Abstract
We examined whether instructions to regulate emotions after a disgust-inducing film clip create an equally costly cognitive load across adulthood. Young and older adults across all instructional conditions initially demonstrated increased working memory performance after mood induction, typical of practice effects. Age-group differences emerged at the second post-induction trial. When instructed to down-regulate disgust feelings, older adults’ performance continually increased, whereas young adults’ performance dropped. Instructions to maintain disgust did not affect working memory performance. Consistent with claims that older adults are more effective at regulating emotions, findings indicate that intentional down-regulation of negative emotions may be less costly in older age.
Evidence is accumulating that emotion regulation is a resource-demanding process that disrupts simultaneously or subsequently performed tasks (Baumeister, Vohs, & Tice, 2007; Richards, 2004). For example, when trying to conceal negative emotions, people’s memory performance suffers (Richards & Gross, 2000). When suppressing forbidden thoughts, people subsequently give up more quickly at solving anagrams (Muraven, Tice, & Baumeister, 1998). When presented with craving-eliciting cues, smokers have prolonged reaction times and their math and language comprehension decreases (C. J. Madden & Zwaan, 2001; Zwaan & Truitt, 1998). So far, studies have examined the link between emotion regulation and cognition primarily in young adults. Thus, the question arises as to whether this detrimental effect of emotion regulation is present in both young and older adults. In other words, does the allocation of resources needed to effectively regulate emotions vary by age?
The aging literature suggests that older adults are more motivated to regulate their emotions and are more effective at doing so than young adults (Birditt & Fingerman, 2005; Blanchard-Fields, Mienaltowski, & Seay, 2007; Carstensen, Pasupathi, Mayr, & Nesselroade, 2000). According to socioemotional selectivity theory (Carstensen, Isaacowitz, & Charles, 1999), older adults’ awareness that lifetime is shrinking motivates them to focus on the present, emphasizing goals related to emotional satisfaction and meaning. Hence, emotion regulation goals are assumed to be chronically activated in older adults, whereas they should be activated in young adults only when the context demands it (Knight et al., 2007; Mather & Carstensen, 2005). There is also evidence that older adults are more effective at regulating emotions. Their self-reported emotional control is higher than that of young adults (e.g., Gross et al., 1997; Lawton, Kleban, Rajagopal, & Dean, 1992), they report fewer interpersonal tensions (Birditt, Fingerman, & Almeida, 2005), and they use more effective emotion regulation strategies to deal with interpersonal tensions (Blanchard-Fields et al., 2007; Blanchard-Fields, Stein, & Watson, 2004). Recent performance-based studies show that older adults are equally or even more effective than young adults in modulating facial expressions or inner experience of emotions (Kunzmann, Kupperbusch, & Levenson, 2005; Magai, Consedine, Krivoshekova, Kudajie-Gyamfi, & McPherson, 2006; Phillips, Henry, Hosie, & Milne, 2008). What has not been studied so far, however, is the cognitive effort necessary for young and older adults to obtain equal (or even higher) levels of emotion regulation.
Given that emotion regulation appears to disrupt young adults’ cognitive performance and older adults appear to be better at regulating emotions, we assumed that emotion regulation may be less effortful and thus less costly in performing tasks for older adults than it is for young adults. We reasoned that the chronic activation of emotion-regulatory goals and the long-term experience, practice, and facility in dealing with emotional situations, as is typical for older adulthood, will render emotion-regulatory processes less effortful for older adults. Consequently, in older adults, emotion regulation should take up less resources that would impede the performance of simultaneously or subsequently performed tasks. This hypothesis may seem in contrast to an array of cognitive research demonstrating older adults’ deficit in executive control (D. J. Madden, 2007). Notably, however, this deficit is not unequivocally found for the processing of negative stimuli. Indeed, older adults seem more efficient than younger adults in inhibiting angry facial expressions in visual search tasks (Hahn, Carlson, Singer, & Gronlund, 2006) and they dwell less on emotionally negative scenes (Rosler et al., 2005).
To investigate our hypothesis, we induced disgust with a short film clip and compared its effect on performance of a working memory task under different emotion-regulatory instructions. We focused on disgust because this emotion can be easily induced in the laboratory, is known to elicit a quick and strong emotional reaction that needs to be regulated (Gross & Levenson, 1995; Rozin & Fallon, 1987), and should impose a comparable load across young and older age groups (Shiota & Levenson, 2008). Young and older adults were randomly assigned to one of four conditions. The experimental group (down-regulation condition) was instructed to down-regulate feelings of disgust as quickly as possible while working on the next task. In a no-instructions control condition, participants simply moved on to the working memory task after the disgust induction. Given that older adults might spontaneously regulate disgust even when not instructed to do so, we further included a maintenance control condition, in which participants were explicitly instructed not to regulate emotions, that is, to maintain feelings of disgust while working on the next task. Finally, in a neutral control condition, participants watched a neutral film clip and then performed the working memory task.
We expected that when instructed to down-regulate disgust in the experimental condition, older adults would show less decrement in working memory performance relative to their baseline performance than young adults. Moreover, among older adults, we expected working memory performance for those in the down-regulation condition and the no-instructions control condition to be equivalent. In contrast, among young adults, those in the down-regulation condition should show larger decrements in working memory performance relative to their baseline performance than those in the no-instructions control condition. We assumed that the experience of disgust per se is not detrimental to working memory performance. Thus, we expected no age differences in change in working memory performance for the maintenance control condition and for the neutral control condition.
Method
Participants
Ninety-one young (20–30 years) and 116 older (60–75 years) adults were recruited from a southeastern metropolitan city. Young adults were college students and participated for course credit. Older adults were well-educated, community-dwelling volunteers who were reimbursed for their time with $20. Fifteen older adults were excluded because they had low cognitive functioning or were unable to understand the working memory task. Young and older adults reported equally good health (1 = poor, 5 = excellent; Myoung = 3.78, SD = .88; Mold = 3.79, SD = .89). Young adults performed better than older adults on a perceptual speed task (Digit Symbol Coding; Wechsler, 1955; Myoung = 62.79, SD = 13.84; Mold = 44.92, SD = 10.49), t (140) = 8.67, p = .001; whereas older adults demonstrated better verbal ability (Shipley Vocabulary Test; Shipley, 1986; Myoung = 17.65, SD = 4.51; Mold = 23.94, SD = 6.54), t (126) = −6.72, p = .001.
Materials and Measures
Mood induction
Disgust was induced by having participants watch a 2:10 minute-long film clip depicting a woman eating horse rectum in order to win money, while describing her experience during this exercise. The neutral film (2:11 min) depicted two men talking about a woman’s dress and subsequently sharing a beer in silence. Both films were piloted in Robert W. Levenson’s lab and had elicited comparable levels of the targeted emotions in young and older adults (Shiota & Levenson, 2008).
Mood assessment
Nine times during the experiment, participants reported to what extent they momentarily felt each of eight emotions (disgusted, sad, frustrated, distressed, angry, happy, content, interested) on a 5-point scale from 1 (very slightly or not at all) to 5 (extremely). Emotions were presented in a different order each time they were rated.
N-back task
Working memory was assessed with the N-Back task. In this task, numbers (0–9) are presented one-by-one on a computer screen and participants have to identify whether the current (randomly generated) number matches the one seen N items previously by pressing one of two keys on the keyboard. We used the 1-Back version of the task (i.e., match to the previously seen number) for practice and the 2-Back version (i.e., match to the number seen two screens earlier) in the remainder of the session. Each block comprised two trials with 22 items each; stimuli were presented for 500ms, followed by a blank screen for 2500ms; 33% of the items in each trial were targets (i.e., matched the letter seen N items previously) and 67% were non-targets (Gray, 2001; Huxhold, Li, Schmiedek, & Lindenberger, 2006). Accuracy (percentage correct) and reaction times were recorded for each item. The N-Back task has been used previously to test the detrimental effects of self-regulation demands on cognitive performance (Gray, 2001), has a sufficient difficulty level to tax cognitive resources, and is not heavily influenced by negative mood. It taps sustained attention and analytical processing without requiring the formation of novel strategies or solutions, which are known to be facilitated by negative mood (Bless, 2003). This was important as our design did not fully disentangle the effects of experiencing disgust from the effects of regulating disgust.
Given the trade-off between accuracy and reaction time (i.e., maximizing accuracy will necessarily result in slower reaction times, and vice versa), we computed a combined performance score for each trial. By definition, the first two numbers in a trial could only be non-targets and were excluded from analyses. Items with reaction times of less than 100ms were treated as errors. Reaction time was calculated only for correct responses and multiplied by −1.00 so that higher values indicated better performance. A combined performance index was then created by averaging single-trial reaction time and accuracy after T-transforming both indices on the basis of the 12 available trials (6 time points × 2 trials; see Huxhold et al., 2006). The resulting combined performance score was again T-standardized and scores for the two trials of each block were averaged (see Table 1 for means by age group and condition).
Table 1.
N-Back Performance (Combined Performance Scores) of Young and Older Adults in the Four Different Conditions Over Time, Given in T-Score Metric
| Age group/ Condition | Baseline | Time 1 | Time 2 | Time 3 | ||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Young adults | ||||||||
| Down-regulate | 53.85 | 5.13 | 56.90 | 4.88 | 55.36 | 5.71 | 57.12 | 4.76 |
| Maintain | 55.41 | 6.76 | 57.05 | 7.45 | 58.04 | 6.12 | 56.98 | 7.36 |
| No instructions | 54.37 | 8.62 | 57.92 | 5.62 | 58.72 | 5.69 | 58.57 | 5.33 |
| Neutral | 54.24 | 6.68 | 56.85 | 6.03 | 57.37 | 4.86 | 57.59 | 5.22 |
| Old adults | ||||||||
| Down-regulate | 45.27 | 4.77 | 49.14 | 4.58 | 51.68 | 4.36 | 52.04 | 5.17 |
| Maintain | 45.89 | 9.04 | 48.04 | 10.61 | 48.11 | 8.74 | 50.13 | 8.79 |
| No instructions | 46.30 | 7.97 | 49.59 | 8.42 | 50.28 | 7.59 | 50.73 | 6.65 |
| Neutral | 40.97 | 6.70 | 46.12 | 8.40 | 46.56 | 7.53 | 47.29 | 6.64 |
Procedure
After signing a consent form, participants reported their momentary emotions and performed one block of practice trials with the 1-back version of the N-Back task, followed by three blocks of trials with the 2-back version of the task. The third block of the 2-back task was used as participants’ baseline performance. After each N-Back block, participants rated their momentary emotions. They next completed a demographics questionnaire and a personality questionnaire irrelevant to this report. The experimenter then started the film clip and left the room for 2 minutes so that participants felt unobserved. Upon the experimenter’s return, participants again rated their momentary emotions. They performed three more blocks of the 2-back task, each followed by another emotion rating. In this way, we measured both persons’ immediate emotional reaction to the mood induction as well as its trajectory over time. The session ended with several cognitive measures and follow-up questions on the effectiveness of the mood induction and motivation to follow instructions. As a manipulation check, the majority (90%) of participants’ faces were videotaped to ensure sufficient attention to the film clip. Coding of facial expressions indicated that 98% of participants who saw the disgust clip and 100% of those who saw the neutral clip were sufficiently attending to the film content.
Participants in the experimental condition and in the maintenance control condition received an emotion regulation instruction immediately before the first 2-back trial after the mood induction. In the down-regulation condition, they were told, “The movie you just saw probably caused you to experience a negative emotional reaction. When working on the next tasks, we would like you to change that negative reaction as fast as you can. Use any strategy you have available to turn your negative feelings into positive ones. At the same time, remember it is important that you do a good job in performing the other tasks.” In the maintenance control condition, they were told, “… When working on the next tasks, we would like you to maintain the intensity of your negative reaction to the film. Just keep your negative feelings going and do not try to change them in any way. ….” Before each subsequent block of the 2-back task, they were reminded of their emotion-regulatory goal. Participants in the remaining two control conditions (no-instruction control, neutral control) did not receive any emotion-regulatory instructions.
Results
We start by describing analyses establishing the effectiveness of the mood induction and investigating potential age differences in the subjective reactivity to and recovery from the mood induction. We then proceed to our main hypotheses regarding age differences in working memory performance under different emotion-regulatory conditions.
Mood Induction Effectiveness
In order to assess mood induction effectiveness, criteria were established to select those people for which the mood induction elicited the targeted emotion: In the three disgust conditions, persons were included for analyses if they had an increase in either their disgust or distress ratings directly after the film clip. The emotion rating preceding the last N-Back task before the mood induction served as baseline. Persons in the neutral control condition were included if they had neither an increase in disgust nor in distress ratings. On the basis of these criteria, 17 young adults and 28 older adults in the three disgust conditions and 2 young adults and 1 older adult in the neutral control condition needed to be excluded, leaving an effective sample of 72 adults per age group for subsequent analyses. This rate of success for negative mood induction is largely comparable to findings in the extant literature (Martin, 1990; Mienaltowski & Blanchard-Fields, 2005).
Subjective Reactivity to and Recovery From the Mood Induction
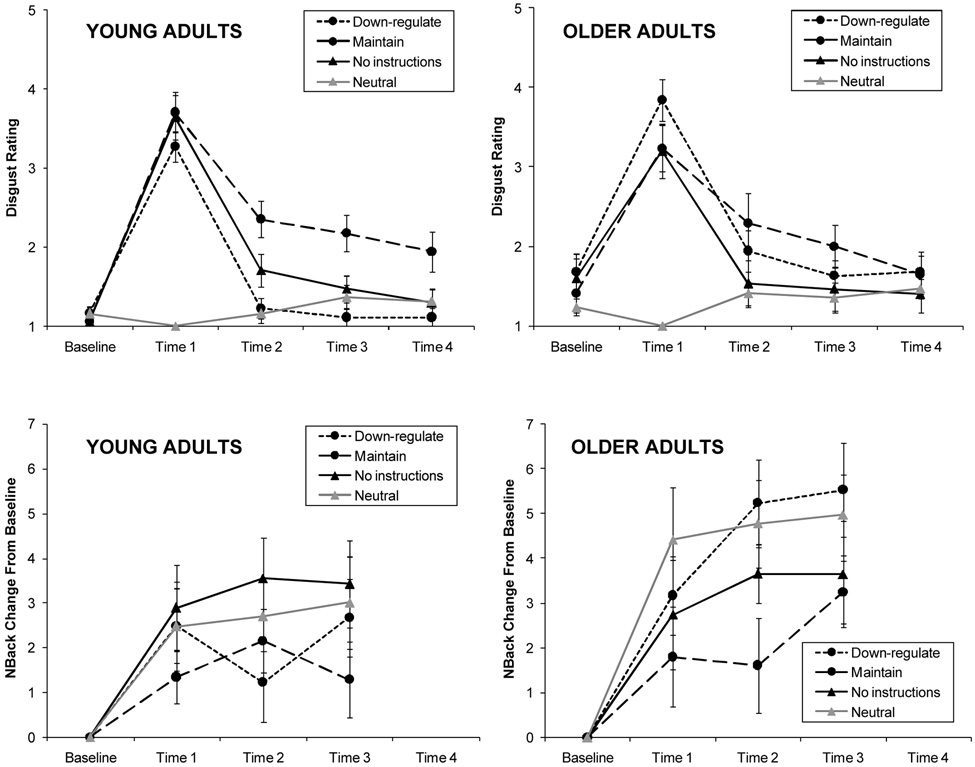
To verify that young and older adults had an equal emotion-regulatory load, we analyzed participants’ disgust ratings at baseline, immediately following the mood induction (T1), and after each subsequent N-Back block (T2–T4; see Figure 1) with a 5 (Time) × 2 (Age) × 4 (Condition) repeated-measures analysis of variance. There were multivariate effects of Time, F (4, 133) = 100.48, p = .001, η2 = .75; Condition, F (3, 136) = 13.94, p = .001, η2 = .24; Time × Condition, F (12, 352.18) = 14.58, p = .001, η2 = .30; and Time × Age, F (4, 133) = 2.69, p = .03, η2 = .08. No effects were found for Age, Condition × Age, and Time × Condition × Age. Follow-up univariate ANOVAs for each time point indicated that age only affected baseline ratings of disgust. Older adults reported slightly elevated disgust at baseline, but not differentially across conditions, F (1, 136) = 10.78, p = .001, η2 = .07; and no Age or Age × Condition effects emerged at subsequent time points.
Figure 1.
Disgust ratings (upper panels) and N-Back performance (lower panels) of young and older adults in the four different conditions over time. Baseline = before mood induction; Time 1 to Time 4 = after mood induction. Disgust ratings were always given before N-Back blocks. N-Back performance is based on a combined performance score (in T-score metric) and is shown relative to baseline performance. Error bars represent standard errors.
The Time × Condition interaction shows the effectiveness of the disgust induction. Seeing the disgust clip produced elevated disgust ratings at T1 relative to baseline, while seeing the neutral clip produced slightly decreased disgust ratings. The down-regulation group and the neutral control group both returned to baseline level of disgust at T2, the no-instruction control group returned to baseline level of disgust at T3, and the maintenance group still had significantly elevated disgust ratings (relative to their baseline) at the final emotion assessment (T4), which was primarily driven by young adults. These trajectories verify the effectiveness of the different emotion regulation instructions.
We further tested whether seeing the neutral film clip elicited any negative emotion other than disgust. Participants in the neutral control condition felt somewhat less frustrated at T1 (M = 1.18) and less distressed at T1, T2, and T3 (M = 1.18 – 1.50) compared to their baseline (Mfrustrated = 1.90; Mdistressed = 1.76, all SEs ≤ .16), probably because the film provided a break from the N-Back task, which was perceived as difficult. No age group effects emerged. Thus, no negative emotion was systematically elevated at any time after seeing the film.
Working Memory Performance
Testing our main hypothesis that emotion regulation instructions would differentially affect young and older adults’ N-Back performance, combined performance scores at baseline (last block of 2-Back task before the mood induction) and at the three time points after the mood induction (T1–T3) were subjected to a 4 (Time) × 2 (Age) × 4 (Condition) repeated-measures analysis of variance. There was a robust multivariate main effect of age: Across time points and conditions, older adults performed worse than young adults; F (1, 136) = 70.01, p = .001, η2 = .34. Furthermore, there was a strong multivariate main effect of time, F (3, 134) = 41.91, p = .001, η2 = .48. Across time, all participants in all conditions increased their performance, with the largest increase from baseline to T1. In line with our a priori hypothesis, age group and condition differences were evident in further performance changes (from T1 to T2 and from T2 to T3), as indicated by a multivariate Time × Age effect, F (3, 134) = 3.00, p = .03, η2 = .06; and a trend for a multivariate Time × Condition × Age effect, F (9, 326.27) = 1.80, p = .07, η2 = .04. The multivariate effects of Condition, Time × Condition, or Condition × Age were not significant. To illustrate, Figure 1 shows individuals’ combined performance scores at the three time points after mood induction (T1–T3) in terms of change from baseline (last block before mood induction).
Planned pair-wise comparisons of conditions were run to disentangle the Time × Condition × Age interaction effect. We used the no-instructions control condition as a reference and compared it with each of the remaining three conditions. To determine if disgust per se had an effect on performance we compared the no-instructions control condition with the neutral control condition. No interaction effects involving age or condition were found. Thus, being confronted with a disgust-eliciting situation relative to an emotionally neutral situation per se did not affect the unfolding trajectory of N-Back performance: According to repeated contrasts, participants in all groups significantly increased performance from baseline to T1 and remained stable thereafter. This confirms our hypothesis of no age differences in change in working memory performance in the neutral condition. In line with hypotheses, there also were no relevant effects when comparing the maintenance control condition with the no-instructions control condition. Trying to maintain feelings of disgust did not disrupt performance in the N-Back task in either age group.
What happened if participants were explicitly instructed to down-regulate their disgust-related feelings? In line with our hypothesis that only young adults’ cognitive performance would be disrupted, we found a significant Time × Age × Condition effect, F (3, 65) = 2.86, p = .04, η2 = .12; and a trend for Time × Age, F (3, 65) = 2.23, p = .09, η2 = .09, when comparing the down-regulation condition with the no-instructions control condition. When intentionally down-regulating disgust, both age groups’ performance initially increased, just as in the other groups. From T1 to T2, however, young adults’ performance dropped, whereas older adults’ performance continued to increase. From T2 to T3, young adults caught up to the level of performance of their age peers in the control conditions. Thus, intentionally down-regulating disgust negatively affected young adults’ working memory performance at the second measurement occasion after the mood induction.
Discussion
We investigated whether regulating negative emotions would disrupt older adults’ performance less than young adults’ performance on a concurrently performed working memory task. To this end, we induced disgust with a short film clip and assessed working memory performance under different emotion-regulatory instructions. After viewing a film that did not induce mood, both young and older adults improved their working memory performance, reflective of typical practice effects (Verhaeghen, Cerella, & Basak, 2004). No further practice gains were found at the two subsequent measurements, suggesting that participants reached asymptotic levels of performance. The most interesting findings occurred for performance following a disgust-evoking event. In this case, instructions to down-regulate emotions differentially affected working memory performance in young and older adults. Similar to past research, when instructed to down-regulate disgust, young adults’ working memory performance was disrupted after the mood induction. Of particular interest is that contrary to findings typical of young adults, working memory performance was unaffected in older adults who were given the same instructions. The trajectory of working memory performance (i.e. increase from baseline to Time 1, and stability thereafter) was unaffected in both young and older adults when they were not given any explicit emotion regulation instruction, or when given the instruction to maintain feelings of disgust. This indicates that the experience of disgust per se did not affect performance on the working memory task.
Again, findings for young adults replicate previous studies showing that regulating emotions (such as suppressing outward signs of emotions or inhibiting unwanted thoughts) has cognitive costs, reducing performance of simultaneously or subsequently performed tasks (Baumeister et al., 2007; Richards, 2004). Importantly, this study is among the first to demonstrate that the costs of emotion regulation may vary across age groups. In line with the literature showing that age is associated with better self-reported and stable or improved performance-based emotional control (Gross et al., 1997; Lawton et al., 1992; Phillips et al., 2008), as well as greater effectiveness in disengaging from negative material (Hahn et al., 2006; Rosler et al., 2005), results suggest that intentionally down-regulating emotions may be less costly for old adults than it is for young adults.
At first blush these findings appear to run contrary to claims that regulating emotion is resource-intensive for older adults (Mather & Knight, 2005). However, a closer examination of Mather and colleagues’ work shows that they assess emotion-related information processing preferences which are assumed to be operating in service of emotion regulation. Unlike the present study, they do not directly assess emotion regulation. Future research needs to establish a direct link between these information processing preferences and effective emotion regulation. At this point our findings support assumptions that to some extent, emotion regulation may become less effortful as we grow older given frequent occasions to exercise the management of negative emotion states over the course of life.
Findings further show how the disruptive effects of emotion regulation unfold over time. We measured emotions and working memory repeatedly over the experimental session to obtain information about the duration of disgust feelings after a disgust-evoking event. We obtained the largest disruptive effect of intentional emotion regulation in young adults at the second measurement occasion, that is, after their subjective feelings of disgust had already returned to baseline levels. This suggests that regulating emotions may negatively affect individuals for a period of time that extends beyond the subjective experience of the emotion. It is possible that the disruptive effect of emotion regulation is cumulative and therefore becomes more evident over time, until the effect of the emotion-triggering event finally fades away.
Notably, performing the working memory task may actually have operated as an emotion regulation strategy itself, making it easier for participants to distract themselves from their memories of the disgust-evoking film. Distraction is known to be a very efficient emotion regulation strategy (Nolen-Hoeksema & Morrow, 1993; Van Dillen & Koole, 2007). Indeed, when asked in the follow-up questionnaire which strategy they used to regulate their emotions, many participants spontaneously reported that they focused on the cognitive task. Possibly, older adults’ lower costs of emotion regulation can be attributed to the fact that they engaged in distraction more than young adults, given that distraction is probably not very cost-intensive. Future research should examine more directly how older adults achieve the same emotion-regulatory goal with less cognitive effort.
The present study also carries some limitations. Participants did not reach maximum levels of performance before receiving instructions, which potentially confounded practice effects with the effects of emotion regulation. Although this does not explain differential effects of instructions on the amount of practice gains, future studies should seek to obtain maximum levels of performance before introducing the emotional task. Another limitation concerns the emotion regulation instructions, which may have been somewhat ambiguous. The down-regulation instruction did not clearly distinguish down-regulation of negative feelings from up-regulation of positive feelings, and the maintenance instruction confounded no-regulation with actual maintenance. Instructions should therefore be improved in future research.
It remains an open question whether the present findings apply to all types of cognitive tasks, or to all types of negative emotions. There are a few studies in which induced sadness disrupted older adults’ performance more so than young adults in making causal attributions (Mienaltowski & Blanchard-Fields, 2005) and in problem solving tasks (Phillips, Smith, & Gilhooly, 2002). Sadness is an emotion that is highly relevant to old age (Kunzmann & Grühn, 2005) and therefore may impose a higher emotion regulation load on older adults than disgust does. Moreover, the tasks used in the above studies required the formation of new strategies and creative solutions, which can be facilitated by negative mood (Bless, 2003). In these contexts, young adults may have had more of an advantage than older adults in performing the cognitive tasks. Importantly, emotion regulation goals were not explicitly manipulated in these studies.
In conclusion, adopting a lifespan perspective provides a more complete picture when cognitive costs of emotion regulation are observed. Motivation and long-term practice in regulating emotions can decrease the amount of resources necessary to maintain or regain emotional well-being, while performing well at other tasks. Growing older has the adaptive potential to reduce the cognitive costs of emotion regulation, further corroborating findings of higher emotional control with age.
Acknowledgments
Susanne Scheibe is now at Stanford University. This research was supported by National Institute on Aging grant R01 AG015019 awarded to Fredda Blanchard-Fields. Susanne Scheibe was supported by a research fellowship from the German Research Foundation (DFG) during preparation of this manuscript. We gratefully acknowledge the invaluable help of Fong Hum in running the study, as well as the assistance of Michelle Horhota, Abby Heckman, Bina Ali, Jonathan Hertzog, Daniel Pierce, Liz Piper, Erin Pridgen, and Katy Riddle. We also thank Ulman Lindenberger and Andrew Mienaltowski for fruitful discussions.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/pag/
Contributor Information
Susanne Scheibe, Max Planck Institute for Human Development, Berlin, Germany and Stanford University.
Fredda Blanchard-Fields, Georgia Institute of Technology, Atlanta.
References
- Baumeister RF, Vohs KD, Tice DM. The strength model of self-control. Current Directions in Psychological Science. 2007;16:351–355. [Google Scholar]
- Birditt KS, Fingerman KL. Do we get better at picking our battles? Age group differences in descriptions of behavioral reactions to interpersonal tensions. Journal of Gerontology: Psychological Sciences. 2005;60B:P121–P128. doi: 10.1093/geronb/60.3.p121. [DOI] [PubMed] [Google Scholar]
- Birditt KS, Fingerman KL, Almeida DM. Age differences in exposure and reactions to interpersonal tensions: A daily diary study. Psychology and Aging. 2005;20:330–340. doi: 10.1037/0882-7974.20.2.330. [DOI] [PubMed] [Google Scholar]
- Blanchard-Fields F, Mienaltowski A, Seay RB. Age differences in everyday problem-solving effectiveness: Older adults select more effective strategies for interpersonal problems. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2007;1:61. doi: 10.1093/geronb/62.1.p61. [DOI] [PubMed] [Google Scholar]
- Blanchard-Fields F, Stein R, Watson TL. Age differences in emotion-regulation strategies in handling everyday problems. Journal of Gerontology: Psychological Sciences. 2004:P261–P269. doi: 10.1093/geronb/59.6.p261. [DOI] [PubMed] [Google Scholar]
- Bless H. The consequences of mood on the processing of social information. In: Tesser A, Schwarz N, editors. The Blackwell handbook of social psychology: Intraindividual processes. Malden, MA: Blackwell Publishers; 2003. pp. 391–411. [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;79:644–655. [PubMed] [Google Scholar]
- Gray JR. Emotional modulation of cognitive control: Approach-withdrawal states double-dissociate spatial from verbal two-back task performance. Journal of Experimental Psychology: General. 2001;130:436–452. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Gotestam-Skorpen C, Hsu AYC. Emotion and aging: Experience, expression, and control. Psychology and Aging. 1997;12:590–599. doi: 10.1037//0882-7974.12.4.590. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotion elicitation using films. Cognition and Emotion. 1995;9:87–108. [Google Scholar]
- Hahn S, Carlson C, Singer S, Gronlund SD. Aging and visual search: Automatic and controlled attentional bias to threat faces. Acta Psychologica. 2006;123:312–336. doi: 10.1016/j.actpsy.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Huxhold O, Li S-C, Schmiedek F, Lindenberger U. Dual-tasking postural control: Aging and the effects of cognitive demand in conjunction with focus of attention. Brain Research Bulletin. 2006;69:294–305. doi: 10.1016/j.brainresbull.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion. 2007;7:705–714. doi: 10.1037/1528-3542.7.4.705. [DOI] [PubMed] [Google Scholar]
- Kunzmann U, Grühn D. Age differences in emotional reactivity: The sample case of sadness. Psychology and Aging. 2005;20:47–59. doi: 10.1037/0882-7974.20.1.47. [DOI] [PubMed] [Google Scholar]
- Kunzmann U, Kupperbusch CS, Levenson RW. Behavioral inhibition and amplification during emotional arousal: A comparison of two age groups. Psychology and Aging. 2005;20:144–158. doi: 10.1037/0882-7974.20.1.144. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Kleban MH, Rajagopal D, Dean J. Dimensions of affective experience in three age groups. Psychology and Aging. 1992;7:171–184. doi: 10.1037//0882-7974.7.2.171. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Zwaan RA. The impact of smoking urges on working memory performance. Experimental and Clinical Psychopharmacology. 2001;9:418–424. doi: 10.1037//1064-1297.9.4.418. [DOI] [PubMed] [Google Scholar]
- Madden DJ. Aging and visual attention. Current Directions in Psychological Science. 2007;16(2):70–74. doi: 10.1111/j.1467-8721.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magai C, Consedine NS, Krivoshekova YS, Kudajie-Gyamfi E, McPherson R. Emotion experience and expression across the adult life span: Insights from a multimodal assessment study. Psychology and Aging. 2006;21:303–317. doi: 10.1037/0882-7974.21.2.303. [DOI] [PubMed] [Google Scholar]
- Martin M. On the induction of mood. Clinical Psychology Review. 1990;10:669–697. [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults' emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mienaltowski A, Blanchard-Fields F. The differential effects of mood on age differences in the correspondence bias. Psychology and Aging. 2005;20:589–600. doi: 10.1037/0882-7974.20.4.589. [DOI] [PubMed] [Google Scholar]
- Muraven M, Tice DM, Baumeister RF. Self-control as a limited resource: Regulatory depletion patterns. Journal of Personality and Social Psychology. 1998;74:774–789. doi: 10.1037//0022-3514.74.3.774. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. Effects of rumination and distraction on naturally occurring depressed mood. Cognition and Emotion. 1993;7:561–570. [Google Scholar]
- Phillips LH, Henry JD, Hosie JA, Milne AB. Effective regulation of the experience and expression of negative affect in old age. Journals of Gerontology: Series B: Psychological Sciences. 2008;63B:P138–P145. doi: 10.1093/geronb/63.3.p138. [DOI] [PubMed] [Google Scholar]
- Phillips LH, Smith L, Gilhooly KJ. The effects of adult aging and induced positive and negative mood on planning. Emotion. 2002;2:263–272. doi: 10.1037/1528-3542.2.3.263. [DOI] [PubMed] [Google Scholar]
- Richards JM. The Cognitive Consequences of Concealing Feelings. Current Directions in Psychological Science. 2004;13:131–134. [Google Scholar]
- Richards JM, Gross JJ. Emotion regulation and memory: The cognitive costs of keeping one's cool. Journal of Personality and Social Psychology. 2000;79:410–424. doi: 10.1037//0022-3514.79.3.410. [DOI] [PubMed] [Google Scholar]
- Rosler A, Ulrich C, Billino J, Sterzer P, Weidauer S, Bernhardt T, et al. Effects of arousing emotional scenes on the distribution of visuospatial attention: changes with aging and early subcortical vascular dementia. Journal of the Neurological Sciences. 2005;229–230:109–116. doi: 10.1016/j.jns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Rozin P, Fallon AE. A perspective on disgust. Psychological Review. 1987;94:23–41. [PubMed] [Google Scholar]
- Shiota MN, Levenson RW. Normal aging and the effectiveness of reappraisal-based emotion regulation. 2008 Manuscript in preparation. [Google Scholar]
- Shipley WC. Shipley Institute of Living Scale. Los Angeles: Western Psychological Services; 1986. [Google Scholar]
- Van Dillen LF, Koole SL. Clearing the mind: A working memory model of distraction from negative mood. Emotion. 2007;7:715–723. doi: 10.1037/1528-3542.7.4.715. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J, Basak C. A working memory workout: How to expand the focus of serial attention from one to four items in 10 hours or less. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30:1322–1337. doi: 10.1037/0278-7393.30.6.1322. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale. Oxford, England: Psychological Corporation; 1955. [Google Scholar]
- Zwaan RA, Truitt TP. Smoking urges affect language processing. Experimental and Clinical Psychopharmacology. 1998;6:325–330. doi: 10.1037//1064-1297.6.3.325. [DOI] [PubMed] [Google Scholar]