Abstract
Senescence is a stable proliferative arrest induced by various stresses such as telomere erosion, oncogenic or oxidative stress. Compelling evidence suggests that it acts as a barrier against tumour development. Describing new mechanisms that favour an escape from senescence can thus reveal new insights into tumorigenesis. To identify new genes controlling the senescence programme, we performed a loss-of-function genetic screen in primary human fibroblasts. We report that knockdown of the M-type receptor PLA2R (phospholipase A2 receptor) prevents the onset of replicative senescence and diminishes stress-induced senescence. Interestingly, expression of PLA2R increases during replicative senescence, and its ectopic expression results in premature senescence. We show that PLA2R regulates senescence in a reactive oxygen species–DNA damage–p53-dependent manner. Taken together, our study identifies PLA2R as a potential new tumour suppressor gene crucial in the induction of cellular senescence through the activation of the p53 pathway.
Keywords: senescence, PLA2R, p53
Introduction
Senescence is a permanent form of cell-cycle arrest that was first described in primary human fibroblasts (HDFs) that had reached their proliferative lifespan (Hayflick & Moorhead, 1961). It can also be induced by other stimuli such as oxidative or oncogenic stress (Serrano & Blasco, 2001). Senescing cells remain metabolically active and show characteristic changes in their gene expression and morphology (Campisi & d'Adda di Fagagna, 2007). Flattened and enlarged, they show positive senescence-associated β-galactosidase (SA-β-gal) activity (Dimri et al, 1995).
Activated in the early stages of tumorigenesis, senescence has recently been described as a tumour suppression mechanism that prevents malignant transformation. This suggests that escape from senescence leads to a progression of malignancy (Braig et al, 2005; Chen et al, 2005). Understanding why a cell under different stresses enters a senescent state and what genetic events might impede this phenomenon therefore seems to be a necessary step towards understanding tumour development.
Various intracellular proteins are known to regulate cellular senescence mainly through the perturbation of the p53 and/or p16/Rb (Rb for retinoblastoma protein) pathways (Pearson et al, 2000; Gil et al, 2004; Sun et al, 2007). Recently, secreted factors such as insulin-like growth factor binding protein 7 (IGFBP7) and chemokines have been reported to be crucial regulators of senescence (Acosta et al, 2008; Kuilman et al, 2008; Wajapeyee et al, 2008), but so far few receptors have been identified as potential regulators of senescence. To our knowledge, only the chemokine receptors CXCR2 (chemokine (CXC) receptor 2) and IL6R (interleukin 6 receptor) are known to control senescence in primary human cells (Acosta et al, 2008; Kuilman et al, 2008). Here, we have identified another kind of receptor, the type I transmembrane glycoprotein receptor PLA2R (phospholipase A2 receptor), as a regulator of senescence. PLA2R is also known as the multifunctional M-type 180-kDa receptor, which belongs to the C-type lectin superfamily and specifically binds to several secreted phospholipase A2 (sPLA2) enzymes (Lambeau & Gelb, 2008).
Results And Discussion
Downregulation of PLA2R bypasses senescence
We performed a loss-of-function genetic screen using the Netherlands Cancer Institute's retroviral short hairpin RNA (shRNA) library that targets approximately 8,000 human genes (Berns et al, 2004). The screen was designed to identify genes that, when downregulated, extend the lifespan of near senescent primary HDFs. Indeed, after a growth phase, HDFs, with a limited growth potential, enter a senescent state. In some cases, outgrowing colonies were observed among cells that had been exposed to library pools. The genomic DNA was isolated from these colonies and the inserted shRNA was sequenced. By using this strategy, an shRNA directed against the M-type receptor PLA2R was identified, along with four other hits that are now under investigation. Positive controls such as shRb and shp53 were also identified. The shRNA identified during the screen was cloned into the pRS vector (shPLA2R) along with two other shRNAs targeting PLA2R messenger RNA (mRNA) at different regions (shPLA2R-6 and shPLA2R-9). In HDFs stably infected with shPLA2R, shPLA2R-6 or shPLA2-9, PLA2R mRNA levels were found to be knocked down by approximately 90% compared with control infected cells (Fig 1A). Then, we assessed cell growth to confirm the effect of the different shRNA-targeting PLA2R. Control, shPLA2R-, shPLA2R-6- or shPLA2R-9-infected cells were seeded at low densities and a colony formation assay was performed. Although control HDFs entered growth arrest, the shRNA-infected cells (shPLA2R, shPLA2R-6 and shPLA2R-9) continued to grow (Fig 1B).
Figure 1.
The downregulation of PLA2R induces a bypass of replicative- and stress-induced senescence. (A) After infection of WI38 cells by control (Ctrl) or shPLA2R-encoding vectors and selection, RNAs were prepared. PLA2R mRNA levels were analysed in control cells and in various shPLA2R-infected cells by using QRT–PCR. (B) Colony formation assay. Control, shPLA2R-, shPLA2R-6- or shPLA2R-9-expressing WI38 cells were seeded at low densities. After 2 weeks, the cells were fixed and stained with crystal violet. (C) Growth curve analysis. WI38 cells were seeded at the same density, split every week and counted. The population doublings were calculated at each passage. (D) Control and shPLA2R WI38 cells were analysed for their SA-β-gal activity and the percentage of positive cells in each condition was calculated. (E) A colony formation assay of control versus shPLA2R-infected cells was performed in various primary human cells. The cells were seeded at low densities and stained 2 weeks later with crystal violet. (F) Control, shPLA2R or shp53 WI38 cells were plated at low densities, pulsed every 2 days with H2O2 (50 μM during 30 min) and stained 2 weeks later with crystal violet. (G) Control, shPLA2R or shp53 cells were treated with H2O2 as described above, until control cells entered a senescence-like morphology after which the cells were subjected to SA-β-gal analysis. For both experiments, untreated controls were also used in parallel. HMEC, human mammary epithelial cells; mRNA, messenger RNA; PLA2R, phospholipase A2 receptor; QRT–PCR, quantitative reverse transcription–PCR; SA-β-gal, senescence-associated β-galactosidase; sh, short hairpin.
To confirm the growth difference observed, we performed a growth curve analysis. Control and shPLA2R-infected HDFs were seeded at the same density, split and counted every week. Control cells proliferated at a much slower rate than the shPLA2R-infected cells (Fig 1C). Finally, to determine whether downregulation of PLA2R causes a bypass of senescence, we checked the SA-β-gal activity. The proportion of senescing cells was higher among control cells than among the different shPLA2R-infected cells, confirming that the shPLA2R-containing cells escaped senescence (Fig 1D).
Next, we wondered whether the effect of PLA2R downregulation was cell dependent or could be reproduced in other primary human cells. Post-stasis primary human mammary epithelial cells (HMECs) and other primary HDFs (IMR90) were infected with the shPLA2R construct or with a control construct. Although control cells were unable to form colonies, both shPLA2R-expressing primary cells tested continued to proliferate (Fig 1E). Interestingly, all these cells (IMR90, WI38 and post-stasis HMEC) were immortalized by hTERT (human telomerase reverse transcriptase) expression showing that the depletion of PLA2R affected telomere-induced senescence (supplementary Fig 1 online; Acosta et al, 2008).
These results suggest that PLA2R knockdown has important effects on replicative senescence. Next, we investigated whether the depletion of PLA2R could favour bypass from stress-induced senescence. Various studies indicate that reactive oxygen species (ROS) participate in the induction of replicative senescence (Parrinello et al, 2003), as well as in the induction of oncogene-induced senescence (Lee et al, 1999), by eventually triggering a DNA damage response resulting in a premature senescence state (d'Adda di Fagagna, 2008). shPLA2R, shp53 (positive control) and young WI38 control cells were seeded at low density, subjected to H2O2 treatment and stained 2 weeks later. For this experiment, untreated young control cells were also used. Although untreated control and H2O2-treated shPLA2R or shp53 cells continued to proliferate, control H2O2-treated cells entered a growth arrest state (Fig 1F). In parallel, an SA-β-gal activity experiment indicated that shPLA2R cells escaped stress-induced senescence (Fig 1G).
Taken together, these results reveal that the downregulation of PLA2R both delays the onset of replicative senescence and diminishes stress-induced senescence in primary human cells.
Ectopic PLA2R expression induces premature senescence
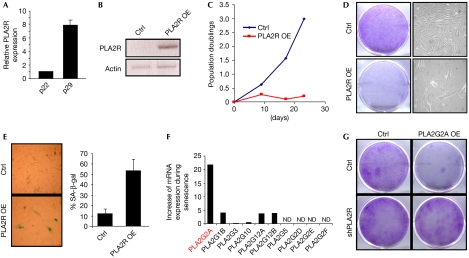
In the light of these results suggesting that PLA2R might be important in controlling senescence, we sought to determine whether the endogenous PLA2R level increases during senescence. PLA2R transcript levels were measured in young proliferating (passage 22) HDFs and compared with old senescing (p29) HDFs. They were found to peak at p29 when most cells were senescing (Fig 2A).
Figure 2.
The ectopic expression of PLA2R triggers premature senescence. (A) RNAs from WI38 HDFs at p22 (proliferating) and p29 (senescing) were prepared. After retro-transcription, Q PCR against PLA2R mRNA and GAPDH mRNA were performed. The relative levels of mRNA PLA2R are shown. (B) After infection, selection and RNA preparation, ectopic expression of PLA2R was verified by RT–PCR in WI38 cells. (C) Growth curve analysis of control versus PLA2R-overexpressing WI38 cells. (D) A colony formation assay was performed to illustrate the growth difference of control and PLA2R-overexpressing WI38 cells. (E) Control and PLA2R-overexpressing WI38 cells were seeded and SA-β-gal activity was analysed. The number of positive cells was counted and the percentage was calculated for both conditions. (F) RNAs were prepared as in (A) and the expression of the indicated sPLA2 was analysed at p22 and p29. The results are presented as a fold increase during senescence (ND stands for not detected). (G) WI38 cells were first infected with a control or an shPLA2R-encoding vector, and G418 was selected. The cells were subjected to a second round of infection with a control or a PLA2G2A-encoding vector and puromycin was selected. The cells were then seeded at low densities and stained 2 weeks later with crystal violet. Ctrl, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HDF, human fibroblasts; mRNA, messenger RNA; OE, overexpression; PLA2R, phospholipase A2 receptor; QRT–PCR, quantitative reverse transcription–PCR; SA-β-gal, senescence-associated β-galactosidase.
To corroborate the function of PLA2R in senescence, we tested the effect of ectopic PLA2R expression on cellular senescence. A retroviral vector expressing PLA2R was generated and young HDFs were transduced with this vector. Ectopic overexpression of PLA2R was verified by reverse transcription–PCR (RT–PCR; Fig 2B). Then, we assessed the growth of control and PLA2R-overexpressing WI38 cells (Fig 2C,D). Cell growth blockade was observed in growth curve analysis and colony formation assay when PLA2R was overexpressed (Fig 2C,D), and was mainly due to senescence induction, as PLA2R-overexpressing cells showed a strong SA-β-gal activity (Fig 2E).
To examine whether some sPLA2 (Lambeau & Gelb, 2008) could be involved in the effect observed when manipulating PLA2R levels, we first assayed the expression levels of various human sPLA2 during senescence. The sPLA2 PLA2G2A mRNA expression was found to increase by more than 20-fold in senescent cells (Fig 2F). Interestingly, constitutive expression of PLA2G2A induced premature senescence (Fig 2G), an effect reminiscent of PLA2R overexpression. To confirm further that PLA2GA could act through PLA2R to induce senescence, we analysed the effect of PLA2G2A constitutive expression in PLA2R-depleted WI38 cells. Interestingly, the growth inhibition induced by PLA2G2A overexpression was reverted in PLA2R-depleted WI38 cells (Fig 2G). These results indicate that PLA2R mediates the effect of PLA2G2A. Murine PLA2G2A seems to be a ligand of murine PLA2R, but this does not hold for human PLA2G2A and human PLA2R (Cupillard et al, 1999). Hence, we propose that PLA2G2A regulates senescence through a pathway that remains to be elucidated and that might involve PLA2R, at least partly, through direct or indirect interplay.
Taken together, these results indicate that genetically modifying the expression of PLA2R has an important impact on the senescence of primary human cells. Next, we went on to investigate how PLA2R might regulate the outcome of senescence.
PLA2R activates ROS production to induce senescence
Senescence induced by the CXCR2 receptor is thought to possibly rely on the production of ROS (Acosta et al, 2008). Previous results also suggest that arachidonic acid can be produced in a PLA2R-dependent manner (Fonteh et al, 2000), and it has been shown that arachidonic acid leads to the production of ROS (Muralikrishna Adibhatla & Hatcher, 2006). Therefore, we investigated whether PLA2R could produce ROS and, if so, whether the production of ROS was necessary for the induction of senescence by PLA2R.
By using H2DCFDA (2′,7′-dichlorodihydrofluorecein diacetate), a cell-permeant indicator for ROS, we examined whether PLA2R could induce the production of ROS. Young HDFs were infected with either an empty control vector or a PLA2R-encoding vector. After selection, cells were loaded with H2DCFDA and the fluorescence was examined. PLA2R-overexpressing cells produced greater fluorescence than control cells (about five times more), indicating a higher concentration of intracellular ROS (Fig 3A). We also infected near senescing HDFs with an empty control or an shPLA2R-encoding vector. When control cells started to enter senescence, the fluorescence of both populations was analysed. The shPLA2R-infected cells were found to contain ROS in lower amounts than senescing control cells (three times less), indicating that PLA2R has an impact on the intracellular levels of ROS (Fig 3A).
Figure 3.
PLA2R induces senescence through the production of reactive oxygen species. (A) Young WI38 cells were infected with control (Ctrl) or PLA2R-expressing vectors. After 2 weeks, the cells were loaded with H2DCFDA and the fluorescence was analysed by microscopy and flow cytometry. Pictures and relative mean fluorescence are shown (upper panel). WI38 cells approaching senescence were infected with a control vector or a shPLA2R construct, and when control cells entered senescence, the cells were loaded with H2DCFDA and the fluorescence was analysed as above (lower panel). (B) After infection, 2.5 mM N-acetyl-cysteine (NAC) was added or not from day 1 after infection and renewed every 2 days. The cells were stained with crystal violet 2 weeks later or pictures were taken after 10 days. (C) WI38 cells were analysed for their SA-β-gal activity, and the percentage of positive cells in each condition was calculated. OE, overexpression; PLA2R, phospholipase A2 receptor; ROS, reactive oxygen species; SA-β-gal, senescence-associated β-galactosidase; sh, short hairpin.
Next, we investigated the function of ROS production in the induction of senescence by PLA2R. Control and PLA2R-expressing cells were treated with or without the antioxidant N-acetyl-cysteine (NAC) just after infection (Catalano et al, 2005; Takahashi et al, 2006). We performed a colony formation assay to assess proliferation in the presence or absence of NAC. PLA2R-overexpressing cells stopped growing in the absence of the ROS scavenger, but not in its presence (Fig 3B). Accordingly, the senescence-like cell morphology—flattened and enlarged—observed in control PLA2R-overexpressing cells was suppressed by treatment with NAC (Fig 3B). Finally, the NAC treatment strongly decreased SA-β-gal labelling in PLA2R-overexpressing cells (Fig 3C). PLA2R thus seems to induce senescence through the production of ROS. Numerous studies have shown that ROS can induce various cellular stresses; they notably favour a DNA break, inducing a DNA damage response and cellular senescence (Lee et al, 1999; Macip et al, 2003). Therefore, we wondered whether PLA2R could activate a DNA damage response.
PLA2R regulates senescence in a p53-dependent manner
The presence of DNA strand breaks was assessed by immunofluorescence staining of the phosphorylated histone H2AX (γH2AX), a protein associated with damaged DNA (Rogakou et al, 1998). Control and shPLA2R-infected cells were cultured until the control cells entered senescence, after which both populations were immunolabelled. Control senescing cells showed more γH2AX-positive cells (29%) than the shPLA2R-infected cells (13.7%; Fig 4A). A similar experiment was carried out, but this time young HDFs were infected with a control or a PLA2R-expressing vector. About 32% of PLA2R-overexpressing cells showed γH2AX labelling against 14% in control cells, indicating that PLA2R triggers DNA damage (Fig 4B). Interestingly, the ROS scavenger treatment diminished the appearance of the γH2AX labelling in PLA2R-overexpressing cells (Fig 4B), showing the link between ROS production and the accumulation of DNA damage.
Figure 4.
PLA2R regulates senescence through the p53 pathway. (A,B) Old control (Ctrl) and shPLA2R-infected WI38 cells or young control and PLA2R-infected WI38 cells were subjected to γH2AX immunofluorescence, and the percentage of γH2AX-positive cells was determined. (C) WI38 cells were cultured until control cells entered senescence. Both control and shPLA2R-infected cells were lysed and western blot analysis was performed. (D) When the growth arrest induced by the overexpression of PLA2R was visualized, control and PLA2R-infected WI38 cells were lysed and western blot analysis was performed. (E) WI38 cells were first infected with E6- or E7-encoding vectors or both. Cells were then infected with control or PLA2R-encoding vectors. After selection, cells were seeded at low densities and the ability to form colonies was visualized by crystal violet staining. NAC, N-acetyl-cysteine; OE, overexpression; PLA2R, phospholipase A2 receptor; sh, short hairpin.
We next checked whether the changes observed in the DNA damage level had any impact on the p53 pathway activity. Interestingly, in shPLA2R-infected HDFs, p53, and its targets p21 and human double minute 2 (HDM2), decreased when compared with control cells. Phospho-Rb increased, suggesting that the cells were proliferating (Fig 4C). Conversely, when PLA2R was ectopically expressed, the levels of p53, p21 and HDM2 increased when compared with control senescing cells and phospho-Rb was found to decrease (Fig 4D).
Finally, to confirm functionally that PLA2R regulates senescence through the p53 pathway, we engineered HDFs to express E6 to inhibit p53 (Scheffner et al, 1990), E7 to inhibit Rb (Dyson et al, 1989), E6E7 to inhibit both. In those HDFs, we ectopically expressed PLA2R or a control green fluorescent protein (GFP). Although E7 did not prevent the growth arrest induced by the expression of PLA2R, E6 was able to efficiently do so according to the colony formation assay (Fig 4E). Nevertheless, the simultaneous inhibition of p53 and Rb reverted completely the growth arrest induced by PLA2R, suggesting that the Rb pathway contributed slightly to the PLA2R effect (Fig 4E). Taken together, these results reveal that PLA2R, through the production of ROS and mainly through the activation of the DNA damage–p53 pathway, regulates the senescence of primary human cells.
Despite the recent discovery of new genes controlling senescence (Acosta et al, 2008; Kuilman et al, 2008), further work is still needed to understand in more depth the molecular mechanism underpinning this phenomenon. By performing a genetic screen using an shRNA library, we have identified PLA2R to be a crucial regulator of both replicative- and stress-induced senescence. The downregulation of PLA2R prevents the onset of senescence, whereas its overexpression triggers premature senescence. We found that PLA2R regulates cellular senescence through the production of ROS and the activation of the DNA damage pathway. Interestingly, ROS-induced senescence was overcome by the depletion of PLA2R, suggesting the existence of a feedback loop between them. Such a feedback loop has already been observed between ROS- and telomere-induced senescence (Richter & Proctor, 2007). PLA2R could thus be a crucial factor regulating replicative- (owing to short telomeres) and stress-induced senescence.
To our knowledge, except for the two recently identified chemokine receptors (Acosta et al, 2008; Kuilman et al, 2008), PLA2R is the only receptor that, when downregulated, allows normal human cells to bypass senescence. Our results indicate that PLA2R generates the production of ROS to affect senescence. Interestingly, the recently identified cytokine receptor CXCR2 is also potentially regulating senescence through ROS production (Acosta et al, 2008). CXCR2 is a G-coupled receptor, whereas PLA2R has not been formally described to bind to any signalling proteins. So whether or not the production of ROS results from a common or a different mechanism thus remains an open question. PLA2R could induce the release of arachidonic acid (Fonteh et al, 2000) and the activation of the MAPK pathway (Kinoshita et al, 1997; Silliman et al, 2002). These pathways have been described as activators of ROS production and senescence occurrence (Lee et al, 1999; Iwasa et al, 2003; Catalano et al, 2005). Our preliminary results, however, suggest that PLA2R, although having an impact on the cell cycle (see levels of p53, p21 and cyclin A), does not have a crucial impact on ERK and p38 kinases in our experimental settings (supplementary Fig 2 online). Alternatively, PLA2R could regulate senescence by controlling the production of cytokines, as PLA2R is able to regulate cytokines (Lambeau & Gelb, 2008) and cytokines are involved in senescence outcome (Acosta et al, 2008; Kuilman et al, 2008). Taken together, our data have identified PLA2R to be a new crucial regulator of senescence in human primary cells.
Methods
Cell culture and retroviral infection. Normal human diploid fibroblasts WI38, IMR90 (American Type Culture Collection (ATCC), Manassas, VA, USA) and GP293 cells (Clontech, Mountain View, CA, USA) were cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone Perbio, Brackley, UK) in the presence of gentamicin at a final concentration of 80 μg/ml (Invitrogen, Carlsbad, CA, USA). HMECs (Clonetics, Basel, Switzerland) were cultured in mammary epithelial cell growth medium (Promocell, Heidelberg, Germany). Cells were maintained at 37°C under a 5% CO2 atmosphere. GP293 packaging cells were used as recommended by the manufacturer (Clontech).
Genetic screening. WI38 cells at p23 (cells are senescing at p30) were infected with the control or pools of the Netherlands Cancer Institute's shRNA library (Berns et al, 2004). Each pool is targeting 96 genes, each gene being targeted by three independent shRNAs. An shRNA pool is used to infect 500,000 cells. We set up the infection efficiency at 30% to have, in most cases, one retroviral particle per infected cell. Cells were selected and split every week (one into three) until proliferation stopped. The emerging clones (without clonal selection) were amplified and genomic DNA was purified. Cells were lysed in TNE buffer (Tris–HCl 10 mM pH 8.0, 100 mM NaCl, EDTA 10 mM pH 8.0) with 0.5% SDS and incubated at 37°C for 2 h in the presence of 50 μg/ml of RNAse A (9707-B; Euromedex, Souffelweyersheim, France). Proteinase K at a concentration of 100 μg/ml (EU0090-B; Euromedex) was then added and the lysate was incubated overnight at 45°C. A phenol–chloroform–isoamyl alcohol (25/24/1) extraction was performed, followed by an isopropanol precipitation. shRNA inserts were amplified by using nested PCR. The first pair of primers used to amplify the 600-bp sequence was: pRS out forward 5′-CCCTTGAACCTCCTCGTTCGACC-3′ and pRS out reverse 5′-GAGACGTGCTACTTCCATTTGTC-3′. An aliquot of 5 μl of the PCR product was used to perform a second PCR with the primers: pRS in forward 5-ACCTCCTCGTTCGACCC-3′ and pRS in reverse 5′-TGTGAGGGACAGGGGAG-3′. The PCR products were purified using the Jet Quick kit (GENOMED, Lohne, Germany) and cloned into the pGEM-T-easy vector (Promega, Madison, WI, USA). The pRS forward was used to sequence the shRNA insert (GenoScreen, Lille, France).
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Informations
Acknowledgments
We thank Julie Bertout, Emeric Deruy, Nicolas Malaquin and all the members of the laboratory for their helpful comments and help. This study was supported by the ‘Association pour la Recherche sur le Cancer' for G.L. and D.B., and also by the ‘Comité du Pas de Calais de la Ligue Nationale contre le Cancer' for D.B.
Footnotes
The authors declare that they have no conflict of interest.
References
- Acosta JC et al. (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018 [DOI] [PubMed] [Google Scholar]
- Berns K et al. (2004) A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428: 431–437 [DOI] [PubMed] [Google Scholar]
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA (2005) Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436: 660–665 [DOI] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8: 729–740 [DOI] [PubMed] [Google Scholar]
- Catalano A, Rodilossi S, Caprari P, Coppola V, Procopio A (2005) 5-Lipoxygenase regulates senescence-like growth arrest by promoting ROS-dependent p53 activation. EMBO J 24: 170–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z et al. (2005) Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436: 725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupillard L et al. (1999) Both group IB and group IIA secreted phospholipases A2 are natural ligands of the mouse 180-kDa M-type receptor. J Biol Chem 274: 7043–7051 [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F (2008) Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer 8: 512–522 [DOI] [PubMed] [Google Scholar]
- Dimri GP et al. (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92: 9363–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N, Howley PM, Munger K, Harlow E (1989) The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243: 934–937 [DOI] [PubMed] [Google Scholar]
- Fonteh AN, Atsumi G, LaPorte T, Chilton FH (2000) Secretory phospholipase A2 receptor-mediated activation of cytosolic phospholipase A2 in murine bone marrow-derived mast cells. J Immunol 165: 2773–2782 [DOI] [PubMed] [Google Scholar]
- Gil J, Bernard D, Martinez D, Beach D (2004) Polycomb CBX7 has a unifying role in cellular lifespan. Nat Cell Biol 6: 67–72 [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25: 585–621 [DOI] [PubMed] [Google Scholar]
- Iwasa H, Han J, Ishikawa F (2003) Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells 8: 131–144 [DOI] [PubMed] [Google Scholar]
- Kinoshita E, Handa N, Hanada K, Kajiyama G, Sugiyama M (1997) Activation of MAP kinase cascade induced by human pancreatic phospholipase A2 in a human pancreatic cancer cell line. FEBS Lett 407: 343–346 [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS (2008) Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133: 1019–1031 [DOI] [PubMed] [Google Scholar]
- Lambeau G, Gelb MH (2008) Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem 77: 495–520 [DOI] [PubMed] [Google Scholar]
- Lee AC, Fenster BE, Ito H, Takeda K, Bae NS, Hirai T, Yu ZX, Ferrans VJ, Howard BH, Finkel T (1999) Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J Biol Chem 274: 7936–7940 [DOI] [PubMed] [Google Scholar]
- Macip S, Igarashi M, Berggren P, Yu J, Lee SW, Aaronson SA (2003) Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol Cell Biol 23: 8576–8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralikrishna Adibhatla R, Hatcher JF (2006) Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med 40: 376–387 [DOI] [PubMed] [Google Scholar]
- Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J (2003) Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol 5: 741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson M et al. (2000) PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 406: 207–210 [DOI] [PubMed] [Google Scholar]
- Richter T, Proctor C (2007) The role of intracellular peroxide levels on the development and maintenance of telomere-dependent senescence. Exp Gerontol 42: 1043–1052 [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273: 5858–5868 [DOI] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63: 1129–1136 [DOI] [PubMed] [Google Scholar]
- Serrano M, Blasco MA (2001) Putting the stress on senescence. Curr Opin Cell Biol 13: 748–753 [DOI] [PubMed] [Google Scholar]
- Silliman CC et al. (2002) Presence of the M-type sPLA(2) receptor on neutrophils and its role in elastase release and adhesion. Am J Physiol Cell Physiol 283: C1102–C1113 [DOI] [PubMed] [Google Scholar]
- Sun P et al. (2007) PRAK is essential for ras-induced senescence and tumor suppression. Cell 128: 295–308 [DOI] [PubMed] [Google Scholar]
- Takahashi A, Ohtani N, Yamakoshi K, Iida S, Tahara H, Nakayama K, Nakayama KI, Ide T, Saya H, Hara E (2006) Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat Cell Biol 8: 1291–1297 [DOI] [PubMed] [Google Scholar]
- Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR (2008) Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132: 363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Informations