Abstract
BACKGROUND:
Antibiotic-associated diarrhea is an important problem in hospitalized patients. The use of probiotics is gaining interest in the scientific community as a potential measure to prevent this complication. The main objective of the present study was to assess the efficacy and safety of a fermented milk combining Lactobacillus acidophilus and Lactobacillus casei that is widely available in Canada, in the prevention of antibiotic-associated diarrhea.
METHODS:
In this double-blind, randomized study, hospitalized patients were randomly assigned to receive either a lactobacilli-fermented milk or a placebo on a daily basis.
RESULTS:
Among 89 randomized patients, antibiotic-associated diarrhea occurred in seven of 44 patients (15.9%) in the lactobacilli group and in 16 of 45 patients (35.6%) in the placebo group (OR 0.34, 95% CI 0.125 to 0.944; P=0.05). The median hospitalization duration was eight days in the lactobacilli group, compared with 10 days in the placebo group (P=0.09). Overall, the lactobacilli-fermented milk was well tolerated.
CONCLUSION:
The daily administration of a lactobacilli-fermented milk was safe and effective in the prevention of antibiotic-associated diarrhea in hospitalized patients.
Keywords: Antibiotic-associated diarrhea, C difficile, Lactobacillus, Probiotic
Abstract
HISTORIQUE :
La diarrhée associée à la prise d’antibiotiques est un important problème chez les patients hospitalisés. L’utilisation de probiotiques gagne en popularité dans la communauté scientifique, à titre de mesure potentielle de prévention de cette complication. Le principal objectif de la présente étude était d’évaluer l’efficacité et l’innocuité d’un lait fermenté alliant Lactobacillus acidophilus et Lactobacillus casei, facile à se procurer au Canada, pour la prévention de la diarrhée associée à la prise d’antibiotiques.
MÉTHODES :
Lors de cette étude à double insu et randomisée, des patients hospitalisés ont été assignés aléatoirement soit à un lait fermenté par lactobacilles, soit à un placebo, sur une base quotidienne.
RÉSULTATS :
Parmi les 89 patients randomisés, la diarrhée associée à la prise d’antibiotiques est survenue chez 7 patients sur 44 (15,9 %) du groupe sous lactobacilles et chez 16 patients sur 45 (35,6 %) du groupe sous placebo (RR 0,34, IC 95 % 0,125 à 0,944; p = 0,05). La durée médiane de l’hospitalisation a été de huit jours dans le groupe sous lactobacilles, contre dix jours dans le groupe sous placebo (p = 0,09). Dans l’ensemble, le lait fermenté par lactobacilles a été bien toléré.
CONCLUSION :
L’administration quotidienne d’un lait fermenté par lactobacilles s’est révélée sécuritaire et efficace pour la prévention de la diarrhée associée à la prise d’antibiotiques chez les patients hospitalisés.
Diarrhea is one of the most frequent side effects of antibiotic use. The incidence of antibiotic-associated diarrhea (AAD) varies between 5% and 30%, and has increased over the past years with the larger use of wide-spectrum antibiotics (1–3). Approximately 10% to 20% of all AAD cases are caused by Clostridium difficile (1,3–5). The severity of AAD may range from benign, uncomplicated diarrhea to C difficile pseudomembranous colitis or toxic megacolon (3,4). Patient discomfort, discontinuation of primary antibiotic therapy, longer hospitalization time, and even the need to readmit patients are serious consequences of AAD (6). Therefore, identifying preventive measures to curtail the occurrence of AAD is required. Among these, the use of probiotics is gaining interest in the scientific community (7).
The Canadian Natural Health Products Regulations (8) define a probiotic as a monoculture or mixed-culture of live microorganisms that benefit the microbiota indigenous to humans. A probiotic is limited to nonpathogenic microorganisms (8). The most frequently used species are Lactobacillus species, Bifidobacterium species and Saccharomyces species (6,9–12). Their efficacy could be explained by various mechanisms such as the production of antimicrobial substances, competition for gastrointestinal (GI) colonization and available nutrients, and immunomodulation (7,9–11,13–15).
Several studies have investigated the efficacy of prophylactic probiotics in preventing AAD but the heterogeneity of the strains and formulation used limit their external validity (5,6,9–12,15–19). The results of some of these studies were also limited by their design, imprecise definition of end points and a lack of patient follow-up after discontinuing antibiotic therapy. A meta-analysis by D’Souza et al (20), including only randomized double-blind placebo-controlled trials, has shown an overall benefit. Because results related to one specific probiotic cannot be extrapolated to another (7), we chose to study a product readily available in Quebec: a fermented milk combining the CL1285 Lactobacillus acidophilus patented strain, and Lactobacillus casei. It is noteworthy that lactobacilli have a long record of safety and have been used for many years in the fermentation process of multiple food products (21,22).
The goal of the present study was to assess the efficacy and safety of a once-daily administration of a lactobacilli-fermented milk compared with placebo, in the prevention of AAD in hospitalized adults.
PATIENTS AND METHODS
Study design and treatment
A prospective, randomized, double-blind, placebo-controlled study was conducted from September 2003 to May 2004 at Maisonneuve-Rosemont Hospital (Montreal, Quebec), a 700-bed tertiary care hospital. The internal review board of the institution approved the study protocol, and written informed consent was obtained from each patient.
Patients were randomly assigned to receive either a lactobacilli-fermented milk or a placebo (a lactoserum devoid of microorganisms), administered once daily. The active preparation was a lactobacilli-fermented milk, combining at least 50×109 colony forming units of L acidophilus CL1285 and L casei (Bio-K+ CL1285, Bio-K+ International Inc, Canada). Both preparations were provided in identically labelled containers; their taste and texture were similar. The administration schedule was 49 g (one-half of a container) once per day for two days, followed by 98 g (one container) once per day to cover the entire duration of the antibiotic treatment. If patients were discharged before the completion of their antibiotic treatment, they were provided with the study containers needed to complete the prophylaxis at home. For GI tolerability purposes, both preparations were administered at a lower dosage for the first two days. Prophylaxis was discontinued if patients developed diarrhea. All patients were instructed to avoid the use of any other probiotic and any type of yogurt for the duration of the study. The follow-up period was planned to end 21 days after the last administered antibiotic dose, unless AAD occurred before that time.
Study population
The trial population consisted of hospitalized patients who were anticipated to take at least three days of any systemic antibiotic. Prophylaxis with the study drug or placebo began within the first 48 h of antibiotic treatment. Exclusion criteria included active diarrhea at enrollment, diagnosis of C difficile-associated diarrhea (CDAD) within the previous three months, antibiotic treatment with vancomycin or an aminoglycoside in monotherapy (23), confirmed lactose intolerance, underlying chronic GI tract disease, patients with stomas, use of parenteral nutrition or tube feeding (1,24), regular probiotic intake, immunocompromised patients, and patients with artificial or damaged cardiac valves (14,20,25).
Outcome measures
The primary outcome of the present study was the incidence of AAD during the study period. AAD was defined as three or more liquid stools in a 24 h period. In case of diarrhea, a medical investigation was performed, including a C difficile cytotoxin assay to eliminate other potential causes. Stool frequency and consistency were retrieved from medical records every third day during antibiotic treatment. This information was also gathered seven, 14 and 21 days after the antibiotic and probiotic regimens were completed. Data for patients discharged before the end of the study were obtained by a telephone call using a standardized questionnaire. At discharge, patients were provided with a diary to optimize data collection. If AAD occurred after discharge, a medical consultation at the hospital’s infectious diseases clinic was organized.
Secondary outcomes included the occurrence of CDAD, the duration of hospitalization and the adverse events associated with the lactobacilli-fermented milk. To assess the safety of the study preparation, patients were questioned about the occurrence of any adverse event on day 5 and at the end of the prophylaxis.
To control for confounding variables, the following data were collected: demographic data; previous history of AAD and/or CDAD; use of antibiotics in the month before enrolment; indication of antibiotic therapy; type, duration and number of prescribed antibiotics; hospitalization on a medical ward with a high rate of nosocomial CDAD; use of proton pump inhibitors, laxatives and narcotics; yogurt intake; and severity of patient medical condition during hospitalization. The severity of the patient’s medical condition was obtained using the All Patient Refined Diagnosis Related Groups (APR-DRG) classification software version 12.0 (3M Health Information Systems, USA).
Statistical analysis
Analyses of the primary and secondary outcomes were based on the intention-to-treat principle. Descriptive statistics were reported for all study variables and included the mean ± SD for continuous variables and frequency distributions for categorical scale variables. Between-group comparisons for categorical variables were based on the χ2 statistic and on the Independent Sample Student’s t test for continuous variables. A multivariate analysis using binary logistic and general linear models were used to evaluate the adjusted between-group differences for the primary outcome measure. The confounding variables mentioned above were included in this multivariate model. All reported P-values were two-sided, and a type I error level of 5% was used. All analyses were performed with SPSS statistical software version 13.0 (SPSS Inc, USA).
Assuming that the incidence of AAD would be 30% in the placebo group and 15% in the study preparation group, we determined that an enrolment of 120 patients per group would give the study a statistical power of 80% (two-sided α=0.05) to detect a significant difference between the two groups.
RESULTS
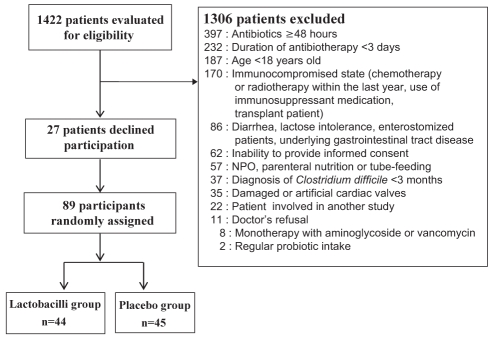
Patient flow is summarized in Figure 1, and baseline characteristics are summarized in Table 1.
Figure 1).
The flow of patients through the study. NPO Nil per os (nothing by mouth)
TABLE 1.
Demographic and baseline characteristics of randomly assigned patients
| Characteristics | Lactobacilli group (n=44) | Placebo group (n=45) | P |
|---|---|---|---|
| Age, years | 68.8±14.5 | 72.9±13.4 | 0.16 |
| Sex | |||
| Male | 20 (45.5) | 23 (51.1) | 0.67 |
| Diagnosis | |||
| Respiratory infection | 40 (90.9) | 41 (91.1) | 1.00 |
| Other* | 4 (9.1) | 4 (8.9) | |
| Hospitalization on a medical ward with a high rate of nosocomial CDAD | 30 (68.2) | 34 (75.6) | 0.49 |
| Previous history of CDAD | 2 (4.5) | 4 (8.9) | 0.68 |
| Previous history of AAD | 8 (18.2) | 9 (20.0) | 1.00 |
| Proton pump inhibitor use | 15 (34.1) | 18 (40.0) | 0.66 |
| Laxative use | |||
| None | 34 (77.3) | 30 (66.7) | 0.39 |
| Occasional | 5 (11.4) | 5 (11.1) | |
| Regular | 5 (11.4) | 10 (22.2) | |
| Narcotic use | |||
| None | 32 (72.7) | 31 (68.9) | 0.51 |
| Occasional | 10 (22.7) | 9 (20.0) | |
| Regular | 2 (4.5) | 5 (11.1) | |
| Yogurt intake | 5 (11.4) | 9 (20.0) | 0.38 |
| Antibiotic use 1 month before enrolment | 9 (20.5) | 9 (20.0) | 1.00 |
Data are reported as number (%) or mean ± SD.
Includes urinary tract, skin and soft tissue infections. AAD Antibiotic-associated diarrhea; CDAD Clostridium difficile-associated diarrhea
Participants were well matched between the two study groups regarding demographic and baseline characteristics. As for the antibiotic and prophylaxis course during the study period, both groups were comparable except for the use of beta-lactam antibiotics, which were prescribed more often in the placebo group (66.7% versus 40.9%; P=0.02) (Table 2). A logistic regression model excluded any effect of this variable on the incidence of AAD.
TABLE 2.
Antibiotics administration and probiotic prophylaxis
| Variable | Lactobacilli group (n=44) | Placebo group (n=45) | P |
|---|---|---|---|
| Number of antibiotics per patient | 2.0±1.0 | 2.3±1.2 | 0.13 |
| Duration of antibiotic therapy, days | 8.8±3.6 | 9.8±4.4 | 0.29 |
| Duration of prophylaxis, days | 7.6±4.3 | 7.3±4.2 | 0.74 |
| Antibiotics received during study* | |||
| Beta-lactams† | 18 (40.9) | 30 (66.7) | 0.02 |
| Macrolides | 27 (61.4) | 25 (55.6) | 0.67 |
| Quinolones | 25 (56.8) | 28 (62.2) | 0.67 |
| Other | 6 (13.6) | 7 (15.6) | 1.00 |
Data are reported as mean ± SD or number (%).
Some patients received more than one antibiotic;
Includes penicillin and cephalosporin antibiotics
Among 44 patients receiving the lactobacilli-fermented milk, seven developed AAD during the study (15.9%). Among 45 patients receiving the placebo, 16 developed AAD (35.6%). The difference in the rate of occurrence of AAD between the two study groups was statistically significant (OR 0.343, CI 0.125 to 0.944; P=0.05) (Table 3). Most AAD episodes occurred when the antibiotic treatment was complete: in 71.4% of patients in the lactobacilli group and 75.0% of patients in the placebo group. The mean delay between the end of antibiotic therapy and AAD occurrence was not significantly different between the lactobacilli and the placebo groups: 4.2 and 8.5 days, respectively (P=0.16).
TABLE 3.
Outcomes according to study group (n=89)
| Outcome | Lactobacilli group (n=44) | Placebo group (n=45) | OR | 95% CI | P |
|---|---|---|---|---|---|
| AAD occurrence | 7 (15.9) | 16 (35.6) | 0.343 | 0.125–0.944 | 0.05 |
| CDAD occurrence | 1 (2.3) | 7 (15.6) | 0.126 | 0.020–1.109 | 0.06 |
| Mean hospitalization duration | 12.2±9.6 | 16.4±16.5 | 0.15 | ||
| Median hospitalization duration | 8 (6–16.8) | 10 (8–19) | 0.09 |
Data are reported as number (%), mean ± SD or median (interquartile range). All analyses were performed according to the intention-to-treat principle. AAD Antibiotic-associated diarrhea; CDAD Clostridium difficile-associated diarrhea
Among all study patients, one patient in the lactobacilli group (2.3%) and seven patients in the placebo group (15.6%) developed CDAD (OR 0.126, 95% CI 0.020 to 1.109; P=0.06). Of note, C difficile cytotoxin assay was performed in only two patients (28%) presenting with AAD in the lacto-bacilli group and in 13 patients (81%) presenting with AAD in the placebo group. Median hospitalization duration was shorter for the lactobacilli group than for the placebo group: eight and 10 days, respectively, although this difference was not statistically significant (P=0.09).
At least one adverse event was reported by 21 patients in the lactobacilli group (47.7%) and by 20 patients in the placebo group (44.4%). There was no significant difference between the lactobacilli and placebo groups with respect to the incidence of treatment-related adverse events (Table 4). The majority of the adverse events concerned the GI tract. Adverse events were reported as the reason for withdrawal for four patients in the lactobacilli group (9.1%) and in nine patients in the placebo group (20.0%). This difference was not significant (P=0.25). During the study, three patients in the lactobacilli group died. However, none of those deaths could be related to the use of the study preparation.
TABLE 4.
Adverse events reported during the study
| Adverse event | Lactobacilli group (n=44) | Placebo group (n=45) |
|---|---|---|
| Gastrointestinal adverse events | ||
| Softened stools (no diarrhea) | 8 (18.2) | 9 (20.0) |
| Taste disorder | 6 (13.6) | 7 (15.6) |
| Abdominal cramping | 4 (9.1) | 5 (11.1) |
| Bloating | 3 (6.8) | 3 (6.7) |
| Gastroesophageal reflux | 2 (4.5) | 2 (4.4) |
| Constipation | 2 (4.5) | 1 (2.2) |
| Flatulence | 2 (4.5) | 1 (2.2) |
| Modified stool colour | 1 (2.3) | 2 (4.4) |
| Nausea | 0 (0.0) | 4 (8.9) |
| Vomiting | 0 (0.0) | 1 (2.2) |
| Foul-smelling stools | 0 (0.0) | 1 (2.2) |
| Other adverse events | ||
| Hallucination | 0 (0.0) | 1 (2.2) |
| Rash | 0 (0.0) | 1 (2.2) |
| Pruritus | 1 (2.3) | 0 (0.0) |
| Presence of at least one adverse event | 21 (47.7) | 20 (44.4) |
Data are reported as number (%). For all adverse events, no statistically significant differences were observed between groups (P>0.05)
DISCUSSION
The present study demonstrated that a fermented milk, combining L acidophilus and L casei at a daily dose of 50×109 colony forming units administered to hospitalized patients receiving antibiotics is effective in the prevention of AAD.
We found that the lactobacilli preparation was effective in reducing the incidence of AAD when compared with a placebo, even though only 89 patients were recruited in the predesignated study period. This study protocol was conducted as part of a pharmacy residency program and was therefore limited to nine months. In spite of a relatively small group, we were able to reach a statistical conclusion by witnessing a higher baseline risk of AAD (35.6% in the placebo group) than initially expected. A previous meta-analysis (20) has shown an overall benefit of administering probiotics as a preventive method for AAD. However, of the nine studies retained for the analysis, only three studies showed some statistically significant advantage; two involved a Saccharomyces species, and one involved a Lactobacillus species. The latter was one of the studies with the biggest weight in the global analysis. Those studies were heterogenous in the duration of treatment, dosage and antibiotics being used. Because our study was placebo-controlled and randomized, it strengthened the role of lactobacilli-based probiotics as prophylactic agents in the prevention of AAD.
Furthermore, the proportion of AAD due to C difficile infection was very high compared with that observed in previous studies (43.6% [seven of 16 cases] in the placebo group versus 10% to 20%) (1,3–5). This observation concurs with the 2003 to 2004 fourfold increase in the number of CDAD cases that affected the Montreal area (26,27). The prevention of CDAD is an important outcome to consider, because this condition has been associated with an increased mortality and morbidity (2). Although there was a trend in the reduction of the incidence of CDAD in the lactobacilli group compared with the placebo group, this difference was not statistically significant. However, the present study was not specifically designed to assess this as a primary outcome and too few patients were randomized to adequately detect such an effect of the lactobacilli preparation.
The lactobacilli-fermented milk did not have any impact on the hospital length of stay. It is worth mentioning that 12 of 23 AAD cases (52.1%) occurred after discharge from the hospital. The long-term follow-up of patients after their antibiotic courses enabled us to witness that the majority of all the episodes occurred for both groups after the end of antibiotic therapy.
It would have been useful to determine the efficacy of the lactobacilli preparation with regard to specific antibiotics classes, because the incidence of AAD may have varied according to which antibiotic was prescribed. However, it was not feasible because of the variety of antibiotic combinations used concurrently, and the relatively small number of patients with diarrhea. In the present study, the use of beta-lactams was more frequent in the placebo group. However, this disparity was not found to affect the AAD occurrence according to a logistic regression analysis adjusting for potential confounders.
Almost one-half of the patients in both groups reported at least one adverse event. Because the placebo was a lactoserum and the study preparation a fermented milk, they would be expected to cause GI disturbances. In fact, most of the adverse events were related to the GI tract. No severe or life-threatening adverse event related to the use of the lactobacilli preparation was reported, although there were some withdrawals associated with the occurrence of an adverse event. Because some studies (14) have reported the development of septicemia and endocarditis with the use of lactobacilli, the present study voluntarily excluded immunocompromised patients, patients at risk for aspiration and GI translocation, and patients with damaged or artificial heart valves. Therefore, the fermented milk was well tolerated, and should not cause any problem if given to patients with no potential risk factors.
SUMMARY
Our findings demonstrate that the daily administration of a fermented milk combining L acidophilus CL1285 and L casei is safe and effective in the prevention of AAD. It also suggests a possible protective effect against CDAD, but the study was not designed to evaluate this hypothesis. Therefore, we currently cannot advocate the use of this probiotic as a preventive measure to decrease the risk of CDAD. A study with a larger patient sample has to be conducted for this purpose.
Acknowledgments
The authors thank John S Sampalis PhD from JSS Medical Research Inc, for statistical support and revision.
Footnotes
FINANCIAL SUPPORT: Product and placebo were provided by Bio-K+ International Inc, Laval, Quebec. A research grant was provided by Bio K+ International Inc to cover the pharmacy administration fees.
POTENTIAL CONFLICTS OF INTEREST: No author had a conflict of interest. This work was partially presented as an abstract at the 2004 Annual Meeting of the American College of Gastroenterology: Beausoleil M, Fortier N, Guenette S, et al. Am J Gastroenterology 2004;99(S10):722. (Abst)
REFERENCES
- 1.Högenauer C, Hammer HF, Krejs GJ, Reisinger EC. Mechanisms and management of antibiotic-associated diarrhea. Clin Infect Dis. 1998;27:702–10. doi: 10.1086/514958. [DOI] [PubMed] [Google Scholar]
- 2.Miller MA, Hyland M, Ofner-Agostini M, Gourdeau M, Ishak M, for the Canadian Hospital Epidemiology Committee Morbidity, mortality, and healthcare burden of nosocomial Clostridium difficile-associated diarrhea in Canadian hospitals. Infect Control Hosp Epidemiol. 2002;23:137–40. doi: 10.1086/502023. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JG. Clinical Practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–9. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 4.Poutanen SM, Simor AE. Clostridium difficile-associated diarrhea in adults. CMAJ. 2004;171:51–8. doi: 10.1503/cmaj.1031189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siitonen S, Vapaatalo H, Salminen S, et al. Effect of Lactobacillus GG yoghurt in the prevention of antibiotic associated diarrhoea. Ann Med. 1990;22:57–9. doi: 10.3109/07853899009147243. [DOI] [PubMed] [Google Scholar]
- 6.Cremonini F, Di Caro S, Nista EC, et al. Meta-analysis: The effect of probiotic administration on antibiotic-associated diarrhea. Aliment Pharmacol Ther. 2002;16:1461–7. doi: 10.1046/j.1365-2036.2002.01318.x. [DOI] [PubMed] [Google Scholar]
- 7.Mercenier A, Pavan S, Pot B. Probiotics as biotherapeutic agents: Present knowledge and future prospects. Curr Pharm Des. 2003;9:175–91. doi: 10.2174/1381612033392224. [DOI] [PubMed] [Google Scholar]
- 8.Health Canada. Overview of the natural health products regulations guidance document. <http://www.hc-sc.gc.ca/dhp-mps/prodnatur/legislation/docs/regula-regle_over-apercu_e.html>. (Version current at October 11, 2007).
- 9.Thomas MR, Litin SC, Osmon DR, Corr AP, Weaver AL, Lohse CM. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: A randomized, placebo-controlled trial. Mayo Clin Proc. 2001;76:883–9. doi: 10.4065/76.9.883. [DOI] [PubMed] [Google Scholar]
- 10.Tankanow RM, Ross MB, Ertel IJ, Dickinson DG, McCormick LS, Garfinkel JF. Double-blind, placebo-controlled study of the efficacy of Lactinex in the prophylaxis of amoxicillin-induced diarrhoea. DICP. 1990;24:382–4. doi: 10.1177/106002809002400408. [DOI] [PubMed] [Google Scholar]
- 11.Armuzzi A, Cremonini F, Bartolozzi F, et al. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163–9. doi: 10.1046/j.1365-2036.2001.00923.x. [DOI] [PubMed] [Google Scholar]
- 12.McFarland LV, Surawicz CM, Greenberg RN, et al. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol. 1995;90:439–48. [PubMed] [Google Scholar]
- 13.Isolauri E, Sütas Y, Kankaanpaa P, Arvilommi H, Salminen S. Probiotics: Effects on immunity. Am J Clin Nutr. 2001;73(2 Suppl):444S–50S. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Olmos MI, Oberhelman RA. Probiotic agents and infectious diseases: A modern perspective on a traditional therapy. Clin Infect Dis. 2001;32:1567–76. doi: 10.1086/320518. [DOI] [PubMed] [Google Scholar]
- 15.Surawicz CM, Elmer GW, Speelman P, McFarland LV, Chinn J, van Belle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii. Gastroenterology. 1989;96:981–8. doi: 10.1016/0016-5085(89)91613-2. [DOI] [PubMed] [Google Scholar]
- 16.Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: A randomized study Pediatrics 1999104e64(Abst). [DOI] [PubMed] [Google Scholar]
- 17.Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36:171–4. doi: 10.1016/s0163-4453(98)80008-x. [DOI] [PubMed] [Google Scholar]
- 18.Gotz V, Romankiewicz JA, Moss J, Murray HW. Prophylaxis against ampicillin-associated diarrhea with a lactobacillus preparation. Am J Hosp Pharm. 1979;36:754–7. [PubMed] [Google Scholar]
- 19.Adam J, Barret A, Barret-Bellet C. Essais cliniques contrôlés en double insu de l'Ultra-levure lyophilisée: étude multicentrique par 25 médecins de 388 cas. Gaz Med Fr. 1977;84:2072–8. [PubMed] [Google Scholar]
- 20.D'Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. Probiotics in prevention of antibiotic associated diarrhoea: Meta-analysis. BMJ. 2002;324:1361. doi: 10.1136/bmj.324.7350.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderhoof JA, Young RJ. Use of probiotics in childhood gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 1998;27:323–32. doi: 10.1097/00005176-199809000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Borriello SP, Hammes P, Holzapfel W, et al. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis. 2003;36:775–80. doi: 10.1086/368080. [DOI] [PubMed] [Google Scholar]
- 23.Stoddart B, Wilcox MH. Clostridium difficile. Curr Opin Infect Dis. 2002;15:513–8. doi: 10.1097/00001432-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 24.McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis. 1998;16(5):292–307. doi: 10.1159/000016879. [DOI] [PubMed] [Google Scholar]
- 25.Lewis SJ, Freedman AR. Review article: The use of biotherapeutic agents in the prevention and treatment of gastrointestinal disease. Aliment Pharmacol Ther. 1998;12:807–22. doi: 10.1046/j.1365-2036.1998.00386.x. [DOI] [PubMed] [Google Scholar]
- 26.Valiquette L, Low DE, Pepin J, McGeer A. Clostridium difficile infection in hospitals: A brewing storm. CMAJ. 2004;171:27–9. doi: 10.1503/cmaj.1040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eggertson L, Sibbald B. Hospitals battling outbreaks of C. difficile. CMAJ. 2004;171(1):19–21. doi: 10.1503/cmaj.1040979. [DOI] [PMC free article] [PubMed] [Google Scholar]