Abstract
The interferon-induced, double-stranded RNA-dependent protein kinase (PKR) can play critical roles in inhibiting virus replication and inducing apoptosis. To develop new agents that may inhibit viral replication or induce apoptosis in cancer cells via the PKR signaling pathway, we screened a chemical library for compounds that have differential cytotoxic effects on wild-type [mouse embryonic fibroblast (MEF)/PKR(+/+)] and PKR-knockout [MEF/PKR(-/-)] mouse embryonic fibroblast cells. We identified a synthetic compound, BEPP [1H-benzimidazole1-ethanol,2,3-dihydro-2-imino-a-(phenoxymethyl)-3-(phenylmethyl)-,monohydrochloride], that induces a cytotoxic effect more effectively in MEF/PKR(+/+) cells than in MEF/PKR(-/-) cells. BEPP also relatively effectively inhibited the growth of a human lung cancer cell line overexpressing PKR, compared with other cancer cell lines. In sensitive cells, BEPP induced apoptosis with activation of caspase-3. Treatment with BEPP led to increased phosphorylation of PKR and eIF2α, increased expression of BAX, and decreased expression of Bcl-2. BEPP-induced apoptosis was PKR dependent and was blocked by the adenovector expressing the dominant-negative PKR. Furthermore, pretreatment of HeLa cells at a noncytotoxic dose of BEPP effectively inhibited Vaccinia virus replication. Together, our results suggest that BEPP and its analogs may induce PKR-dependent apoptosis and inhibition of viral replication and that they can be a potential anticancer or anti-virus agent.
The double-stranded RNA (dsRNA)-dependent protein kinase (PKR) is a ubiquitously expressed serine-threonine kinase that is dramatically induced by interferon-γ. Consisting of two dsRNA-binding domains at its N terminus and a conserved kinase domain at its C terminus, PKR is activated by binding to dsRNA or interaction with other proteins, which leads to dimerization and autophosphorylation of PKR (Williams, 2001). Activated PKR phosphorylates the α subunit of protein synthesis initiation factor eIF2 (eIF2α), leading to inhibition of protein synthesis and eliciting antivirus and antitumor activities. Moreover, PKR has been reported to induce apoptosis by modulating activities of eIF2α (Der et al., 1997), nuclear factor κB (Gil et al., 1999), activating transcription factor-3 (Hai and Hartman, 2001), and p53 (Garcia et al., 2006). Because of this, activation of PKR in tumor cells has been proposed as a modality for cancer treatment. We and others (Pataer et al., 2002; Emdad et al., 2007) have found previously that adenoviral-mediated overexpression of the melanoma differentiation-associated gene 7 induced apoptosis in cancer cells by activation of PKR. Blocking PKR activation inhibited adenoviral-mediated overexpression of the melanoma differentiation-associated gene 7-induced apoptosis, suggesting a critical role of PKR activation in such apoptosis. Likewise, PKR plays an essential role in apoptosis induced by tumor necrosis factor (Yeung et al., 1996) and oncolytic viruses (Gaddy and Lyles, 2007). Furthermore, tumor-specific activation of PKR by dsRNA molecules induced apoptosis of glioblastoma cells in vitro and suppressed glioblastoma in vivo (Shir and Levitzki, 2002). It is interesting that expression and autophosphorylation of PKR were increased in several types of cancer, including melanoma, colon cancer, and breast cancer (Kim et al., 2000, 2002).
The fact that PKR plays an important role in apoptosis induction and that its expression is increased in several types of cancers led us to hypothesize that a balance between increased PKR expression and increased antiapoptosis molecules may be established in cancer cells, preventing them from undergoing apoptosis, and that small molecules that can break this balance might be useful for selective induction of apoptosis in cancer cells that overexpress PKR. To search for such small compounds, we used PKR wild-type [PKR(+/+)] and PKR-knockout [PKR(-/-)] mouse embryonic fibroblasts (MEFs) to screen a chemical library from ChemBridge Research Laboratories, Inc. (San Diego, CA) for chemicals that can effectively kill PKR(+/+) MEFs but not PKR(-/-) MEFs. We found that the synthetic compound BEPP can induce cytotoxic effects more effectively in MEF/PKR(+/+) cells than in MEF/PKR(-/-) cells. The apoptosis induction by BEPP, including caspase activation and BAX overexpression, was PKR dependent and was abrogated when PKR activation was blocked with the dominant-negative PKR. Furthermore, we found that BEPP can dramatically suppress Vaccinia virus replication. Thus, BEPP could be a useful PKR inducer and antivirus agent.
Materials and Methods
Cell and Culture Conditions. The PKR wild-type MEFs [MEF/PKR(+/+)] and PKR-knockout MEFs [MEF/PKR(-/-)], provided by Dr. Glen Barber (University of Miami, Miami, FL), have been described previously (Yang et al., 1995). The human cervical cancer cell line HeLa and the human lung cancer cell lines H1299, H460, H226B, and A549 were maintained in our laboratory. The human bronchial epithelial (HBE) cell line was purchased from Clonetics (Walkersville, MD). All cells except for HBE cells were routinely cultured in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum, 100 units/ml penicillin, and 100 mg/ml streptomycin and maintained in the presence of 5% CO2 at 37°C. HBE cells were cultured in serum-free keratinocyte medium (Invitrogen, Carlsbad, CA).
Chemicals and Antibodies. A chemical library with 10,000 compounds, including BEPP and its analog BECC, was obtained from ChemBridge Research Laboratories, Inc.. The chemicals in the library were provided at a concentration of 5 mg/ml in dimethyl sulfoxide (DMSO). The chemical structure of BEPP is shown in Fig. 1. The compound was dissolved in DMSO to a concentration of 10 mM and stored at 4°C as a master stock solution. Antibodies to the following proteins were used for Western blot analysis: BAX, Bcl-2, PKR, cyclin D1, and caspase-3 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); phosphorylated-PKR (Millipore, Billerica, MA); eIF2α, phosphorylated eIF2α, phosphorylated JNK, phosphorylated p38, AKT, and phosphorylated AKT (Cell Signaling Technology Inc., Danvers, MA); and β-actin (Sigma-Aldrich, St. Louis, MO).
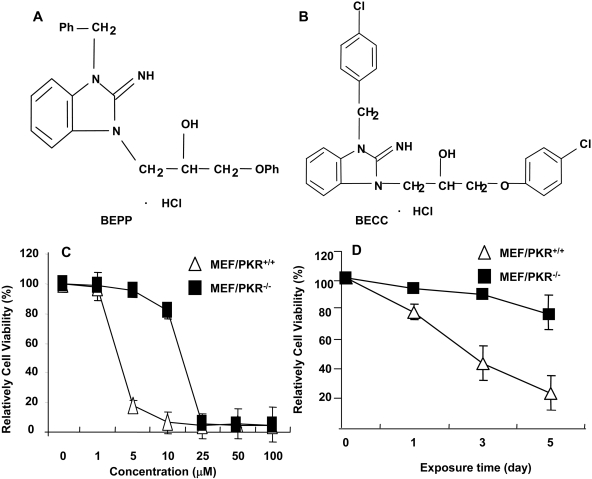
Fig. 1.
Chemical structure of BEPP (A) and its analog BECC (B). Effects of BEPP on proliferation of MEF/PKR(+/+) and MEF/PKR(-/-) cell lines. Cells were treated with various concentrations of BEPP for 72 h (C) or with 2.5 μM BEPP at the indicated time points (D). Cell viability was determined by using the SRB assay. Cells treated with DMSO were used as a control, with their viability set at 100%. Each data point represents the mean ± S.D. of three independent experiments.
Cytotoxicity Studies. The viability of the cell lines was determined using the sulforhodamine B (SRB) assay, as described previously (Pauwels et al., 2003). Cells (2-8 × 103 cells in 100 μl culture medium/well) were seeded in 96-well flat-bottomed plates and treated the next day with the agents at the indicated concentrations. After the indicated times, cells were fixed with trichloroacetic acid. The protein was stained with sulforhodamine B, and the optical density at 570 nm was determined. Relative cell viability was determined by setting the viability of the control cells (exposed only to DMSO) at 100% and comparing the viability of the treated cells with that of the controls. The experiments were performed at least three times for each cell line.
Flow Cytometric Analysis. For analysis of the intracellular DNA content, floating and attached cells treated with BEPP were harvested, washed twice in PBS, and fixed in 70% ethanol at 4°C overnight. For detection of apoptosis, fixed cells were suspended in PBS containing 10 μg/ml propidium iodide (Roche Diagnostics, Indianapolis, IN) and 10 μg/ml RNase A (Sigma-Aldrich) at 37°C for 30 min. Cell cycle analysis was performed using an Epics Profile II flow cytometer (Beckman Coulter, Fullerton, CA) with MultiCycle software (Phoenix Flow Systems, San Diego, CA). Accumulation of sub-G1 cells, a known indicator of DNA fragmentation and apoptosis, was used to quantify apoptosis. All experiments were repeated at least thrice.
Western Blot Analysis. For preparation of whole-cell extracts, cells were washed twice in cold PBS, collected, and lysed in lysis buffer (62.5 mM Tris, pH 6.8, 2% SDS, and 10% glycerol) containing 1× proteinase-inhibitor cocktail (Roche Diagnostics). The lysates were spun at 14,000g in a microcentrifuge at 4°C for 10 min, and the resulting supernatants were used as whole-cell extracts. Protein concentrations were determined by using the BCA protein assay kit (Pierce Chemical, Rockford, IL). Equal amounts (30-50 μg) of proteins were used for immunoblotting as described previously (Teraishi et al., 2003).
Viruses and Titer Analysis. The oncolytic Vaccinia virus also has been described previously (Guo et al., 2005). vSP is a modified Vaccinia virus that has deletions in two antiapoptosis serpin genes, SPI-1 and SPI-2 (Guo et al., 2005). Adenoviruses expressing the dominant-negative dsRNA-dependent protein kinase gene (Ad/PKRΔ6) were provided by Dr. Abujiang Pataer (The University of Texas M. D. Anderson Cancer Center, Houston, TX). The expansion, purification, titration, and quality analysis of both vectors were performed at the Vector Core Facility at M. D. Anderson Cancer Center as described previously (Fang et al., 1998). The titer used for Vaccinia virus in this study was the infectious units determined by the 50% tissue culture infectious dose (TCID50) assay (Fang et al., 1998).
Statistical Analysis. Differences among the treatment groups were assessed by analysis of variance using StatSoft statistical software (StatSoft, Tulsa, OK); p < 0.05 was regarded as significant.
Results
Library Screening for Compounds with Differential Cytotoxic Effects for MEF/PKR(+/+) and MEF/PKR(-/-) Cells. We used the MEF/PKR(+/+) and MEF/PKR(-/-) cell lines to screen for compounds that have differential effects on cell growth because of cellular PKR status. Cells seeded in 96-well plates in parallel were treated with each compound at a final concentration of approximately 5 μg/ml. Cells treated with DMSO (final concentration, 1%) were used as controls. A pilot experiment showed that 1% of DMSO did not have obvious impact on viability of the MEF cells. Changes in cell morphology were then observed under a microscope, and cell viability was determined by the SRB assay 2 to 4 days after treatment. The compounds that were initially observed to have differential effects on MEF/PKR(+/+), and MEF/PKR(-/-) cells underwent two additional screenings to confirm the observation. From 3000 compounds screened, we identified a compound (BEPP) that suppressed the growth of MEF/PKR(+/+) cells more effectively than that of MEF/PKR(-/-) cells (Fig. 1A). BEPP killed MEF/PKR(+/+) cells with an IC50 value of 1.4 μM, whereas its IC50 value on MEF/PKR(-/-) cells was approximately 17.4 μM (Fig. 1, B and C), approximately 10-fold higher than the value for MEF/PKR(+/+).
Induction of Apoptosis in MEF/PKR(+/+) by BEPP. To investigate whether the BEPP-mediated difference in cell viability between MEF/PKR(+/+) and MEF/PKR(-/-) cells was caused by suppression of cell growth or by induction of apoptosis, we examined the caspase activation in MEF/PKR(+/+) and MEF/PKR(-/-) cells treated with BEPP. Cells were treated with various concentrations of BEPP for 72 h, and cell lysates were subjected to 8 to 12% SDS-polyacrylamide gel electrophoresis, followed by Western blotting with caspase-3-specific antibodies. Cleavage of caspase-3 was easily detected at concentrations of 2.5 and 10 μM in MEF/PKR(+/+) cells but not in MEF/PKR(-/-) cells, indicating that BEPP can induce apoptosis in MEF/PKR(+/+) but not MEF/PKR(-/-) cells at these concentrations. Western blot analysis of BAX and Bcl-2 showed that, at a high concentration (10 μM), BAX expression was increased, whereas Bcl-2 expression decreased in MEF/PKR(+/+) cells after treatment with BEPP. The significance of those changes in BAX and Bcl-1 levels was not yet clear. However, treatment with BEPP had no detectable effect on Bax or Bcl-2 expression in MEF/PKR(-/-) cells (Fig. 2A).
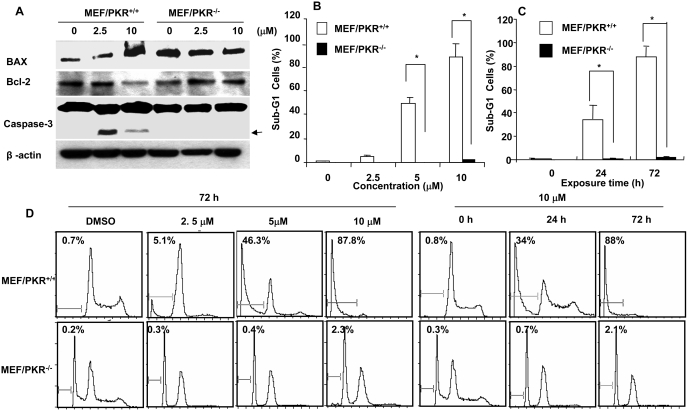
Fig. 2.
Induction of apoptosis in MEF/PKR(+/+) and MEF/PKR(-/-) cell lines. A, MEF/PKR(+/+) and ME/PKR(-/-) cells were treated with the indicated concentrations of BEPP for 72 h, and cell lysates were analyzed by Western blotting. Activation of caspase-3 and expression of Bax and Bcl-2 were determined. β-actin was used as a loading control. Arrowheads, cleaved proteins. B, percentage of cells in sub-G1 phase after treatment with the indicated concentrations of BEPP for 72 h. Each data point represents the mean ± S.D. of data from one of two experiments with similar results. C, percentage of apoptotic cells with 10 μM BEPP at the indicated times. *, p < 0.01. D, histograms derived from flow cytometric analysis.
The apoptosis induction by BEPP in MEF/PKR(+/+) was further confirmed by fluorescence-activated cell sorting analysis. We determined the percentage of apoptosis after MEF/PKR(+/+), and MEF/PKR(-/-) cells were treated with different doses of BEPP for 72 h or after the cells were treated with 10 μM BEPP for different times. Cells were then harvested for quantification of apoptotic subdiploid cells by flow cytometry (Fig. 2, B and C). At 24 and 72 h after treatment with 10 μM BEPP, 32.3 and 84.6% of MEF/PKR(+/+) cells, respectively, were in sub-G1 phase. Likewise, dramatic increases of the sub-G1 portion in MEF/PKR(+/+) cells were observed at doses of 5 and 10 μM. In contrast, only background levels (less than 5%) of sub-G1 cells were seen in MEF/PKR(-/-) cells under the same treatment conditions (Fig. 2, B and C). These results demonstrated that BEPP can induce apoptosis in MEF/PKR(+/+) in a dose- and time-dependent manner.
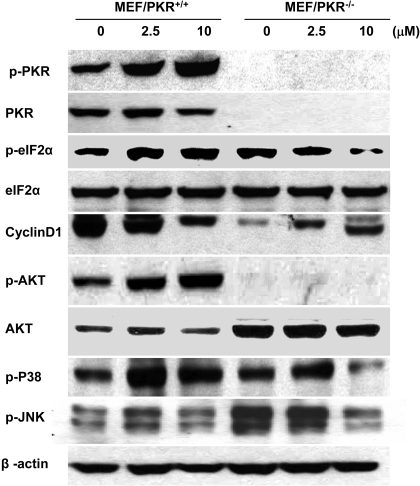
PKR Activation Is Required for BEPP-Induced Apoptosis. The induction of apoptosis observed in MEF/PKR(+/+) but not MEF/PKR(-/-) cells suggested a role for PKR pathways in BEPP-induced apoptosis. Therefore, we investigated the changes in PKR, eIF2α, and cyclin D1 after BEPP treatment. Cells were treated with various concentrations of BEPP for 72 h, and then lysates were analyzed by Western blotting. Control cells were treated with DMSO. The results showed that treatment of MEF/PKR(+/+) cells with BEPP increased the amounts of phosphorylated PKR and eIF2α, which were easily detectable at the concentration of 2.5 μM (Fig. 3). Levels of phosphorylated PKR after treatment of BEPP at the doses of 2.5 and 10 μM were increased approximately 2- and 3.5-fold, respectively, when determined by a densitometric analysis and normalized with that of β-actin (Supplemental Fig. 1). In contrast, treatment with BEPP did not result in detectable changes of phosphorylated eIF2α in MEF/PKR(-/-) cells. Cyclin D1 was decreased after BEPP treatment in MEF/PKR(+/+) cells, which was consistent with eIF2α phosphorylation because phosphorylation of eIF2α results in the inhibition of cyclin D1 translation (Brewer and Diehl, 2000). We also examined the activation of the MAPKs JNK, p38, and AKT after BEPP treatment (Fig. 3). Treatment with BEPP led to an increase in phosphorylated AKT in MEF/PKR(+/+). However, changes in phosphorylated JNK did not differ significantly between MEF/PKR(+/+) and MEF/PKR(-/-) cells. Moreover, BEPP increased the amount of phosphorylation of p38, but this increase did not differ significantly between MEF/PKR(+/+) and MEF/PKR(-/-) cells.
Fig. 3.
Effect of BEPP on PKR, eIF2α, AKT, cyclin D1, and MAPK activity. MEF/PKR(+/+) and MEF/PKR(-/-) cells were treated with the indicated concentrations of BEPP for 72 h. Whole-cell lysates were analyzed for total and active (phosphorylated) PKR, eIF2α, and cyclin D1, total and active (phosphorylated) AKT, active p38, and JNK by Western blotting. β-Actin was used as a loading control.
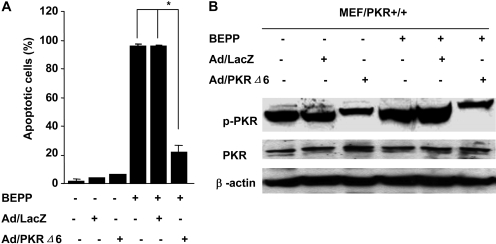
To further investigate the role of PKR activation in BEPP-induced apoptosis, we analyzed the effect of adenovector-mediated transfer of the dominant-negative PKR (Ad/PKRΔ6), which can block PKR activation. Cells treated with Ad/LacZ were used as a mock control. MEF/PKR(+/+) cells were pretreated with 1000 MOI Ad/PKRΔ6 for 24 h and then treated with 10 μM BEPP for 72 h. Cells were harvested for fluorescence-activated cell sorting analysis to quantify apoptotic cells or for Western blotting analysis to determine phosphorylated PKR and PKR expression. Pretreatment with Ad/PKRΔ6 but not the control vector dramatically reduced BEPP-induced apoptosis. The sub-G1 percentage decreased from 84.6 to 21.2% (p < 0.01) (Fig. 4A). Moreover, pretreatment of Ad/PKRΔ6 blocked BEPP-induced PKR activation, as evidenced by marked reductions of phosphorylated PKR compared with that of Ad/LacZ-pretreated cells (Fig. 4B). This result indicates that PKR activation is critical for BEPP-induced apoptosis.
Fig. 4.
Ad/PKRΔ6 inhibited BEPP-induced apoptosis by blocking PKR activation in MEF/PKR(+/+)cells. A, cells were treated with 10 μM BEPP for 72 h after pretreatment with 1000 MOI Ad/PKRΔ6 for 24 h. Ad/LacZ was used as vector control. Cells were then stained with propidium iodide and analyzed by flow cytometry to determine the percentage of apoptotic cells. Columns, mean of three independent experiments; bars, S.D. *, p < 0.01. B, whole-cell lysates treated with the same shown as above were analyzed for total and active (phosphorylated) PKR by Western blotting.
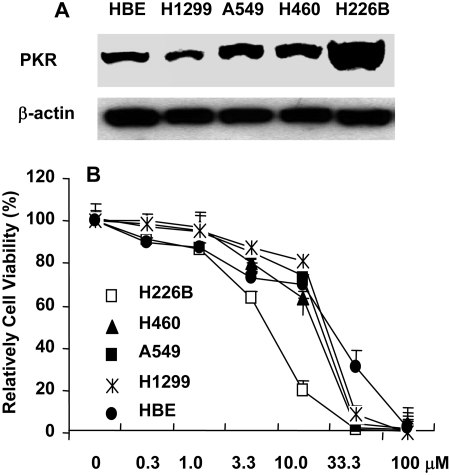
PKR-Overexpressing Cancer Cells Are More Sensitive to BEPP. We then investigated whether levels of PKR expression may affect the susceptibility to BEPP. For this purpose, we evaluated expression of PKR in the human lung cancer cell lines H1299, H460, H226B, and A549 and in normal HBE cells by Western blot analysis. The results showed that H226B cells had higher PKR expression compared with the other four cell lines (Fig. 5A). Next, we determined cell viability of these five cell lines after treatment with BEPP at various concentrations for 72 h. The result showed that BEPP inhibited growth of all five cell lines in a dose-dependent manner (Fig. 5B). The IC50 values at 72 h for H226B, H460, A549, H1299, and HBE were 4.6, 11.5, 13.8, 15.8, and 15.6 μM, respectively. H226B, which has higher PKR expression than the other four cell lines, was more sensitive to BEPP. This result indicates that BEPP or its active analogs might be useful for treatment of cancers with overexpression of PKR.
Fig. 5.
Cytotoxicity of BEPP in four lung cancer cell lines and in normal bronchial epithelial cells. A, total PKR expression was determined by Western blot analysis in these cell lines. B, dose-response to BEPP. Cells were treated with various concentrations of BEPP for 72 h, and cell viability was determined by using the SRB assay. Cells treated with DMSO were used as a control, and their viability was set at 100%. Each data point represents the mean ± S.D. of three independent experiments.
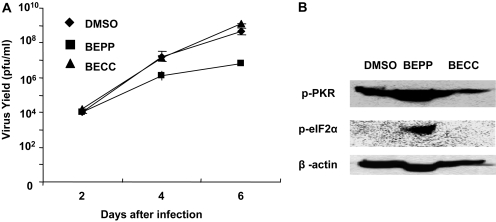
Inhibition of Vaccinia Virus Replication by BEPP. Accumulating evidence has shown that PKR activation is associated with inhibition of virus replication, including the Vaccinia virus (Lee and Esteban, 1993). We hypothesized that a PKR-activating compound might suppress virus replication. To test our hypothesis, we evaluated the effect of BEPP on Vaccinia virus replication in human cervical cancer HeLa cells. We first tested the effect of BEPP in HeLa cells. We found that a BEPP concentration of 7.5 μM was suitable for our study because no obvious cytotoxic effect was observed in HeLa cells up to this concentration (data not shown). Next, we pretreated HeLa cells with 7.5 μM BEPP for 48 h and then infected cells with vSP at a dose of 0.2 MOI. Cell lysates were harvested at different points and titrated for vaccine virus by the TCID50 method. The compound BEEC, which has a structure similar to that of BEPP but showed no differential cytotoxic effect on MEF/PKR(+/+) and MEF/PKR(-/-) cells, was used as a control compound; it also showed no cytotoxic effect on HeLa cells up to a concentration of 7.5 μM (data not shown). The virus yield in the BEPP pretreatment group was much lower than that in the DMSO group or the BECC group (p < 0.01); at days 4 to 6, the difference was approximately 100-fold (Fig. 6A). A similar result was observed when Vaccinia virus and BEPP were added to HeLa cells at the same time (Supplemental Fig. 2). We also harvested cell lysates after infection with vSP for 4 days and tested the status of PKR and eIF2α. BEPP pretreatment resulted in a dramatic increase of phosphorylated PKR and eIF2α (Fig. 6B).
Fig. 6.
Effect of BEPP on vSP replication in HeLa cells. A, cells were pretreated with 7.5 μM BEPP for 48 h. DMSO pretreatment was used as a control, and BECC pretreatment was used as a compound control. Cells were then infected with vSP at a dose of 0.2 MOI. Cell lysates were harvested at the indicated time points and titrated using the TCID50 method. The virus yield in the BEPP group was much lower than in the DMSO or BECC group (p < 0.01) at days 4 to 6. B, cell lysates were harvested after infection of vSP for 4 days. The lysates were analyzed for total and active (phosphorylated) PKR and eIF2α by Western blotting.
Discussion
PKR is one of the well characterized molecules induced by IFN-γ. The well known downstream targets of PKR include eIF-2α and the regulatory subunit of protein phosphatase 2A, B56α. Both eIF-2α and B56α, when phosphorylated by PKR, lead to inhibition of protein synthesis, which is critical for PKR-mediated inhibition of virus replication. Although inhibition of protein synthesis may contribute to PKR-mediated apoptosis, other mechanisms of PKR-mediated apoptosis have also been reported. In addition to inhibition of protein synthesis, PKR has been reported to increase the expression of proapoptotic genes, including Fas and Bax (Balachandran et al., 1998). PKR has also been reported to induce apoptosis by activation of Fas-associated death domain/caspase 8 (Balachandran et al., 1998), by interaction and phosphorylation of p53 and enhancing its transcriptional activity (Cuddihy et al., 1999a,b), and by modulating nuclear factor κB activities (Kumar et al., 1994; Gil et al., 1999). Our results showed that BEPP selectively inhibited cell growth and induced apoptosis in MEF/PKR(+/+) cells compared with MEF/PKR(-/-) cells. The IC50 value for MEF/PKR(+/+) was approximately one tenth of that for MEF/PKR(-/-) cells. Moreover, treatment with BEPP in MEF/PKR(+/+) cells resulted in increased phosphorylation of PKR, and ectopic expression of a dominant-negative PKR can block BEPP-induced apoptosis, demonstrating that BEPP-induced apoptosis is PKR dependent. The fact that BEPP treatment led to an increase of phosphorylation in eIF2α, and increased expression of Bax was also consistent with previous reports of PKR-mediated apoptosis (Der et al., 1997; Gil et al., 2002).
Treatment with BEPP resulted in an increase of phosphorylated PKR but not basal PKR, suggesting that the major mechanism of action is activating, but not induction, of PKR. PKR is known to be activated by binding with dsRNA, a vital cellular antiviral response upon viral infection. In addition to activation by virus infection, PKR is activated through various other stimuli, including cytokine, growth factor, and stress signals. It has been reported that PACT, a cellular protein, acts as a protein activator of PKR in response to diverse stress signals such as serum starvation and peroxide or arsenite treatment (Patel and Sen, 1998; Patel et al., 2000). After exposure of cells to these stress agents, PACT is phosphorylated and associates with PKR with increased affinity. Heterodimerization of PACT with PKR leads to PKR activation in the absence of dsRNA (Li et al., 2006). Moreover, PACT-mediated activation of PKR leads to enhanced eIF2α phosphorylation followed by apoptosis (Patel et al., 2000; Li et al., 2006). Whether PACT is involved in BEPP-mediated PKR activation is not yet clear. Nevertheless, our data suggested treatment with BEPP-induced apoptosis in a PKR-dependent manner, as was evidenced by activation of caspase 3 and dramatic increase of apoptotic cells determined by flow cytometric assay. However, PKR is known to play important role in other cell death mechanisms, such as autophagy (Tallóczy et al., 2002). Whether autophagy is also involved in BEPP-induced cell death remains to be determined.
A small compound that can induce PKR-dependent apoptosis may have two applications. First, such a compound may be useful for treatment of cancers with overexpression of PKR. It has been reported that PKR expression was increased in melanoma, colon cancer, and breast cancer (Kim et al., 2000, 2002). More recently, we have found that PKR played a role in radioresistance (von Holzen et al., 2007). In this study, we tested whether levels of PKR expression in human lung cancer cell lines may affect their sensitivity to BEPP. We found that H226B cells, which have high levels of PKR compared with other cell lines, were more sensitive to BEPP than were other cell lines tested. This result demonstrated that the PKR level could be a marker of cytotoxic effects for BEPP and its active analogs. Second, because most virus infection can cause activation of PKR, induction of PKR-dependent apoptosis may lead to the death of infected cells, thereby preventing replication of the virus. We found that oncolytic Vaccinia virus replication was suppressed as much as 99% by pretreatment host cells with BEPP. However, pretreatment of the Vaccinia virus with BEPP did not have any impact on initial virus infection (data not shown), suggesting that BEPP affects Vaccinia virus replication rather than infection. The finding was consistent with previous reports that PKR activation can inhibit virus replication, such as that of Vaccinia virus, HIV, hepatitis C virus, influenza virus, varicella-zoster, and herpes simplex virus (Adelson et al., 1999; Muto et al., 1999; Bergmann et al., 2000; Pflugheber et al., 2002; Desloges et al., 2005; Chang et al., 2006; Smith et al., 2006; Goodman et al., 2007). Nevertheless, identification of BEPP as a PKR-dependent apoptosis inducer is only the first step in developing novel PKR-targeted therapeutics. Much work remains before BEPP or its analogs can be used as antitumor or antiviral agents.
Supplementary Material
Acknowledgments
We thank Michael Worley for editorial review and Karen M. Ramirez for technical assistance with the flow cytometric analysis.
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant 5R01-CA092487-05, 5P50CA070907-100007 Lung SPORE Developmental Award]; the National Institutes of Health [Grant 3P30-CA016672-32S3]; Lockton grant-matching funds; the Homer Flower Gene Therapy Research Fund; the Charles Rogers Gene Therapy Fund; the Flora and Stuart Mason Lung Cancer Research Fund; the Charles B. Swank Memorial Fund for Esophageal Cancer Research; the George O. Sweeney Fund for Esophageal Cancer Research; the Phalan Thoracic Gene Therapy Fund; and the M.W. Elkins Endowed Fund for Thoracic Surgical Oncology.
doi:10.1124/jpet.108.141754.
ABBREVIATIONS: dsRNA, double-stranded RNA; PKR, double-stranded RNA-dependent protein kinase; MEF, mouse embryonic fibroblast; BEPP, 1H-benzimidazole-1-ethanol,2,3-dihydro-2-imino-a-(phenoxymethyl)-3-(phenylmethyl)-,monohydrochloride; HBE, human bronchial epithelial; BECC, 1H-benzimidazole-1-ethanol,α-[(4-chlorophenoxy)methyl]-3-[(4-chlorophenyl)methyl]-2,3-dihydro-2-imino-,monohydrochloride; DMSO, dimethyl sulfoxide; JNK, c-Jun NH2-terminal kinase; SRB, sulforhodamine B; PBS, phosphate-buffered saline; TCID50, median tissue culture infective dose; MOI, multiplicity of infection; Akt, protein kinase B; PACT, a protein activator of double-stranded RNA-dependent protein kinase.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
References
- Adelson ME, Martinand-Mari C, Iacono KT, Muto NF, and Suhadolnik RJ (1999) Inhibition of human immunodeficiency virus (HIV-1) replication in SupT1 cells transduced with an HIV-1 LTR-driven PKR cDNA construct. Eur J Biochem 264 806-815. [DOI] [PubMed] [Google Scholar]
- Balachandran S, Kim CN, Yeh WC, Mak TW, Bhalla K, and Barber GN (1998) Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J 17 6888-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann M, Garcia-Sastre A, Carnero E, Pehamberger H, Wolff K, Palese P, and Muster T (2000) Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J Virol 74 6203-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JW and Diehl JA (2000) PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci U S A 97 12625-12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KS, Cai Z, Zhang C, Sen GC, Williams BR, and Luo G (2006) Replication of hepatitis C virus (HCV) RNA in mouse embryonic fibroblasts: protein kinase R (PKR)-dependent and PKR-independent mechanisms for controlling HCV RNA replication and mediating interferon activities. J Virol 80 7364-7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddihy AR, Li S, Tam NW, Wong AH, Taya Y, Abraham N, Bell JC, and Koromilas AE (1999a) Double-stranded-RNA-activated protein kinase PKR enhances transcriptional activation by tumor suppressor p53. Mol Cell Biol 19 2475-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddihy AR, Wong AH, Tam NW, Li S, and Koromilas AE (1999b) The double-stranded RNA activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro. Oncogene 18 2690-2702. [DOI] [PubMed] [Google Scholar]
- Der SD, Yang YL, Weissmann C, and Williams BR (1997) A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci U S A 94 3279-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desloges N, Rahaus M, and Wolff MH (2005) Role of the protein kinase PKR in the inhibition of varicella-zoster virus replication by beta interferon and gamma interferon. J Gen Virol 86 1-6. [DOI] [PubMed] [Google Scholar]
- Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sarkar D, Settleman J, and Fisher PB (2007) Combinatorial treatment of non-small-cell lung cancers with gefitinib and Ad.mda-7 enhances apoptosis-induction and reverses resistance to a single therapy. J Cell Physiol 210 549-559. [DOI] [PubMed] [Google Scholar]
- Fang B, Ji L, Bouvet M, and Roth JA (1998) Evaluation of GAL4/TATA in vivo. Induction of transgene expression by adenovirally mediated gene codelivery. J Biol Chem 273 4972-4975. [DOI] [PubMed] [Google Scholar]
- Gaddy DF and Lyles DS (2007) Oncolytic vesicular stomatitis virus induces apoptosis via signaling through PKR, Fas, and Daxx. J Virol 81 2792-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, and Esteban M (2006) Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev 70 1032-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J, Alcamí J, and Esteban M (1999) Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NF-kappaB. Mol Cell Biol 19 4653-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J, García MA, and Esteban M (2002) Caspase 9 activation by the dsRNA-dependent protein kinase, PKR: molecular mechanism and relevance. FEBS Lett 529 249-255. [DOI] [PubMed] [Google Scholar]
- Goodman AG, Smith JA, Balachandran S, Perwitasari O, Proll SC, Thomas MJ, Korth MJ, Barber GN, Schiff LA, and Katze MG (2007) The cellular protein P58IPK regulates influenza virus mRNA translation and replication through a PKR-mediated mechanism. J Virol 81 2221-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZS, Naik A, O'Malley ME, Popovic P, Demarco R, Hu Y, Yin X, Yang S, Zeh HJ, Moss B, et al. (2005) The enhanced tumor selectivity of an oncolytic Vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res 65 9991-9998. [DOI] [PubMed] [Google Scholar]
- Hai T and Hartman MG (2001) The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene 273 1-11. [DOI] [PubMed] [Google Scholar]
- Kim SH, Forman AP, Mathews MB, and Gunnery S (2000) Human breast cancer cells contain elevated levels and activity of the protein kinase, PKR. Oncogene 19 3086-3094. [DOI] [PubMed] [Google Scholar]
- Kim SH, Gunnery S, Choe JK, and Mathews MB (2002) Neoplastic progression in melanoma and colon cancer is associated with increased expression and activity of the interferon-inducible protein kinase, PKR. Oncogene 21 8741-8748. [DOI] [PubMed] [Google Scholar]
- Kumar A, Haque J, Lacoste J, Hiscott J, and Williams BR (1994) Double-stranded RNA-dependent protein kinase activates transcription factor NF-kappa B by phosphorylating I kappa B. Proc Natl Acad Sci U S A 91 6288-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB and Esteban M (1993) The interferon-induced double-stranded RNA-activated human p68 protein kinase inhibits the replication of Vaccinia virus. Virology 193 1037-1041. [DOI] [PubMed] [Google Scholar]
- Li S, Peters GA, Ding K, Zhang X, Qin J, and Sen GC (2006) Molecular basis for PKR activation by PACT or dsRNA. Proc Natl Acad Sci U S A 103 10005-10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto NF, Martinand-Mari C, Adelson ME, and Suhadolnik RJ (1999) Inhibition of replication of reactivated human immunodeficiency virus type 1 (HIV-1) in latently infected U1 cells transduced with an HIV-1 long terminal repeat-driven PKR cDNA construct. J Virol 73 9021-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pataer A, Vorburger SA, Barber GN, Chada S, Mhashilkar AM, Zou-Yang H, Stewart AL, Balachandran S, Roth JA, Hunt KK, et al. (2002) Adenoviral transfer of the melanoma differentiation-associated gene 7 (mda7) induces apoptosis of lung cancer cells via up-regulation of the double-stranded RNA-dependent protein kinase (PKR). Cancer Res 62 2239-2243. [PubMed] [Google Scholar]
- Patel CV, Handy I, Goldsmith T, and Patel RC (2000) PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J Biol Chem 275 37993-37998. [DOI] [PubMed] [Google Scholar]
- Patel RC and Sen GC (1998) PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J 17 4379-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels B, Korst AE, de Pooter CM, Pattyn GG, Lambrechts HA, Baay MF, Lardon F, and Vermorken JB (2003) Comparison of the sulforhodamine B assay and the clonogenic assay for in vitro chemoradiation studies. Cancer Chemother Pharmacol 51 221-226. [DOI] [PubMed] [Google Scholar]
- Pflugheber J, Fredericksen B, Sumpter R Jr, Wang C, Ware F, Sodora DL, and Gale M Jr (2002) Regulation of PKR and IRF-1 during hepatitis C virus RNA replication. Proc Natl Acad Sci U S A 99 4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shir A and Levitzki A (2002) Inhibition of glioma growth by tumor-specific activation of double-stranded RNA-dependent protein kinase PKR. Nat Biotechnol 20 895-900. [DOI] [PubMed] [Google Scholar]
- Smith KD, Mezhir JJ, Bickenbach K, Veerapong J, Charron J, Posner MC, Roizman B, and Weichselbaum RR (2006) Activated MEK suppresses activation of PKR and enables efficient replication and in vivo oncolysis by Deltagamma(1)34.5 mutants of herpes simplex virus 1. J Virol 80 1110-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallóczy Z, Jiang W, Virgin HW 4th, Leib DA, Scheuner D, Kaufman RJ, Eskelinen EL, and Levine B (2002) Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc Natl Acad Sci U S A 99 190-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraishi F, Kadowaki Y, Tango Y, Kawashima T, Umeoka T, Kagawa S, Tanaka N, and Fujiwara T (2003) Ectopic p21sdi1 gene transfer induces retinoic acid receptor beta expression and sensitizes human cancer cells to retinoid treatment. Int J Cancer 103 833-839. [DOI] [PubMed] [Google Scholar]
- von Holzen U, Pataer A, Raju U, Bocangel D, Vorburger SA, Liu Y, Lu X, Roth JA, Aggarwal BB, Barber GN, et al. (2007) The double-stranded RNA-activated protein kinase mediates radiation resistance in mouse embryo fibroblasts through nuclear factor kappaB and Akt activation. Clin Cancer Res 13 6032-6039. [DOI] [PubMed] [Google Scholar]
- Williams BR (2001) Signal integration via PKR. Sci STKE 2001 RE2. [DOI] [PubMed] [Google Scholar]
- Yang YL, Reis LF, Pavlovic J, Aguzzi A, Schäfer R, Kumar A, Williams BR, Aguet M, and Weissmann C (1995) Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J 14 6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MC, Liu J, and Lau AS (1996) An essential role for the interferon-inducible, double-stranded RNA-activated protein kinase PKR in the tumor necrosis factor-induced apoptosis in U937 cells. Proc Natl Acad Sci U S A 93 12451-12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.