SUMMARY
Adiponectin is an abundant plasma protein secreted from adipocytes that elicits protective effects in the vasculature and myocardium. In obesity and insulin-resistant states, adiponectin levels are reduced and loss of its protective effects might contribute to the excess cardiovascular risk observed in these conditions. Adiponectin ameliorates the progression of macrovascular disease in rodent models, consistent with its correlation with improved vascular outcomes in epidemiological studies. The mechanisms of adiponectin signaling are multiple and vary among its cellular sites of action. In endothelial cells, adiponectin enhances production of nitric oxide, suppresses production of reactive oxygen species, and protects cells from inflammation that results from exposure to high glucose levels or tumor necrosis factor, through activation of AMP-activated protein kinase and cyclic AMP-dependent protein kinase (also known as protein kinase A) signaling cascades. In the myocardium, adiponectin-mediated protection from ischemia-reperfusion injury is linked to cyclo-oxygenase-2-mediated suppression of tumor necrosis factor signaling, inhibition of apoptosis by AMP-activated protein kinase, and inhibition of excess peroxynitrite-induced oxidative and nitrative stress. In this Review, we provide an update of studies of the signaling effects of adiponectin in endothelial cells and cardiomyocytes.
Keywords: atherosclerosis, endothelial function, hyperglycemia, ischemia-reperfusion, oxidative stress
INTRODUCTION
Among the many proteins and metabolites secreted by adipocytes, adiponectin serves a unique role as a salutary, antidiabetic, insulin-sensitizing factor that has antiatherogenic and anti-inflammatory properties in the vasculature. Numerous epidemiological studies have shown that reduced adiponectin levels correlate with increased risk of cardiovascular disease in obese individuals and in patients with diabetes and hyperglycemia.1,2 These high-risk patients also have increased concentrations of circulating cytokines such as tumor necrosis factor (TNF). These cytokines exert detrimental effects on endothelial cell function3 by increasing the production of reactive oxygen species (ROS) and triggering an inflammatory signaling cascade that results in increased cell-surface expression of cell adhesion molecules. These cell adhesion molecules enhance leukocyte-endothelium interactions and incite some of the early changes that lead to atherosclerosis.4 In most but not all clinical trials,5-9 increased adiponectin levels have correlated with improved endothelial function. Adiponectin has been shown to ameliorate the progression of macrovascular disease in rodents; neointimal proliferation is attenuated in mechanically injured arteries of adiponectin knock-out (Adipoq-/-) mice,10,11 atherosclerotic lesion formation is decreased in apolipoprotein-E-deficient mice,12,13 and hypertension is ameliorated in obese mice.14 Adiponectin specifically binds to collagens in the vascular intima and has been found to accumulate in the vascular wall of catheter-injured vessels, but not in unharmed control vessels.15 Whether the vasculature-related effects mentioned above are mediated by the same receptors that transduce cellular signals from different forms of adiponectin is not known. In addition to its effects on the vasculature, adiponectin is known to be cardioprotective. Adipoq-/- mice undergo worse myocardial ischemia-reperfusion (MIR) injury than wild type control mice, injury that can be ameliorated by provision of adiponectin.16,17 Rather than focusing on the insulin-sensitizing effects of adiponectin, this Review will explore the effects and underlying signaling mechanisms of adiponectin in the microvasculature and myocardium.
ADIPONECTIN STRUCTURE AND FUNCTION
Adiponectin (∼30 kDa in size) is secreted from adipose tissue and circulates in the bloodstream at high levels, in the range of ∼2-17 μg/ml.1,18 The dramatically high levels of adiponectin in the bloodstream, compared with other hormones and cytokines, are of great interest to many researchers. Adiponectin circulates in large, post-translationally modified complexes that reduce the actual concentration of the individual molecules in plasma and are likely to influence the signaling potential of its various isoforms. Serum levels of adiponectin are approximately twofold higher in females than in males, which probably contributes to sex-related differences in some of its vascular protective effects.
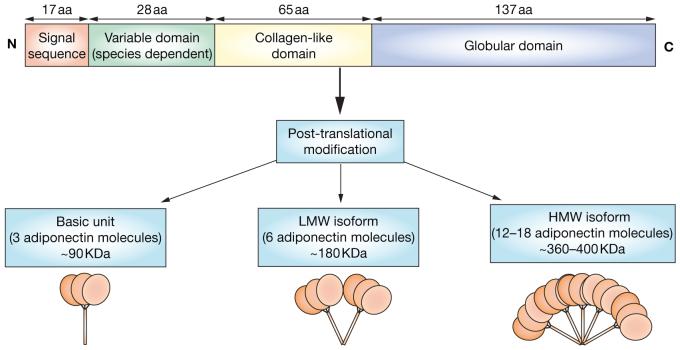
Full-length adiponectin requires post-translational modifications for activity (e.g. hydroxylation and glycosylation)19 and is secreted from adipocytes in three major size classes—trimers that are ∼90 kDa in size (the basic unit), low molecular weight hexamers (∼180 kDa) and high molecular weight isoforms (12-18-mers) that can be more than 400 kDa in size (Figure 1).20,21 Differences in the functions of the various isoforms of adiponectin are currently being elucidated. Both low molecular weight and high molecular weight adiponectin induce apoptosis in nondifferentiated THP1 cells (a human acute monocytic leukemia cell line), reduce expression of macrophage scavenger receptor A messenger RNA, and activate AMP kinase. However, although the low molecular weight form reduces lipopolysaccharide-mediated interleukin-6 release and suppresses nuclear factor κB (NFκB) activation, high molecular weight adiponectin induces interleukin-6 secretion in human monocytes and THP1 cells and does not suppress lipopolysaccharide-induced interleukin-6 secretion.22 These disparate results could reflect cell-type specificity and other experimental differences.
Figure 1.
Structure of adiponectin. Full-length adiponectin requires post-translational modifications for activity (e.g. hydroxylation and glycosylation). Adiponectin molecules are secreted from adipocytes as trimers (∼90 kDa; the basic unit), low molecular weight hexamers (∼180 kDa) and high molecular weight isoforms (12-18-mers; >400 kDa). Abbreviations: aa, amino acids; C, carboxy terminal; HMW, high molecular weight; LMW, low molecular weight; N, amino terminal.
The C-terminal globular domain of adiponectin is a recombinant fragment of 137 amino acids generated in vitro; this fragment has potent pharmacological signaling activity, affects systemic glucose metabolism, and also has effects on the microvasculature and heart.23-25 Whether this fragment is present in notable levels in the circulation, however, remains a point of debate.18,26 The globular and full-length oligomers reportedly differ in metabolic signaling effects;18,27 however, in endothelial cells, they exert comparable effects in suppressing increased ROS production induced by increased glucose levels.28 Further work is needed to characterize the signaling effects of globular adiponectin, as well as those of the major oligomeric forms of full-length adiponectin that occur in the mammalian circulation.
Two structurally related transmembrane receptors for adiponectin have been identified—adiponectin receptor proteins 1 and 2 (ADIPOR1 and ADIPOR2).27 ADIPOR1 and ADIPOR2 belong to a new family of membrane receptors that are predicted to contain seven transmembrane domains but are structurally and topologically distinct from G-protein-coupled receptors. Adiponectin binds to the C-terminal extracellular domain of ADIPOR1; the N-terminal cytoplasmic domain of the receptor interacts with the adaptor protein APPL 1 (adaptor protein containing pleckstrin homology domain, phosphotyrosine-binding domain and a leucine zipper motif 1). ADIPOR1 is abundantly expressed in skeletal muscle and has high affinity for globular adiponectin, whereas ADIPOR2 is predominantly expressed in the liver and has high affinity for full-length adiponectin.27 These receptors are also found in endothelial cells29 and cardiomyocytes;30 they probably have a key signaling role in mediating the vascular and myocardial effects of adiponectin. Considerable evidence exists that adiponectin achieves its biologic function by activation of AMP kinase and PPARα (peroxisome proliferator-activated receptor α), although the precise pathways that lead to their activation remain largely unknown. In addition to these two receptors, T-cadherin is a putative cell-surface binding protein for full-length and oligomeric adiponectin; T-cadherin is found in various cell types, such as those of the myocardium and vasculature.31
ADIPONECTIN AND THE MICROVASCULATURE
The microvasculature is the largest component of the cardiovascular system and contains the greatest amount of vascular endothelium. In addition to regulating systemic arterial blood pressure, the microvasculature is crucial for the homeostasis of organ nutrient supply and organ function throughout the body. The processes that underlie atherosclerosis of conduit vessels have been postulated to be closely tied to microvascular dysfunction; in large arteries, inflammation develops in the abundant plexi of microvessels in connection with their vasa vasorum.32,33 These microvascular networks are likely to contribute to lesion formation, as they provide an abundant surface area for leukocyte trafficking and serve as a portal for entry and exit of leukocytes from established atheroma in the conduit vessels.34,35 Many studies clearly implicate reduced adiponectin levels in macrovascular disease; however, to date, only a few reports have been published on the action of adiponectin on microvascular function.
Reduction of oxidative stress
Levels of circulating adiponectin are inversely correlated with plasma levels of oxidized LDL in patients with type 2 diabetes and coronary artery disease, which suggests that low adiponectin levels are associated with an increased oxidative state in the arterial wall.36 Reduced adiponectin levels also correlate with increased measures of systemic oxidant stress.37 Furthermore, the raised endothelial ROS production that is stimulated by multiple agonists, including oxidized LDL and high glucose levels, is suppressed in vitro by recombinant globular adiponectin in a dose-dependent manner.28,29 As endothelial ROS generation involves both mitochondrial ROS production and NADPH oxidases,3,38,39 adiponectin probably acts on these molecules to reduce oxidative stress.
Anti-inflammatory actions
In metabolic diseases such as diabetes and obesity, inflammation of the systemic microcirculation is widely appreciated to underlie organ damage and other chronic complications. Consistent with the epidemiologic association of reduced adiponectin levels in patients who are obese and in those who have type 2 diabetes, in vitro studies have shown that adiponectin can reverse the deleterious endothelial effects of TNF and other cytokines, as well as high glucose levels,1,2 which trigger an inflammatory signaling cascade, enhance leukocyte-endothelial interactions and, therefore, lead to some of the early processes of atherosclerosis (Figure 2). The microvascular inflammation observed in Adipoq-/- mice24 is thus consistent with the development of cardiovascular complications in individuals with diminished circulating levels of adiponectin, as shown in many epidemiological studies noted above.1,2 In theseAdipoq-/- mice, adiponectin replacement therapy, which used globular adiponectin, reversed the inflammatory phenotype of the microvascular endothelium and thus uncovered a potential therapeutic use of globular adiponectin, and possibly other synthetic forms of adiponectin in vivo.
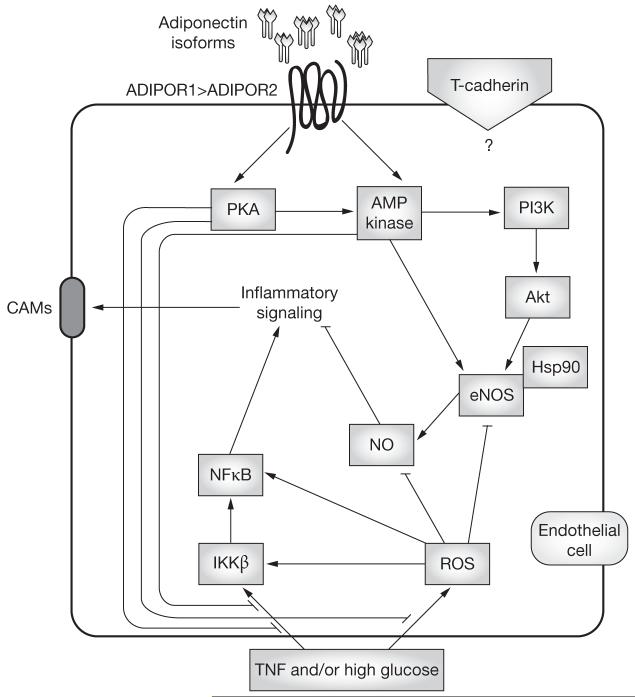
Figure 2.
Adiponectin signal-transduction pathways suppress endothelial cell activation elicited by high glucose levels and agonists such as TNF. Adiponectin isoforms signal via specific cell-surface receptors to elicit anti-inflammatory responses (suppression of IKKβ and NFκB activation) coupled with enhanced NO generation and suppressed production of ROS. Activation of the AMP kinase cascade has been linked to adiponectin’s enhancement of NO generation, and the cAMP-PKA pathway has been shown to mediate adiponectin’s antioxidative and anti-inflammatory protective effects. Abbreviations: ADIPOR1, adiponectin receptor protein 1; ADIPOR2, adiponectin receptor protein 2; AMP kinase, AMP-activated protein kinase; cAMP, cyclic AMP; CAMs, cell adhesion molecules; eNOS, endothelial nitric oxide synthase; Hsp90, heat shock protein 90; IKKβ, IκB kinase; NFκB, nuclear factor κB; NO, nitric oxide; PI3K, phosphatidylinositol 3 kinase; PKA, protein kinase A; ROS, reactive oxygen species; TNF, tumor necrosis factor.
Adiponectin binds to aortic endothelial cells and inhibits TNF-induced expression of cell adhesion molecules.12,40,41 Modulation of vascular inflammation via downregulation of endothelial cell adhesion molecules was one of the first vascular protective actions of adiponectin to be described in vitro.40 Indeed, we expanded on this seminal finding and demonstrated that in the microcirculation of Adipoq-/- mice a considerable increase exists in endothelial expression of the atherogenic cell adhesion molecules E-selectin and vascular cell adhesion molecule 1 (V-CAM 1). These molecules are associated with heightened leukocyte trafficking into mesenteric tissue.24 Adiponectin also protects against endothelial monolayer hyperpermeability induced by angiotensin II or TNF; this effect has been observed for both globular and full-length adiponectin and is associated with amelioration of actin stress fibers, intercellular gap formation and β-tubulin disassembly.42
Most of the anti-inflammatory actions of adiponectin studied in vitro have been linked to activation of endothelial nitric oxide synthase (eNOS) and increased bioavailability of nitric oxide (NO) at the vascular endothelium. Several groups have shown increased endothelial NO availability in response to adiponectin,29,43,44 the mechanism of which involves adiponectin-stimulated binding of regulatory heat shock protein 90 (Hsp90) to eNOS.45 Consistent with these in vitro data, adiponectin inhibition of leukocyte adhesion in TNF-inflamed microvascular networks in vivo is hindered by pharmacologic blockade of eNOS with NΩ-nitro-l-arginine methyl ester.24
A major role for AMP kinase in the effects of adiponectin on glucose and fat metabolism has been amply demonstrated in liver, skeletal muscle and adipose cells.1,18,48 AMP kinase also mediates at least some of the vascular effects of adiponectin in endothelial cells, especially those involving eNOS activation.43,46,47 The enhancement of endothelial NO availability by adiponectin is linked to activation of AMP kinase, as well as signaling through phosphatidylinositol 3′-kinase.43 Indeed, effects of adiponectin on angiogenesis were found to be dependent on adiponectin-stimulated phosphorylation of both AMP kinase and Akt (protein kinase B). AMP kinase was found to act upstream of Akt, since disruption of AMP kinase activation inhibited adiponectin-induced Akt phosphorylation.47 Interestingly, inhibition of AMP kinase does not block globular adiponectin suppression of glucose-induced ROS generation,28 which suggests that this action of globular adiponectin might be largely independent of AMP kinase in endothelial cells.
Pathways that involve protein kinase A (PKA, or cyclic AMP [cAMP]-dependent protein kinase) signaling have also been implicated in the effects of adiponectin in the endothelium.28,41,42,49-51 Ouchi et al. first reported that globular-adiponectin-mediated suppression of TNF-induced activation of NFκB and phosphorylation of inhibitor of NFκB (IκB)α was accompanied by cAMP accumulation and was blocked by inhibitors of adenylate cyclase or PKA in endothelial cells.41 A study published by our group in 2006 showed that the use of oligomers of recombinant globular and full-length adiponectin to suppress ROS production induced by high glucose levels was also accompanied by increased cellular cAMP content and blocked by inhibition of PKA in endothelial cells.28 These findings indicate that the cAMP-PKA pathway is a major signaling system that mediates the effects of adiponectin in endothelial cells to reverse the diverse cellular actions of TNF and high glucose levels.
At the same time as suppressing inflammatory NFκB activation incited by either TNF or high glucose levels, globular adiponectin potently suppresses activation of the upstream enzyme IκB kinase (IKKβ).50 cAMP signaling is implicated in this pathway; the adenylate cyclase activator forskolin mimics the action of globular adiponectin, whereas the adenylate cyclase inhibitor dideoxyadenosine, as well as the PKA inhibitor Rp-cAMP, block the inhibitory effect of globular adiponectin on TNF-induced IKKβ activity.50 Interestingly, although adiponectin-induced AMP kinase and cAMP pathways have been shown to be comparably active in suppressing the TNF-induced increase in IKKβ, activation of AMP kinase is substantially more effective than cAMP signaling in suppressing IKKβ activity induced by increased glucose levels.50 These findings imply that multiple pathways are involved in the suppression of endothelial inflammatory signaling by adiponectin.
The importance of suppression of endothelial ROS generation relative to eNOS stimulation by adiponectin in the balancing of NO availability remains unresolved. However, given that both AMP kinase-eNOS and PKA-ROS-suppression signal transduction pathways are implicated in adiponectin signaling in endothelial cells, potential crosstalk between these two pathways is of great importance and warrants further investigation. To date, the only documented crosstalk has been that upstream AMP kinase kinase LKB1 (serine threonine protein kinase II) can be phosphorylated by PKA.52
Angiogenesis
Interestingly, some studies have also implicated adiponectin in neoangiogenesis. Adiponectin has been shown to stimulate the differentiation of human umbilical vein endothelial cells into capillary-like structures in vitro via an AMP kinase-eNOS-mediated mechanism.47 Furthermore, impaired angiogenesis in hind limbs of Adipoq-/- mice subjected to ischemic stress can be ameliorated by replenishment of adiponectin, in an AMP kinase-dependent manner.53
In contrast to these results, adiponectin has also been reported to function as a negative regulator of angiogenesis; it potently inhibits endothelial cell proliferation and migration, and remarkably prevents new blood vessel growth.54 Globular adiponectin has been shown to reduce endothelial cell proliferation and activation of p42/p44 mitogen-activated protein kinase in vitro.29 Migration of coronary artery endothelial cells, which is induced by vascular endothelial growth factor, is also suppressed by globular adiponectin. This feature is associated with a reduction in ROS generation, diminished activation of mitogen-activated protein kinase and the RhoGTPase RhoA, and reduced formation of actin stress fibers and focal cellular adhesions—all of which are mediated by cAMP-PKA signaling.51 In another vascular model, adiponectin has been shown to inhibit laser-induced choroidal neovascularization in vivo.55
Discrepancies between these results could be explained by different experimental conditions. However, the effect of endogenous adiponectin levels on reparative or maladaptive angiogenesis in cardiovascular disease remains unclear and deserves further investigation.
Localized generation of adiponectin
At the organ level, an emerging concept in the literature is that the microvascular endothelium modulates local levels of adiponectin and thus affects blood flow distribution and metabolism within different sections of the same microcirculation. A 2008 study demonstrated that local production of adiponectin by microvascular endothelial cells might be involved in the regulation of cardiac function in the diabetic rat heart.56 Similar results were also found in humans; adiponectin levels were found to correlate with microvascular coronary function and coronary blood flow in different regions of the myocardium.57 The possibility exists, therefore, that inflamed microvascular networks are not only directly affected by, but also contribute to, the generalized loss of physiological adiponectin levels in metabolic disease states.
A few studies have attempted to correlate adiponectin plasma levels with microvascular complications in patients with diabetes. In 2008, Sharma et al. reported that in renal podocytes adiponectin acted via AMP kinase to ameliorate glomerular albumin permeability as well as suppress renal oxidative stress via reduced glomerular mass of renal NADPH oxidase 4.58 An apparently paradoxical increase in total adiponectin levels has been observed in some patients with Type 1 diabetes who have microvascular complications, such as coronary artery disease.59,60 Other studies have reported increased urinary adiponectin excretion in diabetic patients with nephropathy characterized by microvascular damage.61 Whether increases in adiponectin plasma levels in patients with diabetes are present throughout the entire course of the disease, or whether they are a transient compensatory mechanism meant to offset the loss of adiponectin that results from the hyperpermeable microcirculation that occurs in hyperglycemia remains unclear. Further research on the specific isoforms of adiponectin that circulate in the blood of patients with diabetes is necessary to fully evaluate the influence of changes in plasma and tissue levels of adiponectin in the pathophysiology of diabetes.
ADIPONECTIN AND THE MYOCARDIUM
In vitro studies have demonstrated that adiponectin promotes cell survival and inhibits cell death, which suggests that adiponectin may directly protect cardiomyocytes (in addition to its vascular actions that indirectly protect ischemic-reperfused cardiomyocytes). Indeed, studies have demonstrated that in addition to microvascular defects, Adipoq-/- mice have worse MIR injury—increased myocardial apoptosis and infarct size, and decreased cardiac function—compared with control mice.16,17 In heterozygous (Adipoq+/-) mice, in which circulating adiponectin concentrations are reduced by approximately half, MIR injury was increased compared to controls but the severity was less than that seen in Adipoq-/- animals.17 This finding suggests that adiponectin levels not only correlate inversely with the risk of development of ischemic heart disease (as indicated by the human epidemiological data), but also are inversely related to the severity of a MIR insult. The worsening of MIR injury in Adipoq-/- mice was ameliorated by provision of full-length or globular adiponectin in a dose-dependent manner.16,17 These exciting results suggest that adiponectin might eventually be used as a novel therapeutic agent in the treatment of ischemic cardiac injury.
The antiapoptotic effect of full-length adiponectin in cultured, neonatal cardiomyocytes from Adipoq-/- mice is completely inhibited by transduction of a dominant-negative mutant of AMP kinase,16 which suggests that adiponectin exerts its protective effects in cardiomyocytes largely via an AMP-kinase-mediated signaling pathway (Figure 3). Indeed, Shinmura et al. have shown that the cardioprotective effects of short-term caloric restriction are mediated by adiponectin-stimulated activation of AMP kinase.62 In adult rodent hearts subjected to MIR in vivo, however, a significant amount of adiponectin-mediated cardio-protection remains despite blockade of the AMP kinase signaling system; instead, full-length adiponectin upregulates cyclo-oxygenase 2 (COX-2) expression though activation of sphingosine kinase 1 and inhibits MIR-induced TNF over-production in a COX-2-dependent fashion.63 These seemingly controversial results underscore the complexity of adiponectin signaling and suggest that multiple signaling mechanisms are involved in cardioprotection by adiponectin in adult animals in vivo.
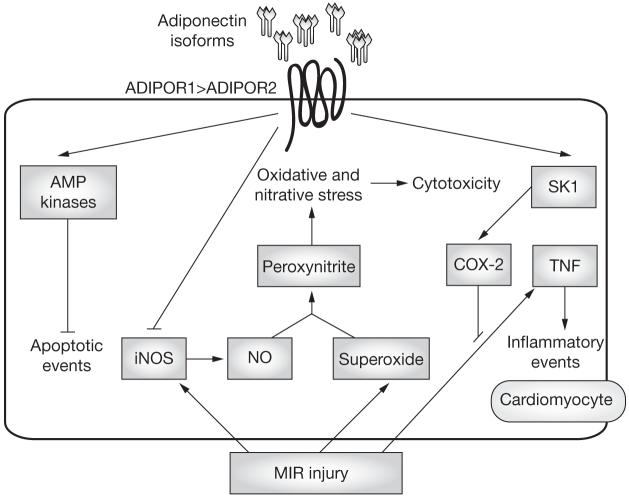
Figure 3.
Adiponectin signaling in cardiomyocytes. Adiponectin isoforms have been shown to exert multiple actions, such as activation of AMP kinases, suppression of TNF signaling via a COX-2-prostaglandin E2-linked cascade, and reduction of oxidative and nitrative stress after MIR injury that is associated with suppression of iNOS induction. Abbreviations: ADIPOR1, adiponectin receptor protein 1; ADIPOR2, adiponectin receptor protein 2; AMP kinase, AMP-activated protein kinase; COX-2, cyclo-oxygenase 2; iNOS, inducible nitric oxide synthase; MIR, myocardial ischemia-reperfusion; NO, nitric oxide; SK1, sphingosine kinase 1; TNF, tumor necrosis factor.
In low concentrations, NO release from eNOS or NO donors is known to be antiapoptotic and cardioprotective,64 and as stated earlier, adiponectin is known to stimulate NO production via AMP-kinase-mediated eNOS phosphorylation.43 Lack of adiponectin, therefore, might conceivably reduce NO production and thus render cardiomyocytes more susceptible to MIR injury. However, this hypothesis is not supported by experimental findings of markedly increased, rather than decreased, total NO production in ischemic-reperfused tissue obtained from Adipoq-/- mice.17 As expected, levels of phosphorylated eNOS are substantially reduced in cardiac tissues obtained from Adipoq-/- mice, compared with controls, and this finding is completely reversed by treatment with globular adiponectin.17 Interestingly, inducible nitric oxide synthase (iNOS) expression is, however, increased in Adipoq-/- mice compared with wild type controls, and treatment with globular adiponectin effectively blocks iNOS expression in the Adipoq-/- animals.17 These novel results indicate that adiponectin differentially regulates NO production by both eNOS and iNOS. Under physiologic conditions, adiponectin stimulates NO production by phosphorylating eNOS and thus contributes to its vasodilatory, anti-inflammatory, vascular-protective actions. By contrast, under pathologic conditions when iNOS is induced, adiponectin might prevent excess NO generation by inhibiting iNOS expression.65
NO itself is not toxic and does not cause serious tissue injury, even at very high concentrations;66 however, NO reacts with superoxide at a nearly diffusion-limited rate. The resultant reaction product, peroxynitrite, is extremely cytotoxic and causes oxidative as well as nitrative stress and tissue injury.67 Of note, the genes that encode NADPH oxidase and iNOS belong to the same inflammatory gene family; therefore, given that adiponectin inhibits iNOS expression, adiponectin might also inhibit NADPH oxidase expression and subsequent superoxide production in ischemic-reperfused cardiomyocytes. Indeed, lack of adiponectin in Adipoq-/- mice results in enhanced expression of gp91phox (the cytochrome b-245 heavy chain subunit of NADPH oxidase) and superoxide production in ischemic-reperfused hearts.17 Treatment of these mice with globular adiponectin effectively inhibits both gp91phox expression and superoxide production.17 Owing to the double inhibitory effect of adiponectin on excess superoxide and NO production induced by ischemia-reperfusion, peroxynitrite formation is intensified in Adipoq-/- mice after ischemia-reperfusion and is inhibited by exogenous adiponectin.17 As peroxynitrite has been shown to be the most important nitrative species in causing severe nitrative stress and tissue injury,68 adiponectin-mediated inhibition of excess peroxynitrite formation indicates that, in addition to its previously reported AMP kinase-mediated metabolic and COX-2-mediated TNF-suppressing actions, adiponectin might exert its anti-ischemic, cardioprotective effects via inhibition of peroxynitrite-induced oxidative and nitrative stress.
FUTURE DIRECTIONS
Numerous epidemiological studies have provided abundant evidence for a potential association between reduced adiponectin levels and cardiovascular disease. When coupled with work in animal models and cultured cells, these studies have elucidated multiple salutary effects of this fascinating, adipose-tissue-derived hormone. Indeed, animal models of adiponectin deficiency have shown that adiponectin has beneficial protective effects, including anti-inflammatory and angiogenic actions on the microvascular endothelium, and that provision of adiponectin can reduce MIR injury. Further work will reveal whether the various oligomeric forms of adiponectin have different effects in the vasculature and heart. Nevertheless, multiple research groups have convincingly demonstrated the beneficial pharmacologic properties of the globular domain of adiponectin, which would be easier to manufacture and administer to patients than its full-length version. Small fragments of adiponectin might also be biologically active, and chemical entities that can activate cell-surface adiponectin receptors should be sought and applied in human trials to test their potential benefit in various clinical settings.
CONCLUSIONS
Adiponectin is an abundant, oligomeric protein secreted from adipose tissue, which exhibits a variety of remarkable salutary responses that affect insulin resistance and vascular inflammation. The varied responses in multiple tissue types involve different signal transduction pathways; notably the AMP kinase and the PKA cascades in the endothelium, as well as AMP kinase and COX-2 signaling in cardiomyocytes. The resultant cellular effects of adiponectin include enhanced NO production, and inhibition of ROS and inflammatory NFκB signaling in the vasculature, as well as reduced MIR injury with suppression of TNF signaling, reduced cardiomyocyte apoptosis and suppression of oxidative and nitrative stress. The many potential benefits of adiponectin and its active derivative forms, such as the C-terminal globular domain, will probably find a place in the future management of endothelial dysfunction and myocardial protection.
KEY POINTS.
-
■
Adiponectin is an abundant plasma protein secreted from adipocytes that elicits salutary effects in the vasculature and myocardium
-
■
The mechanisms of adiponectin signaling are multiple and vary among its cellular sites of action
-
■
In endothelial cells, adiponectin enhances nitric oxide, suppresses oxidative stress and suppresses the inflammatory signaling cascades via AMP-activated protein kinases and the cyclic AMP-protein kinase A-linked pathway
-
■
In the myocardium, adiponectin reduces ischemia-reperfusion injury via COX-2-mediated suppression of tumor necrosis factor signaling, AMP-activated protein kinases, and inhibition of excess peroxynitrite-induced oxidative and nitrative stress
-
■
Further work is necessary to elucidate the role of oligomeric forms of adiponectin in the heart and vasculature and their potential therapeutic value
Footnotes
REVIEW CRITERIA
We searched PubMed for publications published between 1995 and 2008 using the following search terms: “adiponectin” and its synonyms “ACRP30” and “APM1”. All full-text, English-language articles related to vascular or myocardial cellular effects of adiponectin were identified and considered for inclusion in this Review. Some articles were not cited owing to space constraints.
Competing interests
The authors declared no competing interests.
References
- 1.Goldstein BJ, Scalia R. Adipokines and vascular disease in diabetes. Curr Diab Rep. 2007;7:25–33. doi: 10.1007/s11892-007-0006-6. [DOI] [PubMed] [Google Scholar]
- 2.Zhu W, et al. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci (Lond) 2008;114:361–374. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- 3.Jay D, et al. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–192. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Granger DN, et al. Modulation of the inflammatory response in cardiovascular disease. Hypertension. 2004;43:924–931. doi: 10.1161/01.HYP.0000123070.31763.55. [DOI] [PubMed] [Google Scholar]
- 5.Ouchi N, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42:231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- 6.Shimabukuro M, et al. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab. 2003;88:3236–3240. doi: 10.1210/jc.2002-021883. [DOI] [PubMed] [Google Scholar]
- 7.Tan KC, et al. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab. 2004;89:765–769. doi: 10.1210/jc.2003-031012. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Real JM, et al. Adiponectin is associated with vascular function independent of insulin sensitivity. Diabetes Care. 2004;27:739–745. doi: 10.2337/diacare.27.3.739. [DOI] [PubMed] [Google Scholar]
- 9.Halperin F, et al. The role of total and high-molecular-weight complex of adiponectin in vascular function in offspring whose parents both had type 2 diabetes. Diabetologia. 2005;48:2147–2154. doi: 10.1007/s00125-005-1901-5. [DOI] [PubMed] [Google Scholar]
- 10.Kubota N, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda M, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto Y, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi T, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 14.Ohashi K, et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto Y, et al. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res. 2000;32:47–50. doi: 10.1055/s-2007-978586. [DOI] [PubMed] [Google Scholar]
- 16.Shibata R, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMP kinase- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao L, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 18.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, et al. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem. 2002;277:19521–19529. doi: 10.1074/jbc.M200601200. [DOI] [PubMed] [Google Scholar]
- 20.Pajvani UB, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 21.Schraw T, et al. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology. 2008;149:2270–2282. doi: 10.1210/en.2007-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neumeier M, et al. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006;79:803–808. doi: 10.1189/jlb.0905521. [DOI] [PubMed] [Google Scholar]
- 23.Fruebis J, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouedraogo R, et al. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest. 2007;117:1718–1726. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopkins TA, et al. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2006;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waki H, et al. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790–796. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- 27.Kadowaki T, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouedraogo R, et al. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006;55:1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- 29.Motoshima H, et al. Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem Biophys Res Commun. 2004;315:264–271. doi: 10.1016/j.bbrc.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 30.Ding G, et al. Adiponectin and its receptors are expressed in adult ventricular cardiomyocytes and upregulated by activation of peroxisome proliferator-activated receptor γ. J Mol Cell Cardiol. 2007;43:73–84. doi: 10.1016/j.yjmcc.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hug C, et al. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams JK, et al. Vasa vasorum in atherosclerotic coronary arteries: responses to vasoactive stimuli and regression of atherosclerosis. Circ Res. 1988;62:515–523. doi: 10.1161/01.res.62.3.515. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann J, et al. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res. 2001;51:762–766. doi: 10.1016/s0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 34.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 35.Moulton KS. Angiogenesis in atherosclerosis: gathering evidence beyond speculation. Curr Opin Lipidol. 2006;17:548–555. doi: 10.1097/01.mol.0000245261.71129.f0. [DOI] [PubMed] [Google Scholar]
- 36.Lautamaki R, et al. Low serum adiponectin is associated with high circulating oxidized low-density lipoprotein in patients with type 2 diabetes mellitus and coronary artery disease. Metabolism. 2007;56:881–886. doi: 10.1016/j.metabol.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 37.Furukawa S, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 39.Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 40.Ouchi N, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 41.Ouchi N, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 42.Xu SQ, et al. Adiponectin protects against angiotensin II or tumor necrosis factor alpha-induced endothelial cell monolayer hyperpermeability: role of cAMP/PKA signaling. Arterioscler Thromb Vasc Biol. 2008;28:899–905. doi: 10.1161/ATVBAHA.108.163634. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, et al. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 44.Hattori Y, et al. Globular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia. 2003;46:1543–1549. doi: 10.1007/s00125-003-1224-3. [DOI] [PubMed] [Google Scholar]
- 45.Xi W, et al. Stimulated HSP90 binding to eNOS and activation of the PI3-Akt pathway contribute to globular adiponectin-induced NO production: vasorelaxation in response to globular adiponectin. Biochem Biophys Res Commun. 2005;332:200–205. doi: 10.1016/j.bbrc.2005.04.111. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi H, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouchi N, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadowaki T, et al. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2007;582:74–80. doi: 10.1016/j.febslet.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 49.Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 50.Wu X, et al. Adiponectin suppresses IkappaB kinase activation induced by tumor necrosis factor-alpha or high glucose in endothelial cells: role of cAMP and AMP kinase signaling. Am J Physiol Endocrinol Metab. 2007;293:E1836–E1844. doi: 10.1152/ajpendo.00115.2007. [DOI] [PubMed] [Google Scholar]
- 51.Mahadev K, et al. Adiponectin inhibits vascular endothelial growth factor-induced migration of human coronary artery endothelial cells. Cardiovasc Res. 2008;78:376–384. doi: 10.1093/cvr/cvn034. [DOI] [PubMed] [Google Scholar]
- 52.Collins SP, et al. LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem J. 2000;345:673–680. [PMC free article] [PubMed] [Google Scholar]
- 53.Shibata R. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of AMP-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 54.Brååkenhielm E, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bora PS, et al. Expression of adiponectin in choroidal tissue and inhibition of laser induced choroidal neovascularization by adiponectin. FEBS Lett. 2007;58:1977–1982. doi: 10.1016/j.febslet.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 56.Natarajan R, et al. Hypoxia inducible factor-1 upregulates adiponectin in diabetic mouse hearts and attenuates post-ischemic injury. J Cardiovasc Pharmacol. 2008;51:178–187. doi: 10.1097/FJC.0b013e31815f248d. [DOI] [PubMed] [Google Scholar]
- 57.Date H, et al. Adiponectin produced in coronary circulation regulates coronary flow reserve in nondiabetic patients with angiographically normal coronary arteries. Clin Cardiol. 2006;29:211–214. doi: 10.1002/clc.4960290508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma K, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frystyk J, et al. Increased serum adiponectin levels in type 1 diabetic patients with microvascular complications. Diabetologia. 2005;48:1911–1918. doi: 10.1007/s00125-005-1850-z. [DOI] [PubMed] [Google Scholar]
- 60.Schalkwijk CG, et al. Adiponectin is inversely associated with renal function in Type 1 diabetic patients. J Clin Endocrinol Metab. 2005;91:129–135. doi: 10.1210/jc.2005-1117. [DOI] [PubMed] [Google Scholar]
- 61.Koshimura J, et al. Urinary adiponectin excretion is increased in patients with overt diabetic nephropathy. Biochem Biophys Res Commun. 2004;316:165–169. doi: 10.1016/j.bbrc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 62.Shinmura K, et al. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 63.Ikeda Y, et al. Cyclooxygenase-2 induction by adiponectin is regulated by a sphingosine kinase-1 dependent mechanism in cardiac myocytes. FEBS Lett. 2008;582:1147–1150. doi: 10.1016/j.febslet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao F, et al. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation. 2002;105:1497–1502. doi: 10.1161/01.cir.0000012529.00367.0f. [DOI] [PubMed] [Google Scholar]
- 65.Li R, et al. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am J Physiol Endocrinol Metab. 2007;293:E1703–E1708. doi: 10.1152/ajpendo.00462.2007. [DOI] [PubMed] [Google Scholar]
- 66.Kim YM, et al. Nitric oxide as a bifunctional regulator of apoptosis. Circ Res. 1999;84:253–256. doi: 10.1161/01.res.84.3.253. [DOI] [PubMed] [Google Scholar]
- 67.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 68.Ferdinandy P, et al. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]