Abstract
B cells can influence T cell responses by directly presenting antigen or by secreting antibody that binds to antigen to form immunogenic complexes. Conflicting evidence suggests that persisting antigen/antibody complexes propagate long-term T cell memory; yet other data indicate that memory cells can survive without specific antigen or MHC. Here, the roles of B cells and antigen/antibody complexes in T cell responses to lymphocytic choriomeningitis virus (LCMV) infection were investigated using B cell-deficient or B cell-competent mice. Despite normal lymphocyte expansion after acute infection, B cell-deficient mice rapidly lost CD4+ T cell memory – but not CD8+ T cell memory – during the contraction phase. To determine whether antigen/antibody complexes sustain CD4+ T cell memory, T cell responses were followed in B cell-transgenic (mIg-Tg) mice that have B cells but neither LCMV-specific antibody nor LCMV-immune complex deposition. In contrast to B cell-deficient mice, mIg-Tg mice retained functional T-helper cell memory, indicating that B cells selectively preserve CD4+ T cell memory independently of immune-complex formation. An in vivo consequence of losing CD4+ T cell memory was that B cell-deficient mice were unable to resolve chronic virus infection. These data implicate a B cell function other than antibody production that induces long-term protective immunity.
Keywords: T cells, B cells, Memory, Viral, Antibodies, Cytokines
Introduction
Long-term CD4 and CD8 T cell memory is seen in many different antigenic systems under both natural and experimental situations (1). The accelerated responses seen upon re-exposure to antigen are due to quantitative (increased precursor frequency) and qualitative changes in memory T cells (1, 2) that are transmitted epigenetically through subsequent generations of cells. T cell memory is maintained by the cytokines IL-7 and IL-15 through their positive effects on memory cell selection, survival, and periodic homeostatic proliferation (3–7). Of particular interest is the role of antigen and B cells in maintaining long-term T cell memory (8). Antigen-antibody complexes persisting on follicular dendritic cells (FDC) have been implicated in sustaining T cell memory (9, 10). Since FDC do not process and present antigen efficiently it has been postulated that B cells pick up this trapped antigen and present it to T cells (11–16); although, lymphoid DC may also pick it up and present to T cells (17). Thus B cells produce specific antibody needed for antigen deposition on FDC (18), and they subsequently present the trapped antigen to T cells. Additionally, B cells provide co-stimulatory signals to T cells (19) and are a source of cytokines (20–22) that modulate T cell responses (22–24). B cell expressed lymphotoxin and TNF, in particular, are essential for normal lymphoid organogenesis and development of FDCs (25).
Antigen/antibody complexes persist for extended periods of time (10), and a body of evidence indicates that exogenous antigens can be presented via cross-priming mechanisms to CD8 T cells (26), so it was proposed that CD8 memory would be affected by the presence or absence of B cells and antibody. However, B cell-deficient mice maintain CD8 memory as well as wildtype mice (27–30), and it has become clear from these studies and others that CD8 memory can be maintained not only in the absence of specific antigens (31–34) but also in the absence of cross-reactive antigens and MHC-I (35).
The requirements for CD4+ T cell memory are less clearly resolved, and there are conflicting reports on the durability of CD4+ memory (1, 36). Given the close proximity of B cells and CD4+ T cells and the ability of B cells to express MHC-II, it has been proposed that B cells prime CD4+ T cells and that memory B cells present persisting antigen to maintain memory CD4+ T cells (11–13). Initial CD4+ T cell responses require specific processing by B cells for some soluble antigens (37–39), but not others (17, 40–43). Peptide and ovalbumin did not require B cells to prime CD4+ T cells, whereas conalbumin required B cells but not necessarily their expression of MHC-II (43). So the results vary depending on the protein, and in models where B cells are required, it is not clear whether antigen/antibody complexes are needed.
Studies of CD4+ T cell memory following infection have also yielded conflicting data. Reduced levels of memory were found in B cell-deficient mice after lung infection with Chlamydia (44), but normal levels of CD4+ T cell memory were seen after genital tract infection (45). B cells and/ or antibodies play a key role in sustaining the CD4 response to Plasmodium infection. B cells appear to be required for CD4 memory generation following Pneumocystis carinii lung infection (46). CD4+ T cell responses to Listeria monocytogenes are defective in B cell-deficient mice even though B cells do not express listerial antigens (47), implying that there may be some other B cell function that is key for memory CD4+ T cell responses. In contrast to these studies, B cell-deficient mice generated CD4 memory responses to influenza A virus infection (41); however, the IL-2 limiting dilution method used in this study may have introduced error by, for example, in vitro expansion of contaminating IL-2+ve CD8 T cells. Given the variable nature of infections, including the cell types infected, the differing nature of APCs at sites of infection, and the chronicity/kinetics of infections, it is difficult to understand in general terms how B cells and antigen/antibody complexes affect CD4 memory.
Some studies that have employed non-infectious models of T cell differentiation find reduced levels of CD4+ T cell memory in the absence of B cells (17, 39); although, in one case, the reduced levels may have been related to a weak primary response to KLH immunization (39). In another study, primed CD4+ T cells did not survive when transferred to recipients in the absence of antigen (9), which led to the hypothesis that immune complexes are required for T helper cell memory maintenance. Moreover, B cells and dendritic cells were implicated in the maintenance of CD4 memory against soluble KLH immunization where B cells produced the antibodies that resulted in persistent antigen/antibody complexes that were in turn acquired by DC (interdigitating, CD11c+ve) and presented to memory T cells (17). However, it is uncertain whether these antigen/antibody complexes served to rescue CD4 T cells or if B cells themselves were responsible by, for example, providing a cytokine or other signal to maintain them. In view of data showing the persistence of memory in the absence of specific antigen and evidence that MHCII is not required for the survival of memory CD4+ T cells (6, 48, 49), it remains unclear whether persisting antigen/antibody complexes are needed for CD4+ T cell memory.
In the current study, the requirement of B cells and antigen-antibody complexes for T cell memory against LCMV was investigated in B cell-deficient mice and B cell-transgenic (mIg-Tg) mice (18) using assays that do not require extensive in vitro culture and which accurately reflect the numbers of cells in vivo. Moreover, CD4+ T cell responses to specific MHC-II restricted epitopes were followed, which eliminates potential contamination by CD8 T cells. All of the B cells in the mIg-Tg mice express only a membrane-bound form of immunoglobulin-M that use a H-chain commonly found in the response to the hapten NP (4-hydoxy-3-nitrophenyl)-acetyl. These mice have normal proportions of B cells but a restricted repertoire, severely reduced antibody, and undetectable immune-complex formation on FDCs (18). We report that B cell-deficient mice (which lack B cells and antigen-antibody immune complexes) generated normal primary CD4+ T cell responses, but CD4+ T cell memory was short-lived. In contrast, mIg-Tg mice established and maintained T helper cell memory. As these mice do not form LCMV-antigen/antibody complexes, these findings indicate that a B cell function other than antibody production sustains CD4+ T cell memory.
Materials and Methods
Mice and Virus
C57BL/6 (H-2b) mice were purchased from Jackson Laboratory, Bar Harbor, Maine and were used as controls for the B cell-deficient mice. The µMT−/− (B cell-deficient) mice (50) were obtained from Jackson Laboratory and bred at Emory University. The mIg-Tg mice have been described elsewhere (51). They are transgenic for a rearranged VDJ-heavy chain with a µ-heavy chain lacking the secretion sequence. They were maintained on the JH-knockout BALB/c genetic background (18), to prevent any expression of endogenous immunoglobulin. The transgene is not on the same chromosome as the other heavy chain constant regions, so there is no heavy chain switching. Since the transgene only affects the heavy chain, any immunoglobulin diversity would result from light-chain rearrangements. These mice have normal proportions of B cells in the periphery but do not secrete antibody. CB.17 mice were used as controls for the mIg-Tg mice. C57BL/6 and BALB/c carrier mice used in these experiments were generated and bred at Emory University as previously described (52). Adult experimental mice (6–8 weeks old) were infected with 2 × 105 PFU Armstrong CA 1371 strain of LCMV (53). Where indicated, mice were given 2 × 106 PFU LCMV-t1b, LCMV-A22, or LCMV-Clone13 to generate chronic infection (54). The level of virus in the serum and tissues was quantitated by plaque assay on Vero cells (53). All animal experiments were approved by the Emory or TSRI Animal Care and Use Committees.
Flow Cytometry
Spleen cells were surface stained with antibodies from BD-Pharmingen, La Jolla CA that recognize CD8 (clone 53-6.7), CD4 (clone RM4-5), CD44 (clone IM7), or B220 (clone RA3-6B2) using a concentration of 1mg antibody / 106 cells. Anti-mouse Ig(H+L) was purchased from Caltag (Burlingame, CA) and used as recommended by the manufacturer. Tetramer staining was done as described (55) using APC-conjugated Db33-41, Kb34-41, Db396-404 made at Emory University.
CD4+ T cell Enrichment
CD4+ T cell enrichment by negative selection was done using CD4 enrichment columns. Mouse CD4 Subset Column Kits were purchased from R & D systems (Minneapolis, MN) and the manufacturer’s suggested protocol was used. CD4 T cells were >80% pure using this protocol and the number of CD8 T cells was <0.5% as indicated by flow cytometry. As an additional check on the level of CD8+ T cell contamination NP396-404, an MHCI-restricted LCMV epitope, was added to CD4 enriched cultures in some experiments and the number of virus-specific CD8+ T cells was quantified by ELISPOT. By this test, there was little to no detectable CD8+ T cell contamination above background levels in the CD4 T cell enriched cultures.
Quantitation of virus-specific interferon-γ secreting CD8+ and CD4+ T cells
The method for quantitating T cell responses by staining T cells for intracellular IFN-γ has been described previously (56). Spleen cells were stimulated in vitro with media or with GP61-80 (for CD4+ cells) or GP33-41 (for CD8+ cells) for five hours with Brefeldin A (GolgiplugTM, BD-Pharmingen, La Jolla CA). They were then surface stained with anti-CD4 or anti-CD8 and stained for intracellular IFN-γ using Cytofix / Cytoperm staining kit (BD-Pharmingen, La Jolla, CA) as per manufacturer’s recommended protocol. For intracellular IFN-γ stain, FITC conjugated monoclonal rat anti-mouse IFN-γ (clone XMG1.2) and its control isotype antibody (rat IgG1) were used. All antibodies were purchased from BD-Pharmingen, La Jolla, CA. In some experiments, virus-specific CD8+ and CD4+ T cell responses were measured by IFN-γELISPOT assay using whole spleen cells or CD4 purified preparations from mice immunized with LCMV as has been described previously (56). Either carrier mouse spleen cells or purified LCMV peptides (NP396-404, GP33-41, GP276-286 for H-2b mice or NP118 for H-2d mice) that bind to MHC class I were used to stimulate CD8 T cell responses. LCMV peptides that bind to MHC class II (NP309-328 and GP61-80 of Armstrong) were used to stimulate I-Ab-restricted CD4 T cell responses (56).
Quantitation of virus-specific IL-2 Secreting CD4+ T cells
CD4+ T cell production of IL-2 was measured by cytokine-specific ELISA kits purchased from Genzyme Diagnostics (Cambridge, MA) and were performed and analyzed as recommended by the manufacturer. The ELISAs were read using a BioRad Microplate Reader 3550 (BioRad, Hercules, CA) using appropriate filters.
B cell Assays
Levels of serum antibody determined by LCMV-specific ELISA and quantitation of the number of LCMV-specific antibody secreting cells by ELISPOT assay have been described previously (57).
Results
Primary T cell response to LCMV in B cell-deficient mice
The role of B cells in generating anti-viral T cell responses was investigated using B cell-deficient mice (µMT−/−) infected with LCMV-Armstrong. At day 8, spleen cells were recovered and direct killing activity was measured on infected cells. Consistent with previous reports (27, 28, 58), normal levels of anti-viral CTL developed (60–70% specific lysis at 50:1 effector : target ratio) and virus was eliminated as indicated by plaque assay (both groups had <1.7 log10 PFU / ml serum and <2.7 log10 PFU / gram of brain, kidney, liver, lung, or spleen). The frequency of CD8+ T cells specific to each epitope was quantified by intracellular staining for IFN-γ In B cell-deficient mice, 15% of the CD8+ T cells were specific to GP33-41, and in +/+ mice 14% were specific to this dominant epitope (Figure 1A). These frequencies indicated that B cell-deficient mice had 8 × 106 and +/+ had 9 x 106 GP33-41-specific CD8+ T cells per spleen (Figure 1B). The magnitude of the responses to NP396-404, GP276-286, and NP205-212 were similar in both groups of mice (data not shown). These assays confirm earlier limiting dilution analysis of CTL-precursor frequencies in these mice (27) and show that expansion of LCMV-specific CD8 T cells specific to dominant (GP33-41, NP396-404) and subdominant (GP276-286, NP205-211) epitopes does not depend upon B cells.
Figure 1. Preferential loss of CD4 memory in B cell-deficient mice.
B cell-deficient (µMT−/−) and +/+ mice were infected with 2 × 105 PFU of LCMV (strain Armstrong), and LCMV-specific CD8 and CD4 T cell responses were quantified in the same mice by intracellular IFN-γ staining. Primary responses were measured at day 8 after infection, and memory responses were measured at days 70–154. (A) An example of the CD8 response to LCMV peptide GP33-41 with numbers indicating the frequency of CD8+ T cells that are GP33-specific. (B) The number of GP33-specific CD8+ T cells per spleen at various times after infection. (C) The CD4 response at days 8 and 154 with numbers indicating the frequency of CD4 T cells specific to GP61-80. (D) The number of GP61-80 specific CD4 T cells per spleen at day 8 or memory time points. In panels B and D, the numbers in parenthesis indicate the fold reduction in cell number between primary and memory time points. The dashed lines depict the limits of detection by this assay, and error bars represent standard deviations of 3 mice from a representative experiment.
Early LCMV-specific CD4+ T cell responses were followed in B cell-deficient mice. Robust CD4+ T cell responses were found in B cell-deficient mice during the primary phase of the T cell response (Figure 1C). On the H-2b background, a major component of the CD4 response is directed against the LCMV epitope GP61-80 presented on I-Ab. The frequency of GP61-80-specific splenic CD4+ T cells was 2% in B cell-deficient mice and 5% in +/+ mice, corresponding to 2.3 × 105 cells per spleen in B cell-deficient mice and 7 × 105 cells per spleen in +/+ mice (Figure 1D). Analysis of lymph nodes by IL-2 ELISA indicated that CD4+ T cell priming was normal there (data not shown). In uninfected B cell-deficient mice, the number of CD4+ T cells in the spleen (~ 5 × 106 per spleen) is 1/2 to 1/3 of that found in uninfected wild type mice. After infection, the B cell-deficient mice generate a virus-specific CD4+ T cell response that continues to be ~30–40% of controls; therefore, the peak antiviral response in the B cell-deficient mice is proportionate to the starting number of CD4+ T cells.
Memory T cell response to LCMV in B cell-deficient mice
Because the B cell-deficient mice generated good early T cell responses, the effects of B cells and antigen/ antibody complexes on memory T cell levels could be determined independently of their influences on the primary response. Therefore, LCMV-specific T cells were quantified in immune B cell-deficient mice at later time points. At day 154 post-infection, a large frequency of splenic CD8+ T cells specific for GP33-41 could be found in +/+ mice and B cell-deficient mice (Figure 1A). Similar frequencies were also observed by tetramer analysis (not shown). These percentages correspond to ~ 1 × 106 LCMV-specific CD8+ T cells in +/+ and 2 × 105 in B cell-deficient mice (Figure 1B). B cell-deficient mice also showed strong CD8 memory responses to other LCMV epitopes and the level of CD8 memory was found to be stable from 2 months to > 6 months post-infection (not shown). These results confirm previous reports that CD8+ T cell memory can form and be maintained in the absence of B cells (27–29).
In these very same mice, memory CD4 responses were analyzed by the same assays. While 0.5–1.0% of CD4 T cells were specific for GP61-80 in +/+ mice, B cell-deficient had levels at or below the detection limit (<0.01%) by flow cytometry (Figure 1C). In immune mice there were 8.5 × 104 -specific CD4+ T cells in +/+ mice, but there was an over 95% reduction to ~ 2 × 103-specific CD4+ T cells in B cell-deficient mice (Figure 1D). Similar results were obtained with IFN-γ ELISPOT analysis. The results shown in Figure 1 demonstrate a dichotomy between CD8 and CD4 memory: when analyzed in the same mice and by the same assays, B cell-deficient mice had approximately normal levels of CD8 memory but lost CD4 memory.
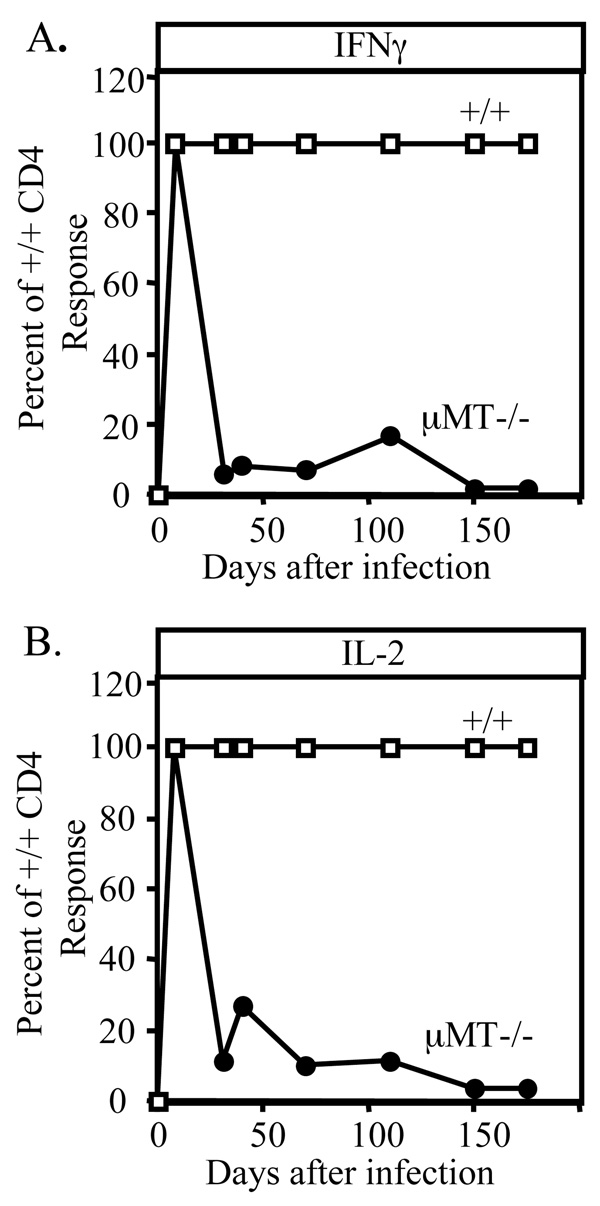
Since the most prominent defect in these mice was the loss of CD4 memory, it was of interest to determine when this occurred (Figure 2). While initial virus-specific IL-2 and IFNγ producing CD4+ T cells were normal, these responses began to wane as early as the second week after infection, and most of the CD4+ T cell response was gone by day 30. This rapid decay might explain discrepancies in prior studies regarding B cell dependent priming of CD4+ T cells: a response may or may not be found depending on the time point studied. These data indicate that B cell function is revealed during the contraction phase of the response in promoting the establishment and maintenance of CD4 T cell memory.
Figure 2. Kinetics of CD4 memory loss in the absence of B cells.
CD4 responses were quantified at various times after infection in +/+ and B cell-deficient mice. CD4 T cells were column purified, cultured for 24 hours with virus re-stimulation, and then cytokine levels in the supernatants were measured by ELISA. The relative amount of IFN-γ (A) or IL-2 (B) made by B cell-deficient CD4 T cells is shown as a percentage of what was made by +/+ CD4 cultures.
Long-term CD8 and CD4 memory in mIg-Tg mice
Since B cell-deficient mice lack serum antibody, one hypothesis is that absence of antigen-antibody complexes leads to the loss of CD4 memory. To investigate this possibility, 0.3ml of hyper-immune serum from LCMV-immune mice was transferred into B cell-deficient mice at days 5 and 15 after infection to form immune complexes. In principle, the anti-LCMV antibody in the serum should bind to LCMV antigens in vivo to form the complexes. The memory CD4 responses in the B cell-deficient mice were quantified at day 30. In some of the B cell-deficient mice, the serum transfer increased the frequency of virus-specific CD4+ T cells 2-fold compared to untreated B cell-deficient mice, implying that under these circumstances, immune complexes stimulate virus-specific CD4+ T cells. Nevertheless, the CD4 response in the treated B cell-deficient mice remained 4- 5-fold lower than in un-manipulated +/+ mice (data not shown).
Another possibility is that B cells provide (directly or indirectly) a factor that maintains CD4 memory. To see if B cells rescue the CD4 response, CD4-depleted spleen cells from immune wildtype mice (which contain memory B cells) or from naive wildtype mice were adoptively transferred into B cell-deficient mice at days 0 or 8 after infection, and the level of CD4 memory was quantified at day 30. In a number of experiments analyzed, the B cell-deficient recipients did not efficiently retain the transferred B cells. Nonetheless, mice given immune spleen cells at day 8 showed a modest enhancement of CD4 memory. While these experiments do not distinguish between antibody-dependent or B cell-dependent mechanisms, they link humoral immune responses to T-helper cell longevity in the LCMV model.
As an alternative approach to investigate this question, mIg-Tg mice were infected with LCMV. In these mice, the µ-chain has a deletion in the secretion domain that prevents antibody secretion while permitting normal mIgM synthesis and expression on the surface (18, 51). These mice contain normal numbers of B cells and have FDCs, but they produce no secreted antibody and, therefore, do not deposit antigen/antibody complexes on follicular dendritic cells (18). Figure 3 shows that mIg-Tg mice have B cells, but essentially no LCMV-specific serum antibody after infection. In addition, the number of LCMV-specific antibody secreting cells was < 2 / 106 spleen cells as determined by antibody ELISPOT. By comparison, wildtype mice had ~600 / 106 spleen cells at this time. In the bone marrow, the frequency of antibody secreting cells was below detection in mIg-Tg mice whereas +/+ mice had ~20–40 / 106 cells (57, 59).
Figure 3. mIg-Tg mice have B cells but no LCMV-specific serum antibody.
Spleen cells from control and mIg-Tg mice were stained for B cells 8 days after infection (A). The level of LCMV-specific IgG in the serum was determined by ELISA at days 8 or 18 after infection (B).
To determine if direct effector responses were affected by the absence of antibody, CTL responses were measured by 51Cr release assay in mIg-Tg mice. These mice had normal CTL responses and the infection was controlled in the serum, liver, lung, and kidney by day 8 after infection (Table 1). The early CD8+ T cell response was quantified by IFN-γ ELISPOT (Figure 4A), and these data indicate that there was a normal expansion of LCMV-specific CD8+ T cells after infection, which resulted in 8 × 106 NP118-128 specific cells per spleen in the mIg-Tg mice. Furthermore, elevated numbers of LCMV-specific CD8 T cells persisted during the memory phase with 1–5 × 105 per spleen in mIg-Tg mice and ~1 × 105 per spleen in +/+ mice. The generation of LCMV-specific CTLp was not affected in mIg-Tg mice, as both +/+ and mIg-Tg mice produced ~1×104 per spleen at day 8 (Figure 4B). Moreover, high numbers of CTLp remained during the memory phase with both groups containing 3–4 × 103 per spleen. Consistent with what was seen in B cell-deficient mice, CD8+ T cell memory was not affected by the absence of serum antibody and antigen / antibody complexes.
Table 1.
Normal anti-viral CTL responses in mIg-Tg mice.
| Percent specific 51Cr-release from balb/cl.7 (H-2d) targets at indicated E/T ratio |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Infected |
Uninfected |
LCMV titer (Log10 PFU/ ml of serum or gm of tissue) |
||||||||
| Mice | 50:1 | 17:1 | 6:1 | 50:1 | 17:1 | 6:1 | Serum | Liver | Lung | Kidney |
| +/+ | 89 | 63 | 30 | 1 | 2 | 0 | <1.7 | <2.6 | <3.0 | <2.6 |
| +/+ | 87 | 64 | 30 | 1 | 3 | 1 | <1.7 | <2.6 | <3.0 | <2.6 |
| mIg-Tg | 68 | 42 | 15 | 0 | 0 | 0 | <1.7 | <2.6 | <3.0 | <2.6 |
| mIg-Tg | 72 | 39 | 15 | 0 | 0 | 0 | <1.7 | <2.6 | <3.0 | <2.6 |
Figure 4. Primary and memory T cell responses in mIg-Tg mice.
mIg-Tg and +/+ mice were infected with 2 × 105 PFU of Armstrong, and T cell responses were analyzed at various times after infection. (A) Numbers of LCMV-specific CD8+ T cells were quantified by IFN-γ ELISPOT assay following stimulation with LCMV peptide NP118-126. The averages of 2–3 mice per time point are shown. (B) CTLprecursors were quantified by limiting dilution assay at day 8 (primary) or days 34, 60, and 68 (memory). The bars represent the average of 4–7 mice per group. (C) CD4+ T cells were column purified and then stimulated with LCMV-carrier spleen cells in an IFN-γ ELISOT assay. The line graphs show the total number of CD4+ T cells per spleen that produced IFN-γ in this assay. The averages of 2–3 mice per time point are shown. (D) Purified CD4+ T cells were cultured with virus-infected carrier spleen cells for 24 hours. ELISA was used to measure the levels of IL-2 in the supernatants. The bars represent the average of 4–7 mice per group. Dashed lines indicate the limits of detection for each assay and the error bars represent standard deviations.
CD4+ T cell responses were followed in the spleens of mIg-Tg mice at various times after infection. There was expansion of LCMV-specific CD4+ T cells at day 8 (Figure 4C). In +/+ mice, there were 2–3 × 104 LCMV-specific CD4+ T cells per spleen at day 8 and mIg-Tg mice had 9 × 104 specific splenic CD4+ T cells. This result confirms that antigen/antibody complexes are not needed for early responses, consistent with what was seen in B cell-deficient mice. To determine whether these immune complexes are needed for T-helper cell memory, virus-specific CD4+ T cell responses were analyzed at later times in the mIg-Tg mice. At days 30 and 60 after infection, there remained 3–4 × 103 LCMV-specific CD4 T cells in +/+ mice, and 5 × 103 in mIg-Tg mice. Virus-specific CD4+ T cells from mIg-Tg mice made normal levels of IL-2 at day 8 and two months later (Figure 4D). These results show that immune complexes with LCMV antigen are not required to establish and maintain CD4+ T cell memory, and in the absence of these complexes, memory CD4+ T cells retain their capacity to secret IFNγ and IL-2.
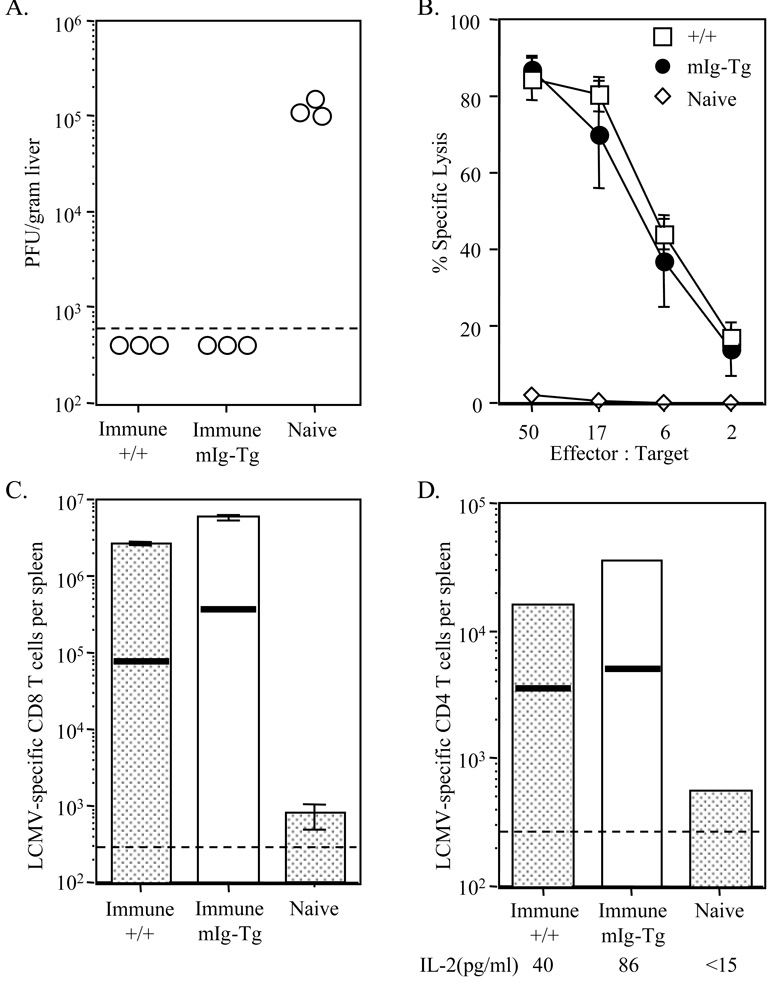
As a more rigorous test of memory, +/+ and mIg-Tg mice immunized 90 days earlier were re-challenged with a high dose infection (1 × 106 PFU LCMV Armstrong). Three days after the re-challenge, levels of virus were quantified by plaque assay (Figure 5A). While naive mice had very high levels of virus in the liver, immune +/+ and mIg-Tg mice controlled the infection quickly and reduced the viremia to levels below detection. Direct ex vivo 51Cr-release assay indicated that immune +/+ and mIg-Tg mice had elevated levels of CTL activity whereas infected naive mice had <5% specific killing (Figure 5B). The number of NP118-126-specific CD8 T cells expanded from 1 × 105 in immune +/+ mice to 2.5 × 106 per spleen at day 3 after reinfection (Figure 5C). Likewise, LCMV-specific CD8 T cells expanded from 4 × 105 in immune mIg-Tg mice to 5 × 106 per spleen at day 3 after re-infection. In contrast, naive mice generated only 1 × 103 per spleen by this time.
Figure 5. Anamnestic responses in immune mIg-Tg mice.
Wildtype and mIg-Tg mice immunized 90 days earlier with LCMV were re-challenged with 106 PFU of LCMV (strain-Armstrong) and at day 3 after the re-infection, levels of virus and T cell responses were quantified. (A) The level of virus infection in the liver was determined by plaque assay. The amount of infection in the liver of naive mice is shown for comparison. (B) Levels of CTL in each group were measured by direct ex vivo 51Cr-release assay. Average specific lysis for immune mIg-Tg, +/+, and naive mice are shown with standard deviation error bars. (C) The number of LCMV-specific CD8+ T cells was determined by IFN-γ ELISPOT following NP118-126 stimulation. Horizontal lines represent the number of memory cells found in immune mice before re-infection, and the bars show the number found after re-infection. Bars represent the average of 2–3 mice. (D) CD4+ T cells from 2–3 mice were purified and the number of LCMV-specific CD4+ T cells was determined by IFN-γ ELISPOT following stimulation with LCMV-infected carrier spleen cells. The horizontal lines represent the number of memory CD4+ T cells found in immune mice before re-infection. Purified CD4+ T cells from each group were also analyzed by ELISA for IL-2 secretion following virus stimulation in vitro. The limit of detection for this assay was 15pg/ml. Dashed lines depict limits of detection for each assay.
There was also secondary expansion of memory CD4 T cells in immune +/+ and mIg-Tg mice. CD4 T cells were purified from immune mice before and after challenge infection and analyzed by IFN-γ ELISPOT (Figure 5D). The number of antigen-specific CD4 T cells expanded from 4 × 103 to 1.6 × 104 per spleen 3 days after the infection in +/+ mice. Similarly, the number of antigen-specific CD4 T cells in mIg-Tg mice expanded from 5 × 103 to 3.5 × 104 per spleen. By comparison, naive mice generated only 500 specific cells during the same period. The responding CD4+ T cells were also capable of producing IL-2. These data show that memory CD8+ and CD4+ T cells were functionally intact in mIg-Tg mice, and they were able to respond to challenge infection by increasing in number and producing cytokine. These findings establish that serum antibody and LCMV-antigen / antibody complexes are not required to maintain CD4 or CD8 memory.
B cell-deficient mice are impaired in their ability to resolve chronic LCMV infection
Previous studies revealed an important role for CD4 T cells during chronic viral infection. When CD4 T cell responses are missing due to gene ablation, transient depletion with antibody or lack of costimulation, CD8-dependent clearance of chronic virus infections becomes impaired (60–62). Similar associations of CD4+ T cell loss with diminished antiviral CTL are seen after HIV, HBV, HCMV, and HCV infections in humans (63–65).
Previous studies in mice examined situations in which no CD4 response (or a severely impaired response) was present during all phases of the chronic infection. So it is unclear whether CD4+ T cells function early on, perhaps to qualitatively or quantitatively enhance CTL development and memory CD8 development (66–68), or if they function later, perhaps to maintain antiviral CTL (69). Since B cell-deficient mice generate strong primary antiviral CD8 and CD4 responses, but then lose the CD4 response over the following 2–3 weeks, it was of interest to examine whether loss of T-helper cell responses would compromise CTL-mediated clearance of chronic LCMV infection. Therefore, B cell-deficient mice were infected with various strains of LCMV that normally persist in the serum in +/+ mice for 3 weeks to 4 months, depending on the variant. These variants spread throughout the body, but the viral load was eventually reduced in mice capable of maintaining strong CTL responses (54, 55, 70). While +/+ mice eventually controlled these infections, B cell-deficient mice never reduced the level of virus in the blood, liver, kidney, or lung (Figure 6A, and data not shown). Even when the level of virus was similar in magnitude early on (Clone13, A22), only the +/+ mice cleared the infection in the blood.
Figure 6. B cell-deficient mice are unable to limit disseminated LCMV infection.

The ability of mice to control chronic LCMV infection was followed as a stringent measure of their immune competence. (A) B cell-deficient (•) and +/+ (■) mice were infected with 3 different strains of LCMV that cause persistent infections. Mice were retro-orbitally bled at various times after infection and serum levels of virus were determined by plaque assay. (B-F) A separate set of B cell-deficient and +/+ mice were given LCMV-t1b, and 44 days later, the antiviral T cell responses in their spleens and viral load were measured. (B) An example of the CD8 response in mice as measured by intracellular IFNγ staining. The oval identifies the GP33-specific cells among all spleen cells and the numbers represent their frequency among all CD8 T cells. (C) The number of epitope-specific CD8+ T cells in wildtype (shaded bars) and B cell-deficient (open bars) was determined by intracellular IFNγ staining (top) and by tetramer staining (bottom). (D) The GP61-80-specific CD4+ T cell response was determined in these same mice by intracellular IFNγ staining. The dot plot is gated on all spleen cells and the oval identifies the IFNγ+ve CD4+ T cells. The number indicates the percentage of IFNγ +ve CD4+ T cells among all CD4+ T cells. (E) The total number of GP61-80-specific CD4+ T cells per spleen in these mice (top). Purified CD4+ T cells from two B cell-deficient and +/+ mice that were given chronic virus infection were restimulated in vitro with virus-infected carrier spleen cells; the bars indicate the amount of IL-2 produced by these cells as measured by ELISA (bottom). (F) The bar graphs show the level of virus found in the LCMV-t1b-infected mice at day 44 as determined by plaque assay. (G-H) Spleen cells (2–3 × 107) from LCMV immune +/+ or B cell-deficient mice were adoptively transferred to congenitally infected LCMV-carrier mice. (G) The recipients were retro-orbitally bled at various times after transfer, and serum levels of virus were measured by plaque assay. The average and standard deviation of 4 mice per group are shown. (H) Spleen cells from the carrier mice were analyzed at day 160 post-transfer for LCMV-specific CTLprecursors by limiting dilution assay. While CTLp were detectable in mice that received immune +/+ cells, the number of CTLp in mice that received immune B cell-deficient cells was below detection. The dashed lines indicate the limits of detection of the assays.
To determine whether the lack of viral control in B cell-deficient mice correlated with the reduced frequency of LCMV-specific T cells, T cell responses were analyzed in a separate set of mice that were given LCMV-t1b infection 44 days before. While wildtype mice contained an elevated frequency of GP33-41-specific CD8+ T cells, infected B cell-deficient mice had a very low frequency (Figure 6B). The total number of epitope-specific CD8+ T cells in B cell-deficient mice that were able to make IFNγ was 1% of that found in +/+ mice (Figure 6C, top). Tetramer staining confirmed that there were fewer epitope-specific CD8+ T cells in the B cell-deficient mice compared to the infected wildtype mice (Figure 6C, bottom). During this chronic virus infection, LCMV-specific CD4 responses were also lost in B cell-deficient mice (Figure 6D and 6E). In contrast to +/+ mice, IL-2 production by CD4+ T cells was severely diminished in B cell-deficient mice (Figure 6E, bottom). The defective antiviral T cell responses seen in the B cell-deficient mice correlated with elevated viral loads in the livers, lungs, and kidneys of the same mice; the wildtype mice that contained functional T cell responses had a lower viral load at this time (Figure 6F). Hence, chronic virus infection in mice devoid of B cells results in the loss of virus-specific T helper cells and the exaggerated deletion of virus-specific CD8+ T cell responses.
A subset of CD8+ T cells can be programmed early to differentiate into memory cells (71–74). It is unknown what signals are required for this differentiation event that occurs within the first week of infection, although CD4+ T cells contribute to this process (66, 67, 69). A primary CD4+ T cell response is made in LCMV-Armstrong infected B cell-deficient mice during this programming period (Figure 1), and they generate normal numbers of memory CD8 T cells that can resolve a re-challenge infection ((27, 30) and Figure 1). To test whether the virus-specific memory CD8+ T cells found in acutely infected B cell-deficient mice are capable of controlling a widespread infection, spleen cells from LCMV-Armstrong immunized B cell-deficient mice were transferred into LCMV-carrier mice that are congenitally infected and immunologically tolerant to the virus; virus reduction in these LCMV-carrier mice is due to the transferred cells (52, 75, 76). Consistent with an earlier report (75), the level of virus in the blood of recipient mice was reduced to below the limits of detection by splenocytes from the immune +/+ mice; however, the cells from the immunized B cell-deficient mice (Figure 6G) did not do so. This difference in viral load was reflected by the number of virus-specific CTLp surviving later (Figure 6H): carrier mice that received wildtype immune cells and controlled the infection had 4 × 103 CTLp per spleen whereas carrier mice that received immune B cell-deficient spleen cells had levels of CTLp that were below detection (<400 per spleen). Even though Armstrong immune +/+ and B cell-deficient mice had memory CTL (27), those present in immune B cell-deficient mice could not confer protection to infected mice. The results indicate that B cells and memory CD4 T cells play important roles for maintaining CTL in conditions of chronic infection and that having CD4 help early on is not sufficient to limit these infections.
Discussion
CD8 memory is maintained in the absence of specific antigen and even selecting MHC molecules, and consistent with these findings, CD8 memory does not require B cells or antigen/antibody complexes for maintenance ((27, 29, 30) and Figure 1 and Figure 4). Evidence also indicates that CD4+ memory T cells survive in the absence of MHC (6, 48). However, CD4+ T cell memory maintenance requires B cells in some systems, but not in others; no clear picture has emerged to explain these differences. Importantly, in cases where B cells affect CD4+ T cell memory, the mechanisms have not been elucidated. Here, we examined the role of B cells and antigen / antibody complexes in the induction and maintenance of T cell responses to LCMV, including the relevance of such responses to viral clearance and secondary protection. We learned that a primary CD4 response is formed to LCMV in the absence of B cells; however, it decays rapidly and is essentially undetectable by thirty days post infection. Also, the role of B cells in this process, contrary to predictions, was independent of antibody secretion.
A major finding of this study is that B cells play a pivotal role during the contraction phase to generate CD4 memory. It was previously thought that immune complexes on FDC were acquired by B cells (11) or by dendritic cells (17), which then process the complex and present antigenic peptide via MHCII for memory T cells. However, our results with mIg-Tg mice show that CD4 memory does not depend upon these complexes, since these mice do not secrete the antibody required for their formation. Other evidence indicates that CD4 memory cells survive in the absence of specific antigen and MHCII (6, 48), although in one case they became non-functional without these signals (49). We find that not only do memory cells persist in the absence of antigen/antibody complexes in mIg-Tg mice, these cells are functional in cytokine production and fully capable of undergoing secondary re-call responses in vivo (Figure 5). An important caveat is that while the B cells in these mice have a transgene coding for the heavy chain of Ig, it is possible that variation in the light chain of Ig leads to virus-specific B cells, since this H chain in combination with various L chains can contribute to a variety of autoantibodies (51) and responses to a variety of proteins ((18) and unpublished). This raises an interesting possibility, for which there is no precedent: virus-specific B cells could serve as APCs by retaining antigen via B cell receptor / antigen complexes and then re-presenting antigenic peptide via MHCII. B cells can retain processed antigen for 1 day (77), but whether they can retain antigen longer remains to be seen. B cells can clearly present antigens to CD4+ T cells and they can also transport particulate antigens to the follicles (15).
B cells serve a vital role during persistent viral infections, as B cell-deficient mice are less able to eliminate these infections (Figure 6 and (28, 58)). However, our interpretation of why B cell-deficient mice are impaired in their responses to chronic infection differs from that proposed by earlier studies that did not quantify CD4 responses and perhaps for this reason favored the conclusion that neutralizing antibody is vital for the eventual resolution of chronic infection. In view of the fact that B cell-deficient mice lose CD4 memory and that depletion of CD4+ T cells even transiently during the acute phase of the response impairs the ability of CTL to resolve infection (54, 78, 79), an additional mechanism is that CTL responses are lost during chronic infection due to short-lived virus-specific CD4 help. Perhaps CD4-dependent IL-2 production or CD4 induction of co-stimulatory molecule expression on APCs allows CD8+ T cells to endure high antigen load over time. Our results highlight the importance of B cells and CD4 memory for sustaining CD8 CTL during chronic infection, which is particularly relevant to successful vaccine design in humans. Another clinical implication from our study is that B cell-depleting drugs may inadvertently lead to reduced memory T cells responses and increase patient susceptibility to viral infections. Indeed, there is an increased incidence of viral, bacterial and fungal infections in patients that have received B cell-depleting drugs (80).
CD8+ and CD4+ T cell memory are differentially regulated (1). The expansion of the CD8 response is much greater (>20 fold) than the CD4 response. In addition, the contraction phase of the CD8 response is quick, occurring mostly within the second week after infection, whereas that of the CD4 response is protracted and occurs over a month (81, 82). The resulting levels of memory remain greater for CD8+ T cells than for CD4+ T cells. Once established, CD8 memory is stable over the lifetime of the mouse. CD4 memory is generally found to be long-lived, although some evidence indicates that it slowly declines in geriatric mice (36, 82). Hence, there are regulatory mechanisms that differentially affect the homeostatic levels of CD8 and CD4 memory over the long term (1). Without B cells, the contraction phase of the CD4 response accelerates and becomes more severe, resulting in a complete loss of memory CD4 T cells 2 weeks after the peak response (Figure 2), whereas CD8 memory was maintained stably up to 400 days (the last time point measured) after infection in the same mice. Hence, during the generation of CD4+ T cell memory, B cells function early, at least by the contraction phase of the response; however, they may act even earlier than this by programming T cell survival during the cognate interactions that stimulated T cell proliferation. Even signals received during the first few days of a response may be sufficient to activate and differentiate effector and memory CD8 cells (71, 72) and influence contraction (83), so it may be that B cells further these earliest differentiation signals to improve CD4 memory development. Qualitative differences in the way they stimulate CD4 T cells – for example via cytokines (84) and/or costimulatory molecule expression could be crucial for selecting a pool of CD4 T cells that survive on into the memory phase. In this scenario, the unique roles of B cells may be due to B cell-specific combinations of local cytokines (e.g. LTα, LTβ, TNF) as well as costimulatory molecules (e.g. B7-RP, OX40L)(85, 86) that act directly on CD4 memory cells; indeed B cells differ from dendritic cells markedly in these respects. B cells might act through indirect ways to augment CD4+ T cell responses. B cells facilitate the movement of antigen presenting dendritic cells within lymphoid organs (87, 88), which may impact the quality of antigen presentation to CD4+ T cells. B cells also influence T cell trafficking and accumulation within the lympoid organs (88). B cell effects on stromal cell number or organization might affect T cell survival or the basal IL-7 levels that are key for CD4 memory (3, 4). Thus, B cells most likely contribute to the quality of CD4 T cell memory via multiple mechanisms. Our data indicate that optimal vaccine strategies will recruit B cells to engage CD4+ T cells, which in turn will directly affect CD4 T cell memory and indirectly affect CD8 T cell effector function, particularly in the face of chronic infection.
Acknowledgments
We thank Rita J. Concepcion and Morry Hsu for excellent technical assistance.
Footnotes
This work was supported by National Institutes of Health R-01 grants AI074862 to J.K.W, AI43603 to M.J.S, and AI-30048 to R.A.; a Burroughs-Wellcome Fund grant 1004313 and National Institutes of Health R-01 grants AI066232-01 and CA038350 to S.M.K; an Associate Investigator Award from the Department of Veterans Affairs and a postdoctoral fellowship from the National Multiple Sclerosis Society to M.S.A.; and a National Institutes of Health Training Grant AI07019 and a Richard K. Gershon Predoctoral Fellowship to L.G.H.
References
- 1.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 2.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–2346. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 3.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 6.Seddon B, Tomlinson P, Zamoyska E. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 7.Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, Homann D. IL-7 regulates basal homeostatic proliferation of antiviral CD4+T cell memory. Proc Natl Acad Sci U S A. 2004;101:9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray D. A role for antigen in the maintenance of immunological memory. Nature reviews. 2002;2:60–65. doi: 10.1038/nri706. [DOI] [PubMed] [Google Scholar]
- 9.Gray D, Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tew JG, Mandel TE. Prolonged antigen half-life in the lymphoid follicles of specifically immunized mice. Immunology. 1979;37:69–76. [PMC free article] [PubMed] [Google Scholar]
- 11.Gray D, Kosco M, Stockinger B. Novel pathways of antigen presentation for the maintenance of memory. Int Immunol. 1991;3:141–148. doi: 10.1093/intimm/3.2.141. [DOI] [PubMed] [Google Scholar]
- 12.Szakal AK, Kosco MH, Tew JG. A novel in vivo follicular dendritic cell-dependent iccosome-mediated mechanism for delivery of antigen to antigen-processing cells. J Immunol. 1988;140:341–353. [PubMed] [Google Scholar]
- 13.Kosco MH, Szakal AK, Tew JG. In vivo obtained antigen presented by germinal center B cells to T cells in vitro. J Immunol. 1988;140:354–360. [PubMed] [Google Scholar]
- 14.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr., Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 17.van Essen D, Dullforce P, Brocker T, Gray D. Cellular interactions involved in Th cell memory. J Immunol. 2000;165:3640–3646. doi: 10.4049/jimmunol.165.7.3640. [DOI] [PubMed] [Google Scholar]
- 18.Hannum LG, Haberman AM, Anderson SM, Shlomchik MJ. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J Exp Med. 2000;192:931–942. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annual review of immunology. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 20.Ware CF, Crowe PD, Grayson MH, Androlewicz MJ, Browning JL. Expression of surface lymphotoxin and tumor necrosis factor on activated T, B, and natural killer cells. J Immunol. 1992;149:3881–3888. [PubMed] [Google Scholar]
- 21.Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol. 2004;172:3422–3427. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- 22.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 23.Suresh M, Lanier G, Large MK, Whitmire JK, Altman JD, Ruddle NH, Ahmed R. Role of lymphotoxin alpha in T-cell responses during an acute viral infection. J Virol. 2002;76:3943–3951. doi: 10.1128/JVI.76.8.3943-3951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puglielli MT, Browning JL, Brewer AW, Schreiber RD, Shieh WJ, Altman JD, Oldstone MB, Zaki SR, Ahmed R. Reversal of virus-induced systemic shock and respiratory failure by blockade of the lymphotoxin pathway. Nat Med. 1999;5:1370–1374. doi: 10.1038/70938. [DOI] [PubMed] [Google Scholar]
- 25.Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annual review of immunology. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- 26.Heath WR, Carbone FR. Cytotoxic T lymphocyte activation by cross-priming. Curr Opin Immunol. 1999;11:314–318. doi: 10.1016/s0952-7915(99)80050-8. [DOI] [PubMed] [Google Scholar]
- 27.Asano MS, Ahmed R. CD8 T cell memory in B cell-deficient mice. J Exp Med. 1996;183:2165–2174. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brundler MA, Aichele P, Bachmann M, Kitamura D, Rajewsky K, Zinkernagel RM. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur J Immunol. 1996;26:2257–2262. doi: 10.1002/eji.1830260943. [DOI] [PubMed] [Google Scholar]
- 29.Di Rosa F, Matzinger P. Long-lasting CD8 T cell memory in the absence of CD4 T cells or B cells. J Exp Med. 1996;183:2153–2163. doi: 10.1084/jem.183.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen H, Whitmire JK, Fan X, Shedlock DJ, Kaech SM, Ahmed R. A specific role for B cells in the generation of CD8 T cell memory by recombinant Listeria monocytogenes. J Immunol. 2003;170:1443–1451. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- 31.Bruno L, Kirberg J, von Boehmer H. On the cellular basis of immunological T cell memory. Immunity. 1995;2:37–43. doi: 10.1016/1074-7613(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 32.Hou S, Hyland L, Ryan KW, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 33.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 34.Mullbacher A. The long-term maintenance of cytotoxic T cell memory does not require persistence of antigen. J Exp Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 36.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and Memory CD4+ T Cell Survival Controlled by Clonal Abundance. Science. 2006 doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Wu Y, Ramarathinam L, Guo Y, Huszar D, Trounstine M, Zhao M. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int Immunol. 1995;7:1353–1362. doi: 10.1093/intimm/7.8.1353. [DOI] [PubMed] [Google Scholar]
- 38.Constant SL. B lymphocytes as antigen-presenting cells for CD4+ T cell priming in vivo. J Immunol. 1999;162:5695–5703. [PubMed] [Google Scholar]
- 39.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 40.Epstein MM, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Topham DJ, Tripp RA, Hamilton-Easton AM, Sarawar SR, Doherty PC. Quantitative analysis of the influenza virus-specific CD4+ T cell memory in the absence of B cells and Ig. J Immunol. 1996;157:2947–2952. [PubMed] [Google Scholar]
- 42.Phillips JA, Romball CG, Hobbs MV, Ernst DN, Shultz L, Weigle WO. CD4+ T cell activation and tolerance induction in B cell knockout mice. J Exp Med. 1996;183:1339–1344. doi: 10.1084/jem.183.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams GS, Oxenius A, Hengartner H, Benoist C, Mathis D. CD4+ T cell responses in mice lacking MHC class II molecules specifically on B cells. Eur J Immunol. 1998;28:3763–3772. doi: 10.1002/(SICI)1521-4141(199811)28:11<3763::AID-IMMU3763>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Brunham RC. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998;161:1439–1446. [PubMed] [Google Scholar]
- 45.Johansson M, Lycke N. Immunological memory in B-cell-deficient mice conveys long-lasting protection against genital tract infection with Chlamydia trachomatis by rapid recruitment of T cells. Immunology. 2001;102:199–208. doi: 10.1046/j.1365-2567.2001.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lund FE, Hollifield M, Schuer K, Lines JL, Randall TD, Garvy BA. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J Immunol. 2006;176:6147–6154. doi: 10.4049/jimmunol.176.10.6147. [DOI] [PubMed] [Google Scholar]
- 47.Matsuzaki G, Vordermeier HM, Hashimoto A, Nomoto K, Ivanyi J. The role of B cells in the establishment of T cell response in mice infected with an intracellular bacteria, Listeria monocytogenes. Cell Immunol. 1999;194:178–185. doi: 10.1006/cimm.1999.1503. [DOI] [PubMed] [Google Scholar]
- 48.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 49.Kassiotis G, Garcia S, Simpson E, Stockinger B. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat Immunol. 2002;3:244–250. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 50.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 51.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmed R, Jamieson BD, Porter DD. Immune therapy of a persistent and disseminated viral infection. J Virol. 1987;61:3920–3929. doi: 10.1128/jvi.61.12.3920-3929.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitmire JK, Asano MS, Murali-Krishna K, Suresh M, Ahmed R. Long-term CD4 Th1 and Th2 memory following acute lymphocytic choriomeningitis virus infection. J Virol. 1998;72:8281–8288. doi: 10.1128/jvi.72.10.8281-8288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995;69:1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomsen AR, Johansen J, Marker O, Christensen JP. Exhaustion of CTL memory and recrudescence of viremia in lymphocytic choriomeningitis virus-infected MHC class II-deficient mice and B cell-deficient mice. J Immunol. 1996;157:3074–3080. [PubMed] [Google Scholar]
- 59.Whitmire JK, Slifka MK, Grewal IS, Flavell RA, Ahmed R. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J Virol. 1996;70:8375–8381. doi: 10.1128/jvi.70.12.8375-8381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khanolkar A, Fuller MJ, Zajac AJ. T cell responses to viral infections: lessons from lymphocytic choriomeningitis virus. Immunologic research. 2002;26:309–321. doi: 10.1385/IR:26:1-3:309. [DOI] [PubMed] [Google Scholar]
- 61.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams MA, Onami TM, Adams AB, Durham MM, Pearson TC, Ahmed R, Larsen CP. Cutting edge: persistent viral infection prevents tolerance induction and escapes immune control following CD28/CD40 blockade-based regimen. J Immunol. 2002;169:5387–5391. doi: 10.4049/jimmunol.169.10.5387. [DOI] [PubMed] [Google Scholar]
- 63.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 65.Lieberman J, Manjunath N, Shankar P. Avoiding the kiss of death: how HIV and other chronic viruses survive. Curr Opin Immunol. 2002;14:478–486. doi: 10.1016/s0952-7915(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 66.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 67.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 68.Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J Immunol. 2004;172:2834–2844. doi: 10.4049/jimmunol.172.5.2834. [DOI] [PubMed] [Google Scholar]
- 69.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whitmire JK, Flavell RA, Grewal IS, Larsen CP, Pearson TC, Ahmed R. CD40-CD40 ligand costimulation is required for generating antiviral CD4 T cell responses but is dispensable for CD8 T cell responses. J Immunol. 1999;163:3194–3201. [PubMed] [Google Scholar]
- 71.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 73.van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 74.Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 75.Homann D, Tishon A, Berger DP, Weigle WO, von Herrath MG, Oldstone MB. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice. J Virol. 1998;72:9208–9216. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oldstone MB, Blount P, Southern PJ, Lampert PW. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986;321:239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- 77.Gondre-Lewis TA, Moquin AE, Drake JR. Prolonged antigen persistence within nonterminal late endocytic compartments of antigen-specific B lymphocytes. J Immunol. 2001;166:6657–6664. doi: 10.4049/jimmunol.166.11.6657. [DOI] [PubMed] [Google Scholar]
- 78.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW, Zinkernagel RM. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gea-Banacloche JC, Weinberg GA. Monoclonal antibody therapeutics and risk for infection. The Pediatric infectious disease journal. 2007;26:1049–1052. doi: 10.1097/INF.0b013e31815a044f. [DOI] [PubMed] [Google Scholar]
- 81.Kamperschroer C, Quinn DG. Quantification of epitope-specific MHC class-II-restricted T cells following lymphocytic choriomeningitis virus infection. Cell Immunol. 1999;193:134–146. doi: 10.1006/cimm.1999.1458. [DOI] [PubMed] [Google Scholar]
- 82.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 83.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 84.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Linton PJ, Bautista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, Padrick RC, Bradley LM. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 87.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J Exp Med. 2001;194:1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]