Abstract
Resistance to leishmaniasis in C57BL/6 mice depends on Th1/Tc1 cells. BALB/c mice preferentially develop Th2 immunity and succumb to infection. We now assessed the role of IL-17 in cutaneous leishmaniasis. During the course of L. major infection, BALB/c CD4 cells and neutrophils produced increased amounts of IL-17 as compared to cells from C57BL/6 mice. This was associated with significantly increased IL-23 release from L. major-infected BALB/c dendritic cells (DC), whereas IL-6 and TGF-β1 production by BALB/c and C57BL/6 DC were comparable. Interestingly, lesion sizes in infected IL-17-deficient BALB/c mice were dramatically smaller and failed to progress as compared to those in control mice. Similar amounts of IL-4, IL-10 and IFNγ were produced by T cells from IL-17−/− mice and control mice consistent with development of Th2-predominant immunity in all animals. Improved disease outcome was associated with decreased CXCL2-accumulation in lesion sites and decreased neutrophil recruitment into lesions of infected IL-17−/− mice confirming prior observations that enhanced neutrophil recruitment contributes to disease susceptibility in BALB/c mice. This study excludes an important facilitating role for IL-17 in Th1/Th2 development in L. major-infected BALB/c mice, and suggests that IL-23 production by L. major-infected DC maintains IL-17+ cells that influence disease progression via regulation of neutrophil recruitment.
Keywords: Parasitic-Protozoan, T cells, Th1/Th2 cells, cytokines
Introduction
In murine experimental cutaneous leishmaniasis, protective immunity that develops in most strains depends on IFN-γ producing CD4+ Th1 and CD8+ Tc1 cells. IFNγ promotes host defense against intracellular pathogens, including Leishmania, by activating murine macrophages (MΦ) to kill parasites via NO (1). CD11c+ dendritic cells (DC) from skin facilitate development of protective immunity after uptake of Leishmania major (L. major) via FcγRI and FcγRIII (2) and infected DC release factors critical for Th1 development (e.g. bioactive interleukin (IL)-12 (3)). However, BALB/c mice succumb to infection due to uncontrolled Th2 development despite production of IL-12 by L. major-infected DC (3). Additional factors that influence disease susceptibility of BALB/c mice include decreased release of DC-derived IL-1α/β (4, 5) and increased production of inhibitory IL-12p40 homodimer (6).
Recently, a new subset of Th cells has been described (Th17 cells; for reviews see (7–10)). IL-17 is a proinflammatory cytokine that is secreted primarily by activated T cells (CD4+ > CD8+) (11) and that has been implicated in several important inflammatory human diseases, e.g. rheumatoid arthritis, psoriasis, multiple sclerosis, inflammatory bowel disease and asthma (7–10). IL-17 stimulates a variety of cells (e.g. fibroblasts, MΦ, endothelial cells) to produce inflammatory mediators including IL-1, TNFα and chemokines. These events ultimately lead to recruitment of neutrophils and other leukocytes that is the hallmark of inflammatory diseases.
IL-17 production appears to be downstream of IL-1α/β in several pathological conditions (12–14). Using IL-1Ra-deficient mice, we previously demonstrated that excessive levels of IL-1 led to increased IL-17 production and destructive arthritis due to enhanced IL-1 signalling (12). In addition, in Th1/Th17-mediated EAE we observed that the IL-1/IL-1Ra system is crucial for induction of autoantigen-specific T cells (13). Finally, in the EAE model, Th17 induction was abrogated in IL-1R type I−/− mice (15).
Since we and others have previously reported that DC-derived IL-1 is important for efficient Th1 induction in leishmaniasis (4, 5), we sought to elucidate the role of IL-17 in the pathogenesis of leishmaniasis. We hypothesized that IL-17 production would be decreased in susceptible BALB/c mice. Surprisingly, we determined that BALB/c mice produced increased levels of IL-17 after infection with L. major and that IL-17−/− BALB/c mice exhibited dramatically attenuated disease despite typical Th2 development. This finding correlated with reduced accumulation of neutrophils in lesional tissue of IL-17−/− BALB/c mice and dramatically reduced CXCL2 levels in infected skin. Increased IL-17-dependent neutrophil recruitment into lesions of L. major-infected BALB/c mice has previously been shown to significantly contribute to disease outcome (16, 17). We also demonstrate that elevated levels of IL17A in BALB/c mice were associated with increased production of IL-23, but not IL-6 and TGF-β1, by infected DC.
Material and Methods
Animals, parasites and infections
Six to 8 week old wild type C57BL/6, BALB/c and IL-17−/− (BALB/c) mice (backcrossed to BALB/c >10 times (12) confirmed by SNP analysis) were obtained from the Central Animal Facility of the University of Mainz. All animals were housed and used in experiments in accordance with institutional guidelines.
Infectious-stage metacyclic promastigotes were isolated from stationary cultures of L. major clone V1 (MHOM/IL/80/Friedlin) by positive selection using a biphasic Ficoll gradient (10%/20%). Infections were initiated intradermally in ear skin of C57BL/6, BALB/c or IL-17−/− mice using standard high dose (2 × 105) or physiological low dose inocula with 1 × 103 metacyclic promastigotes per ear. Lesion development was assessed weekly for up to 5 months in three dimensions using a calliper, calculated as ellipsoids ([a/2 × b/2 × c/2] ×4/3 π) and expressed as mean ± SEM (5). Parasite burdens in lesional tissue were estimated at weeks 3, 6 and 8 as indicated using a limiting dilution assay as described previously (5). In some experiments, ear skin was injected intradermally with 0.5 or 1 μg of recombinant IL-17A (R&D Systems).
Determination of cytokine production in lymph nodes
For measurement of cytokine production, 1 × 106 retroauricular lymph node (LN) cells from infected or uninfected mice were added in 200 μl to 96-well plates in the presence or absence of SLA (25 μg/ml) or Staphylococcal enterotoxin B (SEB, 1 μg/ml, Sigma, Deisenhofen, Germany). Antigen-specific IFNγ, IL-4, IL-10 and IL-17A production was determined after 48 h via ELISA (R&D Systems, Bad Nauheim, Germany, and BD Pharmingen, Heidelberg, Germany).
In selected experiments, γδ TCR+, Gr-1+, CD8+ and CD4+ cells were isolated sequentially from draining LN of infected mice using microbeads (Miltenyi, Bergisch Gladbach, Germany). Cells were then plated at 5 × 105 cells/100 μl for 48 h at 37°C together with L. major-infected bone marrow-derived DC (BMDC) at a ratio of 10 cells/1 DC. Cytokine release was determined in 18 h supernatants using ELISAs specific for IFNγ, IL-4 and IL-17. To determine single-cell cytokine production, T cells were incubated as indicated above and Brefeldin A was added for the last 4 hrs. After harvesting, cells were permeabilized and stained with anti-IL-17A and anti-IL-10 (both Pharmingen).
Detection of DC-derived IL-6, IL-23 and TGF-β1
Bone marrow-derived DC were generated within 6 days as described previously (18) using GM-CSF and IL-4. Cytokine release by DC stimulated with L. major amastigotes (parasite/cell ratio 10:1, 2 × 105 cells/ml) or LPS (100 ng/ml) was determined in 18 hr supernatants by ELISA (5, 6, 19–21). For determination of cytokine release in vivo, groups of ≥3 C57BL/6 or BALB/c mice were infected with 2 × 105 L. major into ear skin. At the time points indicated, cells were harvested from draining LN and plated at 1 × 106/200 μl to 96-well plates in the presence or absence of SLA (25 μg/ml). Supernatants were collected after 48 hrs. Cytokine content was determined using ELISAs specific for IL-6, IL-12p40, TGF-β1 (all Becton Dickinson), and IL-23p19 (eBiosciences).
Lesional cytokine production and characterization of inflammatory infiltrate
At several time points, infected ears were collected and inflammatory cells were isolated using liberase (Roche, Mannheim, Germany) and mechanical disruption (2). To determine IL-17 and chemokine levels in lesional tissue, cells were resuspended in buffer containing a Complete Mini Protease Inhibitor Cocktail (Roche, Manheim, Germany), homogenized using a pellet pestle (Pharmingen) and vigorously vortexed. Supernatants were assayed for the presence of IL-17, CXCL1, CXCL2 and CXCL5 by ELISA (R&D Systems).
In some experiments, cells were counted and the frequencies of various inflammatory cells were determined using flow cytometry via staining with CD4 (L3T4 GK1.5), CD8 (Ly-2 53–6.7), F4/80 (CI:A-3), CD11c (N418), CD19 (1D3), Gr-1 (RB6–8C5) all obtained from BD Pharmingen (Heidelberg, Germany), and NIMP-R14 (DH6, Caltag, Hamburg, Germany) and absolute numbers of cell populations were calculated. Stained cells were analyzed using a FACScan flow cytometer equipped with CellQuest software (Becton Dickinson, Heidelberg, Germany).
Statistical analysis
Statistical significance was determined using the unpaired Student’s-t-test using StatView® for Windows. Results are expressed as mean ± SEM.
Results
CD4+ T-cells from L. major-infected BALB/c mice produce more IL-17 than C57BL/6 CD4 T cells
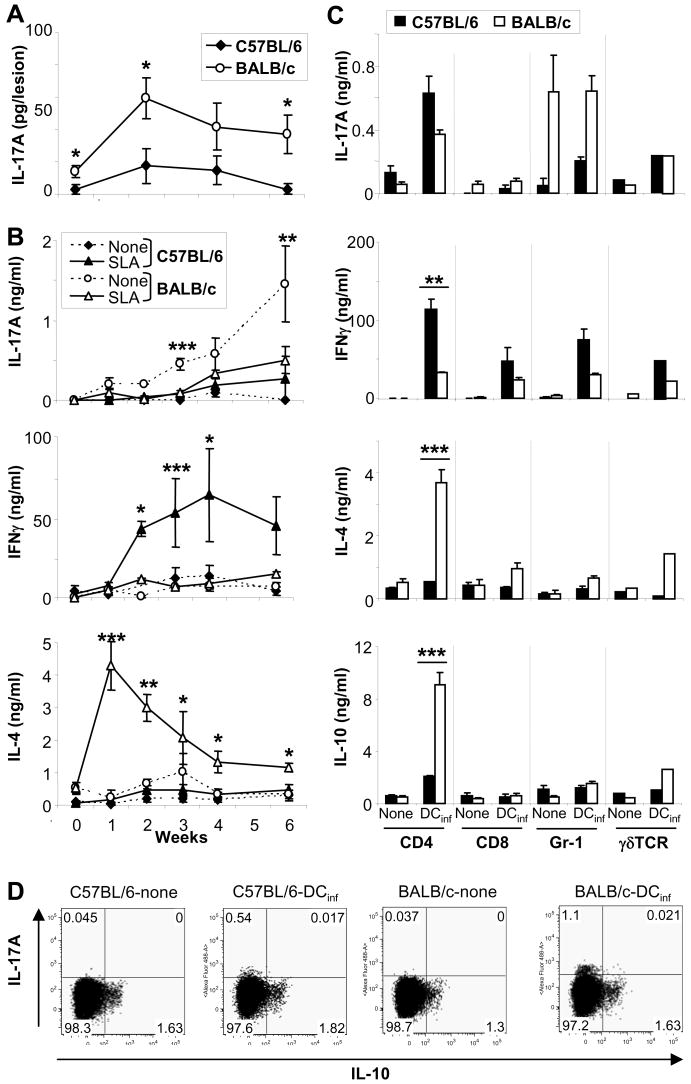
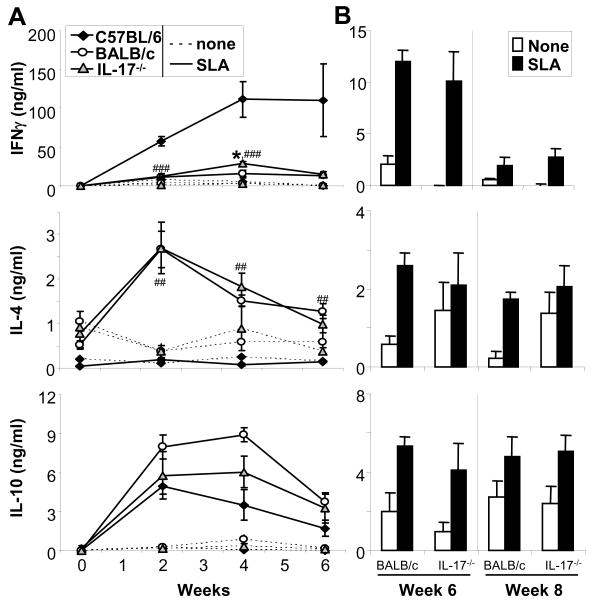
Previously, we and others reported that increased production of IL-1α (and β) by infected C57BL/6 CD11c+ DC as compared to infected cells from BALB/c DC was associated with enhanced Th1 priming in resistant C57BL/6 mice (4, 5, 22, 23). Intending to study downstream effects of IL-1, we analyzed lesions and lymph nodes (LN) of L. major-infected mice for the production of IL-17. Ears of infected mice were harvested at several time points and IL-17 levels were determined in supernatants of ear homogenates by ELISA (Fig. 1A). Lesional IL-17 production was maximal 2 weeks post infection. Interestingly, we found increased levels of IL-17 in lesions of BALB/c mice as compared to C57BL/6 mice over the course of several weeks. We next analyzed supernatants of LN cell cultures for the production of IL-17 (Fig. 1B). We found ~5-fold increased levels of IL-17 in LN cultures prepared from L. major-infected BALB/c mice as compared with cultures from C57BL/6 mice. Differences in IL-17 production by cultured BALB/c and C57BL/6 LN cells were evident beginning in week 1 and increased thereafter (up to 6 weeks). Interestingly, addition of soluble Leishmania-antigen (SLA) to trigger maximal antigen-dependent cytokine responses led to downregulation of IL-17 in the cultures; as shown previously, inhibition of SLA-induced IFNγ or IL-4 release by antibodies abrogated this effect (data not shown). As expected, we detected increased amounts of IFNγ in LN cultures of C57BL/6 mice over the course of several weeks post infection, whereas high levels of IL-4 were detected in LN cultures from infected BALB/c mice. The latter two cytokines were strongly triggered upon additional stimulation with exogenous antigen (SLA).
Figure 1. Leishmania major-infected, susceptible BALB/c mice show increased production of IL-17 as compared to resistant C57BL/6 mice.
Groups of ≥5 BALB/c or C57BL/6 mice were infected with 2 × 105 L. major. A, Homogenates of lesional inflammatory cells were assayed for the presence of IL-17 by ELISA (mean ± SEM, n=5). B, Draining LN cell suspensions were restimulated with soluble Leishmania antigen (SLA) and cytokine production was quantified. C, Isolated CD4+, CD8+, Gr-1+, and γδTCR+ cells from LN from 4 wk-L. major infected mice were restimulated using infected bone marrow-derived dendritic cells (DCinf, ratio 10:1). Cytokine production was assessed after 48 (B, left panels) or 18 hrs (C, right). Cytokine values are shown as mean ± SEM (n ≥ 3), strain-dependent differences in cytokine production are designated *(p ≤ 0.05), **(p ≤ 0.005) and ***(p ≤ 0.002). D, Cytokine production of CD4+ T cells was determined by intracellular flow cytometry after 48 hrs. One representative staining of n=3 experiments is shown.
To identify the cells that produce IL-17 in infected mice, CD4+, CD8+, Gr-1+ and γδTCR+ cells were enriched to high purity (>95%) using microbeads (Fig. 1C). The most abundant cell types in LN of L. major-infected mice were, as expected, CD4 T cells (20% in C57BL/6, 30% in BALB/c), followed by CD8 T cells (no strain difference). Only few neutrophils (3% in C57BL/6, 10% in BALB/c) and a small number of γδT cells (0.3%, no strain difference) were found. All cells were re-stimulated using L. major-infected DC (T cell/DC ratio 10:1). The majority of IL-17 was released by activated CD4 cells and neutrophils. Interestingly, CD4+ T cells from both mouse strains produced comparable levels of IL-17 after antigen-specific restimulation. IL-17 levels were also unrelated to levels of IFNγ or IL-4, consistent with the existence of a distinct IL-17-producing Th subset (7–10). Interestingly, BALB/c neutrophils produced significantly larger amounts of IL-17A as compared to C57BL/6 neutrophils and IL-17A production was Ag-independent. Significant production of IL-17A by CD8 or γδ T cells was not detected. Taking the numbers of each cell type in the LN into account, the majority of IL-17A in vivo appears to be produced by CD4+ T cells (both strains) and neutrophils (predominantly BALB/c).
Recently, McGeachy et al. showed differences between IL-23 versus IL-6/TGF-β1 derived IL-17 producing cells in terms of inducing or limiting immunopathology [24]. We thus assessed the ability of CD4+ T cells that produce IL-17A in cutaneous leishmaniasis to produce IL-10. Isolated CD4 cells were stimulated with infected DC and cytokine production was assessed by intracellular flow cytometry (Fig. 1D). IL-17A staining was detected primarily in BALB/c derived T cells and, to a lesser extent, in C57BL/6 cells. IL-10 production was also detected in CD4 cells from both strains, but cells co-producing both cytokines were not found.
Increased release of IL-23 from BALB/c DC in vitro and in vivo
We next attempted to identify the underlying mechanism explaining the elevated Th17 levels in L. major-infected BALB/c mice. Several cytokines have recently been implicated in the induction of Th17 cells from naïve CD4+ T cells (e.g. IL-1, TGFβ, IL-6, IL-23) (7, 9, 15, 25–27). Even though we and others have previously shown that IL-1α/β synthesis is decreased in DC from BALB/c mice relative to C57BL/6 mice and linked this observation with impaired Th1 education in susceptible animals (4, 5), the observation that IL-17 production is higher in L. major-infected BALB/c mice than in comparable C57BL/6 suggests that IL-1 is not an important regulator of IL-17 in this setting. Thus, we assessed the accumulation of IL-6, TGF-β1 and IL-23 in supernatants of DC and LN cell restimulation cultures at several time points after L. major infection.
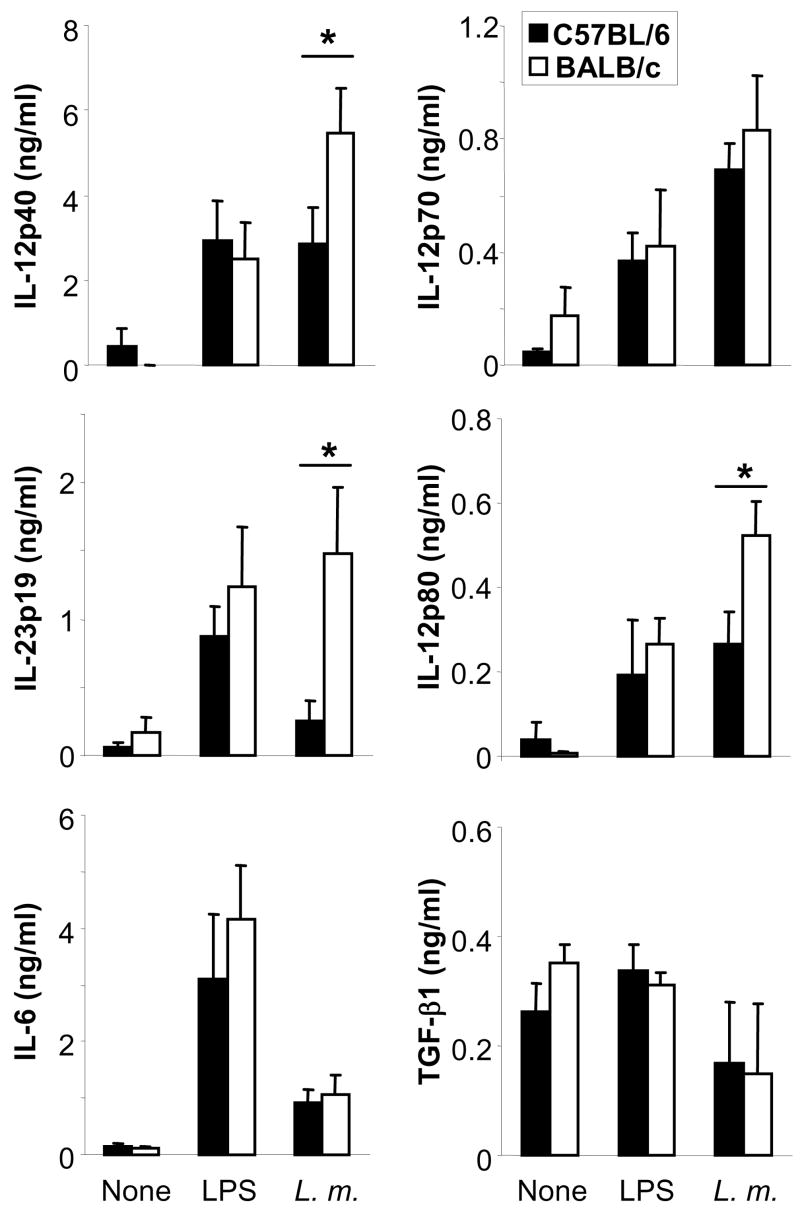
C57BL/6 or BALB/c bone marrow-derived DC were harvested as immature DC on day 6 of culture and stimulated with amastigotes of L. major (10 parasites per cell) or control LPS (100 ng/ml). After 18 hrs, the supernatants were harvested and assayed for the presence of cytokine by ELISA. As described previously, DC from both mouse strains released IL-12p40, IL-12p70, IL-12p80 and IL-6 after infection with L. major parasites or stimulation with LPS (6, 20,21). In addition, L. major-dependent induction of IL-23p19 was observed. L. major-specific synthesis of bioactive TGF-β1 was not found (Fig. 2). Interestingly, strain-dependent differences were found only with regard to IL-12p40, IL-23p19 and IL-12p40 homodimer production. As observed previously, BALB/c DC produced more IL-12p40 and IL-12p80, whereas we observed no difference in the production of bioactive IL-12p70 (6, 21). Interestingly, the production of IL-23p19 was ~5-fold higher in infected BALB/c DC as compared to C57BL/6 DC.
Figure 2. Dendritic cells from BALB/c mice produce more IL-23 upon infection with L. major.
Bone marrow-derived dendritic cells (DC) were generated from BALB/c or C57BL/6 mice, plated at 2 × 105 cells/ml and stimulated with amastigotes of L. major (10 parasites/cell) or LPS (100 ng/ml). After 18 hrs, IL-12p40, IL-12p70, IL-12p80, IL-23p19, IL-6 and TGF-β1 levels were determined in supernatants using commercially available ELISA. The data is expressed as mean ± SEM (n=5, *=p<0.05).
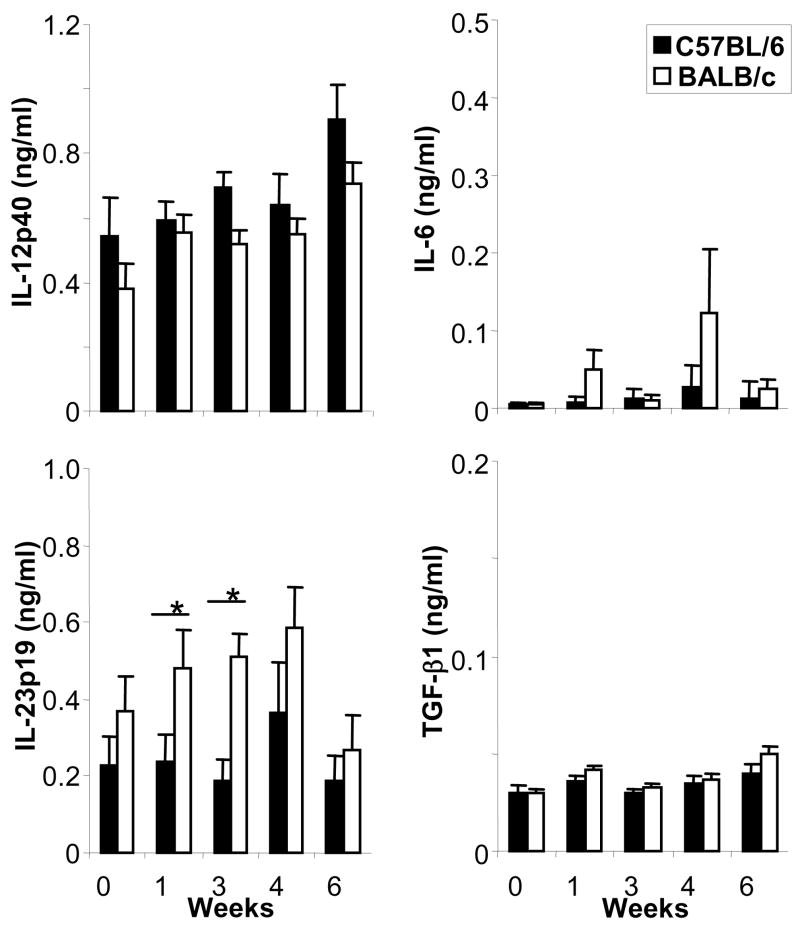
Next, we assessed the production of Th17-inducing cytokines in vivo by determining the cytokine amounts in supernatants of antigen-specifically restimulated LN cells at different time points post infection (Fig. 3). Similar to the findings with DC generated in vitro, we found significantly higher levels of IL-23 in supernatants of BALB/c LN cells than in C57BL/6 mice especially at early time points post infection (week 0–3, including baseline levels). Both IL-12p40 and IL-23p19 in these LN supernatants were DC-derived since CD11c+ DC depletion from LN cells at week 3 abrogated this effect (data not shown). No significant differences were identified in the production of IL-12p40, IL-6 and TGF-β1.
Figure 3. Increased production of IL-23 in draining lymph nodes in Leishmania-susceptible as compared to -resistant mice.
Mice of the Leishmania-susceptible BALB/c or –resistant C57BL/6 strains were infected with 2 × 105 L. major. At the indicated time points post infection, lymph node cells were harvested and restimulated with soluble Leishmania lysate (1 × 106 cells/ml). Production of IL-12p40, IL-23p19, IL-6 and TGF-β1 was assessed after 48 hrs by ELISA (mean ± SEM, n ≥ 5, *=p<0.05).
IL-17 is required for development of progressive cutaneous leishmaniasis in BALB/c mice
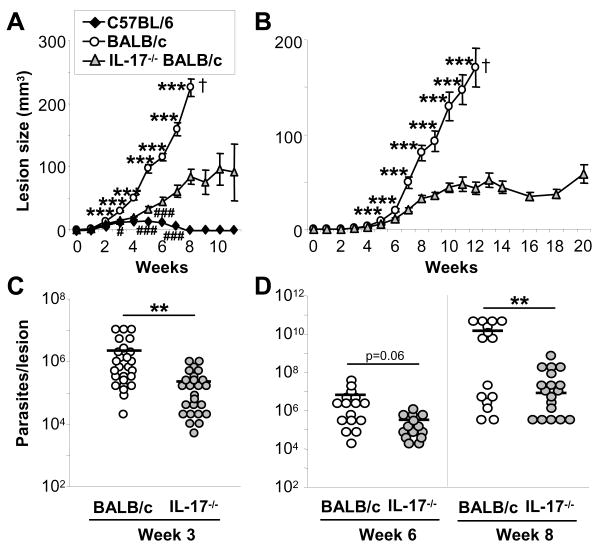
To further characterize the role of IL-17 in leishmaniasis, we studied IL-17−/− mice on a genetically susceptible BALB/c background (12). IL-17-deficient and wild type BALB/c as well as control C57BL/6 ears were infected intradermally with standard high doses (2 × 105) or physiologically relevant low doses (103) of L. major metacyclic promastigotes, and lesion development was monitored over the course of ≥4 months. We found significantly smaller lesion volumes in IL-17-deficient mice as compared to wild type BALB/c mice beginning in week 2 (high dose model, Fig. 4A) or in week 4 (low dose, Fig. 4B). As expected, lesions in susceptible wild type mice progressed and became necrotic. Infected control BALB/c mice had to be euthanized in week 8 (high dose) or week 12 (low dose). In contrast, lesions in IL-17−/− mice were significantly smaller and remained stable for at least 1 (high dose) or 2 (low dose) additional months. Later time points were not examined. However, unlike resistant C57BL/6 mice, BALB/c IL-17−/− mice did not resolve their lesions.
Figure 4. IL-17-deficient mice exhibit decreased lesions volumes and lower parasite burdens after infection with L. major.
Infections were initiated in IL-17−/− and control BALB/c and C57BL/6 mice with 2 × 105 (A, C) or 1,000 (B, D) L. major metacyclic promastigotes and lesion volumes ere assessed weekly (A, B, mean ± SEM; n ≥ 10). At week 3 (high dose, C) and week 6 and 8 (low dose, D), mice were euthanized and lesional parasite loads were determined using limiting dilution assays. Dots represent numbers of parasites in single ears; bars show the arithmetic mean of all mice/group. Statistical differences between BALB/c strains are indicated by *, between IL-17−/− and C57BL/6 mice as # (*,#=p ≤ 0.05, **,##=p ≤ 0.005, ***,###=p ≤ 0.002).
Lesion progression in wild type and IL-17−/− mice correlated with lesional parasite burdens (Fig. 4C+D). In both high and low dose infection models, smaller lesions in IL-17−/− mice coincided with significantly decreased numbers of parasites in infected ears. After infection with 2 × 105 parasites, lesions of 3 week infected BALB/c mice contained ~ 2.2 × 106 parasites, whereas infected ears of IL-17−/− mice showed 10-fold fewer parasites (2.1 × 105, p ≤ 0.005). Wild type mice infected with physiologically low dose inocula harbored 1.4 × 1010 parasites in week 8 lesions, whereas IL-17-deficient ears contained 7.6 × 106 organisms (p ≤ 0.005).
Th1/Th2 ratios are not altered in infected IL-17−/− BALB/c mice
Generally, improved disease outcome in cutaneous leishmaniasis correlates with enhancement of Th1-predominant cytokine responses in draining LN (1). To analyze antigen-specific cytokine profiles, we obtained submandibular LN from infected mice at several time points and restimulated cell suspensions with SLA (Fig. 5A+B). Surprisingly, despite the presence of dramatically smaller lesion volumes in infected IL-17−/− BALB/c mice, we did not detect significant differences in the release of IFNγ, IL-4 or IL-10 as compared to control BALB/c cells. Cytokine responses remained Th2 predominant in both IL-17−/− and L. major-infected wild type BALB/c mice. For comparison, the Th1 cytokine profile of resistant C57BL/6 control mice is shown in Fig. 5A.
Figure 5. Cytokine profiles in L. major-infected BALB/c and IL-17−/− BALB/c mice.
Groups of ≥5 BALB/c, BALB/c IL-17−/− or C57BL/6 mice were infected with 2 × 105 (A) or 103 (B) L. major. A+B, Cytokine profiles were determined by restimulation of draining LN cells with soluble Leishmania antigen (SLA). Cytokine production was assessed after 48 hr by ELISA (mean ± SEM; n ≥ 4 independent experiments). Differences between IL-17−/− BALB/c and BALB/c were designated as *, between IL-17−/− BALB/c and C57BL/6 as # (*=p ≤ 0.05, **,##=p ≤ 0.005, ***,###=p ≤ 0.002).
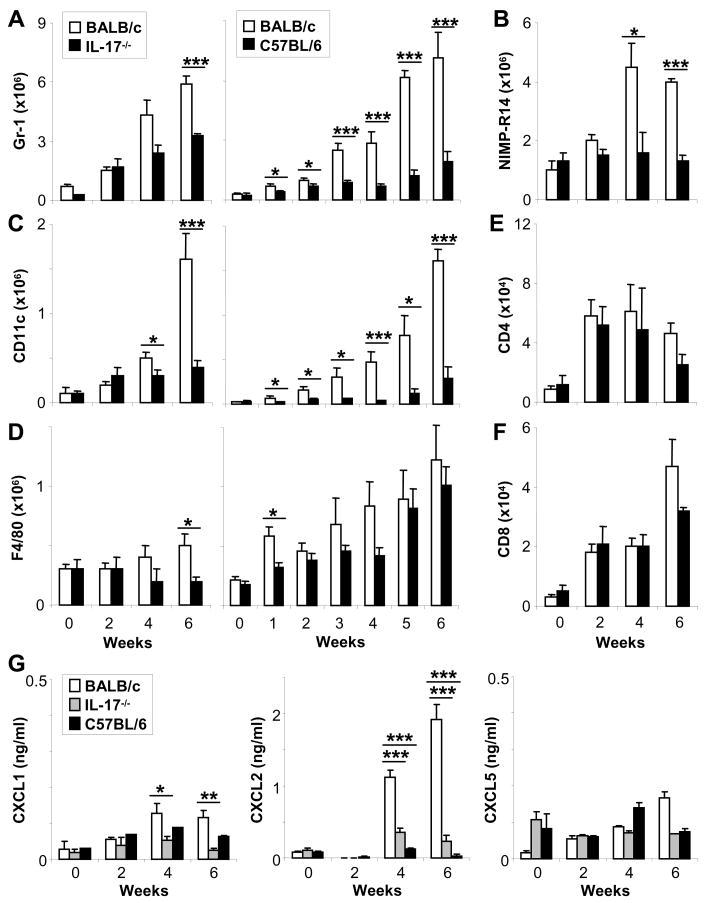
Lesions of infected IL-17−/− mice contained fewer neutrophils and DC
IL-17 has been reported to contribute to inflammatory immune responses by recruiting neutrophils, e.g. in experimental autoimmune encephalitis (EAE) and arthritis (7–10). In L. major infections, previous studies have demonstrated that enhanced recruitment of neutrophils is associated with disease susceptibility in BALB/c mice (16, 17). Importantly, depletion of neutrophils from infected BALB/c mice induced a healing, resistant phenotype (16). Therefore, we analyzed the lesional inflammatory cell infiltrates in IL-17−/− and susceptible BALB/c mice in more detail (Fig. 6). We also compared the inflammatory cells in lesions of BALB/c mice with those in resistant C57BL/6 animals (Fig. 6A, C, D, right panels). To accomplish this, ears of infected mice were obtained at the time points indicated; lesional cells were isolated as described (2) and then analyzed using flow cytometry.
Figure 6. Decreased recruitment of neutrophils and dendritic cells into skin lesions of infected IL-17−/− BALB/c mice and genetically resistant C57BL/6 mice.
Groups of BALB/c, C57BL/6 or IL-17−/− BALB/c mice were infected with 2 × 105 parasites. Lesional inflammatory cells were isolated and characterized by flow cytometry (A+B: Gr-1+ and NIMP-R14+ neutrophils, C: CD11c+ dendritic cells (DC), D: F4/80+ macrophages (MΦ), E+F: CD4+ and CD8+ T-cells). Cell numbers were calculated and are presented as mean ± SEM (n ≥ 3 independent experiments with ≥3 mice/group). G, Homogenates of lesional inflammatory cells were assayed for the presence of chemokine via ELISA (mean ± SEM, n=4). A-G, *=p ≤ 0.05, **=p ≤ 0.005, ***=p ≤ 0.002.
We enumerated neutrophils in Leishmania lesions using anti-Gr-1 (Ly 6G, Fig. 6A) or NIMP-R14 (Fig. 6B), an antibody that reacts only with BALB/c neutrophils (16). Using both antibodies, we found significantly decreased recruitment of neutrophils into lesions of L. major-infected IL-17−/− mice. We also detected decreased numbers of CD11c+ DC and F4/80+ MΦ in lesions of IL-17−/− mice (Fig. 6C+D). Reduced recruitment of inflammatory DC in IL-17-deficient mice as compared to control mice was significant at weeks 4 and 6 post infection (Fig. 6C). Levels of DC recruitment into Leishmania lesions in IL-17−/− mice were similar to those found in resistant C57BL/6 mice. Similar but less striking differences were found with regard to inflammatory MΦ recruitment (Fig. 6D). We did not detect differences in the numbers of recruited T cells (CD4+ and CD8+) in lesions of infected IL-17−/− as compared to wild type BALB/c (Fig. 6E+F) or C57BL/6 mice (data not shown).
Our findings are consistent with the concept that IL-17 is a key cytokine with regard to neutrophil recruitment (7–10), most likely via induction of chemokines (7, 28). Several prior studies suggested that chemokines (including CXCL1, CXCL2 and CXCL5) induced by IL-17 are responsible for neutrophils recruitment (29. 30). Thus, we next quantified levels of Groα (KC, CXCL1), LIX (CXCL2) and MIP-2 (CXCL5) in infected ear skin. Lysates of inflammatory ear cells were assessed for the chemokine content by ELISA (Fig. 6G). Interestingly, we found that CXCL2 was dramatically upregulated in BALB/c ears in response to IL-17 (since it was not seen in IL-17−/− BALB/c mice) as compared to C57BL/6 skin. In addition, CXCL1 is also more abundant in ears of susceptible BALB/c as in C57BL/6 ears, albeit at lower levels. Thus, both CXCL2 and CXCL1 may mediate the effect of IL-17 and facilitate pathological neutrophil recruitment to lesions of L. major-infected BALB/c mice.
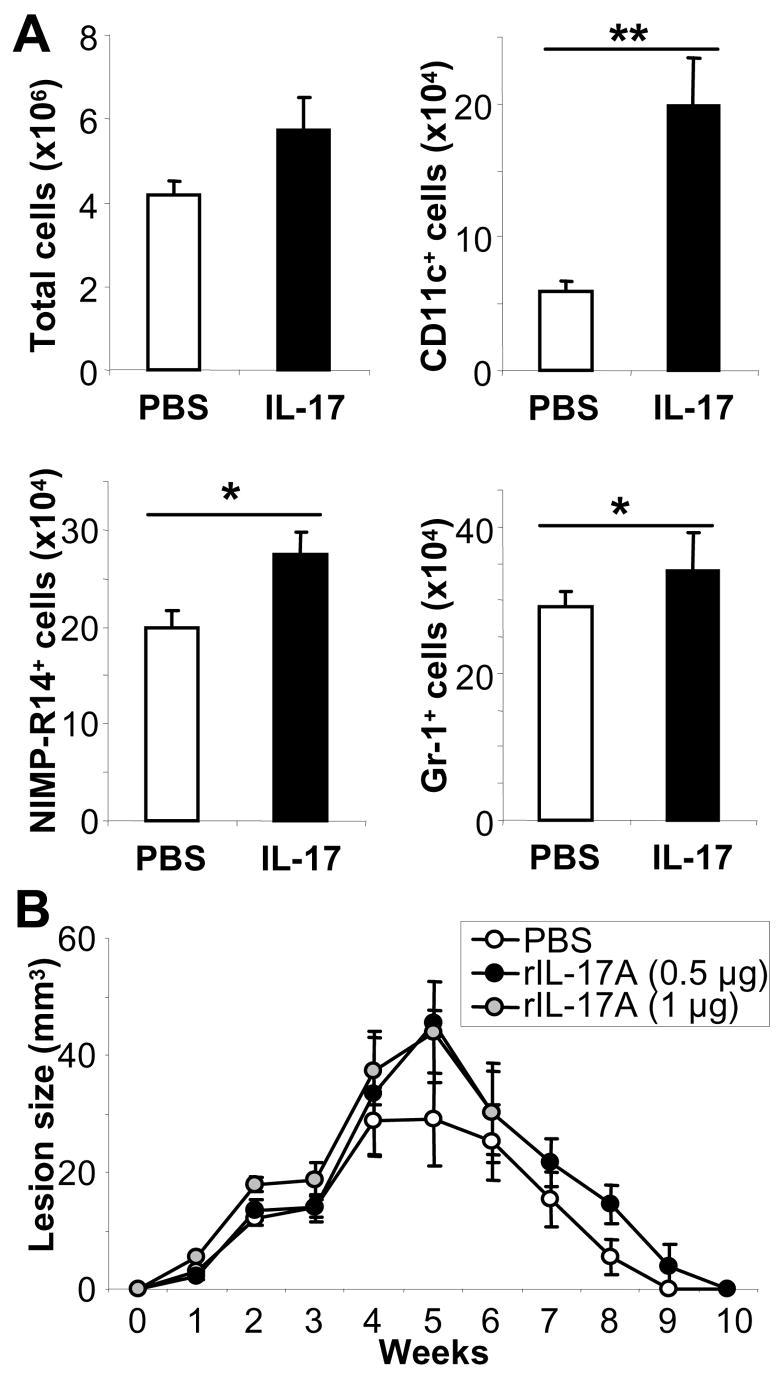
Recruitment of DC to lesions of infected BALB/c mice may also be downstream of IL-17. To test this hypothesis, we injected recombinant IL-17 intradermally into ear skin of naïve mice on 3 consecutive days and found significantly increased immigration of neutrophils and especially CD11c+ DC on day 5 (Fig. 7A). To determine if increased levels of IL-17 might alter disease outcome in genetically resistant mice because of enhanced neutrophil recruitment, we also performed treatment protocols of infected C57BL/6 mice. Mice were injected with either 0.5 or 1 μg/ear every second day between days 7–18 (Fig. 7B). Despite the fact that IL-17 also induced enhanced immigration of neutrophils and DC to sites of application in resistant mice (data not shown), the course of disease was not modified.
Figure 7. IL-17-mediated increased recruitment of DC and neutrophils into skin.
A, Groups of 4 naïve BALB/c mice were injected intradermally with recombinant murine IL-17 (0.5 μg/mouse) of into ear skin on 3 consecutive days. On day 5, inflammatory ear cells were isolated and DC and neutrophils identified by FACS staining with anti-CD11c, anti-NIMP and anti-Gr-1, as appropriate (n=8, *=p ≤ 0.05, ***=p ≤ 0.002). B, C57BL/6 mice were infected with 2 × 105 parasites and treated with 0.5 or 1 μg of rIL-17A as indicated every second day starting on d7 until d19. Lesions are expressed as mean ± SEM, n=2.
Discussion
IL-17 production by Th17 cells has been associated with inflammation in several autoimmune diseases (7). The present study reveals an unexpected role for IL-17 in promotion of disease in a model in which susceptibility has been associated with Th2 immunity. Whereas we previously observed attenuated Th2-associated airway hypersensitivity in IL-17-deficient mice (7), IL-17−/− BALB/c mice exhibited resistance to progressive leishmaniasis that could not be ascribed to alterations in Th2 responses. Thus, in cutaneous leishmaniasis, disease susceptibility is not only associated with uncontrolled Th2 immunity (1), but also with excessive IL-17 release.
In the present study, we detected increased amounts of IL-17 in LN cells of BALB/c as compared to C57BL/6 mice. Interestingly, addition of soluble Leishmania-antigen (SLA) to trigger maximal antigen-dependent cytokine responses led to downregulation of IL-17 in the cultures. In parallel to the LN cells stimulated with SLA, maximal restimulation of isolated BALB/c and C57BL/6 CD4+ T cells with L. major-infected DC in vitro resulted in similar levels of IL-17 in the supernatants. This is consistent with the concept that both Th1 and Th2 effector cytokines, which are strongly upregulated by addition of supra-optimal doses of exogenous antigen such as SLA, are capable of downregulating Th17 cells (7–10). In addition to CD4+ Th17 cells neutrophils were another relevant source of IL-17A in cutaneous leishmaniasis. Release of IL-17 from these cells was not dependent on antigen presenting cells or antigen and was strain-dependent (BALB/c > C57BL/6).
Several cytokines contribute to the induction (e.g. IL-1α/β, IL-6, TGF-β1) and maintenance (IL-23) of Th17 cells (7–10, 31–33). Although DC-derived IL-1α/β production does not correlate with induction of IL-17 in murine cutaneous leishmaniasis, other DC-derived factors in L. major-infected BALB/c mice appear to be relevant (14, 31, 32). Both in DC in vitro and LN supernatants in vivo, we found significantly higher production of IL-23 by BALB/c than by C57BL/6 cells. In contrast, we did not observe differences in the production of the other Th17-inducing cytokines, i.e. IL-6 and TGF-β1. We also did not detect IL-23 release from L. major-infected MΦ in vitro (data not shown). Consistent with the results of McGeachy et al., the finding that IL-17Apos/IL-10neg Th17 cells mediate inflammation leading to susceptibility in leishmaniasis is consistent with their induction by excessive amounts of IL-23 in BALB/c mice (24). To ultimately prove that DC-derived IL-23 is responsible for the induction of detrimental Th17 generation in BALB/c mice, IL-23−/− mice will be studied when they become available on a BALB/c background.
Although neutrophils are among the first cells recruited to L. major lesions and neutrophils have been shown to play some role in inducing protection (33, 34), several reports previously demonstrated that persistent inflammation characterized by increased neutrophil infiltration promoted disease susceptibility in BALB/c mice (16, 17, 35). Increased numbers of neutrophils were identified in established lesions in susceptible mice as compared to those of genetically resistant animals (36, 37). Additionally, depletion of neutrophils from L. major-infected BALB/c mice suppressed Th2 development, reduced parasite loads and improved disease outcome (16, 17). A prior study showed that interaction of MΦ with dying neutrophils from infected BALB/c mice induced TGF-β and PGE2 production, both of which enhanced parasite accumulation (17). In contrast, in the same study, MΦ from C57BL/6 mice phagocytosing dying neutrophils released TNFα and thus facilitated lesion resolution. Genetically determined differences in the behaviour of neutrophils (17), macrophages that phagocytose these neutrophils (17) and DC (5, 6) may account for the inability of recombinant IL-17 to alter the course of disease in resistant mice.
Even though it is well accepted that neutrophil accumulation in BALB/c mice contributes to susceptibility, the mechanism responsible for enhanced neutrophil accumulation in leishmaniasis lesions has not been previously elucidated. Herein, we show that increased levels of IL-17 are responsible for neutrophil immigration, most likely via CXCL2 (and CXCL1), and confirm the deleterious role that IL-17-dependent recruitment of neutrophils into leishmaniasis lesions of infected BALB/c mice plays. Interestingly and in contrast to what might have been predicted (16, 17), Th1/Th2 development was not altered in L. major-infected IL-17−/− BALB/c mice.
The importance of IL-12 and IFNγ for host defence against intracellular pathogens, e.g. L. major, has been clearly demonstrated (1). Recent studies also implicate IL-23 and IL-17 in immunity against extracellular pathogens, e.g. bacteria (Klebsiella pneumoniae), Toxoplasma gondii parasites and fungi (Cryptococcus neoformans) (7, 38, 39). In these models, IL-17 likely mediates recruitment of lesional neutrophils (27, 38), whereas IFNγ is responsible for activating MΦ to kill intracellular pathogens. In Schistosoma mansoni infections, increased levels of IL-23 and IL-17 are associated with disease exacerbation (40). Our results suggest that IL-17-mediated neutrophil recruitment is also detrimental in L. major infections.
In summary, the present study suggests that DC-derived IL-23, in addition to IL-1α and IL-12p80, contributes to disease susceptibility in BALB/c mice. IL-23-mediated IL-17 induction appears to be responsible for persisting and detrimental infiltration of Leishmania lesions with inflammatory neutrophils in as much as prior studies have convincingly demonstrated that disease progression in BALB/c mice is linked with an exuberant neutrophil response. These data indicate that in addition to it’s important role in the pathogenesis of autoimmune diseases, IL-17 is also an important modulator of an infection with an important human pathogen.
Acknowledgments
The authors thank Dr. K. Reifenberg and staff for excellent animal care, and Drs. Ari Waisman, Burkhard Becher, Markus Neurath and Helmut Jonuleit for helpful discussions.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG, SFB 490, and SFB548) and the Berliner Stiftung für Dermatologie to E. von Stebut. M.C. Udey is supported by the Intramural Program of the NCI, Center for Cancer Research, NIH.
Abbreviations used
- LN
lymph node
- MΦ
macrophages
- SLA
soluble Leishmania antigen
- L. major
Leishmania major
Contributor Information
Susanna Lopez Kostka, Department of Dermatology, Johannes Gutenberg-University, Mainz, Germany.
Stephanie Dinges, Department of Dermatology, Johannes Gutenberg-University, Mainz, Germany.
Klaus Griewank, Department of Dermatology, Johannes Gutenberg-University, Mainz, Germany.
Yoichiro Iwakura, Center for Experimental Medicine, Institute of Medical Science, University of Tokyo, Japan and.
Mark C. Udey, Dermatology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, USA
Esther von Stebut, Department of Dermatology, Johannes Gutenberg-University, Mainz, Germany.
References
- 1.Sacks D, Anderson C. Re-examination of the immunosuppressive mechanisms mediating non-cure of Leishmania infection in mice. Immunol Rev. 2004;201:225–238. doi: 10.1111/j.0105-2896.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 2.Woelbing F, Lopez Kostka S, Moelle K, Belkaid Y, Sunderkoetter C, Verbeek S, Waisman A, Nigg AP, Knop J, Udey MC, von Stebut E. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J Exp Med. 2006;203:177–188. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Stebut E, Udey MC. Requirements for Th1-dependent immunity against infection with Leishmania major. Microbes Infect. 2004;6:1102–1109. doi: 10.1016/j.micinf.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Filippi C, Hugues S, Cazareth J, Julia V, Glaichenhaus N, Ugolini S. CD4+ T cell polarization in mice is modulated by strain-specific major histocompatibility complex-independent differences within dendritic cells. J Exp Med. 2003;198:201–209. doi: 10.1084/jem.20021893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Stebut E, Ehrchen JM, Belkaid Y, Lopez Kostka S, Mölle K, Knop J, Sunderkötter C, Udey MC. IL-1α promotes Th1-differentiation and inhibits disease progression in Leishmania major-susceptible BALB/c mice. J Exp Med. 2003;198:191–199. doi: 10.1084/jem.20030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nigg AP, Zahn S, Ruckerl D, Holscher C, Yoshimoto T, Ehrchen JM, Wolbing F, Udey MC, von Stebut E. Dendritic cell-derived IL-12p40 homodimer contributes to susceptibility in cutaneous leishmaniasis in BALB/c mice. J Immunol. 2007;78:7251–7258. doi: 10.4049/jimmunol.178.11.7251. [DOI] [PubMed] [Google Scholar]
- 7.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynn TA. T(H)-17: a giant step from T(H)1 and T(H)2. Nat Immunol. 2005;6:1069–1070. doi: 10.1038/ni1105-1069. [DOI] [PubMed] [Google Scholar]
- 9.Tato CM, O’Shea JJ. Immunology: what does it mean to be just 17? Nature. 2006;441:166–168. doi: 10.1038/441166a. [DOI] [PubMed] [Google Scholar]
- 10.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 11.Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003;48:594–601. doi: 10.1002/art.10816. [DOI] [PubMed] [Google Scholar]
- 12.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100:5986–590. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuki T, Nakae S, Sudo K, Horai R, Iwakura Y. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int Immunol. 2006;18:399–407. doi: 10.1093/intimm/dxh379. [DOI] [PubMed] [Google Scholar]
- 14.Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS, Cho YG, Yoon CH, Park SH, Sung YC, Kim HY. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652–5661. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 15.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tacchini-Cottier F, Zweifel C, Belkaid Y, Mukankundiye C, Vasei M, Launois P, Milon G, Louis JA. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol. 2000;165:2628–2636. doi: 10.4049/jimmunol.165.5.2628. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro-Gomes FL, Otero AC, Gomes NA, Moniz-De-Souza MC, Cysne-Finkelstein L, Arnholdt AC, Calich VL, Coutinho SG, Lopes MF, DosReis GA. Macrophage interactions with neutrophils regulate Leishmania major infection. J Immunol. 2004;172:4454–4462. doi: 10.4049/jimmunol.172.7.4454. [DOI] [PubMed] [Google Scholar]
- 18.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flohe SB, Bauer C, Flohe S, Moll H. Antigen-pulsed epidermal Langerhans cells protect susceptible mice from infection with the intracellular parasite Leishmania major. Eur J Immunol. 1998;28:3800–3811. doi: 10.1002/(SICI)1521-4141(199811)28:11<3800::AID-IMMU3800>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.von Stebut E, Belkaid Y, Jakob T, Sacks DL, Udey MC. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J Exp Med. 1998;188:1547–1552. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Stebut E, Belkaid Y, Nguyen BV, Cushing M, Sacks DL, Udey MC. Leishmania major-infected murine Langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous leishmaniasis. Eur J Immunol. 2000;30:3498–3506. doi: 10.1002/1521-4141(2000012)30:12<3498::AID-IMMU3498>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Shibuya K, Robinson D, Zonin F, Hartley SB, Macatonia SE, Somoza C, Hunter CA, Murphy KM, O’Garra A. IL-1 alpha and TNF-alpha are required for IL-12-induced development of Th1 cells producing high levels of IFN-gamma in BALB/c but not C57BL/6 mice. J Immunol. 1998;160:1708–1716. [PubMed] [Google Scholar]
- 23.Ji J, Sun J, Soong L. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect Immun. 2003;71:4278–4288. doi: 10.1128/IAI.71.8.4278-4288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 25.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 27.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 28.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, Evans RT, Gaffen SL. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109:3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CM, Wright JF, Fouser LA. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 32.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, To W, Wagner J, O’Farrell AM, McClanahan T, Zurawski S, Hannum C, Gorman D, Rennick DM, Kastelein RA, de Waal Malefyt R, Moore KW. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 33.Lima GM, Vallochi AL, Silva UR, Bevilacqua EM, Kiffer MM, Abrahamsohn IA. The role of polymorphonuclear leukocytes in the resistance to cutaneous leishmaniasis. Immunol Lett. 1998;64:145–151. doi: 10.1016/s0165-2478(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Zhang ZH, Watanabe T, Yamashita T, Kobayakawa T, Kaneko A, Fujiwara H, Sendo F. The involvement of neutrophils in the resistance to Leishmania major infection in susceptible but not in resistant mice. Parasitol Int. 2005;54:109–118. doi: 10.1016/j.parint.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs T, Andra J, Gaworski I, Graefe S, Mellenthin K, Kromer M, Halter R, Borlak J, Clos J. Complement C3 is required for the progression of cutaneous lesions and neutrophil attraction in Leishmania major infection. Med Microbiol Immunol (Berl) 2005;194:143–149. doi: 10.1007/s00430-004-0229-y. [DOI] [PubMed] [Google Scholar]
- 36.Beil WJ, Meinardus-Hager G, Neugebauer DC, Sorg C. Differences in the onset of the inflammatory response to cutaneous leishmaniasis in resistant and susceptible mice. J Leukoc Biol. 1992;52:135–142. doi: 10.1002/jlb.52.2.135. [DOI] [PubMed] [Google Scholar]
- 37.Muller K, van Zandbergen G, Hansen B, Laufs H, Jahnke N, Solbach W, Laskay T. Chemokines, natural killer cells and granulocytes in the early course of Leishmania major infection in mice. Med Microbiol Immunol (Berl) 2001;190:73–76. doi: 10.1007/s004300100084. [DOI] [PubMed] [Google Scholar]
- 38.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 39.Kleinschek MA, Muller U, Brodie SJ, Stenzel W, Kohler G, Blumenschein WM, Straubinger RK, McClanahan T, Kastelein RA, Alber G. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J Immunol. 2006;176:1098–1106. doi: 10.4049/jimmunol.176.2.1098. [DOI] [PubMed] [Google Scholar]
- 40.Rutitzky LI, Lopes da Rosa JR, Stadecker MJ. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J Immunol. 2005;175:3920–3906. doi: 10.4049/jimmunol.175.6.3920. [DOI] [PubMed] [Google Scholar]