Abstract
Our previous studies demonstrate that chronic testosterone treatment augments vascular tone under physiological conditions and exacerbates endotoxin-induced inflammation in the cerebral circulation. However, testosterone can be metabolized by aromatase to estrogen, evoking a balance between androgenic and estrogenic effects. Therefore, we investigated the effect of the non-aromatizable androgen receptor agonist, dihydrotestosterone (DHT), on the inflammatory NFκB pathway in cerebral blood vessels. Cerebral arteries were isolated from orchiectomized male rats treated chronically with DHT in vivo. Alternatively, pial arteries were isolated from orchiectomized males and were exposed ex vivo to DHT or vehicle in culture medium. DHT treatment, in vivo or ex vivo, increased nuclear NFκB activation in cerebral arteries and increased levels of the proinflammatory products of NFκB activation, cyclooxygenase-2 (COX-2) and inducible NO synthase (iNOS). Effects of DHT on COX-2 and iNOS were attenuated by flutamide. In isolated pressurized middle cerebral arteries from DHT-treated rats constrictions to the selective COX-2 inhibitor NS398 or the selective iNOS inhibitor L-nil were increased, confirming a functional consequence of DHT exposure. In conclusion, activation of the NFκB mediated COX-2/iNOS pathway by the selective androgen receptor agonist, DHT, results in a state of vascular inflammation. This effect may contribute to sex-related differences in cerebrovascular pathophysiology.
Keywords: dihydrotestosterone, androgen, cerebral arteries, vascular tone, inflammation, rats
Introduction
Effects of sex steroids on cerebral vascular function and cerebrovascular pathophysiology are complex. Experimental stroke models in rodents demonstrate that androgen treatment exacerbates brain injury following ischemic insult (Cheng et al 2007, Hawk et al 1998). In contrast, 17β-estradiol significantly reduces ischemic injury (Alkayed et al 1998, Simpkins et al 1997). Studies have demonstrated that 17β-estradiol decreases cerebrovascular tone by enhancing endothelial-dependent pathways (Geary et al 1998, Krause et al 2006). On the other hand, androgenic compounds such as testosterone have the opposite effect; they enhance tone of cerebral vessels (Gonzales et al 2004). Another example of different effects of estrogen and androgen is observed with vascular inflammation. Using lipopolysaccharide to induce inflammation, chronic testosterone treatment augments levels of the proinflammatory enzymes, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), in male rat brain blood vessels (Razmara et al 2005). In contrast, this same study demonstrated that treatment of male rats with 17β-estradiol significantly suppresses cerebrovascular levels of COX-2 and iNOS protein after an inflammatory stimulus. Taken together these data suggest that androgens, unlike estrogens, may influence ischemic injury by enhancing pro-inflammatory processes in the vasculature.
Certainly, the brain vasculature plays a central role in initiation of inflammation after brain ischemia (Del Zappo and Mabuchi 2003, Nedergaard et al 2003), and inflammation is a critical process in the outcome of conditions such as stroke (Dinagl et al 1999, Emsley and Tyrrell 2002). However, it is important to point out that inflammation may have both beneficial and injurious effects (Kempermann and Neumann 2003). Acute inflammatory responses are part of the normal healing process and may be beneficial. However, in the brain, prolonged inflammation is thought to increase edema, decreasing cerebral blood flow and exacerbating neurological dysfunction.
Interestingly, virtually no data have been reported on the influence of androgens on inflammatory responses in the cerebral vasculature under physiologic conditions in the absence of induced inflammation (i.e. ischemic insult or endotoxic exposure). In addition, many of the specific molecular and cellular mechanisms underlying sex differences under both normal and pathophysiological conditions remain unclear; this is especially true of the cerebral circulation. Thus it is important to determine the contribution of androgen actions on the tissue.
Not all reports agree that androgens exacerbate stroke. For example, clinical studies show that the severity of stroke in aged men is inversely proportional to circulating testosterone (Jeppesen et al 1996). An important factor that may account for these discrepancies concerns the ability of testosterone to exert effects either directly on the androgen receptor or by means of metabolism to either the potent androgen, dihydrotestosterone (DHT), or aromatization to estrogen. Previously we have shown that the testosterone metabolizing enzymes, 5-α reductase and aromatase are present in cerebral arteries (Gonzales et al 2007), underscoring the possibility that, due to local tissue differences in the distribution of testosterone metabolizing enzymes, the balance of androgenic and estrogenic effects caused by testosterone may vary regionally.
Therefore, to better understand the nature of androgenic effects on the cerebral vasculature we investigated the influence of DHT, a testosterone metabolite not subject to aromatization and more potent as an agonist at the androgen receptor, on the NFκB pathway and downstream proinflammatory enzymes, COX-2 and iNOS. We first determined whether chronic DHT treatment modulates NFκB activation and levels of COX-2 and iNOS in cerebral arteries. We then evaluated the possible functional consequence of the modulation of these proinflammatory factors by DHT in isolated, pressurized middle cerebral arteries.
Methods
Chronic In Vivo Hormone Treatment
All experimental and surgical protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine. Male Wistar rats (3 mo old; Charles Rivers) were orchiectomized by the supplier and delivered one week post surgery. One day following arrival, rats were anesthetized with isoflurane (1.5%) and aseptically implanted with placebo or 5α-androstan-17α-ol-3-one (DHT; 25 or 45 mg/21day) pellets (Innovative Research of America Inc, Sarasota, FL). Pellets were tunneled 2 to 3 cm underneath the skin, and the incision site was secured with small rodent staples. Past studies from our laboratory demonstrated that 3 to 4 weeks of testosterone treatment alters vascular reactivity. In addition, a study by Cheng et al (2007) demonstrated that DHT enhanced ischemic brain injury and altered a number of genes including COX-2 following only 1-week post hormone treatment. Based on the outcome of these past studies animals were treated with DHT for 3 weeks to assess the biochemical and vascular effects of this potent androgen receptor agonist on the proinflammatory enzymes COX-2 and iNOS.
Post-surgery animals were treated with a single injection (i.m.) of penicillin (penicillin G benzathine/penicillin G procaine, 30,000 U) and a topical triple antibiotic. Following anesthesia recovery, rats were returned to animal housing (12 hr:12 hr light/dark cycle) with fresh water, food, and bedding.
Following DHT or placebo implants, animals were deeply anesthetized with sodium pentobarbital (50 mg/kg i.p.), and body weight recorded. Next the thoracic cavity was exposed, and a direct cardiac puncture was used to collect blood samples for serum DHT levels. Samples were stored at −80°C until DHT levels were measured with an ELISA kit (Alpha Diagnostics, San Antonio, TX; DHT ELISA limit of detection = 6 pg/ml). After blood collection, animals were immediately heparinized, exsanguinated and decapitated. Whole brain was removed and placed in a Sylgard-coated dissection dish containing ice-cold phosphate buffered saline (PBS). Artery segments [middle cerebral artery (MCA) and/or pial arteries] were carefully dissected using a dissecting microscope and prepared for Western blot, nuclear isolation/NFκB activation measurements, or functional contractile studies. Dorsolateral and ventral lobes of the prostate were dissected free from the prostate- urethra-bladder complex via an alba linea excision, and dry weights recorded. Together with serum DHT levels, prostate weights were used to verify efficacy of androgen treatment.
Ex Vivo Hormone Treatment
Under aseptic conditions, whole brain was removed from orchiectomized rats, and pial arteries were dissected and placed in a sterile 12 well incubation plate containing ice cold DMEM (n=1/well). Artery segments were then transferred to wells containing a 1:1 mix of pre-warmed (37°C) phenol red free DMEM (Sigma Chemical, St Louis MO) and serum- and hormone-free Medium 231 (Cascade Biologics, Portland OR). Arteries were equilibrated for 30 min in a 5% CO2 incubator at 37°C before being transferred to wells containing fresh medium (DMEM/Medium 231) supplemented with hormone, drug or vehicle. Artery segments used for NFκB activation studies were incubated for 1 hour at 37°C while artery segments used for Western analysis were incubated for 8 hours at 37°C.
NFκB p65 Activation Measurements
Nuclei were isolated from pial artery segments using a nuclear extraction kit following the manufacturer’s instructions (Active Motif; Carlsbad, CA). All nuclear fractions were stored at −80°C before analysis. Western blot of the nuclear marker histone-1 (Santa Cruz Biotechnology, Santa Cruz, CA) verified separation of nuclear and cytosolic fractions (data not shown). Protein content for nuclear fractions was determined by the bicinchoninic acid (BCA) assay (Pierce, Rockford, IL).
DNA binding activity for NFκB p65 was measured in pial artery nuclear extracts. In brief, pial artery nuclear protein samples (10 μg/well) or pial artery nuclear extract samples exposed to cobalt chloride (2.5 μg/well; positive control) were added to 96-well plates labeled with an immobilized oligonucleotide containing the NFκB consensus site (5′-GGGACTTTCC-3′). Activated NFκB was detected after 1 hr incubation using an anti-NFκB p65 antibody that recognizes an epitope accessible only when NFκB is activated and bound to its target DNA (TransAM NFκB p65 immunoassay-based kit; Active Motif, Carlsbad, CA). Secondary horseradish peroxidase antibody and developing solution exposure completed the reaction. Absorbance values as a result of the colorimetric reaction were measured within 5 min using a spectrophotometer at 450 nm.
Western Blot
Protein levels for the proinflammatory markers, COX-2 and iNOS, were determined using standard immunoblotting methods. Briefly, pial arteries were gently homogenized in ice cold lysis buffer (pH 7.4). Lysis buffer contained β-glycerophosphate (50 mmol/L), sodium orthovanadate (100 μmol/L), magnesium chloride (2 mmol/L), EGTA (1 mmol/L), and Triton X-100 (0.5%) and was prepared fresh. Protease inhibitors (DL-dithiolthreitol 1 mmol/L, phenylmethylsulfonyl fluoride 1 mmol/L, pepstatin 20 μmol/L, leupeptin 20 mmol/L, and aprotinin 0.1 U/mL) were individually prepared as stock solutions dissolved in ddH2O or ethanol and stored according to suppliers suggestion. Following 20 min incubation, homogenates were centrifuged (4500 g for 10 min 4°C), the supernant drawn off, and a small portion of sample was analyzed for protein content using a bicinchoninic acid protein assay (Pierce; Rockford, IL). Samples for Western analysis were dissolved in Tris-glycine sodium dodecyl sulphate (SDS) sample buffer and boiled for 6 min. Equal amounts of protein (ranging from 30 to 40 mg/lane/experiment) were then loaded and separated in 8% polyacrylamide gels using SDS-polyacrylamide gel electrophoresis. Separated proteins were transferred to polyvinylidene difluoride membranes and blocked overnight at 4° C in PBS (0.1% Tween and 6.5% nonfat dry milk). Membranes were probed with antibodies specific for either COX-2 (1:500), iNOS (1:500), or GAPDH (1:20,000) (Santa Cruz Biotechnology, Santa Cruz, CA). Proteins of interest were visualized using either enhanced chemiluminescence in conjunction with a horseradish peroxidase-labeled secondary antibody (1:15,000) or infrared imaging system (LI-COR Biosciences) using a fluorescently labeled secondary antibody (1:20,000; IRDye 680 Goat anti-rabbit, LI-COR, Lincoln, NE). Densitometry of bands was analyzed with UN-SCAN-it software (Silk Scientific; Orem, UT) or the analysis software provided by LI-COR. Each blot included samples from all of the relevant groups along with GAPDH loading controls. Intensity ratio for each band was normalized to the intensity of its corresponding GAPDH loading control. The normalized values were then expressed as a ratio relative to control (placebo or vehicle) within each blot.
Isolated Pressurized Middle Cerebral Artery (MCA) Preparation
A segment of the second order MCA (250 μm dia) was carefully dissected from whole brain, kept in ice-cold PSS containing Ca2+ and aerated with oxygen/carbon dioxide gas mixture. The proximal end of the artery was cannulated with a glass micropipette and secured with a single cotton filament. Residual blood cells were gently rinsed from the lumen, and the distal end of the MCA was cannulated and secured in a small vessel bath (Living Systems, Burlington VT). Each MCA was slowly pressurized to 60 torr with PSS using a servo-controlled peristaltic pump (Living Systems, Burlington VT) while superfusing with aerated PSS (21% O2/%6 CO2, balance N2; pH 7.4) at 37°C. Intraluminal diameters were visualized using an inverted microscope (Nikon, USA) equipped with a CCD camera/computer interface system (IonOptix Corp.; Milton, MA) and measured using an electronic dimension analyzer (Living Systems; Burlington, VT). Changes in intraluminal diameter were recorded using a computer-based data acquisition edge-detection system (IonOptix Corp.; Milton, MA).
Functional Responses
MCA segments were first equilibrated in PSS for 1 hour at 60 torr, and diameter was measured at increasing step pressures (60, 80 and 100 torr). In experiments to measure the effect of COX-2 inhibition, arteries were incubated for 30 min in the presence of the non-selective NOS inhibitor, NG-nitro-L-arginine-methyl ester (L-NAME, 100 μM) to eliminate NO production. Diameters in L-NAME were recorded at 60, 80 and 100 torr. In the continued presence of NOS inhibition, selective COX-2 inhibitor NS398 (10 μM) was added to the superfusate, a 30 min incubation ensued, and artery diameter was again recorded at 60, 80, and 100 torr. In separate experiments to measure the effect of inhibiting iNOS, arteries were exposed to L-N6-(1-Iminoethyl)-lysine (L-nil; 5 μM; 30 min) and diameters at 60, 80 and 100 torr were recorded. At the end of each experiment arteries were incubated in Ca2+-free PSS containing EDTA (3 mM; 30 min), and intraluminal step pressure changes (60, 80, 100 torr) were recorded to obtain passive diameters (Table 2).
Table 2.
Passive diameters in Ca2+ free PSS with EDTA in isolated pressurized middle cerebral arteries.
| Passive Pressure (μm) | |||
|---|---|---|---|
| Groups (n) | 60 mm Hg | 80 mm Hg | 100 mm Hg |
| Placebo (7) | 205±17 | 213±17 | 215±16 |
| DHT 25 mg (5) | 227±11 | 233±11 | 237±12 |
| DHT 45 mg (5) | 223±36 | 230±20 | 232±18 |
Values are means ± SEM; number of animals used is listed in parentheses. Placebo, orchiectomized male rats treated with an inert placebo pellet; DHT, orchiectomized male rats treated with dihydrotestosterone (25 mg pellet/21 days or 45 mg/pellet/21 days). There are not significant differences between groups.
Drugs and Chemicals
PSS bicarbonate/phosphate buffer stock solution contained (in mM): 122 NaCl, 5.1 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25.5 NaHCO3, and 0.03 EDTA, 11.5 mM glucose and 1.6 mM CaCl2. NG-nitro-L-arginine-methyl ester (L-NAME) and L-N6-(1-Iminoethyl)-lysine (L-nil) (Cayman Chemical, Ann Arbor, MI) were dissolved in double distilled H2O. N-[2-Cyclohexyloxy-4-nitrophenyl]-methanesulfonamide (NS389) (Cayman Chemical) and flutamide were dissolved in ethanol. Unless noted otherwise, all drugs and chemicals were purchased from Sigma Chemical (St. Louis, MO).
Statistical Analysis
For group comparisons, all Western blots included samples from each relevant group so that these were analyzed on a single blot. Band densities were compared using analysis of variance (ANOVA) with repeated measures, and the Student-Newman-Keuls test was used post hoc when ANOVA indicated statistical differences. Change in body weight was calculated as: [(final weight post-implant – initial weight pre-implant)/final weight post-implant] × 100]. Changes in body weight were compared using ANOVA and Student-Newman-Keuls post hoc test. P≤0.05 was considered statistically significant for analysis.
Results
Dihydrotestosterone increased body and prostate weights
Increase in body weight over 18–20 days was greater in intact males and orchiectomized males receiving chronic in vivo DHT treatment (25 or 45 mg) compared to animals treated with placebo pellets (Table 1). Measurements for prostate weight and DHT serum levels in intact animals were used as an index for a physiological range of DHT in the hormone replacement groups. Orchiectomy resulted in a significant decrease in both prostate weight and serum DHT levels compared to levels in intact males. Replacement of DHT dose dependently increased both prostate weight and DHT serum levels, resulting with the 45 mg DHT dose in values for prostate weight comparable to those recorded in intact males. Similar to prostate weight, chronic DHT treatment dose dependently increased DHT serum levels in the physiological range (Table 1). Therefore, in the remainder of this study we assessed the effects of DHT itself by comparing orchiectomized animals treated with DHT with animals treated with placebo pellets.
Table 1.
Body weight, prostate weight and dihydrotestosterone (DHT) serum levels.
| Groups | Body Weight (% change) | Prostate Weight (mg) | DHT Serum Level (pg/ml) |
|---|---|---|---|
| Intact | 12.3±1.0* (14) | 196±22* (9) | 712±39*† (26) |
| ORX+Placebo | 4.7±0.6† (26) | 36±9† (9) | 78±5† (26) |
| ORX+DHT 25 mg | 7.7±1.2*† (19) | 172±12*† (9) | 417±32*† (19) |
| ORX+DHT 45 mg | 9.5±0.9* (17) | 214±19* (9) | 910±105* (17) |
Percent body weight was calculated as [(final weight 18–20 days post-implant – initial weight pre-implant)/final weight 18–20 days post-implant] × 100. Orchiectomized male rats (ORX) were treated for 18–20 days with blank pellets (Placebo), or 25 or 45 mg dihydrotestosterone pellets (DHT 25 mg; DHT 45 mg). DHT ELISA limit of detection = 6 pg/ml. Values are means ± SEM; animal number is shown in parentheses.
P ≤ 0.05 vs ORX.
P ≤ 0.05 vs DHT 45 mg.
Dihydrotestosterone enhanced NFκ B activation
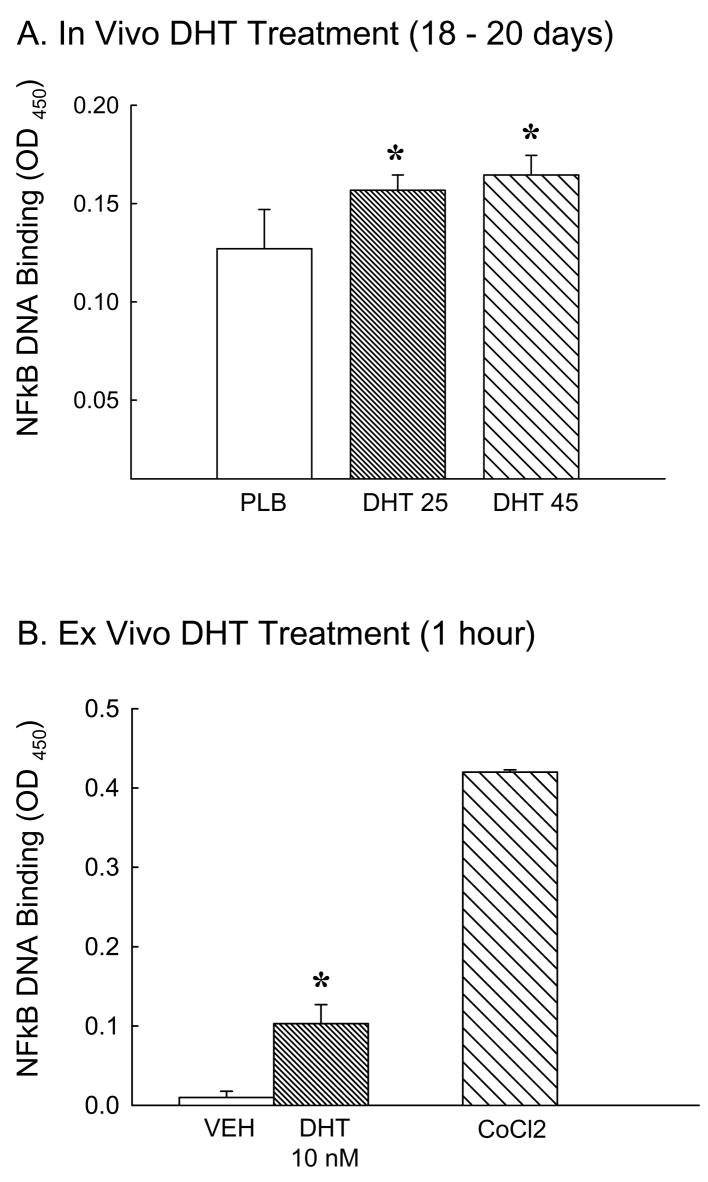
We determined whether chronic DHT treatment, in vivo or ex vivo, activated the NFκB pathway in cerebral arteries. Figure 1A illustrates that chronic treatment with DHT (in vivo) increased nuclear NFκB DNA binding activity in cerebral artery nuclear homogenates compared to nuclear homogenates from placebo treated rats. In DHT groups NFκB DNA binding activity was increased by 26% (DHT 25) and 32% (DHT 45) compared to placebo. Cerebral arteries removed from orchiectomized rats and then incubated with DHT in culture (ex vivo) for 1 hour also showed significant increases in nuclear NFκB DNA binding compared to vehicle treatment (Figure 1B). For ex vivo studies cobalt chloride (CoCl2) stimulated vessels were used as a positive control for NFκB pathway activation.
Figure 1.
Effect of in vivo or ex vivo dihydrotestosterone treatment on NFκB activation. Nuclear NFκB DNA binding activity was measured in pial arteries isolated from orchiectomized male rats A) chronically treated in vivo with placebo (PLB) or dihydrotestosterone (DHT 25 or 45 mg) for 18 to 20 days or B) arteries incubated ex vivo with DHT (10 nM) or vehicle (VEH) for 1 hour. Cobalt chloride (CoCl2) was used as a positive control for NFκB activation. (N = 5 in vivo, 6 ex vivo). *P ≤ 0.05 vs. ORX+PLB or VEH.
Dihydrotestosterone increased cyclooxygenase-2 protein levels
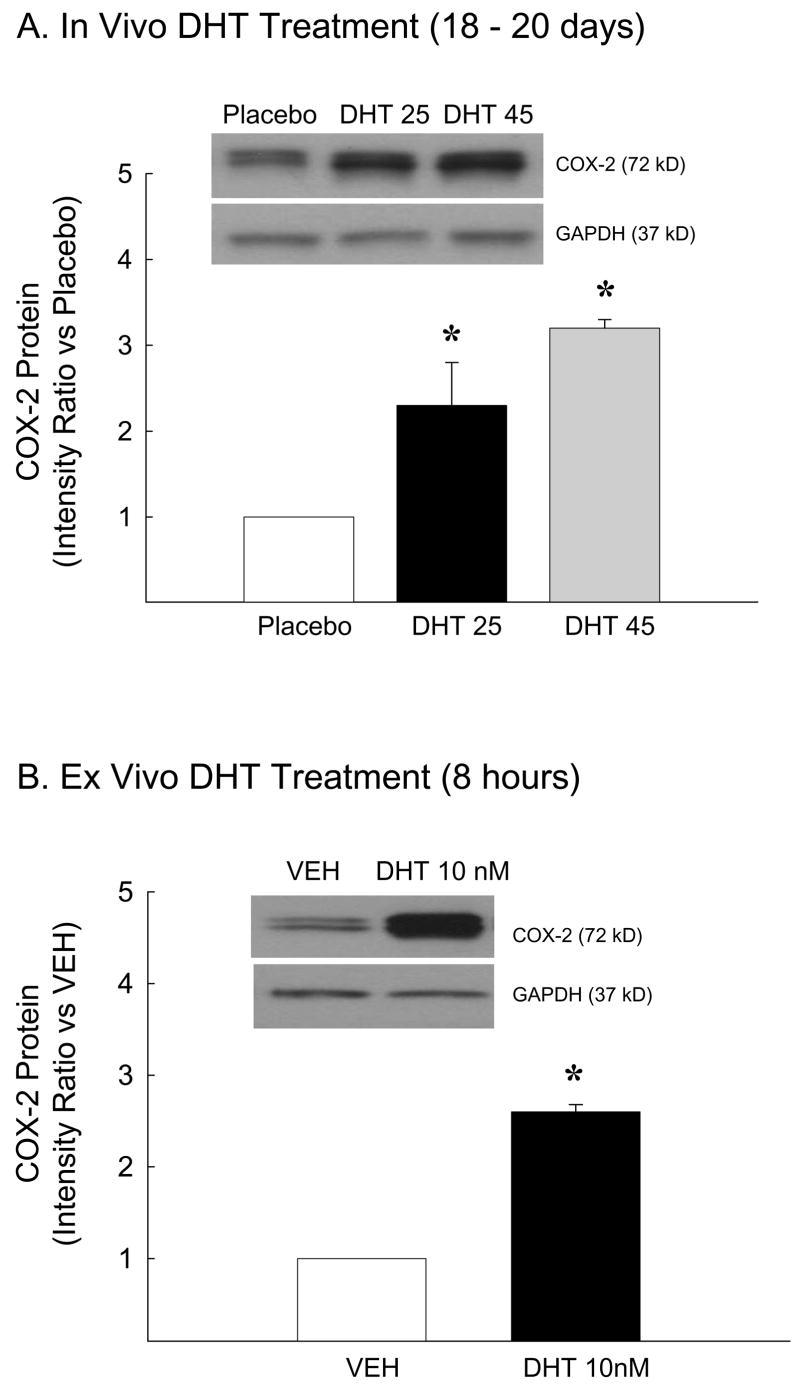
Because DHT increased NFκB activation we assessed whether DHT also increases COX-2, a proinflammatory product of the NFκB transcription pathway. Figure 2A depicts a representative Western blot and levels of COX-2 in cerebral arteries of rats treated chronically with DHT for 18–20 days. In vivo DHT treatment administered at doses of 25 and 45 mg significantly increased levels of COX-2 in cerebral arteries compared to animals receiving placebo (Figure 2A). Consistent with studies of in vivo treatment, ex vivo administration of 10 nM DHT for 8 hours also increased COX-2 protein levels in cerebral arteries (Figure 2B). Together these data suggest that this increase in COX-2 is an androgenic response localized within the cerebrovasculature.
Figure 2.
COX-2 protein levels in pial arteries isolated from orchiectomized rats treated A) in vivo with placebo (PLB) or dihydrotestosterone (DHT 25 or 45 mg) for 18 to 20 days or B) arteries incubated ex vivo with DHT (10 nM) or vehicle (VEH) for 8 hours. GAPDH verified equal total protein loading in each lane (n=5 in vivo; n=8 ex vivo). *P ≤ 0.05 vs. PLB or VEH.
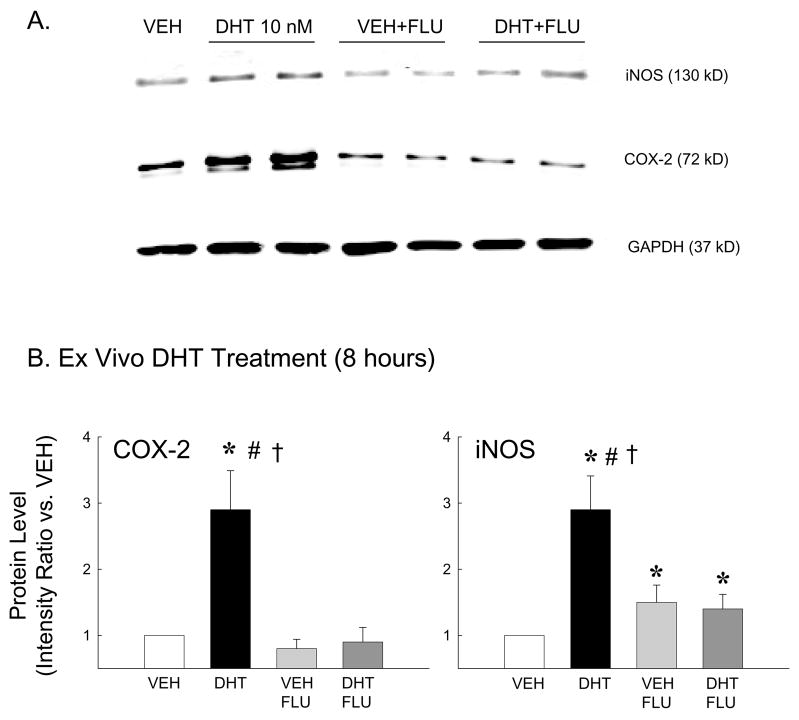
Flutamide blunted dihydrotestosterone-mediated increases in COX-2 and iNOS protein
Because DHT (in vivo or ex vivo) increased levels of the proinflammatory marker, COX-2 (Figure 2), we also investigated whether DHT alters iNOS, another proinflammatory marker known to be regulated by the NFκB pathway. In addition, the effect of DHT to alter COX-2 and iNOS levels via the androgen receptor was assessed using the androgen receptor antagonist, flutamide. Figure 3A illustrates a representative Western blot for both COX-2 and iNOS protein in pial arteries incubated ex vivo (8 hr) with vehicle (VEH), DHT 10 nM, VEH+flutamide (FLU; 10 nM), or DHT+FLU. In each experiment GAPDH (37 kDa) was visualized to verify equal sample loading. Intensity ratio analysis demonstrated that both COX-2 and iNOS levels were significantly increased in cerebral arteries following ex vivo DHT treatment compared to vehicle (Figure 3B). Treatment with flutamide had no effect by itself, but flutamide significantly blunted DHT-induced increases in protein levels of both COX-2 and iNOS.
Figure 3.
COX-2 and iNOS protein levels in pial arteries incubated ex vivo with DHT (10 nM), vehicle (VEH), VEH plus flutamide (10 nM; VEH+FLU) or DHT plus flutamide (DHT+FLU) for 8 hours. GAPDH was used to verify equal protein loading in each lane. (A) Representative Western blot; (B) Mean intensity ratios compared to vehicle treated arteries (n=5 in each treatment group). *P ≤ 0.05 vs. VEH; #P ≤ 0.05 vs. VEH+FLU; †P ≤ 0.05 vs. DHT+FLU.
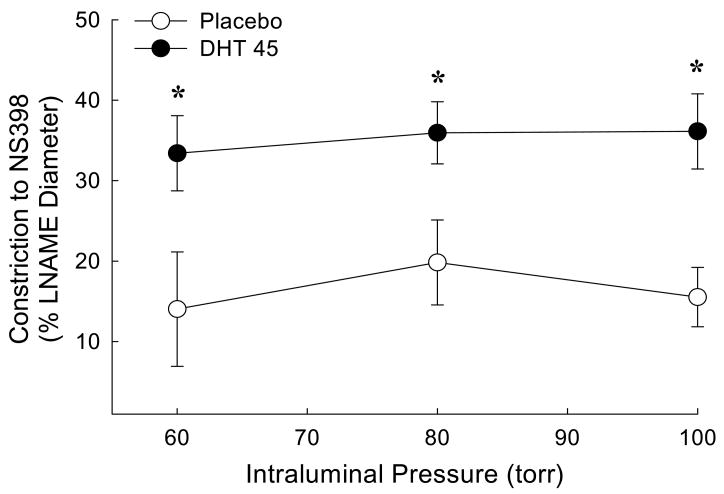
Dihydrotestosterone increased constriction to a COX-2 inhibitor in pressurized middle cerebral artery (MCA)
Since chronic treatment with DHT increased COX-2 protein levels (Figure 2), functional experiments were designed to investigate whether possible metabolites of the COX-2 pathway contribute to MCA tone following chronic in vivo treatment with DHT. In this study we refer to tone as the degree of arterial constriction relative to the maximum dilation in the presence of Ca2+-free PSS. In the presence of Ca2+-free PSS passive diameters were not different among groups (Table 2). Because nitric oxide production has been shown to modulate COX pathways in cerebral vessels (Li et al 2004), vascular responses to COX-2 inhibition were recorded in the presence of L-NAME, the non-selective NOS inhibitor. Data were collected at pressures of 60, 80 and 100 torr. Constriction to L-NAME was not different between groups (data not shown). The addition of the selective COX-2 inhibitor, NS398, elicited further constriction in MCA; this response was significantly greater in MCA from animals treated chronically with DHT (Figure 4).
Figure 4.
Effect of chronic dihydrotestosterone treatment on constriction to selective cyclooxygenase-2 (COX-2) inhibition with NS398 (10 μM). Pressurized middle cerebral artery segments from orchiectomized rats treated with placebo (PLB) or dihydrotestosterone 45 mg pellets (DHT 45 mg) were studied in the presence of the non-selective NG-nitro-L-arginine-methyl ester (LNAME; 100 μM). Constriction to NS398 was calculated as [(diameter in PSS and L-NAME) – (diameter in PSS, L-NAME and NS398)]/(diameter in PSS and L-NAME). The data were expressed as a percent change in constriction to NS398 at 60, 80 and 100 torr. (n = 5). *P ≤ 0.05 comparing DHT to placebo.
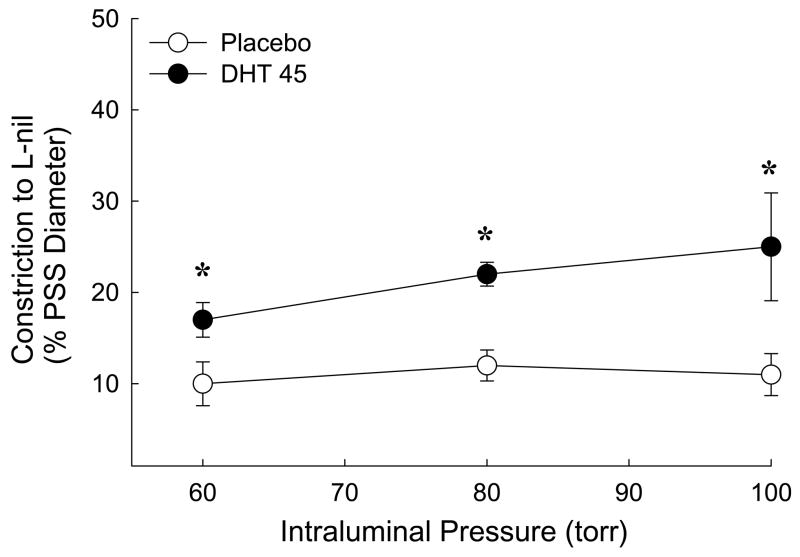
Chronic dihydrotestosterone increased constriction to an iNOS inhibitor in pressurized MCA
Since treatment with DHT also increased iNOS protein levels (Figure 3), we used the selective iNOS inhibitor, L-nil to investigate whether the contribution of iNOS-dependent NO production to cerebrovascular tone is altered following chronic in vivo DHT treatment. Effects of L-nil on MCA isolated from placebo and DHT treated rats were compared. iNOS inhibition caused constriction in MCA from both groups of rats; however, at all intraluminal pressures, constrictor responses were significantly greater in MCA from rats exposed to chronic DHT treatment (Figure 5).
Figure 5.
Constriction to inhibition of inducible nitric oxide synthase (iNOS) with L nil (5 μM) in pressurized middle cerebral artery segments isolated from orchiectomized rats treated with placebo or dihydrotestosterone 45 mg pellets (DHT 45 mg). Constriction to L-nil was calculated as [(diameter in PSS ) – (diameter in PSS and L-nil)]/(diameter in PSS). The data were expressed as a percent change in constriction to L-nil at 60, 80 and 100 torr caused by addition of L-nil (n = 5). *P ≤ 0.05 comparing DHT to placebo.
Discussion
The main objective of this study was to investigate the influence of an androgenic stimulus, DHT, on the NFκB inflammatory pathway in the cerebral vasculature of male rats. We observed that a physiological level of DHT promotes an inflammatory response in cerebral arteries in the absence of another inflammatory stimulus. Specifically, we found that both in vivo and ex vivo DHT treatment enhanced nuclear NFκB DNA binding in cerebral arteries and augmented levels of COX-2 and iNOS, two well-known proinflammatory products of NFκB activation (Baeuerle and Henkel 1994). The effect of DHT to increase COX-2 and iNOS levels was attenuated by the androgen receptor antagonist, flutamide. As a functional consequence of enhanced COX-2 and iNOS levels, we observed that constriction to selective inhibition of either COX-2 or iNOS was greater in isolated pressurized MCA from orchiectomized rats treated chronically with DHT. This suggests that enhanced COX-2/iNOS levels produced by DHT may result in increased basal production of vasoactive factors and modulation of vascular tone.
Using immunostaining and biochemical analysis, past studies from our laboratory show that the cerebral circulation is certainly a target for androgens. We have previously shown that androgen receptors are expressed in both vascular smooth muscle cells and endothelial cells of the cerebral circulation (Gonzales et al 2007). Other investigators have shown similar findings in other vascular tissues such as aorta (Fujimoto et al 1994) and coronary blood vessels (Liu et al 2005). Functional studies show that after chronic in vivo treatment with testosterone, the precursor molecule to DHT, vascular tone in small resistance-sized cerebral arteries is increased (Gonzales et al 2004). In addition, a portion of this enhanced tone appears to result from suppression of an endothelial vasodilator component resembling EDHF (endothelial derived hyperpolarizing factor) and augmentation of the thromboxane synthase pathway (Gonzales et al 2004, Gonzales et al 2005). Other investigations from our laboratory have shown that chronic in vivo testosterone treatment enhances endotoxin-induced inflammation in cerebral blood vessels (Razmara et al 2005). Taken together these findings suggest that androgens may contribute to a predisposed risk for vascular diseases such as stroke.
To date, no published studies have investigated the influence of androgens on the cerebrovascular inflammatory response in the absence of an inflammatory stimulus. In addition, there are very few studies that have investigated effects of the more potent androgen, DHT, on vascular function and inflammation. However, one recent investigation by Cheng et al (2007) demonstrated that DHT enhanced ischemic brain injury following chronic hormone treatment (1 week). They also demonstrated that in a stroke model, middle cerebral artery occlusion/reperfusion, a number of genes are regulated by DHT, including the gene responsible for encoding COX-2.
Interestingly in contrast to our current study using the potent androgen DHT, our previous studies on intact male rats showed very low basal levels of COX-2 and iNOS in cerebral arteries (Razmara et al 2005). Moreover, chronic testosterone treatment of orchiectomized males did not cause any significant effect on markers of inflammation (COX-2 or iNOS) in the absence of an inflammatory stimulus. One explanation for this difference with the present findings using DHT might be that effects of testosterone reflect a balance between androgenic and estrogenic actions. Estrogen treatment of males suppresses the inflammatory response of cerebral vessels (Razmara et al 2005), and DHT, which is not converted into estrogen by aromatase, has purely androgenic effects. As mentioned above, we have previously shown that enzymes responsible for metabolizing testosterone to DHT or estrogen, 5-α-reductase and aromatase, are present in cerebral arteries (Gonzales et al 2007). Thus testosterone can metabolically be converted to estrogen and testosterone, possibly explaining differences between our previous studies of intact and testosterone-treated males and the current study of DHT. Since our strategy was to remove endogenous hormones and then determine the effect of DHT we chose not to include intact or sham animals as a comparison as including such groups would not help us define the androgenic effect, because circulating testosterone can be converted to estrogen as well as DHT. We feel the effects in a sham animal are complicated, reflecting a mix of AR and ER stimulation. As mentioned above, since we have shown previously that estrogen suppresses vascular inflammation and male cerebral vessels express aromatase this suggests a local souce of estrogen production. Therefore, we would not predict that DHT treatment would return the orchiectomized animals to a normal, physiological baseline because the estrogen component is missing. In previous work on cerebrovascular contraction, we have found that neither androgen nor estrogen restores orchiectomized responses to those of an intact animal. Instead the intact animal appears to exhibit a response that is midway between androgen-mediated constriction and estrogen-mediated dilation (Geary et al., 2000).
NFκB has been studied extensively since its first description by Sen and Baltimore (1986) for its role in immunity and stress responses. NFκB is thought to be a central transcriptional mediator of inflammation and can be activated in numerous cell types either by classic cytokine stimulation or in response to oxidative stress (Chen et al 1999, Matsui et al 1999, Schemedtji et al 1997). NFκB is normally stabilized in the cytosol when bound to the repressor phosphoprotein, IκB. However in the presence of an appropriate stimulus, IκB is targeted for ubiquitination and degradation leaving NFκB available for translocation to the nucleus and activation of target genes. Some target genes for NFκB include vascular endothelial growth factor, heme oxygenase-1, COX-2 and iNOS, all capable of modulating blood flow and vascular inflammation. Although NFκB is a transcription factor, it has the ability to interact with multiple other transcription factors and transcriptional cofactors.
Experimental evidence also suggests that sex steroid hormones in combination with their receptors can act as transcription factors to modulate induction of inflammation-induced proteins. An in vitro study using electrophoretic mobility shift assay demonstrated physical association of the NFκB p50 and p65 subunits with a promoter region on the androgen receptor gene (Supakar et al 1993), suggesting interactions between androgen function and inflammation. However, more is known about the effects of estrogens on inflammatory pathways, than is the case for androgens. For example, NFκB-dependent inflammation was shown to be inhibited in brain endothelial cells by estrogen without affecting IκB degradation (Galea et al 2002). Similarly, DNA binding assays revealed that chronic estrogen treatment in ovariectomized female rats suppresses NFκB-dependent cerebral vascular inflammation induced by IL-1 beta (Ospina et al 2004).
Several studies suggest that androgens can modify NFκB transcriptional regulation, but unlike estrogen, which appears to inhibit NFκB, it is still unclear whether androgens have pro- or anti-inflammatory actions. In one study of human umbilical vein endothelial cells stimulated with IL-1β, DHT enhanced NFκB activation leading to increased vascular cell adhesion molecule-1 promoter activity (Death et al 2004). In the current study we observed that DHT increased nuclear NFκB DNA binding in cerebral arteries suggesting that androgens act via NFκB to contribute to a state of proinflammation. However this effect has not been found in all studies. For example, another study in cultured human umbilical cord endothelial cells found DHT decreased endotoxin-induced inflammation by down regulating levels of adhesion molecules, chemokines and protease (Norata et al 2006). The reasons for these discrepancies are not clear but may relate to different conditions of the cells in culture.
None of the previous studies showing DHT can modulate NFκB activation determined a physiological consequence of this effect. Therefore, we investigated the effect of DHT on COX-2 and iNOS levels in cerebral arteries. As mentioned before, previously we have shown that testosterone, an androgenic precursor to DHT, further augments endotoxin-mediated induction of proinflammatory enzymes iNOS and COX-2 in cerebral blood vessels (Razmara et al 2005). In the current study, however, we show that, independent of any inducer of inflammation, chronic administration of physiological doses of DHT by itself augments levels of proinflammatory enzymes in cerebral arteries. This effect is mediated by activation of the androgen receptor as evidenced by use of the androgen receptor antagonist, flutamide. As expected, there was also a DHT-induced change in vasoactive factors produced by the inflammatory enzymes, indicating a functional consequence on cerebrovascular tone.
Clinical studies suggest that testosterone replacement therapy in aged hypogonadal men can modulate immune system responses. One study reported that testosterone replacement to hypogonadal patients has anti-inflammatory properties, resulting in significant reductions in circulating levels of TNF alpha and IL-1 beta when compared with placebo (Malkin et al 2004). An explanation for the reduced levels of these proinflammatory modulators observed in the above study may be due in part to testosterone being further metabolized to estrogen, and this may explain the anti-inflammatory effects. In experimental animal models discrepancies in the effects of androgens on inflammation may result from a variety of sources. In the case of the non-aromatizable DHT, differences may arise from different experimental models (in vivo vs. in vitro) as well as the concentration of DHT used (10−7 M vs. 10−10 M). In our case we show that, in the absence of stimulated inflammation, DHT, whether administered in vivo (physiological dose) or ex vivo (10−8 M), increased NFκB DNA binding in cerebral arteries suggesting that DHT alone can elicit an inflammatory response by activating the NFκB pathway.
In summary, activation of the NFκB mediated COX-2/iNOS pathway by androgens such as DHT elicits a state of vascular inflammation independent of cytokine or any other outside inflammatory stimulus. Clinically, the effects of androgens on proinflammatory enzymes may contribute to further worsening of the detrimental influence of androgenic stimulation on ischemic brain injury. This is critical, because the impact of cerebrovascular inflammation is highly relevant during pathophysiological conditions related to endothelial dysfunction, oxidative stress, hypoxia and stroke. Taken together these data suggest that, in young adults who choose to use androgen supplementation recreationally or for performance enhancement, predisposition for the onset of inflammation may be increased, with significant possible consequences for development of both cerebral and cardiovascular disease.
Acknowledgments
This work was supported by the American Heart Association Scientist Development Award (RJG) and by NIH RO1 NHLBI-50755.
We thank Ms. Bebe Ehsan and Mr. Peter Horvath for their technical assistance with Western blot.
Reference List
- 1.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traysman RJ, Hurn PD. Gender linked brain injury in experimental stroke. Stroke. 1988;29:159–165. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle PA, Henkel T. Function and activation of NF-kB in the immune system. Ann Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 3.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 4.Cheng J, Alkayed NJ, Hurn P. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab. 2007;9:1553–62. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Death AK, McGrath KC, Sader MA, Nakhla S, Jessup W, Handelsman DJ, Celermajer DS. Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-kappaB-dependent pathway. Endocrinology. 2004;145:1889–1897. doi: 10.1210/en.2003-0789. [DOI] [PubMed] [Google Scholar]
- 6.Del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cerebr Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 7.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 8.Emsley HCA, Tyrrell PJ. Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab. 2002;22:1399–1419. doi: 10.1097/01.WCB.0000037880.62590.28. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto R, Morimoto I, Morita E, Sugimoto H, Ito Y, Eto S. Androgen receptors, 5 alpha-reductase activity and androgen-dependent proliferation of vascular smooth muscle cells. J Steroid Biochem Mol Biol. 1994;50:169–174. doi: 10.1016/0960-0760(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 10.Galea E, Santizo R, Feinstein DL, Adamsom P, Greenwood J, Koenig HM, Pelligrino DA. Estrogen inhibits NfκB-dependent inflammation in brain endothelium without interfering with IκB degradation. NeuroReport. 2002;13:1469–1472. doi: 10.1097/00001756-200208070-00024. [DOI] [PubMed] [Google Scholar]
- 11.Geary GG, Krause DN, Duckles SP. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol. 1998;275:H292–300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- 12.Geary GG, Krause DN, Duckles SP. Gonadal hormones affect diameter of male rat cerebral arteries through endothelial-dependent mechansims. Am J Physiol. 2000;279:H610–618. doi: 10.1152/ajpheart.2000.279.2.H610. [DOI] [PubMed] [Google Scholar]
- 13.Gonzales RJ, Duckles SP, Krause DN. Testosterone suppresses endothelium-dependent dilation of middle cerebral arteries. Am J Physiol. 2004;286:H552–H560. doi: 10.1152/ajpheart.00663.2003. [DOI] [PubMed] [Google Scholar]
- 14.Gonzales RJ, Gjaffari AA, Duckles SP, Krause DN. Testosterone treatment increases thromboxane function in rat cerebral arteries. Am J Physiol. 2005;289:H578–H585. doi: 10.1152/ajpheart.00958.2004. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales RJ, Ansar S, Duckles SP, Krause DN. Androgenic/estrogenic balance in the male rat cerebral circulation: metabolic enzymes and sex steroid receptors. J Cereb Blood Flow Metab. 2007;27:1841–1852. doi: 10.1038/sj.jcbfm.9600483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawk T, Zhang Y-Q, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796:296–298. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- 17.Jeppesen LL, Jorgensen HS, Nakayama H, Raaschou HO, Olsen Winther K. Decreased serum testosterone in men with acute ischemic stroke. Arterioscler Thromb Vasc Biol. 1996;16:749–54. doi: 10.1161/01.atv.16.6.749. [DOI] [PubMed] [Google Scholar]
- 18.Kempermann G, Neumann H. Microglia: The enemy within? Science. 2003;302:1689–1690. doi: 10.1126/science.1092864. [DOI] [PubMed] [Google Scholar]
- 19.Krause DN, Duckles SP, Pelligrino DA. The influence of sex steroid hormones on cerebrovascular function. Invited review. J Appl Phyiol. 2006;101:1252–1261. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Geary G, Gonzales RJ, Krause DN, Duckles SP. Effect of estrogen on cerebrovascular prostaglandins is amplified in mice with dysfunctional NOS. Am J Physiol Heart Circ Physiol. 2004;287:H588–H594. doi: 10.1152/ajpheart.01176.2003. [DOI] [PubMed] [Google Scholar]
- 21.Liu PL, Christian RC, Ruan M, Miller VM, Fitzpatrick LA. Correlating Androgen and Estrogen Steroid Receptor Expression with Coronary Calcification and Atherosclerosis in Men without Known Coronary Artery Disease. J of Clinical Endocrinol & Metab. 2005;90:1041–1046. doi: 10.1210/jc.2004-1211. [DOI] [PubMed] [Google Scholar]
- 22.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 23.Matsui H, Ihara Y, Fujio Y, Kunisada K, Akira S, Kishimoto T, Yamauchi-Takihara K. Induction of interleukin (IL)-6 by hypoxia is mediated by nuclear factor (NF)- κB and NF-IL6 in cardiac myocytes. Cardiovasc Res. 1999;42:104–112. doi: 10.1016/s0008-6363(98)00285-5. [DOI] [PubMed] [Google Scholar]
- 24.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Norata GD, Tibolla G, Seccomandi PM, Poletti A, Catapano AL. Dihydrotestosterone decreases tumor necrosis factor-alpha and lipopolysaccharide-induced inflammatory response in human endothelial cells. J Endocrin and Metab. 2006;(2):546–554. doi: 10.1210/jc.2005-1664. [DOI] [PubMed] [Google Scholar]
- 26.Ospina JA, Brevig HN, Krause DN, Duckles SP. Estrogen suppresses IL-1β-mediated induction of COX-2 pathway in rat cerebral blood vessels. Amer J Physiol: Heart Circ Physiol. 2004;286:H2010–H2019. doi: 10.1152/ajpheart.00481.2003. [DOI] [PubMed] [Google Scholar]
- 27.Razmara A, Krause DN, Duckles SP. Testosterone augments endotoxin-mediated cerebrovascular inflammation in male rats. Am J Physiol Heart Circ Physiol. 2005;289(5):H1843–50. doi: 10.1152/ajpheart.00465.2005. [DOI] [PubMed] [Google Scholar]
- 28.Schemedtji JF, Jr, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induced cyclooxygenase-2 via the NF-kappa p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272:601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 29.Sen R, Baltimore D. Multiple nuclear factors interact with the immmunoglobin enhancer sequences. Cell. 1986;46:706–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 30.Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 31.Supakar PC, Song CS, Jung MH, Slomczynska MA, Kim JM, Vellanoweth RL, Chatterjee B, Roy AK. A novel regulatory element associated with age-dependent expression of the rat androgen receptor gene. J Biol Chem. 1993;268(35):26400–26408. [PubMed] [Google Scholar]