Abstract
Objective:
This study tested the differential effects of several cognitive and psychological variables on children's perception of asthma symptoms by use of an Asthma Risk Grid. Children's subjective and objective assessments of PEFR (peak expiratory flow rate) were characterized as representing perceptual accuracy, symptom magnification, and/or underestimation of asthma symptoms.
Design:
Two hundred and seventy children with asthma (ages 7-17) and their primary caregivers completed measures assessing cognitive and psychological factors and a 5-6 week symptom perception assessment.
Main Outcome Measures:
Children's symptom perception scores by use of the Asthma Risk Grid.
Results:
Children's attentional abilities had more of a bearing on their symptom monitoring abilities than their IQ estimates and psychological symptoms. The more time children took on Trails and Cancellation Tasks and the fewer errors they made on these tasks, the more likely they were to perceive their asthma symptoms accurately. More time on these tasks were associated with more symptom magnification scores, and fewer errors were related with fewer symptom magnification scores. More errors and higher total scores on the Continuous Performance Task were associated with a greater proportion of scores in the danger zone.
Conclusion:
Statistical support was provided for the utility of attentional-based instruments for identifying children who may have problems with perceptual accuracy, and who are at risk for asthma morbidity.
Keywords: Asthma, symptom perception, cognitive and psychological factors
Asthma affects over five million children in the United States and is the leading cause of school absences, resulting in an annual loss of over 14 million school days (National Center for Health Statistics, 2002). Asthma places children at risk for functional impairment (e. g., restriction of physical activity; Weil et al., 1999). Given asthma's prevalence in children, it is essential to identify processes that may enhance or impede effective asthma management behaviors. The purpose of this paper is to examine associations among various child-based, cognitive, and psychological processes and an important aspect of asthma management; symptom perception.
Managing asthma effectively involves three critical components, all of which are necessary to maintain normal pulmonary functioning. These components are: 1) consistent use of preventative and as needed medications; 2) avoidance of environmental irritants and allergens; and 3) accurate recognition, response, and monitoring of symptoms (McQuaid et al., 2003; National Heart Lung and Blood Institute, 2002). While recognizing when asthma symptoms occur may be a family's primary method of monitoring the course of asthma and the effectiveness of treatment, many children have difficulty perceiving airway obstruction or bronchodilation accurately (Baker et al., 2000).
Symptom Perception as a Critical Component to Effective Asthma Management
Asthma symptom perception involves the ability to accurately identify pulmonary function compromise and the resulting symptoms. It is a complex skill that includes peripheral sensory capability, the ability to cognitively attend to sensory input, and the capacity to distinguish sensations due to bronchoconstriction from those due to changes in anxiety levels and distress (Fritz et al., 1996). Accurate perception of symptoms can prompt a child to begin the self-management process (e.g., take as needed medication) in a timely fashion. Asthma management behaviors can be guided by misperceptions of the severity of symptoms, which can impact health care utilization and morbidity (Fritz et al., 1996; Klein et al., 2004; McQuaid et al., 2007). For example, children who underestimate their symptoms when symptoms are actually severe (dangerous symptom perception) may be at risk for rapid deterioration in respiratory status. On the other hand, children who overestimate the severity of their symptoms (symptom magnification) may use health care resources and medications unnecessarily. Accurate symptom perception is the desired goal for effective asthma self-management.
Although different methodological paradigms have been used to assess asthma symptom perception in children, this paper will focus on studies including naturalistic paradigms. Such measurements include children's reports of their severity of symptoms using visual analog scales, questionnaires, or symptom diaries. Responses from self-report instruments and actual objective measurements of lung function data (e.g., FEV1; Fritz et al., 1996) have been compared. One study measured children's record of daily symptom scores and compared these data to peak expiratory flow rates (Cabral et al., 2002). Results showed that symptom perception was inaccurate in a substantial number of children with asthma, independent of clinical severity, age, gender, and use of preventative medication (Cabral et al., 2002). Children have also estimated the severity of their asthma symptoms prior to the use of a spirometer and these data have been compared to indicators of pulmonary function (e.g., FEF25-75 and PEFR; (Cabral et al., 2002; Fritz et al., 1996; Yoos, 1999). Findings from these studies demonstrated that good perceptual accuracy in children with asthma is linked with significantly less functional morbidity. Perceptual accuracy has also been associated with more optimal family responses to asthma symptoms(McQuaid et al., 2007).
Results from studies summarized above do not shed light on which specific factors are predictive of poor or accurate symptom perception in children. The individual differences that distinguish children may in part, explain variations in the ability to self-monitor asthma symptoms (Hudgel, 1983). Few studies have shown significant, independent associations between symptom perception and child-based characteristics (Cabral et al., 2002).
Symptom perception may be affected by cognitive factors
Symptom monitoring is a cognitive process, and therefore may be associated with general cognitive ability. Results have demonstrated a positive relation between higher levels of general intelligence and more accurate symptom perception in children (Fritz et al., 1996). It is also possible that specific aspects of intelligence, such as attention, may account for the relation between intellectual ability and perceptual accuracy.
Although studies have shown that children with asthma are at an increased risk for behavioral problems (McQuaid, Penza-Clyve et al., 2001), results fail to support a substantial relation between Attention Deficit Hyperactive Disorder and asthma status (Barkley et al., 1988; Biederman et al., 1994). However, one study assessed the association between asthma severity, asthma status, and attention (Annett et al., 2000). Children with asthma, despite their level of severity, scored between two thirds and one standard deviation below the normative value on a measure of attention assessing impulse control when compared to children without asthma. Data from a recent study demonstrated modest associations between features of ADHD (as measured by parent report and the child CPT task) and asthma functional limitation, suggesting that the presence of problems in attention, concentration, and impulsivity may be related to asthma outcomes (McQuaid et al., 2007). Results from this study also revealed relations between a variety of measures of attention, concentration, and report of ADHD symptoms, and features of family asthma management (specifically, how the family responds to asthma exacerbations). Examining relations between specific attentional correlates and aspects of asthma management such as symptom monitoring in further depth is needed, as difficulty attending to asthma symptoms may increase the risk for experiencing morbidity.
There is significant clinical value for the assessment of specific attentional processes and children's perceptual accuracy of asthma symptoms. Children who attend for longer periods of time may monitor symptoms in a more accurate manner, and formulate and execute a multi-stepped asthma management response (Pontius, 1980). Children who have attentional problems may be challenged when asked to recall the necessary steps of the asthma self-management process at a moment's notice, or to implement a series of steps over time. Such attentional shortcomings may affect how children perceive the severity of their symptoms and impact decisions related to the appropriate next step for treatment. It is important to clarify the nature of children's attention with regard to their symptom perception abilities, as difficulty attending to asthma symptoms has implications for the likelihood of experiencing morbidity. Studies that provide further specificity in measurement on a range of attentional skills are necessary in order to identify specific attention based skills that may be relevant for children's symptom monitoring.
Perceptual accuracy may be affected by psychological factors
Some evidence has documented an association between psychiatric symptoms and asthma exacerbations in children, although this relationship remains complex. It may be that aspects of psychopathology are related to symptom perception ability, however, direct causal pathways between asthma and psychological problems have not been identified (Ortega et al., 2003). Still, a wide range of psychiatric problems is more commonly presented in children with asthma (Bender et al., 2000; Wamboldt et al., 1998). Internalizing the stress associated with having asthma can influence the development of psychological problems (Bender et al., 2000; Mrazek, 1992). Psychiatric symptoms (e.g., panic/fear symptoms) may be associated with asthma exacerbations through hyperventilation (Carr, 1998). Other results indicate that psychiatric problems in children challenge effective asthma management abilities (Weil et al., 1999).
Specifically, studies emphasize a relation between asthma, anxiety, and depressive symptoms (Bennett, 1994; Miller, 1987; Wamboldt et al., 1998). A higher prevalence of anxiety disorders among children with asthma versus healthy controls and children with other chronic illnesses has been demonstrated (Carr et al., 1994; Katon et al., 2004; Ortega et al., 2002; Vila et al., 2000). A recent review showed that in child/adolescent populations with asthma, up to one third met criteria for comorbid anxiety disorders (Brown et al., 2000; Katon et al., 2004; Ortega et al., 2002; Vila et al., 2000), particularly children with severe asthma (Ortega et al., 2002). Additionally, a link between higher levels of global internalizing symptoms and childhood asthma has been shown (Gillaspy et al., 2002; McQuaid, Kopel et al., 2001; Ortega et al., 2002). Relations between depressed mood, poor asthma management, and morbidity in children has also been documented (Galil, 2000; Miller, 1987).
In a population-based epidemiological sample, the parental report of asthma diagnosis in children and attacks was associated with depressive symptoms (Morrison et al., 2002; Mrazek, 2003; Ortega et al., 2004). As with the relations between to stress, anxiety, and asthma status, it is possible that the stress associated with asthma increases the likelihood of the development of depressive symptoms (Mrazek, 2003). The presence of these psychiatric symptoms may distract children's ability to perceive their asthma symptoms accurately or they may influence children's tendency to negatively skew their asthma symptoms. Psychiatric symptoms may also affect children's tendency to misperceive physiological sensations of anxiety as symptoms of asthma. We hypothesize that the presence of anxiety and depressive symptoms may compromise children's ability to accurately perceive their asthma symptoms. Anxiety and depressive symptoms, may separately, and in combination, impair children's asthma perception abilities.
The current study
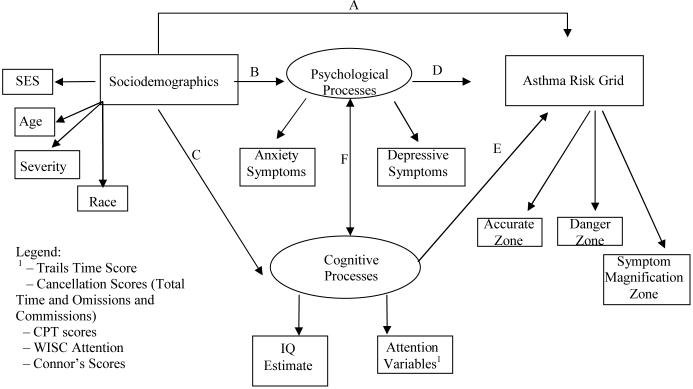
For the current study, associations among various cognitive-based factors (i.e., children's intelligence, different aspects of attention), psychosocial processes (i.e., children's levels of anxiety and depressive symptoms), and the perceptual accuracy of asthma symptoms were examined in a group of children using methods of structural equation modeling (SEM) (see Figure II). A variety of objective and parent-report attention-based instruments were employed to assess different aspects of attention that may have bearing on children's symptom monitoring abilities. Methods that compared children's subjective and actual estimates of lung function were used. This provides a characterization of children's perceptions of asthma symptoms. By this technique, participants provided repeated assessments of pulmonary function in which they estimated their degree of compromise. The overall pattern of results reveals the extent to which children's perception of the severity of asthma symptoms are actually accurate.
Figure II.
Hypothesized Model Testing Associations Between Cognitive and Psychological Processes and Children's Asthma Grid Zone Scores
It was expected that higher IQ estimate scores and lower levels of attentional problems, as well as lower levels of depressive symptoms and anxiety, would be associated with more accurate perception of asthma symptoms. Based on previous research (Strunk et al., 1985), we hypothesized that a pattern of underestimating asthma symptoms (dangerous symptom perception) would be associated with higher levels of depressive and anxiety symptoms. It was also expected that lower IQ estimate scores and attentional skills would be related to dangerous symptom perception. We anticipated that higher levels of anxiety and depressive symptoms would be associated with more symptom magnification, due to the potential for an increased focus on symptoms in children with internalizing problems. We had no a priori hypotheses regarding symptom magnification and cognitive/attentional abilities.
Methods
Participants
Data for this study were collected as part of a larger project assessing methods of perceptual accuracy and correlates of symptom perception in children with asthma(Fritz et al., 2005). The sample included 270 children with physician-diagnosed asthma and their caregivers. In the majority of cases the participating caregiver was the mother (91%), although in some cases it was the father (6%) and grandmother (3%). Demographic characteristics of the study sample are presented in Table I.
Table I.
Demographic Characteristics of Participants
| Variable | % of sample | Mean | SD | Range |
|---|---|---|---|---|
| Child's age | 11.8 years | 2.4 years | 7.0 - 17.0 years | |
| Child's gender | ||||
| Female | 46% | |||
| Male | 54% | |||
| Child's race/ethnicitya | 65% White, Non-Hispanic | |||
| 20% Black, Non-Hispanic | ||||
| 6% Hispanic | ||||
| 1% American Indian | ||||
| 8% Biracial | ||||
| Socioeconomic Status (Occupational Prestige) |
50.89 | 14.50 | 20.05(maid)- 86.05(physician) |
|
Design and Procedures
Data collection for this study occurred at three sites: Brown University Medical School (RI), University of Texas Medical School (Tyler, TX), and National Jewish Medical and Research Center (CO) (22 of the participants were enrolled at the RI site, 21% at the Texas site, and 57% at the Colorado site). The Institutional Review Board at each participating site approved the study. Participants were recruited using several different sources including the waiting areas of urgent care clinics, primary care and asthma specialty practices, an asthma summer camp, and advertising. The following conditions were required for study eligibility: 1) child was between the ages of 7 and 17 years old; 2) child had physician-diagnosed asthma for at least six months prior to study participation, 3) child had an active prescription for a brochodilator such as Albuterol, and 4) child and parent were able to complete the protocol in English. Participants completed two research sessions separated by a five to six week period. During the initial session, parents and children provided informed consent and assent. Clinician-trained research assistants administered cognitive and psychosocial assessments to children in an interview-based format. Adult participants completed parent-report measures. Measurements were administered in the same order to all participants. Assignment of a computerized hand-held spirometer (“AMII”, Jaeger) that measured peak expiratory flow rate (PEFR) occurred at the end of the first visit. A trained research assistant instructed participants on the proper use of the AMII using a standardized script and coached children to perform valid spirometric maneuvers until the proper technique was achieved. Subjects were instructed to use the device for five to six weeks, twice a day, plus whenever they were experiencing asthma symptoms. Participants received periodic phone prompts to encourage daily use of the device. At the end of this period families participated in a second research session to complete a follow-up assessment of functional morbidity and return the AMII device. Each child-parent dyad received $75 for their participation in the study.
Measures
All variables listed were measured during the initial research session. For the IQ estimate and attention scores from the WISC subscales, we utilized scaled scores and for the Trails and Cancellation tasks, we converted the time and accuracy (error) scores to standardized z scores based on current available norms that took into consideration children's age.
Demographic Variables
A demographic questionnaire was created for this study and administered to the parent to assess key demographic variables.
Asthma Severity
Severity of asthma was assessed by a study physician who classified each participating child as mild intermittent, mild persistent, moderate persistent, or severe persistent according to National Heart Lung and Blood Institute (NHLBI) guidelines (National Heart Lung and Blood Institute, 1997). Severity ratings were based on children's prescribed asthma medications plus parent report of the child's symptoms and health care utilization in the 12 months prior to the date of the initial study session. A range of severity levels was represented (mild intermittent 7%; mild persistent 61%, moderate persistent 24%, severe persistent 8%).
Socioeconomic Status
Occupational prestige was used as an indicator of socioeconomic status, as it is highly correlated with total family income in the past year (Nakao & Treas, 1992). In the absence of information on family's income, each parent's occupation was coded using the National Opinion Research Council (NORC) coding system (Nakao & Treas, 1992). The highest rating for the family parental occupation was used, as male and female participants' prestige scores did not differ. Higher scores indicate more prestigious jobs.
Cognitive Variables
Estimate of Children's Intelligence
Scores from the Vocabulary and Block-design subtests from the Wechsler Intelligence Scale for Children –Third Edition (WISC–III; Wechsler, 1991) were combined and used to estimate intelligence. This combination of subtests provides an accurate short-form estimate of general intelligence (Sattler, 1982). The WISC-III Vocabulary subtest consists of 32 words which the child defines. On the Block Design subtest, the child is instructed to assemble various designs with colored blocks from pictures of the desired designs. The items on this subtest are timed and points are given based on accuracy and speed.
Children's Attention
A variety of measurements were used to assess children's attention.
Cancellation tasks
Letter, number and symbol cancellation tasks were implemented in this study. These tasks assess specific aspects of children's attention, such as the capacity for sustained attention, visual scanning, and activation and inhibition of rapid responses(Rudel et al., 1978). Children were asked to scan each document and mark an X on all of the specific designated targets (the letters LIF, the numbers 592, a symbol of a diamond). Normative data for these tasks are available with adequate reliability and validity data reported (Rudel et al., 1978). The total time and error scores (omissions and commissions) were used for this study. Time and Error scores on these tasks were determined according to instrument specifications (time to complete the task and number of errors made on the task).
Trail Making Test
Part B of this task (the intermediate form for children aged 9-14), from the Halstead-Reitan Neuropsychological Test Battery, was used. This task assesses visual attention, and visual conceptual and visuomotor tracking (Reitan, 1986). Norms for this task are available (Lezak, 1976). Participants were asked to draw lines to connect consecutively numbered and lettered circles by alternating between the two sequences. Children were urged to connect the circles “as fast as you can” without lifting the pencil from the paper. Errors were pointed out as they occurred. The scoring of this test was based solely on total time to completion, as this is the most common scoring system used (Spreen, 1991). Reported reliability coefficients are good to excellent (Ernst et al., 1987).
Continuous Performance Task
The Conners' Continuous Performance Test (CPT), which is a computer delivered test designed to measure attention difficulties in children aged 6 to adult (Connors, 1995), was administered to children. Children are required to press the space bar immediately following the presentation of a target letter (any letter other than X) while refraining from pushing the button when non-target letters are presented (the letter X). These stimuli (target and non-target letters) are presented in variable time intervals. Raw scores, converted T-scores (mean of 50 and a S.D. of 10), and percentile scores can be provided for numerous scales. For this study, we examined the CPT Total index score, which is an overall summary score, the Commissions T-Score (the commissions score represents the number of times the person incorrectly responded to a non-target letter (“X”) and the Omissions T-Score (the omissions score represents the number of target letters to which the child did not respond). For a detailed explanation of how scores on this task are calculated and a description of normative data available, see Conners' Continuous Performance Test Manual (Connors, 1995).
WISC Attention
Scaled scores from the Arithmetic and Digit-Span subtests from the Wechsler Intelligence Scale for Children –Third Edition (WISC–III; Wechsler, 1991) were combined and used to estimate an index of short-term attention for this study. For the Digit-Span subtest, children were asked to repeat a dictated series of digits (e.g., 4 1 7 9) forwards and another series backwards. Each series begins with two digits and keeps increasing in length, with two trials at each length. For the Arithmetic subtest, children were orally presented with verbally framed math applications problems without paper or, for most problems, any visual aids at all.
Conners' Behavior Report
The Conners' Parent Rating Scale-Revised, Short Form (Connors, 2000) is a norm-based behavior rating scale used to assess areas of attention, conduct, cognition, family, social problems, academics, perfectionism, emotion, anger control, and anxiety in children aged 3-17 (Connors, 2000). Responses are provided by use of a Likert-type scale ranging from 0 (not true at all) to 3 (very much true). Conners' Parent Rating Scale raw scores are converted to T-scores (mean of 50 and a S.D. of 10). In this study, the following subscale variables were used: Oppositional, Cognitive Problems/Inattention, Hyperactivity, and ADHD (Connors, 2000). Internal consistency reliability coefficients for Conners' Parent Rating Scale range from .67 to .95 on the subscale level, the total internal reliability coefficients for the subscales range from .73 to .94.
Psychosocial Variables
Children's levels of anxiety were measured by the Multidimensional Anxiety Scale for Children (MASC; March et al., 1997), which is a standardized 39-item self-report measure yielding four factor scores. Each item is rated on a 4-point Likert-type response scale ranging from 0 (never true) to 3 (often true). The four factor scales include Social Anxiety (9 items), Separation Anxiety (9 items), Harm Avoidance (9 items), and Physical Symptoms (12 items). For this study, a total score for anxiety symptoms was used, higher scores reflecting higher levels of anxiety. Adequate reliability for this scale has been reported (March et al., 1997). Cronbach's alpha for this sample was .90.
Children's current levels of depressive symptoms were assessed by the Children's Depressive Inventory (CDI) (Kovacs, 1981, 1982), a 27-item self-report instrument, measuring cognitive, affective, and behavioral symptoms of depression in children and adolescents. Each item consists of three statements graded in order of increasing severity from 0 to 2. Participants select one sentence from each group that best describes themselves for the past 2 weeks. In nonclinical populations, the measure has relatively high levels of internal consistency, test-retest reliability, predictive utility, convergent, and construct validity (Carey et al., 1987). Cronbach's alpha for this sample was .85. In this study, raw total scores were converted to a T-score.
Assessment of Symptom Perception
The Asthma Risk Grid
The Asthma Risk Grid (Fritz et al., 1996; Klein et al., 2004), which was developed as a clinical tool to compare patients' subjective estimates of asthma symptoms with objective lung function data, was used to assess children's symptom perception (see Figure 1). The Asthma Risk Grid was specifically developed for children with asthma; however, it was adapted from methods used in the blood glucose estimation literature (see Cox et al., 1985). During the initial study visit children and caregivers were oriented to the use of the AM2. Using a standard study script and printed instructions (available upon request), a trained research assistant first demonstrated and then coached children to blow into the device using maximal sustained effort. Children practiced with feedback from study staff until proper technique was achieved A small screen on the device prompted children to enter subjective assessments of their pulmonary functioning at that moment in the form of a peak flow guess (‘guess your peak flow’). Children scrolled up by tens through a numeric scale to the value of their peak flow guess. Once entered the guess was locked into the device memory and could not be altered. Children were then cued to complete three successive “blows” into the device. The highest Peak Expiratory Flow (PEF) value of the three blows was saved with the corresponding subjective responses taken during the five to six week interval between assessments. Assessments were downloaded once the device was returned to the lab.
Figure I. Asthma Risk Grid (Klein et al., 2004).
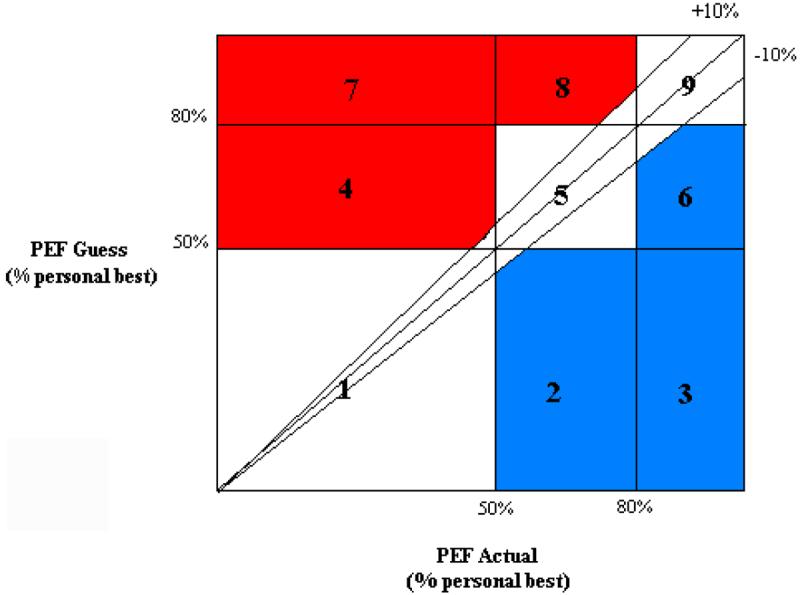
Accurate Zone: boxes 1, 5, 9 & +/− 10% wedge. The Accurate Zone of the Risk Grid includes: a wedge in which the estimate is +/−10% of the actual value; boxes 1 and 5 in which compromise below 50 and 80% of personal best is recognized as such; and box 9 in which adequate function (above 80% of personal best) is recognized.
Danger Zone: boxes 4, 7 & 8. The Danger Zone of the Risk Grid includes : points that fall in boxes 4, 7, and 8, where clinically significant compromised function (below 80% of personal best) is not recognized by the patient.
Symptom Magnification Zone: boxes 2, 3 & 6. The Symptom Magnification Zone includes boxes 2, 3, and 6 and reflects oversensitivity to minor symptoms or exaggeration of symptoms.
Subjective and objective peak flow values were plotted on the Asthma Risk Grid for each participant (Klein et al., 2004) (See Figure 1). Subjective estimates represented children's guess of the impending peak flow value and is depicted by the vertical axis on the Risk Grid. The objective lung function data, indicated on the horizontal axis of the Risk Grid, is represented by children's actual expiratory peak flow value. Values were converted to “percent-of-personal best” units (by dividing the highest PEFR value obtained during the data collection period and multiplying by 100) and represented comparisons of subjective and objective estimates of each child's lung function. Resulting points were characterized as accurate (the accurate zone included a subjective assessment which corresponded appropriately to the objective clinical status), dangerous (the danger zone included points falling in clinically significant compromised function) or magnification of symptoms (the symptom magnification zone included points reflecting oversensitivity to minor or no symptoms). For each child, scores for these three endpoints represented the proportion of blows that ended up in each zone (representing the difference between children's subjective and objective peak blow values). Each zone of the Asthma Risk Grid will be the endogenous variables of interest for this study. Children used the devices an average of 31 out of 46 assigned days. On average, approximately 42 data points were collected per child.
Results
Descriptive Data and Data reduction efforts
Demographic variables describing the study sample were created and are presented in Table I. Table II presents the mean, standard deviation and range of each measure in the current study. Data transformations were applied to variables unlikely to conform to assumptions of normality and homogeneity of variance. Probit transformations were applied to all Asthma Grid zone scores (Accurate Zone, Symptom Magnification Zone, and Danger Zone) to normalize their distributions (Cohen, 1983). Raw Zone scores were included for descriptive purposes. Specific data cleaning procedures for the AM2 were applied and are available upon request. The proportion of missing data from predictor variables is listed in Table II. Site comparisons indicated that basic demographic indicators did not differ across locations.
Table II.
Descriptive Summary of Data for Study Variables
| Variable | Mean (SD) | Range in sample |
Proportion of Missing Data |
|---|---|---|---|
| Cognitive Variables: | |||
| Estimate of IQ (scaled scores) | 9.73 (2.8) | 2.0-17.5 | 1% |
| Letter Canc. Task: Time z- score | −.002 (1.1) | −8.6-2.3 | 4% |
| Letter Canc. Task: Total Errors z- score | 23 (.89) | −3.0-2.8 | 4% |
| Number Canc. Task: Time z- score | −.02 (1.1) | −4.8-2.1 | 4% |
| Number Canc. Task: Total Errors z- score | .10 (.94) | −6.0-2.5 | 4% |
| Symbol Canc. Task: Time z- score | .08 (1.9) | −15.9-2.8 | 5% |
| Symbol Canc. Task: Total Errors z- score | .35 (.82) | −1.3-3.0 | 5% |
| Trails B Time Score z- score | −1.1 (1.9) | −15.9-1.66 | 6% |
| CPT Total Index Score | 9.0 (6.6) | 0-30 | 4% |
| CPT Commissions T-Score | 47.8 (10.3) | 19-75 | 4% |
| CPT Omissions T-Score | 42.0 (8.6) | 15-72 | 4% |
| WISC Attention (scaled scores) | 9.5 (2.7) | 1-18 | 2% |
| Connors Behavior Report T-Scores | 4% | ||
| Oppositionality | 53.9 (11.2) | 31-90 | |
| Cognitions | 53.8 (11.6) | 41-90 | |
| Hyperactivity | 56.0 (11.4) | 42-90 | |
| ADHD | 54.6 (10.7) | 40-90 | |
| Psychological Variables | |||
| Anxiety | 47.4 (18.4) | 9-95 | 6% |
| Depressive Symptoms (T-score) | 44.5 (9.6) | 0-82 | 2% |
We constrained analyses to include data from participants who completed the AMII procedures. All outcome data (GRID scores) for this sample were complete. Participants were excluded from the dataset if they had fewer than 20 subjective/objective paired recordings across the five week symptom assessment period, as extensive data examination judged “20” to be the minimum number necessary to provide reliable estimates of summary index scores. Thirty-three participants were excluded from the dataset based on this criterion. These participants' age, socioeconomic status, ethnicity, and asthma severity level did not significantly differ from the participants' demographic data in the final sample.
For the subjects whose data remained (N=270), Risk Grids were applied to the data as described. Results indicate that across all participants, the average number of blows were higher in the accurate zone (M = 72.7%, range = 3.3 - 100%), than in the symptom magnification (M = 19.2%, range = 0 - 96.7%), and danger zone (M = 8.3%, range = 0-78.1%). It is noteworthy that there was a considerable range of scores across participants and zones.
Statistical Analysis Plan
The predictor variables included in our analyses were the cognitive (the IQ estimate and all of the attention scores) and psychological constructs (anxiety and depressive levels). Given that the purpose of this paper was to determine which predictors best explained the variance in the different Asthma Grid Zone scores, each Grid Zone score functioned as a distinct outcome variable (e.g., the proportion of blows children had in the accurate, symptom magnification, and danger zone). Thus, as described in more detail below, three separate models including each Grid zone score were tested separately. Analyses proceeded in three stages. First, Pearson product moment correlations were conducted to determine whether key demographic variables (e.g., child's age, gender, race, socioeconomic status, severity level) were potential confounders in our subsequent analyses. Results indicated that each demographic variable was significantly related to more than one predictor variable and at least one outcome variable (e.g., the grid scores). Thus, each demographic variable met our criteria for inclusion in subsequent analyses.
Structural equation modeling (Muthen & Muthen, 2004) was used to test the hypotheses of the study, which are captured by Figure II. Initially, the measurement model was evaluated to determine whether our hypothesized latent constructs fit the data well (i.e., the relations between the observed measures indicated in rectangles in Figure II with the latent variables indicated by ovals in Figure II). This was followed by evaluation of whether the association among the latent constructs fit the data well (i.e., the paths denoted by labels A thru F). To summarize, advantages of this method are twofold for the specific purposes of this report. First, SEM allows for the analysis of latent variables (akin to factor scores), which reduces measurement error in estimation of core constructs and provides an efficient means of data reduction. Second, SEM allows for the examination of more complex models, with an overall evaluation of those models. SEM provides methods to achieve more precise measurement accuracy (i.e., less measurement error) in a manner that is statistically sound.
Evaluating the Measurement Model
The measurement model for relevant latent constructs summarizing psychological and cognitive variables was tested in Mplus (3.0). The results indicated that a three-construct model provided a good fit to these data (χ2s(df = 32, N= 270) = 39.05, ps < .18); comparative fit index [CFI] = 0.98; Tucker Lewis Index [TLI] = 0.99. root mean square error of approximation [RMSEA] = 0.03). [A good fitting model includes a CFI and TLI closest to one and an RMSEA closest to zero, which indicates that the hypothesized model did not significantly differ from the data]. It should be noted that the chosen goodness of fit measures were used because the Chi Square statistic is influenced by sample size, such that in small samples there is a risk of accepting a model that truly does not fit the data. While testing models with large samples, on the other hand, there is a risk of rejecting a model that does fit the data. The sample size of the current study is considered an adequate size to conduct Structural Equation Modeling.
The three latent factors that fit the data were: (a) Latent Factor One or Speed of Processing: the standardized time scores from the Trails and Cancellation Tasks; (b) Latent Factor Two or Cancellation Errors: the standardized error scores (commissions and omissions) from the Cancellation Tasks; and (c) Latent Factor Three or Continuous Performance Scores: the total index score and the commission and omission scores from the Continuous Performance Task. All loadings for these indicators were significant. The results of this analysis indicated that the psychological variables did not provide a good fit to this model. In addition, correlations between psychological and cognitive factors included in the models are shown in Table III.
Table III.
Intercorrelations Among Study Predictor Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Est. IQ (SS) | -- | .08 | −.21* | .23 | −.03 | .14* | −.08** | .31** | −.20* | −.24** | −.04 | .55** | −.15* | −.29** | −.28** | −.27** | −.13 | −.17* |
| 2. Letter Canc. Task: Time (z-score) | -- | .13 | −.68** | −.04 | .52** | 0.8 | .18* | −.15* | −.10 | .01 | .20* | −.19* | −.18* | −.14* | −.22** | −.12 | .04 | |
| 3. Letter Canc. Task: Errors (z-score) | -- | .01 | −.12 | .08 | .16* | −.02 | .04 | .15* | .06 | −.10 | .01 | .10 | .11 | .10 | .10 | .08 | ||
| 4. # Canc. Task: Time (z-score) | -- | .02 | .43** | .08 | .24** | −.71** | −.14* | −.08 | .26** | −.15* | −.22** | −.15* | −.22** | −.18* | .09 | |||
| 5. # Canc. Task: Errors (z-score) | -- | −.07 | −.09 | .04 | .06 | −.09 | −.04 | .05 | −.06 | −.02 | −.03 | −.07 | −.05 | −.03 | ||||
| 6. Sym. Canc. Task: Time (z-score) | -- | .23** | .15* | −.07 | −.06 | −.06 | .19* | −.04 | −.14* | −.06 | −.12 | −.08 | −.05 | |||||
| 7. Sym. Canc. Task: Errors (z-score) | -- | .06 | .10 | −.05 | −.06 | .02 | .07 | .01 | .05 | .05 | .01 | .02 | ||||||
| 8. Trails B Time (z-score) | -- | −.09 | −.11* | −.08 | .37** | −.07 | −.26** | −.14 | −.20* | .00 | −.07 | |||||||
| 9. CPT Total Index (T-score) | -- | .56* | .21* | −.21* | .16* | .03 | .20* | .12 | .05 | .08 | ||||||||
| 10. CPT Omm. (T-score) | -- | .37** | −24** | .15* | .25* | .19* | .26* | .16* | .05 | |||||||||
| 11. CPT Comm. (T-score) | -- | −.09 | .06 | .14* | .07 | .12* | .06 | .07 | ||||||||||
| 12. WISC Attention. (SS) | -- | −.12 | −.29** | −.21* | −.24** | −.14 | −.12 | |||||||||||
| 13. Connors Opp. (T-score) | -- | .54** | .60** | .64** | .14* | .15* | ||||||||||||
| 14. Connors Cog. (T-score) | -- | .55** | .90** | .29** | .24** | |||||||||||||
| 15. Connors Hyper. (T-score) | -- | .70** | .18* | .16* | ||||||||||||||
| 16. Connors ADHD (T-score) | -- | .28** | .27** | |||||||||||||||
| 17. Anxiety | -- | .27** | ||||||||||||||||
| 18. Depressive Symp. (T-score) | -- |
Evaluating the Structural Model
Following evaluation of the measurement model, we then turned to evaluating whether the cognitive and psychological factors predicted zone scores (as depicted by Figure II). Three comparable models, each including one of the grid zone scores as the dependent variable were tested. All demographic variables were initially including during each model building process. The latent variables emerging from the measurement model evaluation (described in the section above) were predictors in these three models. Demographic variables that contributed to the overall model fit, and were significantly associated with the grid zone score, were included in the final structural model. An example of one of the three final structural models is represented by Figure III (which depicts the final model containing the Accuracy Grid Zone Scores). Each of the final structural models represented the best fit of the data to explain differential outcomes in each of the Asthma Risk Grid zone scores. Thus, only predictors that significantly accounted for variance in the Asthma Risk Grid zone scores are included in each of the three final models described below. The psychological constructs were included during the initial stages of the structural equation modeling analysis. It was during the development of the final models (described below) where we found that these variables did not improve model fit, and they were removed from each of the final models including each Asthma Grid Zone Score.
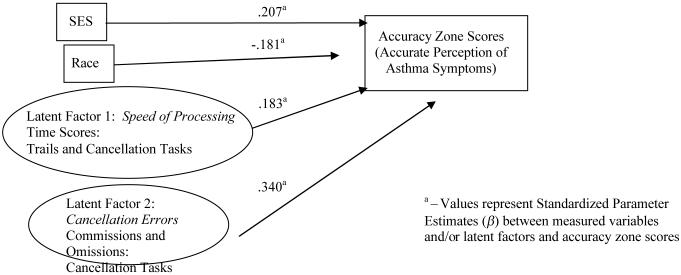
Figure III.
Final Structural Model for Significant Predictors Associated with Children's Accuracy Zone Scores
Model including Proportion of Blows in the Accurate Zone
We first tested the hypothesized model including children's symptom perception scores in the accurate zone as the dependent variable, all of the demographic variables (site, age, gender, race, ses, and severity), and the cognitive and psychological predictors. As indicated in Figure III, the model that best fit the data to explain differential outcomes in the accurate zone scores included SES and race, and Latent Factors One (Speed of Processing) and Two (Cancellation Errors), (χ2s(df = 28, N= 270) = 31.43, ps < .29); [CFI] = 0.99; [TLI] = 0.98. [RMSEA] = 0.02). These predictors accounted for 22% of the variance in the accurate zone scores (r= .46, a medium to high effect size according to (Cohen, 1983). The unstandardized and standardized parameter estimates are presented in Table III. All the model-estimated loadings for the manifest variables were significant. Occupational prestige was positively associated with a greater percentage of scores in the accurate zone, and race was negatively associated with accurate symptom perception (ethnic minority children had lower accuracy scores). The more time taken and fewer errors made on the Trails and Cancellation tasks were significantly associated with more accurate symptom perception scores.
Model including Proportion of Blows in the Symptom Magnification Zone
The model that best fit the data to explain variations in the symptom magnification zone scores included the following predictors; socioeconomic status, race, and Latent Factor One (Speed of Processing) and Two (Cancellation Errors), (χ2s(df = 53, N= 270) = 58.77, ps < .27); [CFI] = 0.98; [TLI] = 0.98. [RMSEA] = 0.02). These predictors accounted for 20% of the variance in the accurate zone scores (r= .45, a medium to high effect size according to (Cohen, 1983). The unstandardized and standardized parameter estimates are presented in Table III. All the model-estimated loadings for the manifest variables were significant. Occupational prestige and race were positively associated with a greater percentage of scores in the symptom magnification zone (ethnic minority children had more scores in the symptom magnification zone). The more time taken on the Trails and Cancellation tasks were significantly associated with more symptom magnification. Finally, the more errors made on these tasks were associated with fewer scores in the symptom magnification zone.
Model including Proportion of Blows in the Dangerous Zone
The model that best fit the data to explain variations in the danger zone scores included the following predictor; Latent Factor Three (Continuous Performance Task Scores), (χ2s(df = 39, N= 266) = 48.19, ps < .18); [CFI] = 0.98; [TLI] = 0.97. [RMSEA] = 0.03). This predictor accounted for 5% of the variance in the accurate zone scores (r= .21, a small to medium effect size according to (Cohen, 1983). The unstandardized and standardized parameter estimates are presented in Table III. All the model-estimated loadings for the manifest variables were significant. Higher total scores and more omission and commission errors on the Continuous Performance Task were positively associated with more blows in the danger zone.
Discussion
Given the centrality of child symptom perception to asthma management, the primary aim of this paper was to examine the differential effects of a variety of psychological and cognitive variables on children's perception of asthma symptoms. Consistent with our hypothesis involving the link between better attentional skills and more accurate symptom monitoring, our results showed that the time and error scores from the Trails and Cancellation tasks strongly predicted children's perceptual accuracy of symptoms (as reflected by pathway E in our Initial Hypothesized Model: Figure II). The more time children took on these tasks and the fewer errors they made on these tasks, the more likely they were to accurately perceive their asthma symptoms. These results suggest that the attention-based processes involving children's visual scanning, planning, cognitive flexibility as assessed by the trails and cancellation tasks, may help children to be more attuned to their asthma symptoms.
The more time that was taken on the Trails and Cancellation tasks was significantly associated with more scores in the symptom magnification zone, and more errors made on these tasks were associated with less symptom magnification. These results indicate that the use of a more careful approach or a perseverative manner may be associated with a tendency to overestimate the severity of symptoms. These findings also suggest that when children are more distracted by stimuli in their environment, they may be less likely to attend to or ruminate about their asthma symptoms, which may minimize the likelihood of overestimating the severity of asthma symptoms.
More errors that were made and higher total scores on the Continuous Performance Task were associated with a greater proportion of scores in the danger zone. When children were more disinhibited and haphazard, they may have had difficulty perceiving severe asthma symptoms that reflected compromised lung function. Commissions and ommisions made on the Continuous Performance Task occur when children identify the wrong target or miss the correctly identified target. Such attentional errors reflect poor perceptual sensitivity, which may place children who face severe symptoms at further risk for morbidity (Oades, 2000).
Statistical support for a significant contribution of children's estimates of intelligence or the psychological variables (anxiety and depressive symptoms reflected by pathway D in Figure II) on the different zone scores did not emerge. When the standardized IQ estimates, as well as the total scores for depressive symptoms and anxiety were added to the model, our hypothesized model fit less well to our data. This provides little evidence of their contribution to the models. It may be that depressive and anxiety symptoms have more of a bearing on other aspects of the asthma treatment process, such as medication adherence or health care utilization (e.g., what children do after they perceive symptoms) or other processes that influence effective asthma control (e.g., self-efficacy to manage symptoms, communication with family members, collaboration with health care provider).
Specific attention skills as assessed by the Trails, Cancellation, and Continuous Performance tasks may play a more significant role in children's ability to detect changes in respiratory functioning in comparison to their psychiatric symptoms or general cognitive ability. Our results suggest that careful attention to internal cues associated with pulmonary compromise is likely to be associated with accurate perception, and excessive attention may result in symptom magnification. These findings are consistent with the clinical impression that children who are more focused and careful may be likely to perceive their symptoms accurately, or, alternatively, they may be highly attuned to their symptoms and over-interpret them. Additionally, self-awareness abilities and attentional effort are executive skills that are related to frontal lobe function (Lezak, 1976). In general, the degree of frontal lobe involvement on a task is related to the degree of attentional effort. Therefore, it may be that children who exhibit better attentional skills may also be more attentive to internal cues and better at discriminating between stimuli. Further, although there is some overlap in the aspects of attention captured by the attention measurements of this study (e.g., both the Cancellation and CPT Tasks test for focused or selective attention abilities), there are unique distinctions in the processes measured by each instrument (e.g., the CPT Task also assesses sustained and divided attention abilities). Our findings suggest that specific attention processes represented by the error scores from the Cancellation tasks may be more relevant for accurately and over estimating symptoms and the specific attention processes represented by the error scores from the CPT Task may be more relevant for under magnifying symptoms.
Utility of the Study and its Findings
Research is still needed to investigate how children's individual characteristics may be related to their asthma symptom perception abilities. Identification of such differences is critical to the effective utilization of self-management training. This study utilized a multi-method approach including objective assessments of various attention constructs and a clinical tool to assess children's symptom perception; the Asthma Risk Grid. Specific measurements of objective, attention-based constructs were better proximal predictors of variation in children's perceptual accuracy abilities than our objective measurement of intelligence and our self-report measurements assessing current depressive or anxiety symptoms. These results lend credence to the use of objective measurements of attention as potential screening modalities for children who may be at risk for poor symptom perception and poor asthma control. Additionally, children who have ADHD may require more structure and support for asthma symptom monitoring. Children that have attention problems might have challenges in specific areas of asthma self-management. Therefore, clinicians and health care providers seeing a child with attention problems may appreciate knowing that this may serve as a risk factor for the symptom perception process.
Limitations and Future Directions
There were several limitations of this study that warrant attention. Although children's age did not contribute to the overall fit of our final models, children's ages did represent a wide range (7-17) in our sample. The association between children's perceptual accuracy of asthma symptoms and attentional skills at different age levels bears further examination in future work. Additionally, it is possible that children with attention problems were less attentive to the protocol (i.e., did not complete the task with focused attention), which could have enhanced the association between attentional skill and symptom perception accuracy. Further, despite the multiple procedures in place to ensure that children were properly trained in using the AMII, it was difficult to distinguish poor effort of the AMII from a poor value. Given that human subjects considerations dictated that children be able to see their actual peak flow after each blow, the possibility existed that children could, by attending to this information, become more accurate during the study monitoring period. However, a previous analysis of the relationship between subjective and objective PEFR in the first half of the study interval versus the second half indicated that no learning affect occurred (Fritz, et al., 1996).
We also did not include a clinical, psychiatric sample, so it is possible that children's anxiety and depressive symptoms were too low to make a meaningful contribution to our models. Although we gathered information on the types of medications children used for the clinician assessment of asthma severity level, we did not systematically enter this information to determine the proportion of children who were using long-term controller medications, nor did we examine children's medication adherence (preventive or as needed) and possible side effects of medications that can affect attention (Bender & Milgrom, 1992; Hederos, 2004). An additional limitation of the Asthma Risk Grid involves its summary of three variants of perceptual accuracy scores, which are non-independent in nature. In other words, an individual with a high proportion of scores in the accurate zone will, by definition, have fewer scores in the danger and symptom magnification zones. Despite these limitations, we find this model of quantifying symptom perception to be useful and clinically meaningful (Klein et al., 2004). Two of the final models indicated that both children's race and socioeconomic status were associated with children's tendency to accurately perceive or over magnify their asthma symptoms. Future studies with larger samples of children from diverse ethnic and socioeconomic backgrounds are needed to more deeply understand how experiences related to ethnicity and SES may be relevant for asthma symptom recognition. Finally, it is unclear whether the results of our study are sample-specific; therefore, additional research is needed to determine whether our findings can be replicated with other samples of children who have asthma.
These findings have practical significance in identifying specific attention-based factors that may impact variations in symptom monitoring, which can inform next steps for treatment. Although attention-based processes may play an important role in children's symptom perception abilities, they are but one element of the complex interplay between individual, family, environmental, and health care system characteristics that are likely implicated in this important aspect of the asthma self-management process.
Table IV.
Parameter Estimates and Associations between Predictor and Outcome Variables in Final Structural Models
| Latent Factor | Unstandardized Parameter Estimatesa |
S.E. | Est./S.E.b | Standardized Parameter Estimates StdYX |
|---|---|---|---|---|
| Model including Predictors of Scores in the Accurate Zone | ||||
| Sociodemographic Variables | ||||
| SES | .013 | .004 | 3.160 | .207 |
| Race | −.137 | .048 | −2.851 | −.181 |
| Latent Factor One: Speed | .318 | .152 | 2.088 | .183 |
| Trails Time Score | 1.000 | .000 | 0.000 | .274 |
| Cancellation Tasks: Time Scores | ||||
| Number Task | 1.658 | .413 | 4.015 | .758 |
| Symbol Task | 2.195 | .570 | 3.852 | .606 |
| Letter Task | 1.888 | .486 | 3.888 | .850 |
| Latent Factor Two: Canc. Err. | .910 | .322 | 2.824 | .340 |
| Cancellation Tasks: Omission and Commission Scores | ||||
| Number Task | 1.000 | .000 | 0.000 | .360 |
| Symbol Task | −.619 | .249 | −2.489 | −.254 |
| Letter Task | −3.214 | 1.321 | −2.432 | −.690 |
| Model including Predictors of Scores in the Symptom Magnification Zone | ||||
| Sociodemographics | ||||
| SES | −.012 | .004 | −2.595 | −.177 |
| Race | .150 | .052 | 2.881 | −.188 |
| Latent Factor One: Speed | −.369 | .167 | −2.202 | −.205 |
| Trails Time Score | 1.000 | .000 | 0.000 | .278 |
| Cancellation Tasks: Time Scores | ||||
| Number Task | 1.649 | .404 | 4.085 | .767 |
| Symbol Task | 2.148 | .551 | 3.899 | .603 |
| Letter Task | 1.840 | .465 | 3.957 | .843 |
| Latent Factor Two: Canc.Err. | −1.061 | .361 | 2.938 | −.364 |
| Cancellation Tasks: Omission and Commission Scores | ||||
| Number Task | 1.000 | .000 | 0.000 | .349 |
| Symbol Task | −.592 | .244 | −2.422 | −.235 |
| Letter Task | −3.478 | 1.383 | −2.516 | −.723 |
| Model including Predictors of Scores in the Danger Zone | ||||
| Latent Factor Three: | .034 | .013 | 2.749 | .214 |
| Continuous Performance Task Scores | ||||
| Total Index | 1.000 | .000 | 0.000 | .593 |
| Commissions | .886 | .143 | 6.199 | .449 |
| Omissions | 6.712 | 1.157 | 5.803 | .932 |
Estimates represent Factor Loadings for the Latent Factors and regression paths for the structural paths of the model. Unstandardized and standardized coefficients above can be interpreted in a similar manner to regression weights in multiple regression analyses.
When Estimated divided by Standard Error is greater than 1.96, the parameter is assumed to be significantly different than 0 at the .05 level.
Acknowledgments
Funds for this study were provided by Grant #2R01HL45157 (G. Fritz, P.I), NHLBI
References
- Annett RD, Aylward EH, Lapidus J, Bender BG, DuHamel T. Neurocognitive functioning in children with milk and moderate asthma in the childhood asthma management program. The childhood asthma management program (camp) group. Journal of Allergy and Clinical Immunology. 2000;105:717–724. doi: 10.1067/mai.2000.105226. [DOI] [PubMed] [Google Scholar]
- Baker RR, Mishoe SC, Zaitoun FH, Arant CB, Lucas J, Rupp NT. Poor perception of airway obstruction in children with asthma. Journal of Asthma. 2000;37:613–674. doi: 10.3109/02770900009090817. [DOI] [PubMed] [Google Scholar]
- Barkley R, Fischer M, Newty R, Breen M. Development of a multimethod clinical protocol for assessing stimulant drug responses in adhd children. Journal of Clinical Child Psychology. 1988;17:14–24. [Google Scholar]
- Bender B, Milgrom H. Theophylline-induced behavior change in children. An objective evaluation of parents' perceptions. Journal of the American Medical Association. 1992;267:2621–2624. [PubMed] [Google Scholar]
- Bender B, Annett RD, Ikle D, DuHamel TR, Rand C, Strunk RC. Relationship between disease and psychological adaptation in children in the childhood asthma management program and their families. Archives of Pediatrics and Adolescent Medicine. 2000;154:706–713. doi: 10.1001/archpedi.154.7.706. [DOI] [PubMed] [Google Scholar]
- Bennett D. Depression among children with chronic medical problems: A meta-analysis. Journal of Pediatric Psychology. 1994;19:149–169. doi: 10.1093/jpepsy/19.2.149. [DOI] [PubMed] [Google Scholar]
- Biederman J, Milberger S, Faraone SV, Guite J, Warburton R. Associations between childhood asthma and ADHD: Issues of psychiatric comorbidity and familiality. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:842–848. doi: 10.1097/00004583-199407000-00010. [DOI] [PubMed] [Google Scholar]
- Brown ES, Khan DA, Mahadi S. Psychiatric diagnoses in inner city outpatients with moderate to severe asthma. International Journal of Psychiatry in Medicine. 2000;30:319–327. doi: 10.2190/7U7P-EJYL-5BKG-6106. [DOI] [PubMed] [Google Scholar]
- Cabral AL, Conceicao GM, Saldiva PH, Martins MA. Effect of asthma severity on symptom perception in childhood asthma. Brazilian Journal of Medical and Biological Research. 2002;35:319–327. doi: 10.1590/s0100-879x2002000300006. [DOI] [PubMed] [Google Scholar]
- Carey MP, Faulstich ME, Gresham FM, Ruggiero L, Enyart P. Children's depression inventory: Construct and discriminant validity across clinical and nonreferred (control) populations. Journal of Consulting and Clinical Psychology. 1987;55:755–761. doi: 10.1037//0022-006x.55.5.755. [DOI] [PubMed] [Google Scholar]
- Carr R. Panic disorder and asthma: Causes, effects and research implications. Journal of Psychosomatic Research. 1998;44:43–52. doi: 10.1016/s0022-3999(97)00137-2. [DOI] [PubMed] [Google Scholar]
- Carr RE, Lehrer PM, Rausch LL, Hochron SM. Anxiety sensitivity and panic attacks in an asthmatic population. Behaviour Research and Therapy. 1994;32:411–418. doi: 10.1016/0005-7967(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Statistical power analysis for the behavioral sciences. Erlbaum; Hillsdale, NJ: 1983. [Google Scholar]
- Connors C. Conners' continuous performance test manual. Multi-Health Systems Inc; 1995. [Google Scholar]
- Connors C. Connors' rating scales- revised, Technical Manual. Multi-Health Systems Inc; 2000. [Google Scholar]
- Cox DJ, Clarke WL, Gonder-Frederick L, Pohl S, Hoover C, Snyder A, et al. Accuracy of perceiving blood glucose in iddm. Diabetes Care. 1985;8:529–536. doi: 10.2337/diacare.8.6.529. [DOI] [PubMed] [Google Scholar]
- Ernst J, Warner MH, Townes BD, Peel JH, Preston M. Age group differences on neuropsychological battery performance in a neuropsychiatric population: An international descriptive study with replications. Archives of Clinical Neuropsychology. 1987;2:1–12. [PubMed] [Google Scholar]
- Fritz G, Adams SK, McQuaid EL, Klein RB, Kopel SJ, Nassau J, et al. A comparison of resistive loading and in vivo approaches to assessing symptom perception in pediatric asthma; Paper presented at the International Society for the Advancement of Respiratory Psychophysiology; Hamburg, Germany. 2005. [Google Scholar]
- Fritz GK, McQuaid EL, Spirito A, Klein RB. Symptom perception in pediatric asthma: Relationship to functional morbidity and psychological factors. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1033–1041. doi: 10.1097/00004583-199608000-00014. [DOI] [PubMed] [Google Scholar]
- Galil N. Depression and asthma in children. Current Opinion in Pediatrics. 2000;12:331–335. doi: 10.1097/00008480-200008000-00008. [DOI] [PubMed] [Google Scholar]
- Gillaspy SR, L HA, Mullins LL, Van Pelt JC, Chaney JM. Psychological distress in high-risk youth with asthma. Journal of Pediatric Psychology. 2002;27:363–371. doi: 10.1093/jpepsy/27.4.363. [DOI] [PubMed] [Google Scholar]
- Hederos C. Neuropsychologic changes and inhaled corticosteroids. Journal of Allergy and Clinical Immunology. 2004;114:451–452. doi: 10.1016/j.jaci.2004.03.045. [DOI] [PubMed] [Google Scholar]
- Hudgel D, Kinsman R. Interactions among behavioral style, ventilatory drive, and load recognition. American Review of Respiratory Disease. 1983;128:246–248. doi: 10.1164/arrd.1983.128.2.246. [DOI] [PubMed] [Google Scholar]
- Katon W, Richardson L, Lozano P, McCauley E. The relationship of asthma and anxiety disorders. Psychosomatic Medicine. 2004;66:349–355. doi: 10.1097/01.psy.0000126202.89941.ea. [DOI] [PubMed] [Google Scholar]
- Klein RB, Walders N, McQuaid EL, Adams S, Yaros D, Fritz GK. The asthma risk grid: Clinical interpretation of symptom perception. Allergy and Asthma Proceedings. 2004;25:1–6. [PubMed] [Google Scholar]
- Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatrica. 1981;46:305–315. [PubMed] [Google Scholar]
- Kovacs M. Prediction of suicidal behaviors. Bibliotheca Psychiatrica. 1982;162 [PubMed] [Google Scholar]
- Lezak M. Neuropsychological assessment. Oxford University Press; New York: 1976. [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The multidimensional anxiety scale for children (masc): Factor structure, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- McQuaid EL, Kopel SJ, Nassau JH. Behavioral adjustment in children with asthma: A meta-analysis. Journal of Developmental and Behavioral Pediatrics. 2001;22:430–439. doi: 10.1097/00004703-200112000-00011. [DOI] [PubMed] [Google Scholar]
- McQuaid EL, Penza-Clyve S, Nassau JH, Fritz GK, Klein R, O'Connor S, et al. Sharing family responsibility for asthma management tasks. Children's Health Care. 2001;30:183–199. [Google Scholar]
- McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: Reasoning, responsibility, and behavior. Journal of Pediatric Psychology. 2003;28:323–333. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- McQuaid EL, Koinis Mitchell D, Walders N, Nassau JH, Kopel SJ, Klein RB, et al. Pediatric asthma morbidity: The importance of symptom perception and family response to symptoms. Journal of Pediatric Psychology. 2007;32:167–177. doi: 10.1093/jpepsy/jsj112. [DOI] [PubMed] [Google Scholar]
- Miller B. Depression and asthma: A potentially lethal mixture. The Joural of Allergy and Clinical Immunology. 1987;80:481–486. doi: 10.1016/0091-6749(87)90080-7. [DOI] [PubMed] [Google Scholar]
- Morrison KM, Goli A, Van Wagoner J, Brown ES, Khan DA. Depressive symptoms in inner-city children with asthma. Primary Care Companion to the Jounral of Clinical Psychiatry. 2002;4:174–177. doi: 10.4088/pcc.v04n0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrazek D. Psychiatric complications of pediatric asthma. Annals of Allergy. 1992;69:285–290. [PubMed] [Google Scholar]
- Mrazek D. Psychiatric symptoms in patients with asthma causality, comorbidity, or shared genetic etiology. Child and Adolescent Psychiatric Clinicals of North America. 2003;12:459–471. doi: 10.1016/s1056-4993(03)00028-2. [DOI] [PubMed] [Google Scholar]
- Muthen BO, Muthen L. Mplus user's guide. Muthen and Muthen; Los Angeles: 2004. [Google Scholar]
- Nakao K, Treas J. The 1989 socioeconomic index of occupations: Construction from the 1989 occupational prestige scores. GSS Methodological Report. 1992;74 [Google Scholar]
- National Center for Health Statistics Current estimates from the national health interview survey, 2002. Vital and Health Statistics. 2002;10 [Google Scholar]
- National Heart Lung and Blood Institute . Asthma statistics, data fact sheet. National Heart Lung and Blood Institute,.; Bethesda, MD: 1997. [Google Scholar]
- National Heart Lung and Blood Institute . Asthma statistics, data fact sheet. National Institute of Health; Bethesda, MD: 2002. [Google Scholar]
- Oades R. Differential measures of ‘sustained attention’ in children with attention-deficit/hyperactivity or tic disorders: Relations to monoamine metabolism. Psychiatry Research. 2000;93:165–178. doi: 10.1016/s0165-1781(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Ortega AN, Huertas SE, Canino G, Ramirez R, Rubio-Stipec M. Childhood asthma, chronic illness, and psychiatric disorders. Journal of Nervous and Mental Disorders. 2002;190:275–281. doi: 10.1097/00005053-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Ortega AN, McQuaid EL, Canino G, Ramirez R, Fritz GK, Klein RB. Association of psychiatric disorders and different measures of asthma in island puerto rican children. Social Psychiatry and Psychiatric Epidemiology. 2003;38:220–226. doi: 10.1007/s00127-003-0623-6. [DOI] [PubMed] [Google Scholar]
- Ortega AN, McQuaid EL, Canino G, Goodwin RD, Fritz GK. Comorbidity of asthma and anxiety and depression in Puerto Rican children. Psychosomatics. 2004;45:93–99. doi: 10.1176/appi.psy.45.2.93. [DOI] [PubMed] [Google Scholar]
- Pontius A, Yudowitz BS. Frontal lobe system dysfunction in some criminal actions as shown in the narratives te. The Journal of Nervous and Mental Disease. 1980;168:111–117. doi: 10.1097/00005053-198002000-00008. [DOI] [PubMed] [Google Scholar]
- Reitan R. Theoretical and methodological bases of the halstead-reitan neuropsychiatric test battery. In: Grant I, Adams K, editors. Neuropsychological assessment of neuropsychiatric disorders. Oxford University; New York: 1986. pp. 3–30. [Google Scholar]
- Rudel RG, Denckla MB, Broman M. Rapid silent response to repeated target symbols by dyslexic and nondyslexic children. Brain and Language. 1978;6:52–62. doi: 10.1016/0093-934x(78)90043-3. [DOI] [PubMed] [Google Scholar]
- Sattler J. Assessment of children's intelligence and special abilities. Allyn and Bacon, Inc; Boston: 1982. [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests : Administration, norms and commentary. Oxford University Press; New York: 1991. [Google Scholar]
- Strunk R, Mrazek DA, Wofson, Fuhrmann GS, LaBreque JF. Physiological and psychological characteristics associated with deaths due to asthma in childhood: A case-controlled study. Journal of the American Medical Association. 1985;254:1193–1198. [PubMed] [Google Scholar]
- Vila G, Nollet-Clemencon C, de Blic J, Mouren-Simeoni MC, Scheinmann P. Prevalence of dsm iv anxiety and affective disorders in a pediatric population of asthmatic children and adolescents. Journal of Affective Disorders. 2000;58:223–231. doi: 10.1016/s0165-0327(99)00110-x. [DOI] [PubMed] [Google Scholar]
- Wamboldt M, Fritz GK, Mansell A, McQuaid EL, Klein RB. Relationship of asthma severity and psychological problems in children. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:943–950. doi: 10.1097/00004583-199809000-00014. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children-revised. Psychological Corporation; New York: 1991. [Google Scholar]
- Weil CM, Wade SL, Bauman LJ, Lynn H, Mitchell H, Lavigne J. The relationship between psychosocial factors and asthma morbidity in inner-city children with asthma. Pediatrics. 1999;104:1274–1280. doi: 10.1542/peds.104.6.1274. [DOI] [PubMed] [Google Scholar]
- Yoos H, McMullen A. Symptom monitoring in childhood asthma: How to use a peak flow meter. Pediatric Annals. 1999;28:31–39. doi: 10.3928/0090-4481-19990101-09. [DOI] [PubMed] [Google Scholar]