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev 20: 2985–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O (2001) RNA silencing as a plant immune system against viruses. Trends Genet 17: 449–459 [DOI] [PubMed] [Google Scholar]
- Voinnet O (2005) Induction and suppression of RNA silencing: insights from viral infections. Nat Rev Genet 6: 206–220 [DOI] [PubMed] [Google Scholar]
- Wang W, Riedel K, Lynch P, Chien CY, Montelione GT, Krug RM (1999) RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA 5: 195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li M, Zheng H, Muster T, Palese P, Beg AA, Garcia-Sastre A (2000) Influenza A virus NS1 protein prevents activation of NF-κB and induction of α/β interferon. J Virol 74: 11566–11573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XH, Aliyari R, Li WX, Li HW, Kim K, Carthew R, Atkinson P, Ding SW (2006) RNA interference directs innate immunity against viruses in adult Drosophila. Science 312: 452–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kato N, Jazag A, Dharel N, Otsuka M, Taniguchi H, Kawabe T, Omata M (2006) Hepatitis C virus core protein is a potent inhibitor of RNA silencing-based antiviral response. Gastroenterology 130: 883–892 [DOI] [PubMed] [Google Scholar]
- Yang N, Kazazian HH Jr (2006) L1 retrotransposition is suppressed by endogenously encoded small interfering RNAs in human cultured cells. Nat Struct Mol Biol 13: 763–771 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Yi R, Cullen BR (2003) MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci USA 100: 9779–9784 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures