Abstract
Background
Diabetes increases the morbidity/mortality of ischemic heart disease, but the underlying mechanisms are incompletely understood. Deficiency of both AMP-activated protein kinase (AMPK) and adiponectin occurs in diabetes, but whether AMPK is cardioprotective or a central mediator of adiponectin cardioprotection in vivo remains unknown.
Methods and Results
Male adult mice with cardiomyocyte-specific overexpression of a mutant AMPKα2 subunit (AMPK-DN) or wild-type (WT) littermates were subjected to in vivo myocardial ischemia/reperfusion (MI/R) and treated with vehicle or adiponectin. In comparison to WT, AMPK-DN mice subjected to MI/R endured greater cardiac injury (larger infarct size, more apoptosis, and poorer cardiac function) likely as a result of increased oxidative stress in these animals. Treatment of AMPK-DN mice with adiponectin failed to phosphorylate cardiac acetyl-CoA carboxylase as it did in WT mouse heart. However, a significant portion of the cardioprotection of adiponectin against MI/R injury was retained in AMPK-DN mice. Furthermore, treatment of AMPK-DN mice with adiponectin reduced MI/R-induced cardiac oxidative and nitrative stress to the same degree as that seen in WT mice. Finally, treating AMPK-DN cardiomyocytes with adiponectin reduced simulated MI/R-induced oxidative/nitrative stress and decreased cell death (P<0.01).
Conclusions
Collectively, our results demonstrated that AMPK deficiency significantly increases MI/R injury in vivo but has minimal effect on the antioxidative/antinitrative protection of adiponectin.
Keywords: adipocytokine, apoptosis, diabetes mellitus, myocardial infarction, signal transduction
Ischemic heart disease is the leading cause of death in patients with diabetes.1 Hyperglycemia and hyperlipidemia not only cause vascular injury that results in myocardial ischemia (MI) but also have a direct adverse impact on ischemic cardiomyocytes causing larger infarct size2,3 and more severe heart failure4 after MI. Identifying the molecular basis that links diabetes with increased morbidity and mortality of ischemic heart disease is not only scientifically important but may also reveal new therapeutic targets against diabetic heart injury.
Clinical Perspective p 844
AMP-activated protein kinase (AMPK) is a heterotrimeric protein kinase expressed in most mammalian tissues including cardiac muscle, where it plays an essential role in the regulation of cellular energy metabolism.5,6 Although the concept of AMPK as a cardioprotective molecule during ischemia is well accepted, whether AMPK activation is beneficial or detrimental during reperfusion remains an actively debated topic, largely because of its putative effect on cardiometabolic regulation and adverse effects such as acidosis and calcium overload.7 Several recent studies in isolated buffer-perfused hearts have demonstrated that AMPK deficiency renders the hearts more susceptible to MI/reperfusion (MI/R injury), as evidenced by increased myocardial cell death8 and poorer cardiac function recovery.9,10 However, many additional factors (including metabolic substrates, inflammatory mediators, and neuronal/humoral factors) are operative in vivo that can influence the action of AMPK. Whether AMPK activation is detrimental or beneficial in clinically relevant animal models of regional MI/R remains largely unknown.
Adiponectin is an adipocytokine secreted from adipose tissue.11 Several clinical observations have demonstrated that plasma adiponectin concentrations are significantly reduced in patients with type II diabetes and are inversely correlated with the risk of MI, indicating that reduced adiponectin function may be a major contributor to increased ischemic heart disease morbidity in diabetic patients.12,13 Moreover, Walsh and colleagues14 and our group15 have recently demonstrated that MI/R injury is markedly increased in adiponectin knockout mice and that exogenous supplementation of adiponectin is cardioprotective, suggesting that reduced adiponectin levels found in diabetic patients may also play a causative role in increased cardiomyocyte death and high mortality in diabetic patients after MI/R. However, discernment of the specific intracellular signaling mechanisms responsible for the cardiomyocyte protective effect of adiponectin remains elusive. Considerable evidence from many investigators has demonstrated that AMPK plays an essential role in adiponectin intracellular signaling.16-18 However, it is well known that AMPK is dramatically activated by MI itself, and whether additional AMPK activation by adiponectin under this specific pathological condition is responsible for the powerful cardioprotection of adiponectin against MI/R injury remains unknown.
Therefore, the aims of the present study were (1) to investigate the impact of AMPK deficiency on the severity of MI/R injury in intact animals; (2) to determine the degree of AMPK involvement in the cardioprotective signaling of adiponectin; and (3) to identify possible mechanisms by which adiponectin may directly protect cardiomyocytes during MI/R independent of AMPK.
Methods
All experiments were performed on adult male mice overexpressing a dominant negative α2 subunit (D157A) of AMPK (AMPK-DN) or male littermate controls expressing wild type of α2 subunit of AMPK (WT). Generation, breeding, phenotype characteristics, and genotyping of these mice have previously been described in detail.10 The experiments were performed in adherence with the National Institutes of Health Guidelines on the Use of Laboratory Animals and were approved by the Thomas Jefferson University Committee on Animal Care.
Experimental Protocols
Mice were anesthetized with 2% isoflurane (total exposure time <5 minutes), and MI was produced as described in our previous studies.15 Twenty minutes after MI, animals were randomized to 1 of the following groups: WT sham MI (n=12), WT MI+vehicle (n=13), WT MI+globular domain of adiponectin (gAPN) (2 μg/g IP; n=13),15 AMPK-DN sham MI (n=12), AMPK-DN MI+vehicle (n=14), and AMPK-DN MI+gAPN (n=14). After 30 minutes of MI, the slipknot was released, and the myocardium was reperfused. After 3 hours (all assays except cardiac function and infarct size) or 24 hours (cardiac function and infarct size only) of reperfusion, the ligature around the coronary artery was retied, and 1 mL of 2% Evans blue dye was injected into the left ventricular cavity. The Evens blue-negative stained part (ischemic/reperfused tissue or area at risk [AAR]) was isolated, and all assays including immunohistological, biochemical, and Western blot were performed with the tissue from AAR.
Determination of Myocardial Apoptosis and Myocardial Infarct Size
Myocardial apoptosis was determined by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining, caspase 3 activity, and DNA fragmentation as described in our previous study.19 Myocardial infarct size was determined by Evans blue-2,3,5-triphenyl tetrazolium chloride (TTC) double staining methods.
Determination of Cardiac Function
Cardiac function was determined by echocardiography as well as left ventricular catheterization methods 24 hours after reperfusion before the chest was reopened, as described in our previous studies.15,19
Quantification of Superoxide Production
Myocardial superoxide content (in AAR) was quantified by lucigenin-enhanced luminescence, and the cellular origin of reactive oxygen species was determined by dihydroethidium staining (Molecular Probes, Carlsbad, Calif), as described previously.15
Determination of Total Nitric Oxide and Nitrotyrosine Content in Cardiac Tissue
The tissue nitric oxide (NO) and its metabolic products (NO2 and NO3) were determined by use of a chemiluminescence NO detector (Siever 280i NO Analyzer), and nitrotyrosine content in MI/R cardiac tissue was quantified by enzyme-lined immunosorbent assay as described previously.15,19
Western Blot Analysis
Proteins were separated on SDS-PAGE gels, transferred to nitrocellulose membranes, and incubated with primary antibodies (monoclonal antibody against phosphorylated acetyl-CoA carboxylase [ACC], inducible NO synthase [iNOS] [Upstate, Chicago, Ill], or gp91phox [Transduction Laboratories, San Jose, Calif] and horseradish peroxidase-conjugated secondary antibody. The blot was developed with a Supersignal Chemiluminescence detection kit (Pierce, Rockford, Ill), and band was visualized with a Kodak Image Station 400 (Rochester, NY).
Adult Mouse Cardiomyocyte Culture
Adult mouse cardiomyocytes were isolated from WT or AMPK-DN mice and subjected to 3 hours of simulated ischemia and 6 hours of reperfusion (SI/R) as described in our previous studies.15 The effect of gAPN (2 μg/mL) on SI/R-induced cell death (determined by LDH release), superoxide (determined by dihydroethidium staining) and NO production, and nitrotyrosine formation was determined as described above.
Statistical Analysis
All values in the text and figures are presented as mean±SEM of n independent experiments. ANOVA across all investigated groups was conducted first, and post hoc pairwise tests for certain group pairs with assessment of statistical significance were then performed after Bonferroni correction of the overall significance level. Western blot densities were analyzed with the Kruskal-Wallis test followed by the Dunn post hoc test. Probabilities of ≤0.05 were considered statistically significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Cardiomyocyte-Specific Overexpression of a Dominant Negative Mutant of AMPKα2 Subunit Increased Susceptibility to MI/R Injury
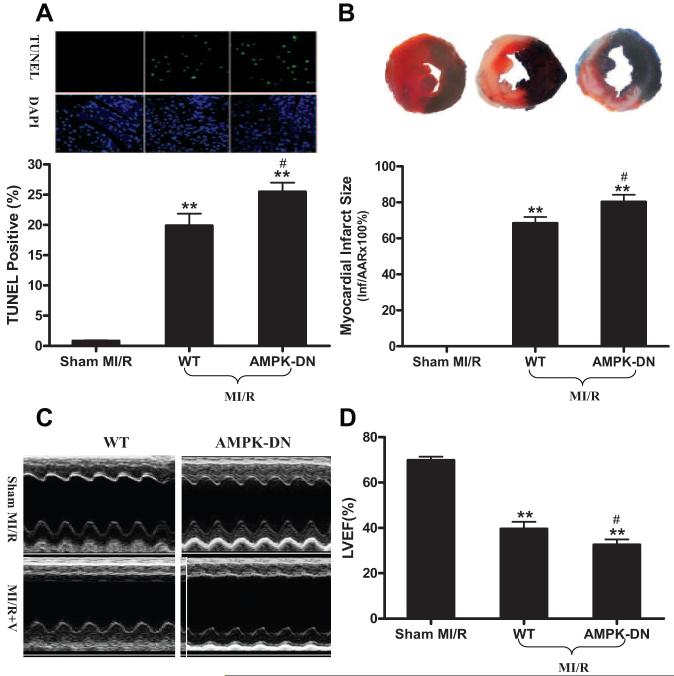
MI followed by reperfusion resulted in significant cardiac injury including cardiomyocyte apoptosis, myocardial infarction, and cardiac contractile as well as diastolic dysfunction in WT littermates (Figure 1, Table). Mice with cardiomyocyte-specific overexpression of a dominant negative mutant of AMPKα2 catalytic subunit developed normally and had normal cardiac function under basal conditions (sham MI/R groups in the Table). However, when subjected to in vivo MI/R, AMPK-DN animals manifested significantly greater cardiac injury than their littermate controls. Specifically, MI/R-induced cardiomyocyte apoptosis was increased (Figure 1A), infarct size was enlarged (Figure 1B), and cardiac function was further depressed (Figure 1C and 1D, Table).
Figure 1.
A, Cardiomyocyte apoptosis determined by TUNEL (n=5 to 6 hearts per group). B, Myocardial infarction determined by Evans blue/TTC double staining (n=12 to 14 hearts per group). C/D, Cardiac function determined by echocardiography (n=12 to 14 animals per group). **P<0.01 vs sham MI/R; #P<0.05 vs WT with MI/R. V indicates vehicle; LVEF, left ventricular ejection fraction.
Table. Effect of gADN Treatment on Hemodynamic Changes in WT and AMPK-DN Mice Subjected to MI/R.
| WT |
AMPK-DN |
|||||
|---|---|---|---|---|---|---|
| Sham MI/R | MI/R+Vehicle | MI/R+gADN | Sham+MI/R | MI/R+Vehicle | MI/R+gADN | |
| Heart rate, bpm | 369±14 | 378±22 | 413±28 | 404±34 | 446±16 | 432±21 |
| LVEDP, mm Hg | 4.5±0.6 | 17.1±2.1† | 6.2±0.5*‡ | 5.0±0.4 | 23.4±1.9† | 11.9±1.5†‡ |
| dP/dtmax, mm Hg/s | 7429±451 | 4055±724† | 5839±288*‡ | 7986±208 | 3458±448† | 6209±359*‡ |
| -dP/dtmax, mm Hg/s | 5877±430 | 3102±557† | 4119±367*‡ | 5199±275 | 2296±320† | 3900±316*‡ |
Treatment with gADN 10 minutes before reperfusion had no effect of heart rate but significantly reduced left ventricular end-diastolic pressure (LVEDP) and increased maximal positive and negative values of the instantaneous first derivative of left ventricular pressure (dP/dtmax and -dP/dtmax) in WT as well as AMPK-DN mice subjected to 30 minutes of MI and 24 hours of reperfusion. Hemodynamic measurements were made by using a 1.2F micromanometer advanced to the left ventricular cavity through the right common carotid artery. The left ventricular pressure was recorded, and heart rate, LVEDP, and dP/dtmax and -dP/dtmax were obtained with the use of computer algorithms and an interactive videographics program.
P<0.05
P<0.01 vs sham MI/R
P<0.05 vs MI/R vehicle.
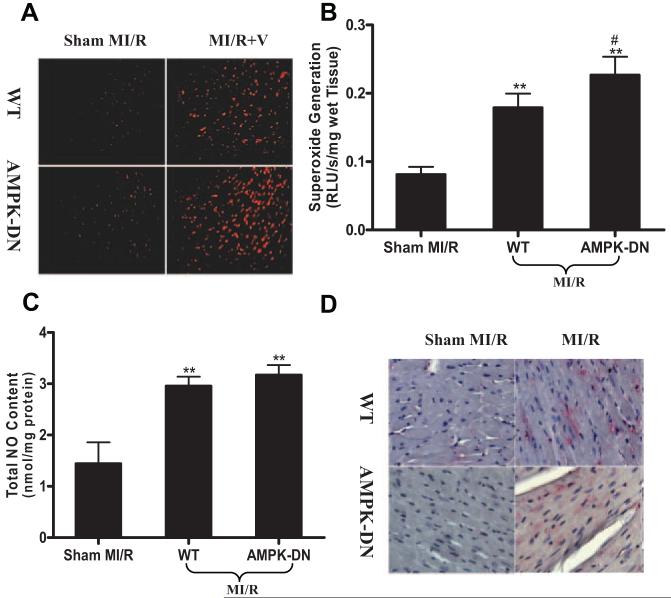
AMPK Deficiency Enhanced MI/R-Induced Oxidative Stress but Had No Significant Effect on MI/R-Induced Nitrative Stress
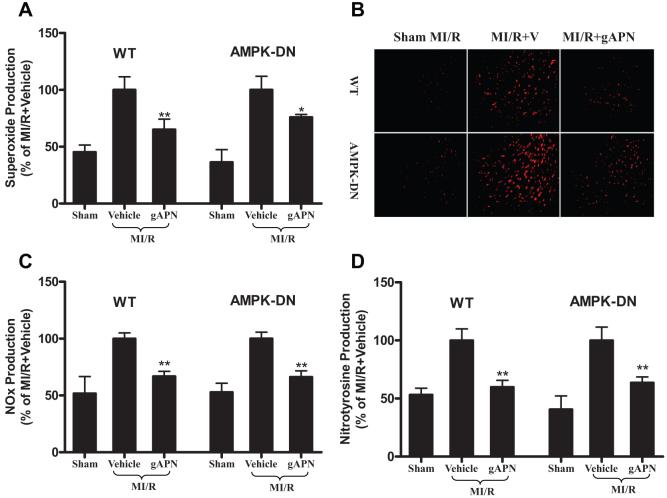
Oxidative and nitrative stresses are primary factors responsible for MI/R injury. As summarized in Figure 2, MI/R-induced superoxide production was further enhanced in AMPK-DN hearts, as evidenced by intensified dihydroethidium staining (Figure 2A) and increased lucigenin-enhanced luminescence signals (Figure 2B). However, MI/R-induced NO overproduction was not significantly changed (Figure 2C) in AMPK-DN hearts, and no significant difference in nitrotyrosine formation was observed between WT and AMPK-DN hearts subjected to MI/R (Figure 2D) with a relatively small simple size included in the present study.
Figure 2.
AMPK deficiency increases superoxide production in MI/R cardiac tissue without altering NO and peroxynitrite formation. A, In situ superoxide detection performed with dihydroethidium (n=4 to 5 hearts per group) staining. B, Superoxide content quantified with lucigenin-enhanced luminescence (n=9 to 12 hearts per group). RLU indicates relative light units. C, Total NO content determined by chemiluminescence NO detector (n=9 to 12 hearts per group). D, Immunohistological sections stained with an antibody against nitrotyrosine to determine the nitrotyrosine formation in the MI/R heart, a footprint of in vivo peroxynitrite formation and an index for nitrative stress (n=5 to 6 hearts per group). **P<0.01 vs sham MI/R; #P<0.05 vs WT with MI/R.
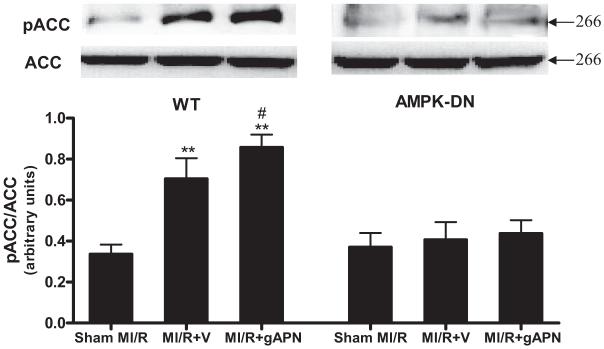
Overexpressing a Dominant Negative Mutant of AMPK α2 Subunit Abolished MI/R-Induced ACC Phosphorylation and Completely Blocked gAPN-Stimulated ACC Phosphorylation
ACC is a major substrate of AMPK, and its phosphorylative inhibition plays an essential role in the metabolic regulation of AMPK. Moreover, previous studies have demonstrated that much of the cardiometabolic effect of adiponectin is conducted by the AMPK-ACC signaling axis.13,20 Consistent with these notions, a >2-fold increase in ACC phosphorylation was observed in WT hearts after MI/R, and treatment with gAPN (1.2-fold; P<0.05) further enhanced ACC phosphorylation modestly (Figure 3, left panel). However, MI/R-induced ACC phosphorylation was lost in AMPK-DN hearts, and, more importantly, treatment with gAPN failed to stimulate ACC phosphorylation in the transgenic hearts (Figure 3, right panel).
Figure 3.
Effect of MI/R and adiponectin treatment on ACC phosphorylation in WT mice (left) and AMPK-DN mice (right). Inserts, Representative Western blots. Bar graph, Density analysis results from 5 to 8 hearts per group. **P<0.01 vs sham MI/R; #P<0.05 vs MI/R+V. V indicates vehicle.
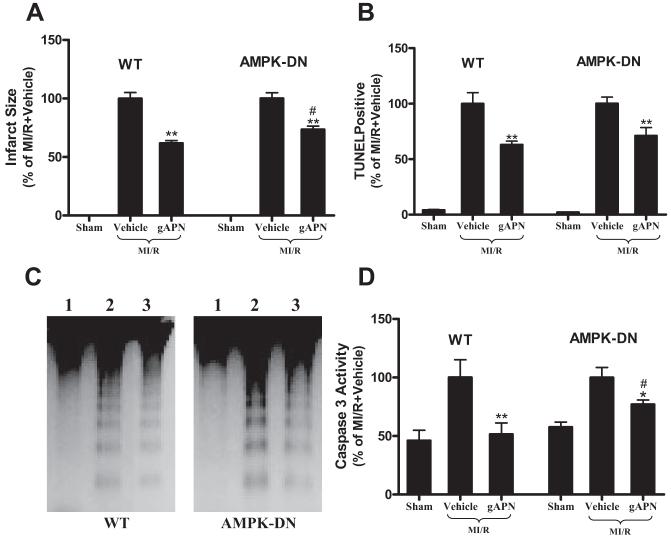
Cardioprotective Effect of gAPN Is Reduced But Not Lost in AMPK-DN Mice
Other investigators have demonstrated that many of the biological functions of adiponectin are completely abolished when AMPK signaling is blocked either genetically or pharmacologically,16-18,21-23 and data from the present study summarized above confirmed the essential role of AMPK in gAPN-induced ACC phosphorylation. We next determined whether AMPK serves as the central mediator for the cardioprotective effects of gAPN. To better compare the cardioprotective effects of gAPN between WT littermates and AMPK-DN mice, data were normalized against the mean value of the respective vehicle group. Consistent with our previous observations,15 treatment of WT animals with gAPN significantly reduced infarct size (Figure 4A left panel), attenuated MI/R-induced apoptosis determined by TUNEL staining (Figure 4B left panel), DNA fragmentation (Figure 4C left panel), and caspase 3 activation (Figure 4D left panel), and improved cardiac functional recovery after reperfusion (Table). However, although the cardioprotective effects of gADN were modestly reduced in AMPK-DN hearts compared with WT hearts (Figure 4; note that the percent reduction of infarct size and apoptosis by gAPN treatment is reduced in AMPK-DN mice compared with WT), a significant portion of cardioprotection from gAPN was retained in AMPK-DN hearts. Specifically, treatment with gAPN significantly reduced infarct size (Figure 4A, right panel), decreased cardiomyocyte apoptosis (less TUNEL-positive labeling in Figure 4B; weaker DNA fragmentation in Figure 4C; and lower caspase 3 activity in Figure 4D), and improved cardiac functional recovery (Table). These novel data suggest that mechanisms exist that are responsible for the cardioprotection of gAPN through signaling pathways other than AMPK.
Figure 4.
Cardioprotective effects of adiponectin are blunted but not lost in AMPK-DN mice. Mean values obtained from vehicle-treated MI/R animals were treated as 100%, and individual values from each animal were normalized against the mean values. Myocardial infarct size was determined by Evans blue/TTC double staining (A, n=12 to 14 hearts per group). Myocardial apoptosis was determined by TUNEL labeling (B, n=5 to 6 hearts per group), DNA ladder formation (C, n=4 to 6 hearts per group; lane 1=sham MI/R, lane 2=MI/R+vehicle, lane 3=MI/R+gAPN), and caspase 3 activation (D, n=10 to 12 hearts per group). *P<0.05, **P<0.01 vs MI/R+vehicle; #P<0.05 vs WT mice treated with adiponectin.
Antioxidative/Antinitrative Effects of gAPN in the MI/R Hearts Are Not Mediated by AMPK
Having demonstrated that gAPN protects hearts against reperfusion injury partially through an AMPK-independent system, we further explored the potential mechanisms that might be responsible for the AMPK-independent cardioprotection of gAPN. We reported recently that gAPN cardioprotection involves the reduction of oxidative/nitrative stress.15 Two series of experiments were thus performed to delineate the relationship between AMPK signaling and oxidative/nitrative stress in MI/R hearts. In the first series of experiments, MI/R-induced myocardial oxidative stress and nitrative stress were determined. As illustrated in Figure 5, MI/R-induced superoxide/NO overproduction (Figure 5A to 5C) and nitrotyrosine formation (Figure 5D) were significantly inhibited when WT and AMPK-DN animals were treated with gAPN. There was no statistically significant difference in the antioxidant and antinitrative effect of gAPN (expressed as percent reduction over their respective vehicle groups) between AMPK-DN and WT animals.
Figure 5.
Treatment with adiponectin reduced superoxide production determined by lucigenin-enhanced luminescence (A, n=9 to 12 hearts per group) and dihydroethidium staining (B, n=4 to 5 hearts per group), reduced nitric oxide (NOx) production (C, n=9 to 12 hearts per group), and decreased peroxynitrite formation (D, n=10 to 12 hearts per group) in WT as well as in AMPK-DN mice. *P<0.05, **P<0.01 vs MI/R+vehicle. V indicates vehicle.
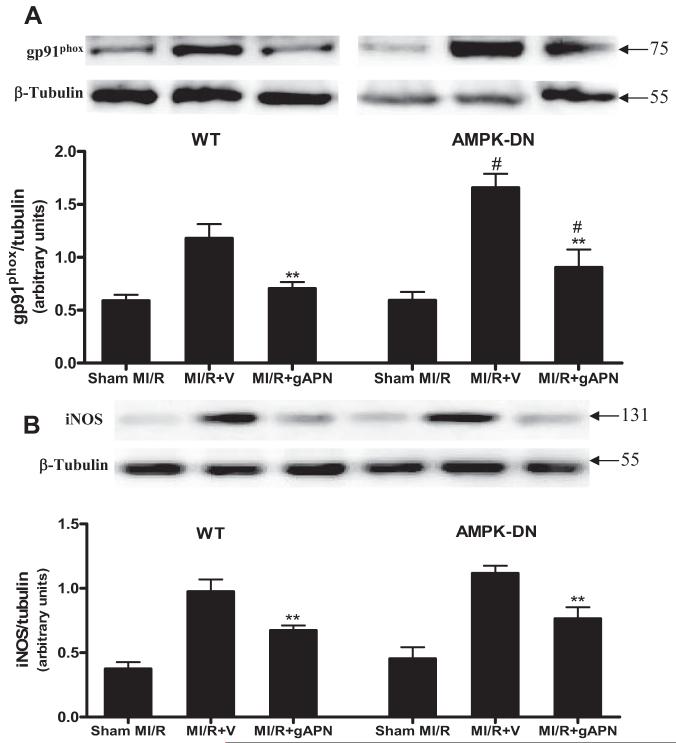
In the second series of experiments, we further determined the molecular sources that might be responsible for the AMPK-independent antioxidant/antinitrative actions of gAPN. As illustrated in Figure 6A, MI/R-induced overexpression of gp91phox was significantly increased in AMPK-DN hearts compared with WT hearts (Figure 6A; lane 2 versus lane 5, #P<0.05). This result is consistent with data presented in Figure 2A and 2B showing that MI/R-induced superoxide overproduction was further enhanced in AMPK-DN hearts. Treatment of WT as well as AMPK-DN mice with gAPN significantly inhibited gp91phox expression (Figure 6A; **P<0.01). Although the absolute level of gp91phox expression remains greater in gAPN-treated AMPK-DN hearts than in gAPN-treated WT hearts (Figure 6A; lane 3 versus lane 6, #P<0.05), the percent inhibitory effect of gAPN over their respective vehicle groups is comparable (40.4% and 44.3% reduction, respectively; P>0.05) between these 2 groups. Together with the data presented in Figure 2, these results demonstrated that MI/R-induced oxidative stress was increased when AMPK signaling is blocked, but the antioxidant effect of gAPN was not mediated by AMPK activation.
Figure 6.
Treatment with adiponectin inhibited gp91phox (A) and iNOS expression (B) in MI/R hearts. Inserts, Representative Western blots. Bar graph, Density analysis results from 5 to 8 hearts per group. **P<0.01 vs MI/R+V; #P<0.05 vs WT mice. V indicates vehicle.
Data presented in Figure 5C and 5D demonstrated that gAPN inhibited MI/R-induced NO overproduction and nitrotyrosine formation in an AMPK-independent fashion. To further determine the NO source inhibited by gAPN, the effect of gAPN on iNOS expression was determined. As illustrated in Figure 6B, AMPK deficiency had no significant effect on MI/R-induced iNOS expression. These data are consistent with that presented in Figure 2C showing that AMPK deficiency had no significant effect on NO overproduction. Moreover, treatment of AMPK-DN hearts with gAPN reduced MI/R-induced iNOS expression to a level comparable to that seen in WT hearts. Together with the data presented in Figure 2, these results demonstrated that AMPK deficiency had no significant effect on MI/R-induced nitrative stress and that gAPN inhibited nitrative stress in an AMPK-independent fashion.
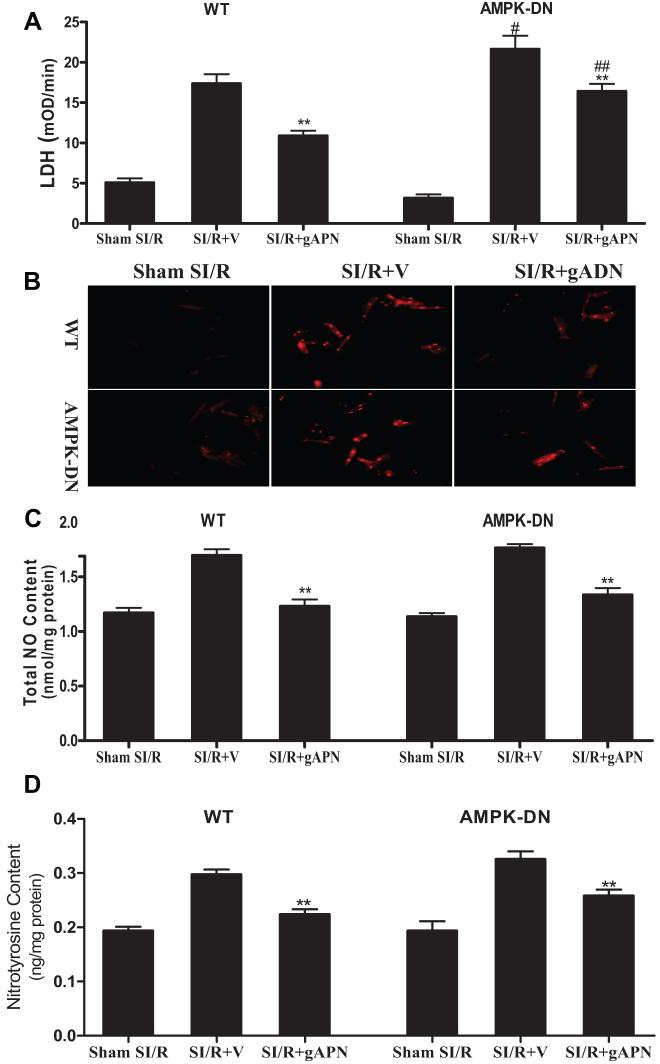
gAPN Directly Inhibited Oxidative/Nitrative Stress in Cardiomyocytes in an AMPK-Independent Fashion
Our aforementioned in vivo experimental results demonstrated that the cardioprotective effects of gADN are partially AMPK independent and that the antioxidant/antinitrative effect of gAPN is not mediated by AMPK. Because adiponectin is a potent anti-inflammatory molecule, it is thus possible that gAPN may achieve its antioxidant/antinitrative effects via an AMPK-mediated inhibitory effect on leukocyte/endothelial interactions in our cardiomyocyte-specific transgenic mice. To directly address this issue, adult cardiomyocytes were isolated from WT or AMPK-DN mice and subjected to SI/R as described in our previous publications.15 Ten minutes before SI, cardiomyocytes were treated with either vehicle or gAPN (2 μg/mL), and the effect of gAPN on SI/R-induced cell death (LDH release) and oxidative/nitrative stress was determined. Consistent with our in vivo results, SI/R-induced cell death was significantly increased in cardiomyocytes isolated from AMPK-DN mice compared with WT mice (Figure 7A). However, SI/R-induced oxidative/nitrative stress was comparable in cardiomyocytes isolated from WT and AMPK-DN hearts (Figure 7B, 7C, 7D). Treatment of cardiomyocytes isolated from WT as well as AMPK-DN mice with gAPN significantly reduced SI/R-induced cardiomyocyte death (Figure 7A), superoxide and NO overproduction (Figure 7B, 7C), and protein nitration (Figure 7D). These results demonstrated that gAPN is capable of inhibiting oxidative/nitrative stress occurring in MI/R cardiomyocytes independent of AMPK and significantly reduced cell death.
Figure 7.
Adiponectin reduced cell death (A, n=12 to 14 dishes isolated from at least 4 hearts per group, the same for all other assays) and inhibited production of superoxide (B), nitric oxide (C), and peroxynitrite (D) in cardiomyocytes isolated from WT as well as from AMPK-DN mice. **P<0.01 vs SI/R+V; #P<0.05 vs WT cardiomyocytes subjected to SI/R treated with vehicle; #P<0.05, ##P<0.01 vs cardiomyocytes isolated from WT. V indicates vehicle.
Discussion
Several important observations have been made in the present study. First, we have demonstrated that cardiomyocyte-specific AMPK deficiency significantly enhanced postischemic myocardial apoptosis, increased infarct size, and worsened cardiac functional recovery in a clinically relevant animal model of in vivo regional MI/R. Moreover, we have demonstrated that NADPH oxidase expression and superoxide production are significantly increased in AMPK-DN hearts after MI/R. These results not only provided additional evidence supporting a study published early this year by Calvert et al24 showing that AMPK deficiency increases infarct size but, more importantly, also provided novel cellular mechanisms by which AMPK deficiency promotes myocardial cell death. Second, we have provided the first direct evidence that gAPN protects MI/R heart in vivo partially through AMPK-independent mechanisms. Finally, we have proved that the antioxidative and antinitrative effects of gAPN in MI/R hearts are not mediated by AMPK.
AMPK and MI/R Injury
Although the protective role of AMPK during cardiac ischemia has been investigated extensively and multiple mechanisms by which AMPK activation protects ischemic heart have been identified,8,10,24,25 the role of AMPK in cardiac reperfusion after ischemia is much more complex. It was generally believed that AMPK activation during reperfusion can cause high rates of fatty acid oxidation that may adversely affect cardiac functional recovery.7 Whereas the evidence from 2 recent in vitro studies8,9 using AMPK-deficient cardiac models appears to disprove the notion that AMPK activation is detrimental to functional recovery during reperfusion, a conclusion that AMPK activation is beneficial to the heart during reperfusion cannot yet be made on the basis of these ex vivo experiments because of many limitations associated with the buffer-perfused heart model. In the present study, we utilized cardiomyocyte-specific AMPKα2 mutant transgenic mice and subjected them to regional MI/R in vivo. Our results demonstrated that compared with WT mice, MI/R in AMPK-DN mice caused greater cardiac injury, including larger infarct size, more apoptosis, and poorer cardiac function recovery (Figure 1 and Table). These results are important in defining the role of AMPK in MI/R injury.
The study by Russell et al8 suggested that AMPK may protect reperfusion injury by limiting apoptotic activity rather than through its metabolic actions. Indeed, activation of AMPK by 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside attenuates endoplasmic reticulum stress and reduces apoptotic cell death in cultured cardiomyocytes.26 However, the manner in which AMPK reduces reperfusion-induced endoplasmic reticulum stress and apoptosis remains unclear. Because overproduction of reactive oxygen species is a central cause of reperfusion-induced endoplasmic reticulum stress and apoptosis, our present study demonstrating increased expression of gp91phox (the essential membrane component of NADPH oxidase that accounts for the large portion of superoxide production in the MI/R heart) and enhanced superoxide production in AMPK-DN hearts provides the first likely mechanism that might be responsible for increased cardiomyocyte apoptosis observed in these animals.
Role of AMPK in Adiponectin Cardioprotection Against MI/R
The biological activities of adiponectin have been investigated extensively in recent years. Four major functions, including metabolic regulation and anti-inflammatory, vasculoprotective, and cardioprotective effects, have been identified.27 Substantial evidence has been accumulated supporting the conception that AMPK plays an essential role in adiponectin intracellular signaling. Specifically, pharmacological inhibition of AMPK activity or genetic inhibition of AMPK expression virtually abolishes the metabolic,16,18 anti-inflammatory,28 and vasculoprotective17,29 effects of adiponectin. However, although the evidence for the cardioprotective effects of adiponectin in MI/R is strong, it is still unclear whether AMPK is the central mediator of this effect.20 In the first study reporting the cardioprotective effect of adiponectin, Shibata et al14 demonstrated that the importance of AMPK in cardiomyocyte injury varies greatly in different models. Specifically, adiponectin blocks hypoxia/reoxygenation-induced neonatal cardiomyocyte apoptosis exclusively through AMPK activation. In contrast, its inhibitory effect on lipopolysaccharide-induced tumor necrosis factor-α production is completely AMPK independent. Moreover, in vivo administration of NS398, a cyclooxygenase-2 inhibitor, abrogated the infarct-sparing action of exogenous adiponectin, suggesting that cyclooxygenase-2 plays a significant role in adiponectin cardioprotection after MI/R. However, 2 very recent ex vivo studies reported that AMPK is essential in the cardioprotective effects of adiponectin. The study by Shinmura et al30 demonstrated that short-term caloric restriction protected the heart by increasing serum adiponectin levels with subsequent AMPK activation. In a more recent study, Gonon et al31 reported that adiponectin protected against MI/R injury via the AMPK/Akt/NO signaling axis. Although the data clearly demonstrated that administration of adiponectin in isolated perfused hearts resulted in AMPK phosphorylation, whether the protective effects were blocked when AMPK was inhibited was not determined, and a cause-effect relationship was not established. It thus remains unclear whether AMPK phosphorylation is a necessary step for adiponectin protection of the MI/R heart. Taken together, currently available data strongly suggest that the degree of AMPK involvement in adiponectin cardioprotection varies significantly under different experimental conditions, and an in vivo study utilizing AMPK gene-manipulated animals is essential to definitively discern the role of AMPK in adiponectin cardioprotection.
Our present study provided direct evidence that a large portion of gAPN cardioprotection is AMPK independent in intact animals. Moreover, our study demonstrated for the first time that gAPN inhibits MI/R-induced oxidative/nitrative stress in an AMPK-independent fashion and that these actions may account for the cardioprotection of gAPN observed in AMPK-DN animals. In a recent study, Ouedraogo32 reported that treatment with gAPN suppressed glucose-induced superoxide overproduction in cultured endothelial cells and that this action cannot be blocked by pretreatment with AMPK inhibitors, indicating that gAPN reduces high glucose-induced superoxide overproduction in an AMPK-independent fashion. Because the same isoform of adiponectin (ie, globular domain of adiponectin) was used in these 2 studies (ie, Ouedraogo’s study and our present study) and similar results were observed, it remains to be determined whether this AMPK-independent antioxidant action of adiponectin is isoform specific (ie, gAPN) or uniform to all isoforms of adiponectin.
It should be noted that AMPKα2 activity was markedly inhibited (78% reduction) but not completely lost and that AMPKα1 activity was unaltered in the transgenic animals used in our study.10 Therefore, it is possible that residual AMPKα2 or AMPKα1 might be responsible for the antioxidant and antinitrative action of adiponectin, as was observed. Although additional studies utilizing AMPKα1/2 double knockout mice are necessary to definitely address this concern, currently available data argue against this possibility. The α2 is the dominant form of AMPK in the heart, and the small amount of α1 present in the α2 mutant transgenic heart is maximally activated at baseline before ischemia.8,10 There-fore, it is highly unlikely that AMPKα1 is responsible for the results of adiponectin treatment given 20 minutes after MI.10 More importantly, our present study demonstrated that adiponectin-induced ACC phosphorylation is completely lost in AMPK-DN mice, indicating that the residual AMPKα2 and small amount of α1 are insufficient to effectively transfer the signaling of adiponectin in the MI/R heart.
AMPK and adiponectin are 2 critical metabolic regulators, and their deficiency exists in obesity and diabetes.33-35 Because of their well-recognized cardioprotective actions, it has been proposed that impaired adiponectin/AMPK signaling might be responsible for increased cardiovascular complications in patients with diabetes.36 In recent years, AMPK has become a major target in the search for effective treatments for metabolic syndromes, including type II diabetes. However, our present experimental results strongly suggest that adiponectin might be a better, more effective therapeutic target in the treatment of ischemic heart injury in diabetic patients. For instance, administration of adiponectin may still be an effective therapy under conditions in which the AMPK signaling-dependent cardioprotective agents become ineffective. Indeed, a recent study by Lefer and colleagues24 demonstrated that the cardioprotective effects of metformin, 1 of the most commonly prescribed antihyperglycemic agents for the treatment of type 2 diabetes, are completely lost in AMPK-DN animals. In addition, numerous studies have demonstrated that physiological or pharmacological concentrations of NO generated from endothelial NOS or NO donors exert significant cardioprotective effects against ischemia and MI/R injury,37 and adiponectin has been shown to stimulate NO production by endothelial NOS phosphorylation.21 More importantly, it is well recognized that simultaneous overproduction of superoxide and NO not only causes the inactivation of a cytoprotective molecule (ie, NO) but also produces a highly cytotoxic molecule, peroxynitrite. Therefore, those therapeutic interventions that prevent concomitant, synchronized NO/superoxide overproduction effectively block peroxynitrite formation and offer great tissue protection. Our present experimental results demonstrated that AMPK activation may have an antioxidant effect via inhibiting NADPH oxidase expression but does not appear to have a significant role in MI/R-induced peroxynitrite formation. In contrast, administration of gAPN reduced synchronized NO/superoxide overproduction from NADPH oxidase and iNOS and effectively blocked peroxynitrite formation in MI/R hearts. As such, interventions with aims of bolstering adiponectin production may hold great potential in reducing diabetic patient mortality in those with ischemic heart disease.
Acknowledgments
Sources of Funding
This research was supported by the following grants: National Institutes of Health 2R01HL-63828, American Diabetes Association 7-05-RA-83, Commonwealth of Pennsylvania Department of Health, American Heart Association GIA0855554D (to Dr Ma), American Diabetes Association 7-06-JF59 (to Dr Tao), and National Institutes of Health DK063018, DK071360 (to Dr Goldstein).
Footnotes
Disclosures
None.
CLINICAL PERSPECTIVE
The incidence of obesity and type 2 diabetes mellitus is increasing worldwide, and both of these conditions increase the risk of cardiovascular disease. Adiponectin is an adipocyte-specific protein that plays a critical role in glucose and lipid metabolism regulation. Prospective studies have demonstrated the association between decreased plasma adiponectin levels and increased cardiovascular morbidity in diabetic patients. Clarification of the mechanisms by which adiponectin acts advantageously may contribute to our understanding of diabetic pathophysiology and provide insight to a potential therapeutic modality. Considerable evidence exists that the metabolic regulatory, anti-inflammatory, and vascular protective effects of adiponectin are largely mediated by activation of AMP-activated protein kinase (AMPK). However, our present study demonstrated for the first time that the anti-ischemic and cardioprotective effect of adiponectin is largely AMPK independent. In recent years, AMPK has become a major target in the search for effective treatments for metabolic syndromes, including type II diabetes. However, our present experimental results strongly suggest that adiponectin might be a better, more effective therapeutic target in the treatment of ischemic heart injury in diabetic patients. For instance, administration of adiponectin may still be an effective therapy under conditions in which the AMPK signaling-dependent cardioprotective agents become ineffective. Moreover, our present experimental results demonstrated that administration of globular domain of adiponectin reduced synchronized nitric oxide/superoxide overproduction from NADPH oxidase and inducible nitric oxide synthase and effectively blocked peroxynitrite formation in ischemic/reperfused hearts. As such, interventions with aims of bolstering adiponectin production may hold great potential in reducing diabetic patient mortality in those with ischemic heart disease.
References
- 1.Norhammar A, Lindback J, Ryden L, Wallentin L, Stenestrand U. Improved but still high short- and long-term mortality rates after myocardial infarction in patients with diabetes mellitus: a time-trend report from the Swedish Register of Information and Knowledge about Swedish Heart Intensive Care Admission. Heart. 2007;93:1577–1583. doi: 10.1136/hrt.2006.097956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marfella R, D’Amico M, Di Filippo C, Piegari E, Nappo F, Esposito K, Berrino L, Rossi F, Giugliano D. Myocardial infarction in diabetic rats: role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia. 2002;45:1172–1181. doi: 10.1007/s00125-002-0882-x. [DOI] [PubMed] [Google Scholar]
- 3.Forrat R, Sebbag L, Wiernsperger N, Guidollet J, Renaud S, De Lorgeril M. Acute myocardial infarction in dogs with experimental diabetes. Cardiovasc Res. 1993;27:1908–1912. doi: 10.1093/cvr/27.11.1908. [DOI] [PubMed] [Google Scholar]
- 4.Jagasia D, McNulty PH. Diabetes mellitus and heart failure. Congest Heart Fail. 2003;9:133–139. doi: 10.1111/j.1527-5299.2002.00901.x. [DOI] [PubMed] [Google Scholar]
- 5.Young LH. AMP-activated protein kinase conducts the ischemic stress response orchestra. Circulation. 2008;117:832–840. doi: 10.1161/CIRCULATIONAHA.107.713115. [DOI] [PubMed] [Google Scholar]
- 6.Dolinsky VW, Dyck JR. Role of AMP-activated protein kinase in healthy and diseased hearts. Am J Physiol. 2006;291:H2557–H2569. doi: 10.1152/ajpheart.00329.2006. [DOI] [PubMed] [Google Scholar]
- 7.Dyck JRB, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol (Lond) 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell RR, III, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvajal K, Zarrinpashneh E, Szarszoi O, Joubert F, Athea Y, Mateo P, Gillet B, Vaulont S, Viollet B, Bigard X, Bertrand L, Ventura-Clapier R, Hoerter JA. Dual cardiac contractile effects of the alpha2-AMPK deletion in low-flow ischemia and reperfusion. Am J Physiol. 2007;292:H3136–H3147. doi: 10.1152/ajpheart.00683.2006. [DOI] [PubMed] [Google Scholar]
- 10.Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative {alpha}2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 11.Hug C, Lodish HF. The role of the adipocyte hormone adiponectin in cardiovascular disease. Curr Opin Pharmacol. 2005;5:129–134. doi: 10.1016/j.coph.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Basu R, Pajvani UB, Rizza RA, Scherer PE. Selective downregulation of the high molecular weight form (HMW) of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from non-diabetic subjects. Diabetes. 2007;56:2174–2177. doi: 10.2337/db07-0185. [DOI] [PubMed] [Google Scholar]
- 13.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16:141–146. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 16.Bruce CR, Mertz VA, Heigenhauser GJ, Dyck DJ. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes. 2005;54:3154–3160. doi: 10.2337/diabetes.54.11.3154. [DOI] [PubMed] [Google Scholar]
- 17.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of AMP-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 19.Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, Christopher TA, Lopez BL, Yodoi J, Koch WJ, Feelisch M, Ma XL. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation. Proc Natl Acad Sci U S A. 2004;101:11471–11476. doi: 10.1073/pnas.0402941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyck JRB. The ischemic heart: starving to stimulate the adiponectin-AMPK signaling axis. Circulation. 2007;116:2779–2781. doi: 10.1161/CIRCULATIONAHA.107.742023. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 22.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CC, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355–1363. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- 24.Calvert JW, Gundewar S, Jha S, Greer JJM, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 25.Tian R, Balschi JA. Interaction of insulin and AMPK in the ischemic heart: another chapter in the book of metabolic therapy? Circ Res. 2006;99:3–5. doi: 10.1161/01.RES.0000233142.26369.f6. [DOI] [PubMed] [Google Scholar]
- 26.Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouchi N, Shibata R, Walsh K. Targeting adiponectin for cardioprotection. Expert Opin Ther Targets. 2006;10:573–581. doi: 10.1517/14728222.10.4.573. [DOI] [PubMed] [Google Scholar]
- 28.Hattori Y, Nakano Y, Hattori S, Tomizawa A, Inukai K, Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-kappaB activation in vascular endothelial cells. FEBS Lett. 2008;582:1719–1724. doi: 10.1016/j.febslet.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 29.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 31.Gonon AT, Widegren U, Bulhak A, Salehzadeh F, Persson J, Sjoquist PO, Pernow J. Adiponectin protects against myocardial ischaemia-reperfusion injury via AMP-activated protein kinase, Akt, and nitric oxide. Cardiovasc Res. 2008;78:116–122. doi: 10.1093/cvr/cvn017. [DOI] [PubMed] [Google Scholar]
- 32.Ouedraogo R, Wu X, Xu SQ, Fuchsel L, Motoshima H, Mahadev K, Hough K, Scalia R, Goldstein BJ. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006;55:1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- 33.Bonnard C, Durand A, Vidal H, Rieusset J. Changes in adiponectin, its receptors and AMPK activity in tissues of diet-induced diabetic mice. Diabetes Metab. 2008;34:52–61. doi: 10.1016/j.diabet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Wan Q, Guan Q, Gao L, Zhao J. High-fat diet feeding impairs both the expression and activity of AMPKa in rats’ skeletal muscle. Biochem Biophys Res Commun. 2006;339:701–707. doi: 10.1016/j.bbrc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 35.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 36.Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67:705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 37.Lefer AM, Lefer DJ. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischaemia-reperfusion. Cardiovasc Res. 1996;32:743–751. [PubMed] [Google Scholar]