Abstract
Autophagy, an evolutionarily conserved process, has functions both in cytoprotective and programmed cell death mechanisms. Beclin 1, an essential autophagic protein, was recently identified as a BH3-domain-only protein that binds to Bcl-2 anti-apoptotic family members. The dissociation of beclin 1 from its Bcl-2 inhibitors is essential for its autophagic activity, and therefore should be tightly controlled. Here, we show that death-associated protein kinase (DAPK) regulates this process. The activated form of DAPK triggers autophagy in a beclin-1-dependent manner. DAPK phosphorylates beclin 1 on Thr 119 located at a crucial position within its BH3 domain, and thus promotes the dissociation of beclin 1 from Bcl-XL and the induction of autophagy. These results reveal a substrate for DAPK that acts as one of the core proteins of the autophagic machinery, and they provide a new phosphorylation-based mechanism that reduces the interaction of beclin 1 with its inhibitors to activate the autophagic machinery.
Keywords: autophagy, beclin 1, Bcl-2, DAPK
Introduction
Autophagy is an evolutionarily conserved process that is characterized by the formation of double-membrane-enclosed autophagosomes that engulf intracellular organelles and cytoplasmic constituents, and deliver them to the lysosomes for degradation. In addition to its cytoprotective functions in stressed cells (Levine & Kroemer, 2008), autophagy can also act as a cell death mechanism under some conditions (Berry & Baehrecke, 2007; Gozuacik & Kimchi, 2007; Maiuri et al, 2007b; Gozuacik et al, 2008).
Beclin 1, a haplo-insufficient tumour suppressor that was initially identified as a Bcl-2-binding protein, is part of a class III phosphatidylinositol-3-kinase (PI(3)K) multiprotein complex that participates in autophagosome nucleation (Cao & Klionsky, 2007). Beclin 1 interacts with several activators (AMBRA1, UVRAG and Bif-1; Liang et al, 2006; Fimia et al, 2007; Takahashi et al, 2007), which positively regulate autophagy by promoting the activation of the PI(3)K protein, Vps34, and the formation of autophagosomes. The autophagy-promoting activity of beclin 1 is suppressed by anti-apoptotic members of the Bcl-2 family through direct binding. It has been reported recently that beclin 1 is a bona fide BH3-domain-only protein, and that the α-helix of its BH3 domain binds to the hydrophobic groove in Bcl-XL domain similarly to the interactions shown previously for the other BH3-domain-only proteins (Pattingre et al, 2005; Erlich et al, 2007; Feng et al, 2007; Oberstein et al, 2007; Maiuri et al, 2007a). Under normal steady-state growth conditions, beclin 1 is bound to various Bcl-2 family members, whereas its dissociation from Bcl-2 mediates autophagy (Pattingre et al, 2005).
Death-associated protein kinase (DAPK) is a calcium/calmodulin (CaM) serine/threonine (Ser/Thr) kinase isolated by a genetic screen for positive mediators of cell death (Cohen et al, 1997). It functions as a tumour suppressor gene, the expression of which is lost in many cancer types, and has been linked to different cell death pathways, including autophagic cell death (reviewed by Bialik & Kimchi, 2006). The positive connection of DAPK to autophagy was established in mammalian cell cultures and in Caenorhabditis elegans (Inbal et al, 2002; Kang et al, 2007; Gozuacik et al, 2008). However, the molecular mechanisms underlying the link of DAPK to autophagy have been little investigated, and the relevant substrate(s) have not been identified. Here, we discovered that DAPK phosphorylates beclin 1 on Thr 119 located at its BH3 domain, and that this phosphorylation promotes the dissociation of beclin 1 from its inhibitor––Bcl-XL. These results show that beclin 1, one of the core proteins of the autophagic machinery, acts as a substrate for DAPK, and they suggest a new phosphorylation-based mechanism for activating beclin 1 to induce autophagy.
Results
Functional interactions between DAPK and beclin 1
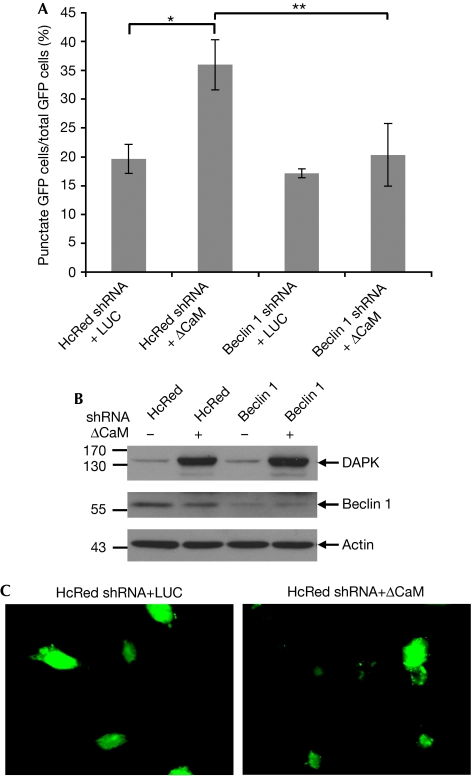
To investigate the molecular mechanisms through which DAPK promotes autophagy, we focused on the early steps of vesicle nucleation in which beclin 1 participates. First, we tested whether the knockdown of beclin 1 inhibits DAPK-induced autophagy by co-transfecting human embryonic kidney 293 (HEK293) cells with ΔCaM DAPK (an activated form of DAPK lacking its CaM-regulatory domain; Cohen et al, 1997) and short hairpin RNA (shRNA)-plasmid-targeting beclin 1. A third co-transfected construct, GFP–LC3 (green fluorescent protein–LC3), was used to assess the autophagy process by scoring LC3 punctate staining (Kabeya et al, 2000). The frequency of cells in which the GFP–LC3 appeared in puncta increased over basal levels when ΔCaM DAPK was introduced to the cells (Fig 1A–C) and was significantly reduced by knocking down beclin 1 (Fig 1A). These results indicate that beclin 1 is required for DAPK-induced autophagy.
Figure 1.
Functional interaction between DAPK and beclin 1. (A) HEK293 cells were transfected with DAPK ΔCaM or with a control vector (pcDNA3-luciferase, LUC), together with shRNAs targeting beclin 1 or HcRed, and with GFP–LC3 plasmid. After 72 h, cells were counted and lysates were prepared. (A) The percentage of cells with punctate GFP–LC3 fluorescence per total GFP–LC3-positive cells was quantified. Data presented are the mean±s.d. from a triplicate of 100 transfected cells. The asterisks denote a significance level of P=0.001. (B) Western blot analysis was performed using the indicated antibodies. (C) Representative GFP–LC3 staining of cells transfected with the control shRNA (HcRed) together with pcDNA3-luciferase or DAPKΔCaM. CaM, calmodulin; DAPK, death-associated protein kinase; GFP, green fluorescent protein; HEK, human embryonic kidney; shRNA, short hairpin RNA.
Beclin 1 is a new substrate of DAPK
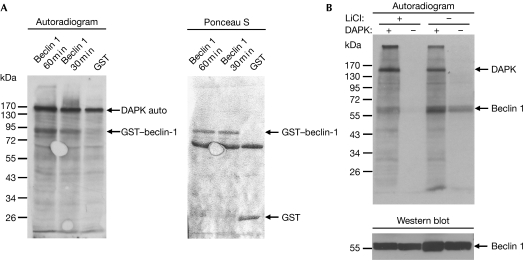
We next examined whether DAPK phosphorylates beclin 1 by in vitro kinase assays in which purified DAPK was incubated with glutathione S-transferase (GST)–beclin 1 in the presence of Ca2+, CaM and [γ-33P]ATP. GST–beclin 1, but not GST alone, was phosphorylated by DAPK (Fig 2A, and see also supplementary Fig S1 online). The autophosphorylation of DAPK indicates that it was active in all samples. The phosphorylation by DAPK was also observed when Flag-tagged beclin 1, immunoprecipitated from HEK293T cells, was used as a substrate (Fig 2B). In this context, beclin 1 pulled down endogenous kinase(s) that induced some background phosphorylation without adding external DAPK (Fig 2B, right lane). This background signal was reduced by washing the beclin 1 immunoprecipitates with high salt concentrations (that is, 0.5 M KCl and LiCl) before the kinase assay (Fig 2B, leftmost two lanes). Taken together, these results indicate that DAPK phosphorylates beclin 1 purified from bacterial or mammalian cells.
Figure 2.
Beclin 1 is a new substrate of DAPK. (A) Flag-tagged DAPK (100 ng) was incubated with GST (900 ng) or GST–beclin-1 (750 ng) in the presence of Ca2+, calmodulin and [γ-33P]ATP for 30 min or 60 min. Phosphorylated proteins were visualized by X-ray film exposure, and GST/GST–beclin-1 levels were visualized by Ponceau S staining. The autophosphorylation of DAPK indicates that its catalytic activity was intact in all samples. (B) Flag-tagged DAPK (60 ng) was incubated with Flag-tagged beclin 1 (250 ng), which was purified from HEK293T cells, and a kinase assay was performed for 60 min. Where indicated (+LiCl), beclin-1-bound beads were first washed stringently in 0.5 M LiCl and 0.5 M KCl. Phosphorylated proteins were visualized by X-ray film exposure, and the levels of beclin 1 were visualized by Western blot analysis using beclin 1 antibodies. DAPK, death-associated protein kinase; GST, glutathione S-transferase; HEK, human embryonic kidney.
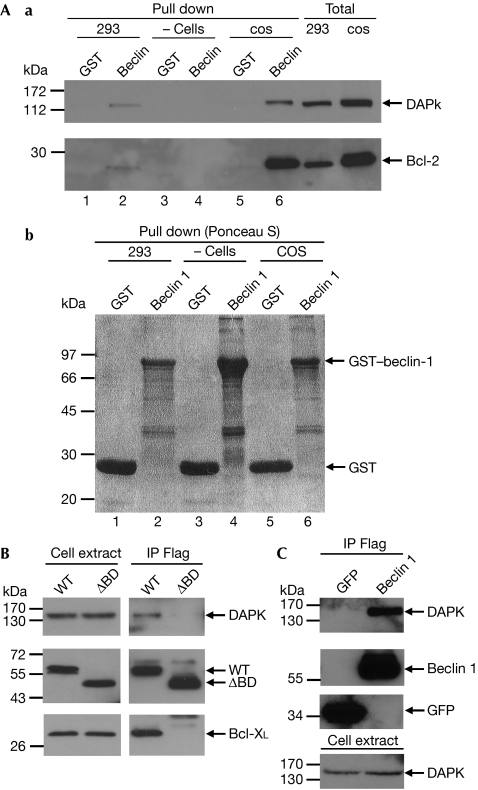
The existence of physical interactions between the two proteins was first documented by showing that GST–beclin 1, but not GST alone, pulled down the endogenous DAPK when added to protein extracts from COS7 cells or HEK293T cells. As expected, Bcl-2 was also pulled down (Fig 3A). In a second set of experiments, Flag-tagged beclin 1 and haemagglutinin (HA)-tagged DAPK were co-expressed in HEK293 cells, beclin 1 was immunoprecipitated with Flag antibodies and eluted from the beads with Flag peptide. Overexpressed Bcl-XL was used as a positive control. It was found that beclin 1 binds the HA-tagged DAPK (Fig 3B, WT). Notably, the Flag-tagged beclin 1 also immunoprecipitated the endogenous DAPK from HEK293 cells (Fig 3C). Interestingly, when beclin 1 lacking the Bcl-2-binding domain (that is, amino acids 88–150) was immunoprecipitated, DAPK could no longer bind to beclin 1 (Fig 3B, ΔBD). Thus, the results imply that DAPK binds to beclin 1, and that the Bcl-2-binding domain is required for this interaction.
Figure 3.
Physical interaction between DAPK and beclin 1. (Aa) Protein extracts from COS7 cells, HEK293T cells, or no extracts were added to bacterially produced GST or GST–beclin-1. The pulled-down proteins, as well as the total cell extracts, were blotted with the indicated antibodies. (Ab) Ponceau S staining of GST and GST–beclin-1 to which the extracts were added. (B) HEK293 cells were co-transfected with Flag-tagged beclin 1 or with Flag-tagged beclin 1 lacking the Bcl-2-binding domain (ΔBD) together with Bcl-XL and HA-tagged DAPK. Beclin 1 was immunoprecipitated using Flag antibodies, and the co-immunoprecipitated proteins, as well as the total cell extracts, were blotted with DAPK, Bcl-XL and beclin 1 antibodies. (C) HEK293 cells were transfected with Flag–beclin-1 or Flag–GFP, and the Flag-tagged proteins were immunoprecipitated using Flag antibodies, and eluted with an excess of Flag peptides. The blot was reacted with DAPK antibodies or with Flag antibodies at different time exposures. DAPK, death-associated protein kinase; GFP, green fluorescent protein; GST, glutathione S-transferase; HA, haemagglutinin; HEK, human embryonic kidney.
DAPK phosphorylates beclin 1 on its BH3 domain
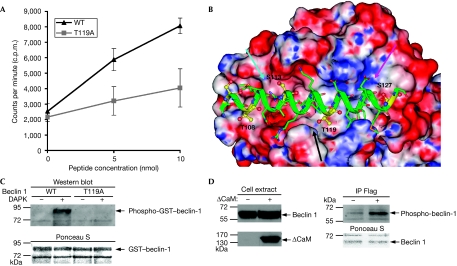
In the light of our finding that the interaction of DAPK with beclin 1 depends on the presence of the Bcl-2-binding domain, we assessed whether DAPK phosphorylates beclin 1 on this region, and more specifically on its BH3 domain. To this end, a peptide corresponding to the BH3 domain of beclin 1 (aa 108–127) was synthesized and subjected to in vitro kinase assay using a bacterially produced catalytic domain of DAPK. It was found that DAPK phosphorylates the BH3 peptide of beclin 1 in a dose-dependent manner (Fig 4A; supplementary Fig S2 online, WT).
Figure 4.
DAPK phosphorylates beclin 1 on Thr 119 located within its BH3 domain. (A) A bacterially purified catalytic domain of DAPK was incubated for 15 min at 30°C with increasing concentrations (5–10 nmol) of a peptide corresponding to the BH3 domain of beclin 1 (aa 108–127) and with the same peptide in which Thr 119 was substituted by alanine. An in vitro kinase assay was performed, and the reactions were applied to Whatman filters. Total levels of TCA-insoluble counts were measured and plotted against substrate concentration. Data are the mean±s.d. of three experiments. (B) A model of the interaction between the BH3 domain of beclin 1, and the hydrophobic pocket of Bcl-XL. The cyan arrow points to the groove in which phosphorylated Ser 113 can be accommodated. The black arrow points to the site that might be disrupted by phosphorylation of Thr 119. The magenta arrow points to the positively charged groove in which phosphorylated Ser 127 can be accommodated. (C) Flag-tagged DAPK (60 ng) was incubated with GST–WT beclin 1 or with GST–T119A beclin 1 (1000 ng) in the presence of Ca2+, calmodulin and ATP for 30 min. GST-beclin 1 levels were visualized by Ponceau S staining, and phosphorylation on Thr 119 was detected by a phosphoThr 119 antibody (Western blot). (D) HEK293 cells were co-transfected with Flag-tagged beclin 1 with or without ΔCaM DAPK. After 24 h, beclin 1 was immunoprecipitated from cells, using Flag antibodies, and the immunoprecipitates were reacted with phosphoThr 119 antibodies. The Ponceau staining shows equal amounts of immunoprecipitated beclin 1. The cell extract blots were reacted with haemagglutinin antibodies or with beclin 1 antibodies. CaM, calmodulin; DAPK, death-associated protein kinase; GST, glutathione S-transferase; HEK, human embryonic kidney; TCA, trichloro-acetic acid.
Next, we examined the available crystal structure of the Bcl-XL/beclin 1 complex (Oberstein et al, 2007) in an attempt to predict in silico which Ser/Thr residues in the BH3 domain of beclin 1 might affect the interaction with Bcl-XL on its phosphorylation. Specifically, we examined each of the four Ser/Thr residues (Thr 108, Ser 113, Thr 119 and Ser 127) located within the BH3 domain of beclin 1 (Fig 4B). Thr 108 is partly buried with its Cγ2 atom interacting with Leu 112 of Bcl-XL, and its Oγ1 atom exposed to the solvent. Phosphorylation at this site does not cause steric clash as the PO3 group points towards the solvent. Ser 113 binds in a shallow pocket of Bcl-XL. Phosphorylation on this residue is not likely to have a strong influence because the PO3 group can be accommodated in a groove next to the Ser 113-binding pocket (cyan arrow). Thr 119 is partly buried. Its Cγ atom has a hydrophobic contact with Phe 97 of Bcl-XL, whereas its Oγ atom has a hydrogen bond with the backbone carbonyl of beclin 1 Arg 115. Phosphorylation on this residue is most likely to cause a severe clash with Bcl-XL and possibly disrupt the beclin 1 helix (black arrow). Ser 127 is partly buried. Its Oγ atom has a hydrogen bond with the hydroxyl oxygen of Bcl-XL Tyr 195. Phosphorylation at this position requires a minor conformation change (rotation of Ser 127 about χ1) to accommodate the PO3 group in a positively charged groove (magenta arrow); the hydrogen bond with Tyr 195 however is disrupted. Tyr 195 of Bcl-XL has another hydrogen bond with beclin 1 Asp 124, which is not affected by the phosphorylation of Ser 127. On the basis of this analysis, we concluded that phosphorylation on Thr 119 might disrupt the binding of beclin 1 to Bcl-XL and therefore should be examined experimentally as a potential target of DAPK.
To this end, we synthesized a mutant BH3 peptide in which Thr 119 was substituted by alanine (T119A), and compared its phosphorylation with the wild-type peptide. Changing Thr 119 to alanine strongly inhibited the extent of peptide phosphorylation (Fig 4A; supplementary Fig S2 online). Notably, this substitution did not completely abolish phosphorylation, and some residual radioactivity was incorporated into the peptide, suggesting that other Ser/Thr sites might be weakly phosphorylated under the conditions used. These experimental data indicate Thr 119 as a major target for phosphorylation by DAPK.
Next, the full-length GST–beclin-1 protein was incubated with DAPK and cold ATP, proteolysed and analysed by mass spectrometry to map the phosphorylation site(s) (supplementary Fig S3 online). Around 76% of the amino acids of beclin 1 were covered in this analysis, out of which two phosphorylation sites were identified with good accuracy, both of which were located within the BH3 domain. The first was located in peptide 103–118, where the fragmentation data could not differentiate between a phosphorylation on Ser 104 or on Thr 108, and the second was identified as Thr 119. Thus, the phosphopeptide mapping confirmed that DAPK phosphorylates beclin 1 on Thr 119.
Finally, phosphoThr 119 antibodies were generated and tested against GST–beclin-1 subjected to in vitro kinase assay with DAPK. These antibodies exclusively recognized the in vitro phosphorylated beclin-1 (Fig 4C, WT). Also, when a mutant GST–beclin-1 in which Thr 119 was substituted by alanine (T119A) was used, the antibodies could no longer detect DAPK-mediated phosphorylation (Fig 4C, T119A), confirming the specificity of these antibodies, and proving that the Thr 119 residue is phosphorylated by DAPK. Similar results were obtained when Flag-tagged beclin 1, immunoprecipitated from mammalian cells, was incubated with DAPK (supplementary Fig S4 online; interestingly, these antibodies also recognized the background phosphorylation caused by the endogenous kinase(s) that co-immunoprecipitated with beclin 1––the rightmost lane).
These antibodies were then used for detecting the phosphorylation of beclin 1 by DAPK in cells. Transfection of HEK293 cells with ΔCaM DAPK strongly increased the phosphorylation state of beclin 1 (Fig 4D), indicating that phosphorylation on Thr 119 occurs by DAPK in cells.
Altogether, the peptide phosphorylation assays, the phospho-peptide mapping by mass spectrometry and the use of the phosphoThr 119 antibodies establish that this site within the BH3 domain of beclin 1 is specifically phosphorylated by DAPK. In the light of our prediction that phosphorylation of this site might dissociate beclin 1 from Bcl-XL, we next turned to study these interactions in more detail.
DAPK promotes the dissociation of beclin 1 from Bcl-XL
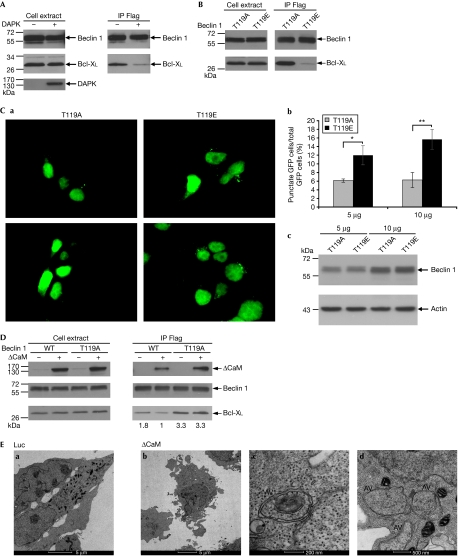
To examine the influence of DAPK on the interaction of beclin 1 with Bcl-XL, HEK293 cells were co-transfected with Bcl-XL and Flag-tagged beclin 1 with or without HA-tagged DAPK. The levels of Bcl-XL, which co-immunoprecipitated with beclin 1, were significantly reduced in the DAPK-transfected cells (Fig 5A), suggesting that DAPK promotes the dissociation of beclin 1 from Bcl-XL. Similar results were obtained when cells were co-transfected with ΔCaM DAPK (supplementary Fig S5 online). To find out whether the phosphorylation at position Thr 119 is causal to the reduced association of beclin 1 with Bcl-XL, we generated a phospho-mimicking mutant of beclin 1 (T119E), and tested its binding to Bcl-XL in these co-transfection assays as compared with the phospho-silencing mutation (T119A). It was found that the threonine to glutamic acid substitution at position 119 strongly reduced the binding to Bcl-XL (Fig 5B). In parallel, we compared the ability of these mutants to induce autophagy by using the GFP–LC3 punctate staining assay. Although the two mutants were expressed to the same extent (Fig 5Cc), the frequency of cells in which the GFP–LC3 appeared in puncta increased in a dose-dependent manner only when the beclin 1 T119E mutant was introduced to the cells (Fig 5Ca,b). Taken together, these results imply that phosphorylation on Thr 119 causes the dissociation of beclin 1 from Bcl-XL, leading to increased formation of autophagosomes. We also found that the suppressive effects of DAPK on the association of beclin-1 with Bcl-XL, shown in Fig 5A and supplementary Fig S5 online, exclusively depend on Thr 119 phosphorylation. As shown in Fig 5D, ΔCaM DAPK had no effect on the amount of Bcl-XL, which co-immunoprecipitated with the T119A beclin 1 mutant, whereas it reduced the amounts of Bcl-XL immunoprecipitated by the wild-type beclin 1. Notably, DAPK was present in both beclin 1 immunoprecipitates (Fig 5D, +(CaM)). In addition, higher levels of Bcl-XL co-immunoprecipitated with T119A beclin 1 than with wild-type beclin 1 in the presence or absence of DAPK (Fig 5D). Thus, a lack of phosphorylation at position Thr 119 leads to a stronger interaction between beclin 1 and Bcl-XL, which becomes resistant to DAPK-dissociating effects. Transmission electron microscopy studies confirmed that, under the specific conditions used in Fig 5D (that is, co-transfection of ΔCaM DAPK with wild-type beclin 1 and Bcl-XL), typical double-membrane autophagosomes accumulated in cells, thus confirming that autophagy was induced in these cellular settings (Fig 5E).
Figure 5.
DAPK promotes the dissociation of beclin 1 from Bcl-XL. (A) HEK293 cells were transfected with Flag-tagged beclin 1 and Bcl-XL with or without haemagglutinin-tagged DAPK. Beclin 1 was immunoprecipitated using Flag antibodies, and the co-immunoprecipitated proteins, as well as the total cell extracts, were blotted using the indicated antibodies. (B) HEK293 cells were co-transfected with Flag-tagged T119A or T119E beclin 1 mutants and Bcl-XL. Beclin 1 was immunoprecipitated using Flag antibodies, and the co-immunoprecipitated proteins, as well as the total cell extracts, were blotted using the indicated antibodies. (C) HEK293 cells were transfected with 5 or 10 μg T119A or T119E beclin 1 mutants together with GFP–LC3 plasmid. After 24 h, cells were counted and lysates were prepared. (a) Representative GFP–LC3 staining. (b) The percentage of cells with punctate GFP–LC3 fluorescence per total GFP–LC3-positive cells was quantified. Data presented are the mean±s.d. from a triplicate experiment with 100 transfected cells. The asterisks denote significance level: *P=0.01; **P=0.005. (c) Western blot analysis was performed using the indicated antibodies. (D) HEK293 cells were transfected with Bcl-XL, Flag-tagged beclin 1 (WT) or Flag-tagged T119A beclin 1 mutant with or without haemagglutinin-tagged activated DAPK (ΔCaM). Beclin 1 was immunoprecipitated using Flag antibodies, and the co-immunoprecipitated proteins, as well as the total cell extracts, were blotted using the indicated antibodies. Levels of Bcl-XL were quantified using NIH image software, and the ratio between immunoprecipitated and expressed Bcl-XL was calculated. (E) Transmission electron micrographs of HEK293 cells 24 h after they were transfected with Bcl-XL, Flag-tagged beclin 1 and ΔCaM or pcDNA3-luciferase as a control. The images in (c) and (d) were taken at higher magnifications of the ΔCaM treatment (see the scale bars). ‘AV' indicates autophagic vacuoles. CaM, calmodulin; DAPK, death-associated protein kinase; GFP, green fluorescent protein; GST, glutathione S-transferase; HEK, human embryonic kidney; NIH, National Institutes of Health.
Discussion
Here, we proved that beclin 1 is a substrate of DAPK, and mapped the phosphorylation site to Thr 119 located within the BH3 domain of beclin 1. DAPK significantly reduced the amounts of Bcl-XL, which were immunoprecipitated by wild-type beclin 1, whereas it failed to reduce Bcl-XL binding to the T119A phospho-silencing mutant, thus conferring a functional role to the phosphorylation event. The T119E phospho-mimicking mutant, conversely, revealed a weaker association with Bcl-XL, and could induce autophagy when overexpressed. Taken together, these data suggest that DAPK phosphorylation on Thr 119 leads to the dissociation of beclin 1 from its inhibitor Bcl-XL, resulting in the induction of autophagy.
Thr 119 is unique to the BH3 domain of beclin 1, as in other BH3-domain-only proteins a hydrophobic residue exists in this site. In these other proteins, this hydrophobic residue acts together with three additional hydrophobic residues present in their BH3 amphipathic α-helix to stabilize the interaction with the hydrophobic residues in the target-binding pocket. The lack of a hydrophobic amino acid at position 119 in beclin 1 might explain why binding of beclin 1 to Bcl-XL is weaker than that of other BH3-domain-only proteins (Feng et al, 2007). Interestingly, it has been shown previously that substituting Thr 119 in beclin 1 by isoleucine enhanced its association with Bcl-XL, confirming that this is a crucial site in the interface between beclin 1 and its inhibitors (Feng et al, 2007). Our current data show that this crucial site is in fact a target of tight regulation by phosphorylation, causing a strong inhibition in the interaction with Bcl-XL. Interestingly, it has been reported recently that phosphorylation of Bcl-2 by Jun amino-terminal kinase 1 (JNK1) at residues Thr 69, Ser 70 and Ser 87 located within the non-structural loop of Bcl-2 is another mechanism that reduces the interaction between beclin 1 and its inhibitor (Wei et al, 2008). The interaction between beclin 1 and its inhibitors is therefore dynamic, subjected to regulation by phosphorylation of either one of the two partners in this complex. Notably, BAD, another BH3-domain-only protein, also dissociates from Bcl-2 and Bcl-XL by phosphorylation on its BH3 domain (Datta et al, 2000; Tan et al, 2000), suggesting that regulation by BH3 phosphorylation might be more general and common to different BH3-domain-only proteins.
In summary, these results functionally map the position of DAPK within the autophagic module, and suggest a new mechanism that reduces the interaction of beclin 1 with its inhibitors thus promoting autophagy.
Methods
Cell cultures and co-immunoprecipitations. HEK293 and HEK293T cells were grown in DMEM medium (Biological Industries, Kibbutz Beit Haemek, Israel) supplemented with glutamine, penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA), and 10% fetal bovine serum (Hyclone, Logan, UT, USA). Cells were transiently transfected by the standard calcium phosphate technique with the indicated plasmids. Cell pellets were lysed in NP-40 buffer or in B buffer (supplementary information online) supplemented with 1 mM Na3VO4, 1 mM DTT and protease inhibitors. When the phosphorylation state of beclin 1 was examined, 1 mM NaF and 50 mM β-glycerol phosphate were added. Following pre-clearance with protein G PLUS-Agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA, USA), the extracts were incubated with anti-Flag M2 beads (Sigma, Rehovot, Israel) for 2 h, eluted with excess Flag peptide (Sigma) and subjected to Western blot analysis.
GFP–LC3 punctate staining assay. HEK293 cells were plated in 9-cm plates containing poly-L-lysine-covered 13-mm glass cover slips, and after 24 h the cells were transfected as indicated below. At different time points of post-transfection, the cells grown on the cover slips were fixed with 3.7% formaldehyde, and the remaining cells were extracted using protein lysis buffer (PLB; supplementary information online) containing protease inhibitors, and subjected to Western blot analysis. To visualize autophagosomes, fixed cells were viewed by fluorescent microscopy (Olympus BX41) with × 60 (NA 1.25) UPlan-Fl oil immersion objectives, and digital images were obtained with a DP50 CCD camera using the cellA software (Olympus, Center Valley, PA, USA). The percentage of cells with punctate GFP–LC3 fluorescence (more than five puncta per cell) per total GFP–LC3-positive cells (n=100) was quantified.
In Fig 1, the cells were transfected with pSUPER-based shRNAs targeting beclin 1 or the fluorescent protein HcRed as a negative control (Reef et al, 2006) together with GFP–LC3 plasmid and DAPK ΔCaM or pcDNA3 vector expressing luciferase. Cells were fixed 72 h post-transfection.
To assess the function of the phosphorylation mutants (Fig 5C), the cells were transfected with 5 or 10 μg T119A or T119E beclin 1 mutants together with GFP–LC3 plasmid. Cells were fixed 24 h post-transfection.
Structure analysis. The structure analysis was based on the X-ray structure of Bcl-XL in complex with beclin 1, taken from the Protein Data Bank (Berman et al, 2000) number 2p1l. Fig 4B was prepared with the program InsightII (Accelrys Inc., San Diego, CA, USA); the electrostatic potential was calculated using the Delphi module of InsightII.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Information
Acknowledgments
We thank B. Levine for the Flag–beclin-1 and Flag–beclin-1 ΔBD plasmids, G. Kroemer for the Bcl-XL plasmid, and N. Mizushima and T. Yoshimori for the GFP–LC3 plasmid. We thank the Smoler Proteomics Center at Technion for performing the mass spectrometry analysis. The electron microscopy studies were conducted at the Irving and Cherna Moskowitz Center for Nano and Bio-Nano Imaging at the Weizmann Institute of Science. This study was supported by the Kahn Fund for System Biology at the Weizmann Institute of Science, and by the Israel Science Foundation. A.K. is the incumbent of Helena Rubinstein Chair of Cancer Research.
Footnotes
The authors declare that they have no conflict of interest.
References
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The Protein Data Bank. Nucleic Acids Res 28: 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DL, Baehrecke EH (2007) Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell 131: 1137–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialik S, Kimchi A (2006) The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem 75: 189–210 [DOI] [PubMed] [Google Scholar]
- Cao Y, Klionsky DJ (2007) Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res 17: 839–849 [DOI] [PubMed] [Google Scholar]
- Cohen O, Feinstein E, Kimchi A (1997) DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J 16: 998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME (2000) 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell 6: 41–51 [PubMed] [Google Scholar]
- Erlich S, Mizrachy L, Segev O, Lindenboim L, Zmira O, Adi-Harel S, Hirsch JA, Stein R, Pinkas-Kramarski R (2007) Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy 3: 561–568 [DOI] [PubMed] [Google Scholar]
- Feng W, Huang S, Wu H, Zhang M (2007) Molecular basis of Bcl-xL's target recognition versatility revealed by the structure of Bcl-xL in complex with the BH3 domain of Beclin-1. J Mol Biol 372: 223–235 [DOI] [PubMed] [Google Scholar]
- Fimia GM et al. (2007) Ambra1 regulates autophagy and development of the nervous system. Nature 447: 1121–1125 [DOI] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A (2007) Autophagy and cell death. Curr Top Dev Biol 78: 217–245 [DOI] [PubMed] [Google Scholar]
- Gozuacik D et al. (2008) DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ 15: 1875–1886 [DOI] [PubMed] [Google Scholar]
- Inbal B, Bialik S, Sabanay I, Shani G, Kimchi A (2002) DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J Cell Biol 157: 455–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19: 5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, You YJ, Avery L (2007) Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes Dev 21: 2161–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132: 27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, Jung JU (2006) Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol 8: 688–699 [DOI] [PubMed] [Google Scholar]
- Maiuri MC et al. (2007a) Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J 26: 2527–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007b) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8: 741–752 [DOI] [PubMed] [Google Scholar]
- Oberstein A, Jeffrey PD, Shi Y (2007) Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem 282: 13123–13132 [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B (2005) Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122: 927–939 [DOI] [PubMed] [Google Scholar]
- Reef S, Zalckvar E, Shifman O, Bialik S, Sabanay H, Oren M, Kimchi A (2006) A short mitochondrial form of p19ARF induces autophagy and caspase-independent cell death. Mol Cell 22: 463–475 [DOI] [PubMed] [Google Scholar]
- Takahashi Y et al. (2007) Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol 9: 1142–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Demeter MR, Ruan H, Comb MJ (2000) BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J Biol Chem 275: 25865–25869 [DOI] [PubMed] [Google Scholar]
- Wei Y, Pattingre S, Sinha S, Bassik M, Levine B (2008) JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell 30: 678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information