Abstract
Multiple sclerosis is a sexually dimorphic, demyelinating disease of the central nervous system, and experimental allergic encephalomyelitis (EAE) is its principal autoimmune model. Young male SJL/J mice are relatively resistant to EAE while older males and SJL/J females of any age are susceptible. By comparing a wide age range of PLP139–151-immunized mice, we found female disease severity remains constant with age. In contrast, EAE disease severity increases with age in SJL/J males, with young males having significantly less severe disease and older males having significantly more disease than equivalently aged females. To determine if the Y-chromosome contributes to this sexual dimorphism, EAE was induced in consomic SJL/J mice carrying a B10.S Y-chromosome (SJL.YB10.S). EAE was significantly more severe in young, male SJL.YB10.S mice compared to young, male SJL/J mice. These studies show that Y-chromosome-linked polymorphism controls the age-dependent EAE sexual dimorphism observed in SJL/J mice.
Keywords: Autoimmunity, EAE/MS, Neuroimmunology, sex chromosomes
Introduction
Multiple sclerosis (MS) is a chronic, demyelinating disease of the central nervous system that results in substantial disability and paralysis. Although MS prevalence is ~ 3 fold higher in women than men, MS in men is characterized by a more rapid clinical course and typically presents as severe progressive disease (1). Interestingly, the female-biased sexual dimorphism has been found to be on the rise within the past fifty years, presumably due to gene-environment interactions (2, 3).
Experimental allergic encephalomyelitis (EAE) is the principal autoimmune model of MS. EAE in the inbred SJL/J mouse strain has been used as a model of the sexual dimorphism seen in MS, as SJL/J females are considered more susceptible to EAE than males. However, this gender bias in the EAE sexual dimorphism of SJL/J mice is unique in that it is age dependent, reflecting changes in EAE susceptibility of male mice, not female mice. Compared to young female SJL/J mice, young (4–8 wk) male SJL/J mice show significantly impaired delayed type hypersensitivity responses (4, 5) and are significantly less susceptible to EAE (6–10). In contrast, EAE susceptibility in older (≥ 12 wk) SJL/J males is equivalent to that seen in 6 wk old SJL/J females (6). Consequently, this gives rise to a female-biased EAE sexual dimorphism when female mice of any age are compared only with young (4–8 wk) males. Both gonadal hormones (11–14) and sex chromosome (Chr) effects (15, 16) have been postulated to cause the sexual dimorphism. In young SJL/J mice, male gonadal hormones (12, 17) have been implicated in EAE resistance because castration of young SJL/J mice significantly increases EAE incidence and severity (6, 14) and induces the female predominant relapsing-remitting form of the disease (12, 17). Collectively, these published reports show that the gender bias of the EAE sexual dimorphism in SJL/J mice is a function of a testes-dependent age-related transition in EAE susceptibility of SJL/J males and is not due to an inherent steady-state increase in the EAE susceptibility of female SJL/J mice.
Using C57BL/6J Y Chr substitution strains (consomic mice), we previously demonstrated that a gene or genes on the Y Chr, termed Yeae, influence EAE (18). This was the first experimental evidence demonstrating the existence of a Y Chr polymorphism capable of modifying EAE susceptibility. Because the age-dependent change in EAE susceptibility of SJL/J mice is male-specific and orchiectomy influences this phenotype, we reasoned that the SJL/J Y Chr may play a role in the genetic control of the SJL/J sexual dimorphism. This hypothesis was tested directly in this study using SJL/J Y Chr consomic mice. We report here that, in fact, the SJL/J Y Chr selectively interacts with the SJL/J background to control the unique EAE sexual dimorphism seen in this strain.
Materials and Methods
Animals
SJL/J, B10.PL-H2u H2-T18a/(73NS)SnJ (B10.PL), and B10.S/SgMcdJ (B10.S), were purchased from The Jackson Laboratory (Bar Harbor, ME). SJL/J.YB10.S and B10.S.YSJL/J mice are consomic strains in which the Y Chr has been replaced with B10.S and SJL/J, respectively. These lines were generated at the University of Vermont and backcrossed for a minimum of 15 generations. Animals were housed in specific pathogen-free conditions under NIH guidelines, and experiments performed in this study were approved by the Animal Care and Use Committee of the University of Vermont.
Mouse spinal cord homogenate (MSCH) and encephalitogen-CFA emulsions
MSCH was generated using retired breeder SJL/J mice (The Jackson Laboratory) as described (19). MSCH-CFA and PLP139–151 (UVM Chemistry Dept., Burlington, VT) emulsions were prepared by sonication (ext-ENC-CFA) and syringe extrusion (int-ENC-CFA), respectively (19).
Induction and evaluation of active EAE
SJL/J mice were injected s.c. in the flanks with 0.2 ml of an emulsion containing 1.0 mg dry weight SJL/J MSCH or 100 µg PLP139–151 in saline and an equal volume of CFA containing 200 µg Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) in the posterior right and left flank (2 × 0.1 ml); one week later mice received the same injection on the right and left flank anterior of the original injection site (20). Each animal received a total of 2.0 mg dry weight MSCH and 200 µg PLP139–151, and 400 µg M. tuberculosis H37Ra. EAE was induced in B10.PL mice with 0.2 ml of an emulsion containing 400 µg MBPAc1-11 (Beckman Institute, Palo Alto, CA) and an equal volume of CFA containing 200 µg Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) as above. On Day 0 and again on Day 2, mice received an i.p. injection of 200 ng pertussis toxin (List Biological Laboratories Inc., Campbell, CA) (21).
EAE was evaluated daily beginning at day 10 after injection as previously reported (20). All animals were considered affected that showed any clinical signs greater or equal to a score of 1 for 2 or more consecutive days. The severity index is the CDS / days affected.
Proliferation and cytokine assays
Ex vivo proliferation and cytokine assays were carried out as previously described (20) using spleen and lymph node cells obtained from mice immunized 10 days earlier with 2× PLP139–151-CFA.
Statistical analyses
A multiple logistic regression analysis was performed to examine the effect of age, season and sex on incidence. Multiple linear regression analyses were performed to examine the effect of the same factors on measures of disease severity. Final models presented include age, month of injection, sex and sex-by-age interaction when significant. The Kruskal-Wallis test followed by Dunn’s multiple comparison test or the Mann Whitney U test were used where applicable. Differences were considered significant at p < 0.05. A repeated-measures ANOVA was performed to examine the group changes in the mean clinical score across time, as well as time by group interactions as previously described (18) using BMDP statistical software (BMDP Statistical Software, Los Angeles).
Results and Discussion
Neuroantigen-complete Freund’s adjuvant emulsion structure modifies the EAE sexual dimorphism in SJL/J mice
We have shown that immunization of young (5–6 wk) SJL/J male mice with MSCH-CFA emulsions composed of particles where the M. tuberculosis and neuroantigen are localized on the phase surfaces (Ext-ENC-CFA), abolished the age-dependent difference in EAE susceptibility of SJL/J male mice (19) In contrast, emulsions in which the bacterial products and encephalitogen were buried inside the water/oil vesicles (Int-ENC-CFA) did not (19). To assess the effect of PLP139–151-CFA emulsion particle structure on EAE susceptibility of young SJL/J males, 5–8 wk old male SJL/J mice were immunized with PLP139–151-CFA emulsions prepared by sonication (Ext-ENC-CFA) or syringe extrusion (Int-ENC-CFA) (19). Young SJL/J mice immunized with Int-MSCH-CFA emulsions were resistant to EAE (0/9) whereas young SJL/J males immunized with Ext-MSCH-CFA emulsions were significantly more susceptible to disease (10/10), consistent with our previous findings. Similarly, young SJL/J male mice had significantly higher disease incidence (12/12 vs. 11/16, Χ2=4.6; p=0.03) and greater CDS (28.3 vs. 10.1; p<0.001) when immunized with Ext-PLP139–151-CFA compared to Int-PLP139–151-CFA. These results are consistent with the seminal observations of Cua et al., (6) showing that the age-dependent changes in susceptibility of SJL/J males to EAE are likely due to a developmental delay in the ability of APC to process and present antigens. Importantly, emulsions in which the M. tuberculosis and encephalitogen are localized on the phase surfaces (Ext-ENC-CFA) effectively increases the antigen specific immunostimulatory activity of APC compared to Int-ENC-CFA vesicles. Ext-PLP139–151-CFA emulsions were used in all subsequent studies.
Season affects EAE incidence and severity in SJL/J mice
Seasonal differences in EAE incidence and severity have been reported in SJL-derived mice (22, 23). In the present studies, both male and female SJL/J mice were more likely to develop EAE in April, May and July compared to November (supplemental Table I and II). Therefore, season was included in the regression model when assessing the effect of age on the EAE sexual dimorphism (supplemental Table III; Fig. 1).
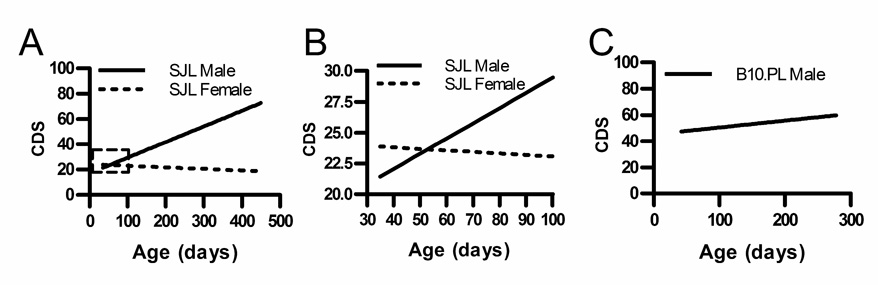
Figure 1. EAE severity increases with increasing age of immunization in male SJL/J mice but not in female SJL/J or male B10.PL mice.
Active EAE was induced in SJL/J mice by immunization with PLP139–151-CFA on days 0 and 7 (A and B) or in B10.PL mice with MBPAc1-11-CFA plus pertussis toxin (C). Linear regression analysis of changes in CDS by age of immunization over the entire course of the study (A) and between 5 and 14 wks of age (B) are shown. The male SJL/J CDS-by-age slope (solid line) changes over time and is different from 0 (p < 0.001, n = 117) but the female CDS-by-age slope (dotted line) is not different from 0 (p = 0.61, n = 101). (C) Male B10.PL CDS-by-age slope is not different from 0 (p = 0.27, n = 45).
SJL/J sexual dimorphism in EAE severity changes with age
SJL/J mice (5–65 wks old) were immunized for EAE and the mean cumulative disease score (CDS) as a function of age was analyzed by linear regression. In female SJL/J mice, the CDS remained constant with age (Fig. 1) whereas in male mice, the CDS increased with age. The slope of the male CDS-by-age regression line (slope = 0.14; p<0.001) indicates that the CDS increased by 0.14 for each day older at the time of immunization, while the regression line for female CDS-by-age held steady (slope = 0.02; p = 0.91). EAE disease severity was not significantly different between 12 wk old SJL/J males and 6 wk old SJL/J females.
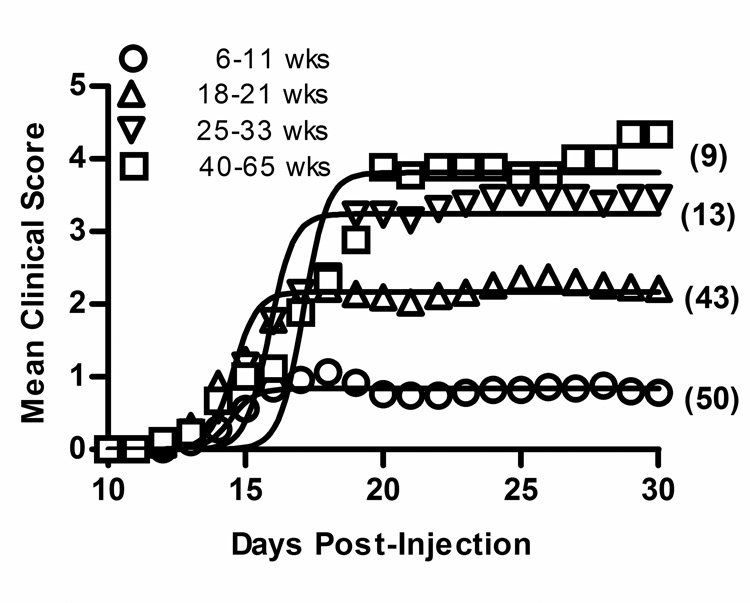
The CDS-by-age regression lines diverged again as the male CDS continued to increase over time while the female CDS remained constant (Fig.1). The effect of age on disease severity was significantly different between males and females, as indicated by the significant sex-by-age interaction (p < 0.01) (supplemental Table III). Peak score, severity index, and days affected also increased in SJL/J males but not in SJL/J females (supplemental Table III). Incidence did not vary with age in male or female mice. When divided into four representative age groups, 6–11 wk old males had significantly less severe EAE than any older age group (Fig. 2 and supplemental Table IV).
Figure 2. Age of immunization influences EAE severity in male SJL/J mice.
Active EAE was induced in male SJL/J mice with PLP139–151-CFA on days 0 and 7. The CDS was significantly lower in mice immunized at 6–11 wks of age compared with mice immunized at 18–21 wks, 25–33 wks and 40–65 wks. Regression analysis revealed that severity of EAE differed significantly among the age groupings (F = 122.0; p < 0.0001) with 6–11 < 18–21 < 25–33 = 40–65.
This age effect does not occur in all mouse strains, as the CDS of B10.PL male mice immunized with MBPAc1-11 does not increase significantly with age (Fig. 1C). Therefore, the commonly held notion that SJL/J females are more susceptible to EAE, applies only when comparing young (4–8 wk) SJL/J mice and reverses with age, with older (≥ 12 wk) males have more severe EAE than older females. This may be a useful model for the later onset and more progressive MS course observed in men (24).
The SJL/J Y Chr controls the age-related transition in susceptibility of SJL/J male mice to EAE
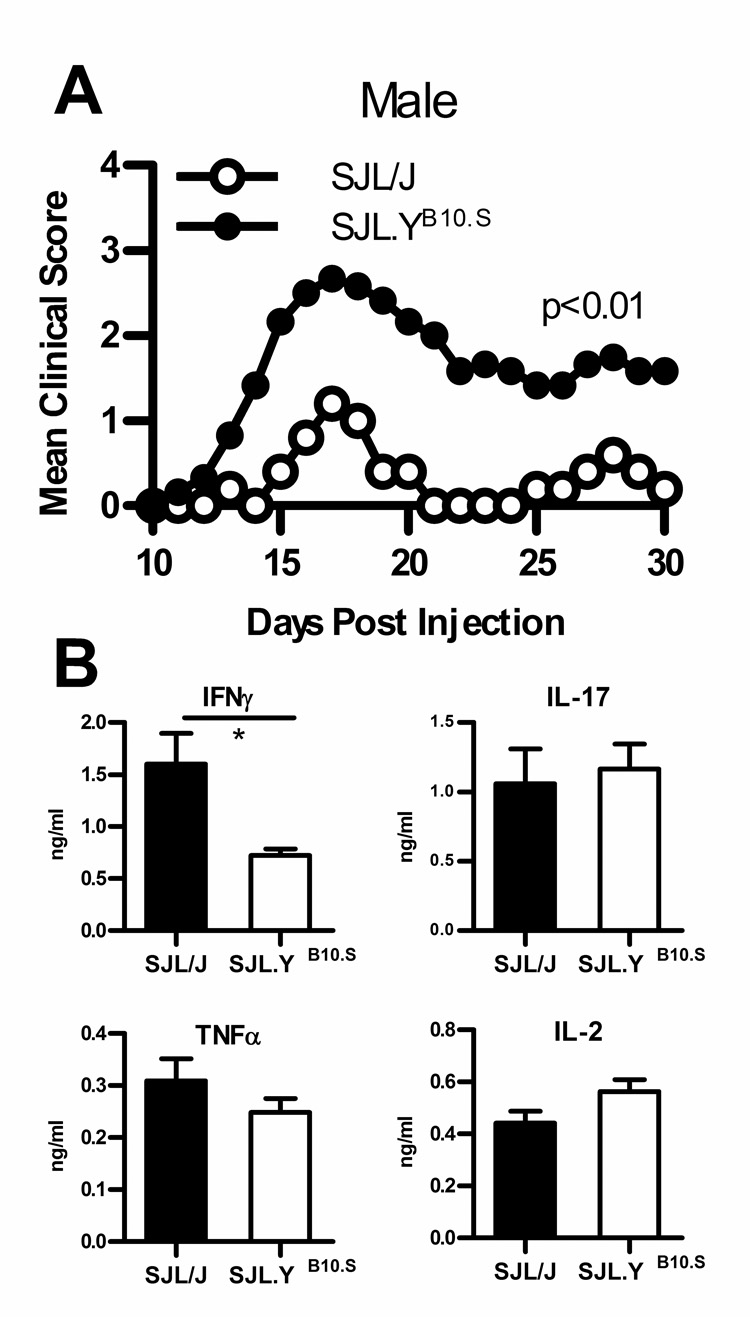
Because the age-dependent change in EAE susceptibility of SJL/J mice is male-specific and orchiectomy influences this phenotype, we reasoned that genes on the Y Chr may play a role in the genetic control of the SJL/J sexual dimorphism. The influence of the SJL/J Y Chr was tested directly by generating Y Chr consomic mice bearing the YB10.S Chr which is of M. m. musculus origin, like that of B10.PL and C57BL/6 (25) on the SJL/J background (SJL.YB10.S). The reciprocal consomic was constructed with the YSJL Chr, which is of M. m. domesticus origin, on the B10.S background (B10.S.YSJL). Consomic mice were immunized for EAE. The clinical disease courses differed significantly between young SJL/J and SJL.YB10.S male mice, with that of SJL.YB10.S males being significantly more severe than that of young SJL/J males (Fig. 3; Table I) and equivalent to that seen in SJL/J and (SJL/J × SJL.YB10.S) F1 females (supplemental Fig. 1). In contrast, B10.S male mice with either the YB10.S or YSJL Chr (incidence: 2/17 and 3/11, respectively) remained resistant to PLP139–151-induced EAE (supplemental Table V). However, additional animals may need to be studied in order to definitively conclude that the YB10.S Chr does not impact resistance in B10.S mice.
Figure 3. The SJL/J Y Chr controls EAE severity in SJL/J males.
Active EAE was induced in 6–8 wk old SJL/J mice with PLP139–151-CFA on days 0 and 7. (A) The clinical course of EAE differed significantly between SJL.YB10.S (n = 20) and SJL/J mice (n = 9) (time effect, p < 0.01; group effect, p < 0.01; interaction term, p < 0.01). (B) Ten days after injection, splenocytes and lymphocytes were stimulated with 50 µg/ml PLP139–151 and cytokine production measured by ELISA. Mean and SD are show. Statistically significant differences by Student’s t-test are indicated (*, p < 0.05; n = 6).
Table I.
Clinical disease traits in male SJL/J and SJL.YB10.S consomic mice following immunization with PLP139–151-CFA.
| Strain | Incidence | DO | CDS | PS | SI | DA | |
|---|---|---|---|---|---|---|---|
| SJL/J | 7/9 | 16.7 | 10.1 | 2.1 | 1.5 | 7.3 | |
| SJL.YB10.S | 20/20 | 13.9 | 31.5 | 3.1 | 2.1 | 13.7 | |
| χ2= | 4.7 | ||||||
| p = | 0.03 | 0.0008 | 0.002 | 0.03 | 0.03 | 0.006 |
Active EAE was induced in 6–8 week old mice with PLP139–151-CFA on days 0 and 7.
We next compared the ex vivo PLP139–151-specific proliferative responses and cytokine production by spleen and lymph node cells from SJL/J and SJL.YB10.S mice immunized with 2× PLP139–151-CFA. The proliferative responses were not significantly difference between the strains (data not shown), nor was the production of IL-2, TNFα and IL-17 (Fig. 3B). However, SJL/J mice produced significantly more IFNγ than did SJL.YB10.S mice. This is consistent with the findings of Staykova et al. (9) who reported that compared to T cells from older SJL/J males, T cells from young SJL/J male make significantly more IFNγ.
Taken together, out data indicate that one or more polymorphic genes on the SJL/J Y Chr, which we previously designated Yeae (18), specifically interact with the SJL/J background to control the developmental delay in susceptibility of SJL/J male mice to EAE and regulate the production of IFNγ, a cytokine that has both pro- and anti-inflammatory effects (26). Y Chr polymorphism in the mouse has also been shown to influence cardiomyocyte size (27) and the number of Vα14+ invariant natural killer T cells (28). In Drosophila melanogaster Y Chr polymorphism affects the expression of large numbers of X-linked and autosomal genes impacting microtubule stability, lipid and mitochondrial metabolism, and the thermal sensitivity of spermatogenesis (29).
The major candidate gene on the Y Chr for Yeae is Sry, the testes determining gene, which interacts in an allele-specific manner with autosomal loci in controlling normal testicular development (30). In the male fetus, the appearance of fetal Leydig cells is preceded by Sry expression in pre-Sertoli cells, and fetal Leydig cell differentiation depends on Sertoli cell-derived factors (31–34). Fetal Leydig cells produce the androgens required for masculinization of the male during embryogenesis (35). Sry polymorphisms may regulate ‘fetal programming’ of susceptibility to EAE in males as a result of variation in the timing and/or intensity of the prenatal testosterone surge. Functionally significant Sry polymorphisms are well documented as transfer of certain Y Chr onto the C57BL/6 background leads to varying degrees of sex-reversal, ranging from normal testis development to permanent sex-reversal (30, 36–39) caused by the presence of particular Sry protein isoforms combined with insufficient Sry expression (40, 41).
In summary, increasing age leads to more severe chronic EAE in SJL/J male mice, and replacement of the SJL/J Y Chr with the B10.S Y Chr on the SJL/J background leads to the development of the relapsing-remitting form of disease similar to that seen in young orchiectomized SJL/J males. Moreover, immunization of young SJL/J males with emulsions in which the M. tuberculosis and encephalitogen are localized on the phase surfaces is capable of overcoming the developmental delay in the ability of young SJL/J male APC to process and present antigens. Taken together, these results reinforce the importance of gene-environment interactions in not only controlling overall susceptibility to inflammatory demyelinating disease of the CNS but in influencing the sexual dimorphisms seen in such diseases.
Supplementary Material
Acknowledgments
This work was supported by the National Multiple Sclerosis Society RG3575 (EPB, CT) and NIH NS36526 (CT, EPB), NS061014 (CT), and NS060901 (CT, EPB).
Non-standard abbreviations
- Chr
chromosome
- CDS
cumulative disease score
- DA
days affected
- DO
day of onset
- EAE
experimental allergic encephalomyelitis
- MS
multiple sclerosis
- MSCH
mouse spinal cord homogenate
- PLP139–151
proteolipid protein peptide 139–151
- PS
peak score
- SI
severity index
References
- 1.Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 2.Alonso A, Hernan MA. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology. 2008;71:129–135. doi: 10.1212/01.wnl.0000316802.35974.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orton SM, Herrera BM, Yee IM, Valdar W, Ramagopalan SV, Sadovnick AD, Ebers GC. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol. 2006;5:932–936. doi: 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]
- 4.Stohlman SA, Matsushima GK, Casteel N, Frelinger JA. The defect in delayed-type hypersensitivity of young adult SJL mice is due to a lack of functional antigen-presenting cells. Eur J Immunol. 1985;15:913–916. doi: 10.1002/eji.1830150909. [DOI] [PubMed] [Google Scholar]
- 5.Matsushima GK, Stohlman SA. Maturation of the delayed-type hypersensitivity response in SJL mice: absence of effector cell induction. Eur J Immunol. 1988;18:1411–1416. doi: 10.1002/eji.1830180917. [DOI] [PubMed] [Google Scholar]
- 6.Cua DJ, Hinton DR, Kirkman L, Stohlman SA. Macrophages regulate induction of delayed-type hypersensitivity and experimental allergic encephalomyelitis in SJL mice. Eur J Immunol. 1995;25:2318–2324. doi: 10.1002/eji.1830250830. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Voskuhl RR. Decreased IL-12 production underlies the decreased ability of male lymph node cells to induce experimental autoimmune encephalomyelitis. J Immunol. 1999;162:5561–5568. [PubMed] [Google Scholar]
- 8.Papenfuss TL, Rogers CJ, Gienapp I, Yurrita M, McClain M, Damico N, Valo J, Song F, Whitacre CC. Sex differences in experimental autoimmune encephalomyelitis in multiple murine strains. J Neuroimmunol. 2004;150:59–69. doi: 10.1016/j.jneuroim.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Staykova MA, Fordham SA, Bartell GJ, Cowden WB, Willenborg DO. Nitric oxide contributes to the resistance of young SJL/J mice to experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;176:1–8. doi: 10.1016/j.jneuroim.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Bebo BF, Jr, Vandenbark AA, Offner H. Male SJL mice do not relapse after induction of EAE with PLP 139–151. J Neurosci Res. 1996;45:680–689. doi: 10.1002/(SICI)1097-4547(19960915)45:6<680::AID-JNR4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Bebo BF, Jr, Schuster JC, Vandenbark AA, Offner H. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol. 1999;162:35–40. [PubMed] [Google Scholar]
- 12.Bebo BF, Jr, Zelinka-Vincent E, Adamus G, Amundson D, Vandenbark AA, Offner H. Gonadal hormones influence the immune response to PLP 139–151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 1998;84:122–130. doi: 10.1016/s0165-5728(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 13.Matejuk A, Adlard K, Zamora A, Silverman M, Vandenbark AA, Offner H. 17 beta-estradiol inhibits cytokine, chemokine, and chemokine receptor mRNA expression in the central nervous system of female mice with experimental autoimmune encephalomyelitis. J Neurosci Res. 2001;65:529–542. doi: 10.1002/jnr.1183. [DOI] [PubMed] [Google Scholar]
- 14.Palaszynski KM, Loo KK, Ashouri JF, Liu HB, Voskuhl RR. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol. 2004;146:144–152. doi: 10.1016/j.jneuroim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Palaszynski KM, Smith DL, Kamrava S, Burgoyne PS, Arnold AP, Voskuhl RR. A yin-yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinology. 2005;146:3280–3285. doi: 10.1210/en.2005-0284. [DOI] [PubMed] [Google Scholar]
- 16.Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR. A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med. 2008 doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fillmore PD, Blankenhorn EP, Zachary JF, Teuscher C. Adult gonadal hormones selectively regulate sexually dimorphic quantitative traits observed in experimental allergic encephalomyelitis. Am J Pathol. 2004;164:167–175. doi: 10.1016/S0002-9440(10)63107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teuscher C, Noubade R, Spach K, McElvany B, Bunn JY, Fillmore PD, Zachary JF, Blankenhorn EP. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc Natl Acad Sci U S A. 2006;103:8024–8029. doi: 10.1073/pnas.0600536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fillmore PD, Brace M, Troutman SA, Blankenhorn EP, Diehl S, Rincon M, Teuscher C. Genetic analysis of the influence of neuroantigen-complete Freund's adjuvant emulsion structures on the sexual dimorphism and susceptibility to experimental allergic encephalomyelitis. Am J Pathol. 2003;163:1623–1632. doi: 10.1016/S0002-9440(10)63519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noubade R, Milligan G, Zachary JF, Blankenhorn EP, del Rio R, Rincon M, Teuscher C. Histamine receptor H1 is required for TCR-mediated p38 MAPK activation and optimal IFN-gamma production in mice. J Clin Invest. 2007;117:3507–3518. doi: 10.1172/JCI32792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polanczyk M, Yellayi S, Zamora A, Subramanian S, Tovey M, Vandenbark AA, Offner H, Zachary JF, Fillmore PD, Blankenhorn EP, Gustafsson JA, Teuscher C. Estrogen receptor-1 (Esr1) and -2 (Esr2) regulate the severity of clinical experimental allergic encephalomyelitis in male mice. Am J Pathol. 2004;164:1915–1924. doi: 10.1016/s0002-9440(10)63752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teuscher C, Bunn JY, Fillmore PD, Butterfield RJ, Zachary JF, Blankenhorn EP. Gender, age, and season at immunization uniquely influence the genetic control of susceptibility to histopathological lesions and clinical signs of experimental allergic encephalomyelitis: implications for the genetics of multiple sclerosis. Am J Pathol. 2004;165:1593–1602. doi: 10.1016/S0002-9440(10)63416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teuscher C, Doerge RW, Fillmore PD, Blankenhorn EP. eae36, a locus on mouse chromosome 4, controls susceptibility to experimental allergic encephalomyelitis in older mice and mice immunized in the winter. Genetics. 2006;172:1147–1153. doi: 10.1534/genetics.105.049049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergamaschi R. Prognostic factors in multiple sclerosis. Int Rev Neurobiol. 2007;79:423–447. doi: 10.1016/S0074-7742(07)79019-0. [DOI] [PubMed] [Google Scholar]
- 25.Wardell BB, Sudweeks JD, Meeker ND, Estes SS, Woodward SR, Teuscher C. The identification of Y chromosome-linked markers with random sequence oligonucleotide primers. Mamm Genome. 1993;4:109–112. doi: 10.1007/BF00290435. [DOI] [PubMed] [Google Scholar]
- 26.Heremans H, Dillen C, Dijkmans R, Grau G, Billiau A. The role of cytokines in various animal models of inflammation. Lymphokine Res. 1989;8:329–333. [PubMed] [Google Scholar]
- 27.Llamas B, Belanger S, Picard S, Deschepper CF. Cardiac mass and cardiomyocyte size are governed by different genetic loci on either autosomes or chromosome Y in recombinant inbred mice. Physiol Genomics. 2007;31:176–182. doi: 10.1152/physiolgenomics.00072.2007. [DOI] [PubMed] [Google Scholar]
- 28.Wesley JD, Tessmer MS, Paget C, Trottein F, Brossay L. A Y chromosome-linked factor impairs NK T development. J Immunol. 2007;179:3480–3487. doi: 10.4049/jimmunol.179.6.3480. [DOI] [PubMed] [Google Scholar]
- 29.Lemos B, Araripe LO, Hartl DL. Polymorphic Y chromosomes harbor cryptic variation with manifold functional consequences. Science. 2008;319:91–93. doi: 10.1126/science.1148861. [DOI] [PubMed] [Google Scholar]
- 30.Eicher EM, Washburn LL, Schork NJ, Lee BK, Shown EP, Xu X, Dredge RD, Pringle MJ, Page DC. Sex-determining genes on mouse autosomes identified by linkage analysis of C57BL/6J-YPOS sex reversal. Nat Genet. 1996;14:206–209. doi: 10.1038/ng1096-206. [DOI] [PubMed] [Google Scholar]
- 31.Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63:1825–1838. doi: 10.1095/biolreprod63.6.1825. [DOI] [PubMed] [Google Scholar]
- 32.Gnessi L, Basciani S, Mariani S, Arizzi M, Spera G, Wang C, Bondjers C, Karlsson L, Betsholtz C. Leydig cell loss and spermatogenic arrest in platelet-derived growth factor (PDGF)-A-deficient mice. J Cell Biol. 2000;149:1019–1026. doi: 10.1083/jcb.149.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brennan J, Tilmann C, Capel B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003;17:800–810. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao HH, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sriraman V, Anbalagan M, Rao AJ. Hormonal regulation of Leydig cell proliferation and differentiation in rodent testis: a dynamic interplay between gonadotrophins and testicular factors. Reprod Biomed Online. 2005;11:507–518. doi: 10.1016/s1472-6483(10)61147-9. [DOI] [PubMed] [Google Scholar]
- 36.Eicher EM, Washburn LL, Whitney JB, 3rd, Morrow KE. Mus poschiavinus Y chromosome in the C57BL/6J murine genome causes sex reversal. Science. 1982;217:535–537. doi: 10.1126/science.7089579. [DOI] [PubMed] [Google Scholar]
- 37.Biddle FG, Nishioka Y. Assays of testis development in the mouse distinguish three classes of domesticus-type Y chromosome. Genome. 1988;30:870–878. doi: 10.1139/g88-140. [DOI] [PubMed] [Google Scholar]
- 38.Nagamine CM, Michot JL, Roberts C, Guenet JL, Bishop CE. Linkage of the murine steroid sulfatase locus, Sts, to sex reversed, Sxr: a genetic and molecular analysis. Nucleic Acids Res. 1987;15:9227–9238. doi: 10.1093/nar/15.22.9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunt PA, Jackson JM, Horan S, Lawson CA, Grindell L, Washburn LL, Eicher EM. The mouse A/HeJ Y chromosome: another good Y gone bad. Chromosome Res. 2008;16:623–636. doi: 10.1007/s10577-008-1216-8. [DOI] [PubMed] [Google Scholar]
- 40.Nagamine CM, Morohashi K, Carlisle C, Chang DK. Sex reversal caused by Mus musculus domesticus Y chromosomes linked to variant expression of the testis-determining gene Sry. Dev Biol. 1999;216:182–194. doi: 10.1006/dbio.1999.9436. [DOI] [PubMed] [Google Scholar]
- 41.Albrecht KH, Young M, Washburn LL, Eicher EM. Sry expression level and protein isoform differences play a role in abnormal testis development in C57BL/6J mice carrying certain Sry alleles. Genetics. 2003;164:277–288. doi: 10.1093/genetics/164.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.