Abstract
Azathioprine, a widely-used immunosuppressant, is also used in the control of inflammatory disorders. These are characterized by the local accumulation of immune effector cells that produce reactive oxygen species (ROS). The DNA of azathioprine treated patients contains 6-thioguanine (6-TG), a base analog that is particularly susceptible to oxidation. Here we demonstrate that 6-TG is vulnerable to ROS produced by chemical oxidants and that cells containing DNA 6-TG are hypersensitive to these oxidants. We also show 6-TG incorporated into the DNA of macrophages sensitizes them to killing by endogenously produced ROS. ROS generated by macrophages are also a hazard for co-cultured non-macrophage cells containing DNA 6-TG. This bystander vulnerability of cells containing DNA 6-TG to oxidation by ROS generated by immune effector cells has implications for the long-term use of azathioprine in the management of inflammatory disorders.
Keywords: 6-Thioguanine, ROS, inflammation, co-culture
INTRODUCTION
Chronic inflammatory disorders are relapsing and remitting conditions that reflect an underlying immune dysfunction. Chronic inflammation can be a risk factor for the development of malignancy, and patients with ulcerative colitis and Crohn's disease have an increased risk of colonic epithelial dysplasia and carcinoma (1). Current treatments for this group of diseases involve nonspecific suppression of the inflammatory processes with drugs, such as NSAIDS and steroids, antibiotics, and modulators of the immune response. In the latter category, the thiopurines azathioprine and 6-mercaptopurine (6-MP), are the treatments of choice for severe inflammatory bowel disease (IBD) and are often used in the management of rheumatoid arthritis (2). Thiopurines are efficacious and 70% of patients with steroid dependent IBD achieve and maintain remission (3). Azathioprine is a prodrug that is cleaved to 6-MP which in turn is metabolized to 6-thioguanine nucleotides (TGN). These are precursors of DNA synthesis and 6-TG becomes incorporated into DNA of patients taking Aza (4, 5).
DNA 6-TG has two properties that distinguish it from the canonical DNA bases. Firstly, it is a UVA chromophore with an absorbance maximum at 340nm and is photochemically susceptible to UVA. One of the photoproducts of DNA 6-TG, guanine-6-sulfonate (GSO3) is a strong block to replication in vitro and in vivo. This lesion can, however, be bypassed by error-prone DNA polymerases (6) in an emergency response that is a potential source of mutation (7, 8). The corresponding photochemical reactions occur in patients taking azathioprine whose skin contains DNA 6-TG and is selectively sensitive to UVA wavelengths (9). The second important feature of DNA 6-TG is its low oxidation potential. The formation of GSO3 reflects the relatively favourable oxidation of 6-TG by reactive oxygen species (ROS) generated photochemically by UVA.
Oxygen-related DNA damage is an inescapable hazard and cells have evolved multiple protective and repair systems to minimize the detrimental consequences of oxidized DNA which include disease and aging. ROS are important signaling molecules and their intracellular levels are normally under careful control (10). Excessive production of ROS, following for example, exposure to oxidizing chemicals such as H2O2, causes oxidative stress and DNA damage. Persistent oxidative DNA damage is linked to human cancer (11). Chronic inflammatory disorders are characterized by the infiltration of a large numbers of neutrophils, monocytes and lymphocytes into the affected sites (for review see ref. 12). These immune effector cells are a significant source of ROS and they generate H2O2 and superoxide (O2·−) as part of their defence against pathogens. The persistence of inflammatory cells, and the associated production of ROS are thought to play an important role in the pathogenesis of IBD. In addition, the duration and severity of inflammation are risk factors in the development of colon cancer in IBD patients (13,14).
Systemic treatment with thiopurines introduces 6-TG into the DNA of all dividing cells, and we were interested to examine whether cells containing DNA 6-TG might be particularly at risk from ROS produced by immune effector cells. Here we show that 6-TG is highly susceptible to oxidation by H2O2 – one of the main components of ROS generated by immune cells – and to KBrO3, another chemical oxidant, and that cells containing DNA 6-TG are hypersensitive to killing by these oxidants. To mimic the infiltration of ROS-generating cells into sites of chronic inflammation, target cells containing DNA 6-TG were co-cultured with an established macrophage cell line. Our results show that ROS produced by macrophages oxidizes the DNA 6-TG of co-cultured target cells and that this results in increased target cell death.
MATERIALS and METHODS
Unless stated, all chemicals were obtained from Sigma-Aldrich (Gillingham, UK).
Cell culture
HCT116 human colon carcinoma, SV40-transformed GM04429f and MRC5-VA human fibroblast, and the murine macrophage precursor J774a.1 cell lines obtained from the CR-UK LRI Central Cell Services, were grown in Dulbecco MEM medium supplemented with 10% FCS (Gibco/Invitrogen, Paisley, Scotland). CCRF-CEM leukemia cells were grown in suspension culture in RPMI medium supplemented with 10% FCS. HCT116, GM04429f and MRC5-VA cells were maintained in 10% CO2, whilst the CCRF-CEM and J774a.1 cells were maintained at 5% CO2 in 37°C humidified incubators.
Thioguanine labelling and oxidant treatment
Cells were routinely cultured for 48h in medium containing 0.5 or 1μM 6-TG. 6-TG treated cells were incubated further in growth medium containing a chemical oxidant; H2O2 (0.01-1mM, 1h), KBrO3 (1-100μM, 3h), or ionizing radiation (IR; 1-10Gy) delivered by a 137Cs γ-radiation source in conjunction with an IBL 437C irradiator (2.2Gy/min; CIS Bio International, Bagnols/Ceze, France). UVC irradiation was delivered from a germicidal UVC lamp at a dose rate of 1 J/m2/s.
Clonogenic survival assay
For adherent cells, survival was determined by clonal assay in triplicate 6-well plates. Surviving colonies were allowed to grow for 7 to 10 days, Giemsa stained, and counted. Each experiment was performed at least three times. Results are expressed as mean ± SEM.
Annexin and PI staining
In some experiments, two-colour flow cytometry was used to assess viability of HCT116 or CCRF-CEM cells. After 48h co-culture with J774a.1, all cells were harvested, washed twice with PBS and incubated with Annexin V-FITC (1μg/ml) and PI (5μg/ml) in binding buffer (10mM Hepes/NaCl pH 7.4, 140mM NaCl, 2.5mM CaCl2) for 15 min at room temperature in the dark. Samples were analysed within 1h of staining using a Beckton Dickinson FACScan. J774a.1 cells were gated and size excluded. Quadrant markers were set on dotplots of unstained HCT116 (viable cell population) and then subsequently applied to other samples. Data acquisition and analysis was performed using CellQuest v3.3 software.
ROS detection
Cells were grown in the presence or absence of 6-TG (1μM) for 24h, washed twice with PBS and incubated with 5 μM CM-H2DCFDA (Invitrogen, Paisley, Scotland) for 20 mins at 37°C. After additional washing with PBS, cells were treated with H2O2, KBrO3 or IR. Treated cells were trypsinized and green fluorescence analyzed by FACS.
Intracellular ROS production by lipopolysaccharide-stimulated (LPS - 10μg/ml; Sigma) macrophages was examined by light microscopy. J774a.1 cells were incubated with 5μM CM-H2DCFDA for 20 mins at 37°C, washed with PBS and examined for green fluorescence by microscopy (Zeiss Axioplan fitted with Hamamatsu C4747-95 digital camera, images were acquired using OpenLab v2.2.5 - Improvision).
ROS released by J774a.1 cells were quantitated by microassay (15). For H2O2, 100μl of assay solution (28mM phenol red, 100U/ml horseradish peroxidase in HBSS) was added to LPS (1-10μg/ml)-stimulated J774a.1 cells in 96-well plates. After incubation for 1h at 37°C, the reaction was stopped by the addition of 10μl 1N NaOH and the A630nm was determined.
For O2−, LPS-stimulated cells in 100μl of assay solution (160μM cytochrome c made fresh in HBSS) were incubated at 37°C for 1h. The reaction was stopped by the addition of 10μl SOD (3000 U/ml) and A550nm was determined.
DNA synthesis
Cells were grown in medium containing 6-TG for 24h, washed and resuspended in PBS before treatment with H2O2 for 1h. After treatment, cells were returned to fresh medium and pulse-labelled with [5′ 3H]-thymidine (1 μCi/ml; 511 GBq/mmol) for 15 mins at 37°C. Trichloroacetic acid insoluble radioactivity in 2 × 106 cells was determined by scintillation counting. All experiments were performed in duplicate.
6-TG incorporation
Cells were treated with 6-TG for 48h, washed with PBS, and DNA was extracted using the Wizard Genomic DNA purification kit (Promega UK, Southampton, UK), according to the manufacturers' instructions. DNA (100μg in 100μl total volume) was digested to nucleosides with 10 units of nuclease P1 (1h, 50°C) followed by 2 units of alkaline phosphatase (1h, 37°C). Deoxynucleosides were separated by HPLC as described (7) using a Waters Symmetry C18 column (Waters, Elstree, UK) equipped with a Waters 2487 Dual Wavelength Absorbance detector and a Waters 2475 Multiwavelength Fluorescence detector. Elution was with a gradient of 10mM KH2PO4, pH 6.7 in methanol. Column eluates were monitored simultaneously by A342 and fluorescence at 410nm. 6-TG substitution was calculated as a percentage of total deoxyguanosine in the same samples. Guanine sulfonate was detected by its fluorescence and quantitated by comparison to an authentic standard. Calibration curves for guanine sulfonate were linear up to 10nmole and the detection limit was approximately 50fmole.
RESULTS
Susceptibility of 6-TG to chemical oxidation
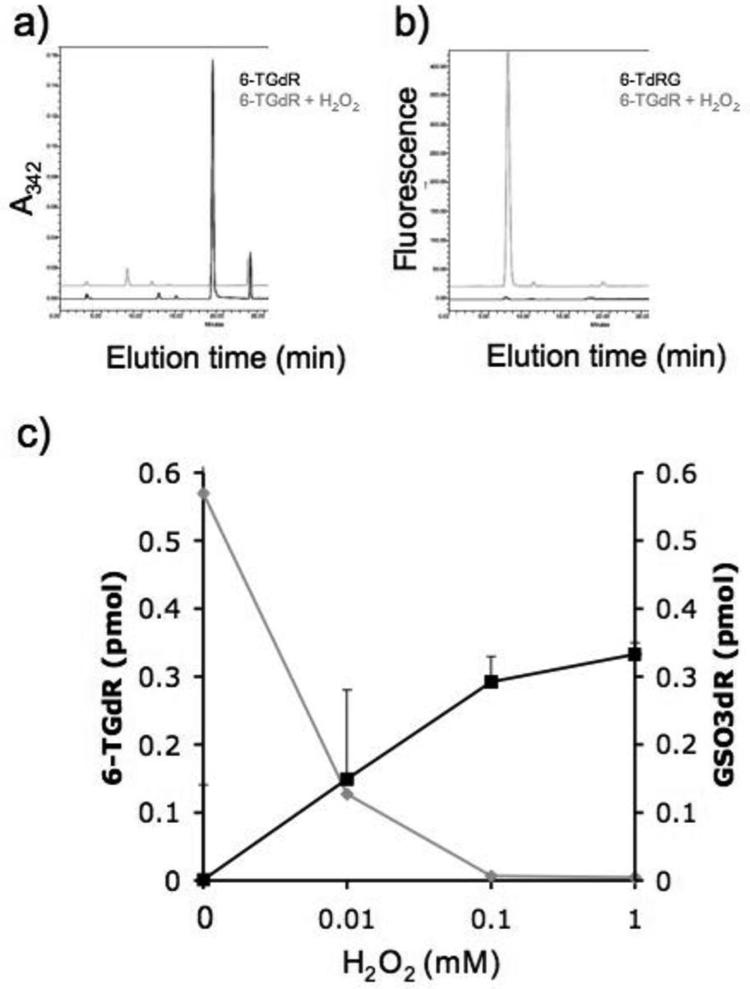
In view of the susceptibility of 6-TG to photooxidation by UVA (7), we examined its chemical oxidation. The deoxyribonucleoside of 6-TG (6-TGdR) was treated with H2O2 and the products analyzed by reverse-phase high-pressure liquid chromatography (HPLC). The 6-TGdR was monitored by its absorbance at 342 nm. Figure 1a shows that treatment with H2O2 destroyed the 6-TGdR as indicated by the loss of A342. The single detectable product was fluorescent (Excitation λmax 324nm, Emission λmax 410nm). This property, together with its elution time (Figure 1b) identified it as the previously characterized guanine-6-sulphonate deoxynucleoside (GSO3dR; (7)). Conversion of 6-TGdR to GSO3dR was dependent on H2O2 concentration between 0.01 and 1mM (Figure 1c). We conclude that 6-TG is susceptible to chemical oxidation to GSO3, a known product of photochemical oxidation.
Figure 1. H2O2 oxidises 6-TGdR to GSO3dR.
An aqueous solution of 6-TGdR (0.1mM) was treated with 1mM H2O2 for 60 min and the reaction products were analyzed by reverse-phase HPLC. Column eluates were monitored by A342 and fluorescence (excitation 324 nm and emission at 410 nm).
a) Untreated (Black trace). 6-TGdR elutes at 20 minutes.
b) H2O2 treated (Grey trace). GSO3dR elutes at 10 minutes.
c) The amounts of 6-TGdR (grey line) and GSO3dR (black line) after treatment with the H2O2 concentrations shown were quantified by HPLC.
DNA 6-TG sensitizes cells to chemical oxidants
HCT116 colorectal carcinoma cells were used to examine whether DNA 6-TG influenced sensitivity to the cytotoxic effects of chemical oxidants. These DNA mismatch repair defective cells incorporate significant levels of DNA 6-TG without detectable toxicity. Following growth for 24h in the presence of 1μM 6-TG, approximately 0.25% of the total DNA guanine was replaced by 6-TG. The 6-TG-treated cells were then exposed to H2O2, KBrO3 or IR and their clonal survival was compared to that of similarly treated cells without DNA 6-TG. Figure 2a shows that DNA 6-TG caused a significant sensitization to H2O2. Formation of intracellular ROS was confirmed using CM-H2DCFDA, a dye that emits green fluorescence on reaction with ROS (Figure 2a, insert). 6-TG-treated HCT116 cells were also more sensitive to KBrO3 (Figure 2b) which also generated measurable levels of intracellular ROS (Figure 2b insert). In contrast, the presence of DNA 6-TG did not significantly alter the sensitivity of cells to ionising radiation (Figure 2c) under conditions that did not detectably increase steady-state levels of ROS (Figure 2c insert). We conclude that DNA 6-TG sensitizes cells to treatments that generate ROS. It is likely that the susceptibility of DNA 6-TG to oxidation underlies this effect.
Figure 2. DNA 6-TG sensitizes cells to killing by chemical oxidants that induce ROS.
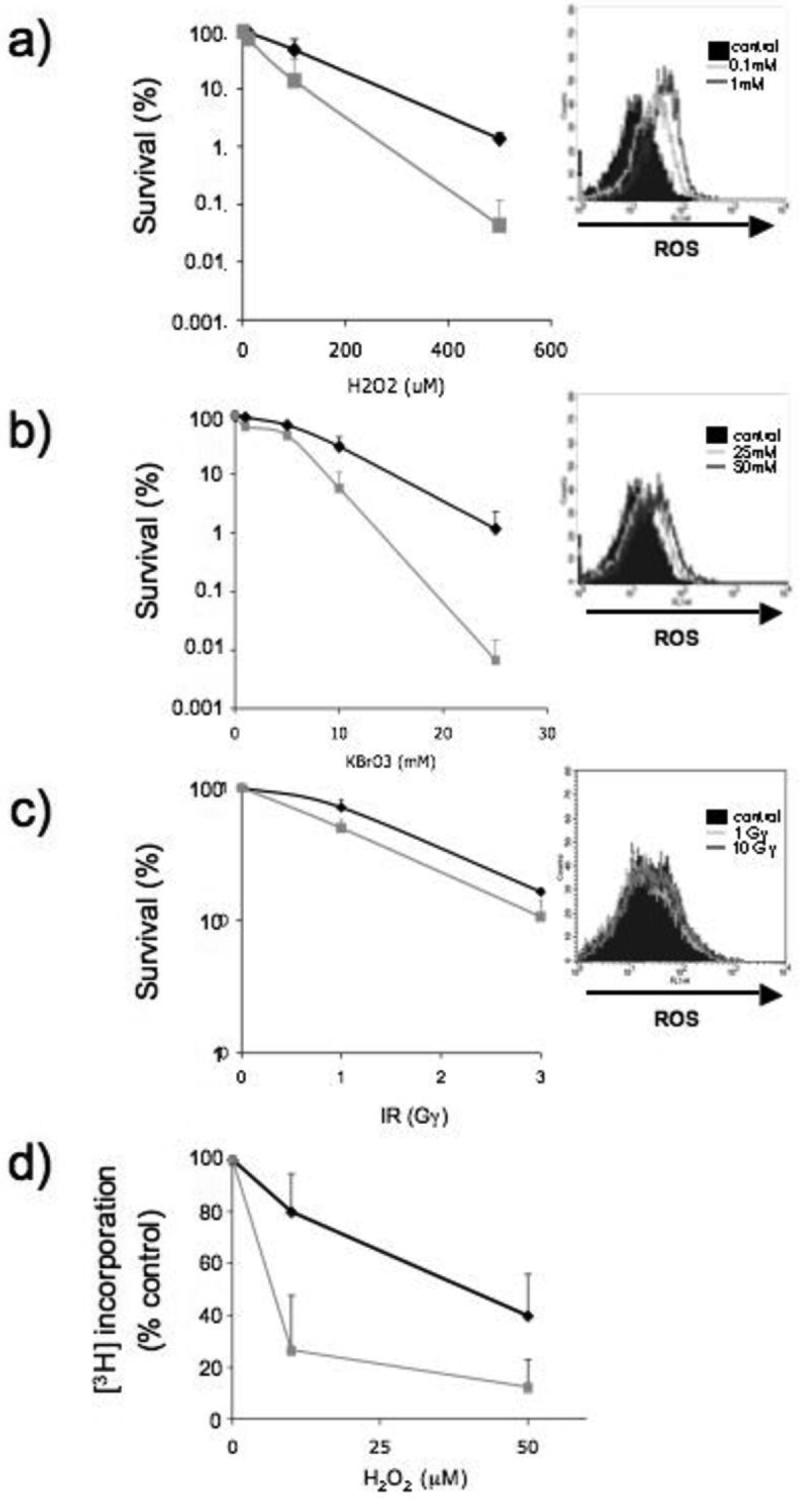
HCT116 cells that had been grown in the presence of 6-TG (1μM) DNA for 24h were treated with (a) H2O2 (1h) or (b) KBrO3 (3h) or (c) γ-irradiated at the doses indicated. Survival of HCT116 in absence of 6-TG (black line) or containing 6-TG in their DNA (grey line) was determined by clonogenic assay (a - c). The data represent the mean ± SD of 4 independent experiments. In parallel, the same HCT116 cells were loaded with the ROS reactive dye CM-H2DCFDA prior to exposure to H2O2, KBrO3 or IR. Changes in fluorescence intensity were analysed by FACS (insert a - c). The solid histogram represents untreated cells.
d) DNA replication. Control (black line) or 6-TG treated HCT116 cells (grey line) were treated with H2O2 for 1h and [3H]-TdR incorporation into TCA-insoluble material was measured. Incorporation is expressed as a percentage of that in untreated cells. Data are the mean ± SD from 2 separate experiments.
The UVA photoproducts of DNA 6-TG, particularly DNA GSO3, are robust inhibitors of DNA replication (8). In agreement with the likely formation of DNA GSO3, H2O2 induced a pronounced replication inhibition in HCT116 cells containing DNA 6-TG (Figure 2d). These data are consistent with the oxidation of DNA 6-TG to replication-blocking DNA lesions, including GSO3, and the enhanced sensitivity of 6-TG treated cells to killing by oxidizing chemicals.
Lethal oxidation products are not excised by nucleotide excision repair
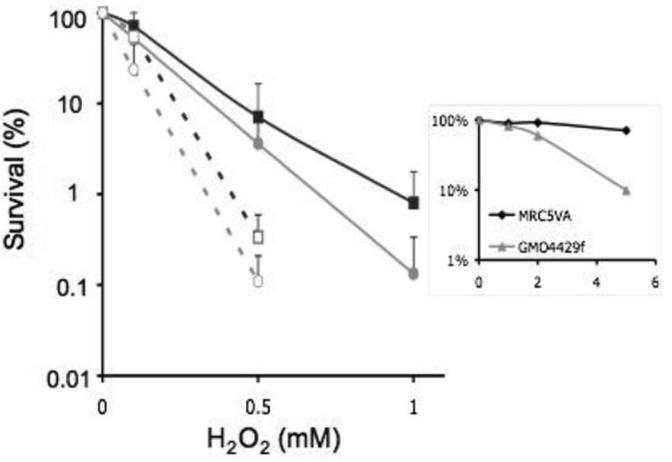
Nucleotide excision repair (NER) can excise potentially lethal lesions which distort the DNA double helix (16). To investigate whether NER could ameliorate the lethal effects of oxidized DNA 6-TG, we compared the sensitivity of cell lines proficient or defective in NER. GM04429f cells, SV40 transformed fibroblasts derived from an XP group A patient, and MRC5-VA - similarly transformed fibroblasts from a clinically normal individual - were cultured for 48h in 0.5μM 6-TG. This resulted in comparable levels of DNA substitution by 6-TG (0.22% DNA guanine in GM04429f and 0.26% in MRC5-VA). The presence of this relatively low level of DNA 6-TG did not detectably affect the viability of either cell line as determined by clonogenic assay (data not shown). Cells were treated with H2O2 and clonal survival was measured. In agreement with published findings (17), GM04429f cells were approximately two-fold more sensitive than MRC5-VA to H2O2 (Figure 3). DNA substitution by 6-TG sensitized GM04429f cells to H2O2 and induced a similar degree of sensitization in MRC5-VA cells. The intrinsic hypersensitivity to UVC of GM04429f cells was confirmed in a parallel experiment in which cells not treated with 6-TG were exposed to UVC (Figure 3 insert). Similar results were obtained with a second XPA cell line, XP12RO, in which DNA 6-TG induced a similar degree of sensitization to H2O2 (data not shown). These findings indicate that NER does not play a significant role in the removal of potentially cytotoxic DNA lesions produced by the chemical oxidation of DNA 6-TG.
Figure 3. Lethal DNA lesions induced by H2O2 oxidation of DNA 6-TG are not excised by NER.
SV40 transformed GM04429f (circles) or MRC5-VA (squares) cells were cultured for 48h in 0.5μM 6-TG then treated with H2O2 for 1h. Clonal survival was determined. Solid lines: no 6-TG treatment. Dashed lines: + 6-TG treatment.
Data are the mean ± SD of 4 independent experiments.
Insert. Confirmation of the XP phenotype. Cells not treated with 6-TG were irradiated with UVC at the doses indicated and survival determined as above.
DNA 6-TG oxidation by ROS from immune effector cells
As a model for ROS-secreting immune effector cells in an intense inflammatory microenvironment, we used the murine macrophage precursor cell line J774a.1. Microassays (15) confirmed that unstimulated J774a.1 cells secrete measurable H2O2 and superoxide (O2.−) and that this is increased following treatment with lipopolysaccharide (LPS) (Figure 4a,b). ROS generation by J774a.1 cells was confirmed by fluorescent microscopy using CM-H2DCFDA dye. Within 1h of treatment with LPS, there was an approximately 10-fold increase in the number of positively staining cells. Even after stimulation, however, only around 10% of cells contained detectable ROS – a representative image is presented in Figure 4c. The percentage of positive cells remained approximately constant for up to 48h after stimulation (Figure 4d) although direct microscopic examination indicated that the secreting cell population changed with time – possibly indicating paracrine signalling. Overall, it was apparent that the high levels of ROS are maintained for at least 48h after LPS stimulation.
Figure 4. ROS production by activated J774a.1 cells.
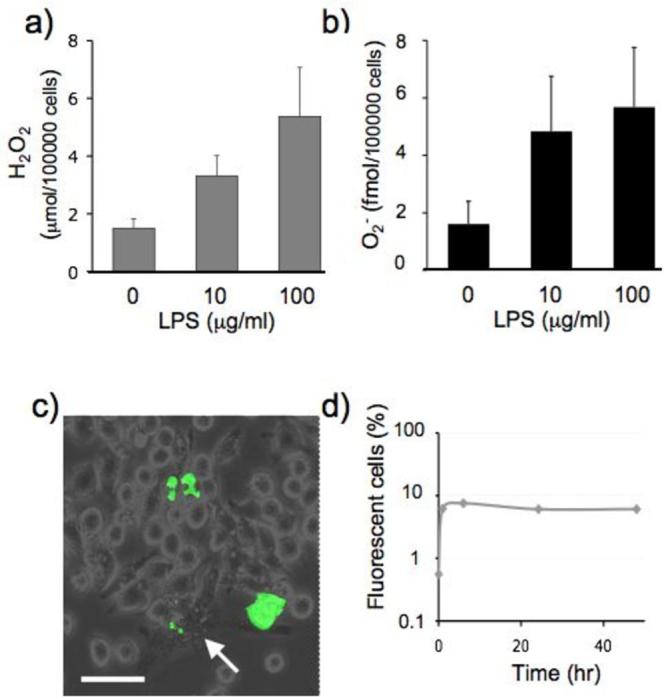
J774a.1 macrophage precursor cells were incubated for 48h in medium containing LPS at the indicated concentrations.
(a) H2O2 accumulated in 60 min was determined by microassay based on the peroxidase dependent oxidation of phenol red.
(b) Superoxide (O2−) accumulated in 60 mins was determined by O2− microassay based on the reduction of cytochrome c.
(c) Cells were loaded with the ROS reactive dye CM-H2DCFDA prior to activation with LPS (100μg/ml) and analysed by fluorescent microscopy 24h later. Following LPS activation, macrophages differentiated to produce a varied cell morphology which included a number of large and very granular cells (arrow). Fluorescent staining for ROS reflected this variation in morphology and cells displaying punctated cytoplasmic staining as well as whole cell staining were observed. Scale bar is 50μm. The fraction of ROS positive cells (d) includes all these predominant staining patterns.
To examine whether DNA was vulnerable to oxidation by endogenous ROS, we compared DNA 6-TG oxidation in J774a.1 and HCT116 cells. Cells were cultured in the presence of 6-TG for 48h, DNA was extracted and the extent of DNA substitution by 6-TG and the fraction of incorporated 6-TG that had been oxidized to GSO3 was determined by HPLC. In the ROS-producing J774a.1 cells, 0.4 ± 0.16% of incorporated 6-TG had been converted to DNA GSO3. This value is 5-fold higher than the 0.08 ± 0.05% DNA GSO3 associated with a similar level of 6-TG substitution in HCT116 cells. The increased ROS production that accompanied LPS treatment doubled the extent of DNA 6-TG oxidation in J774a.1 cells and GSO3 comprised 0.85% of incorporated 6-TG in stimulated cells (Figure 5a).
Figure 5. Oxidation of J774a.1 DNA 6-TG by endogenous ROS and sensitization to killing.
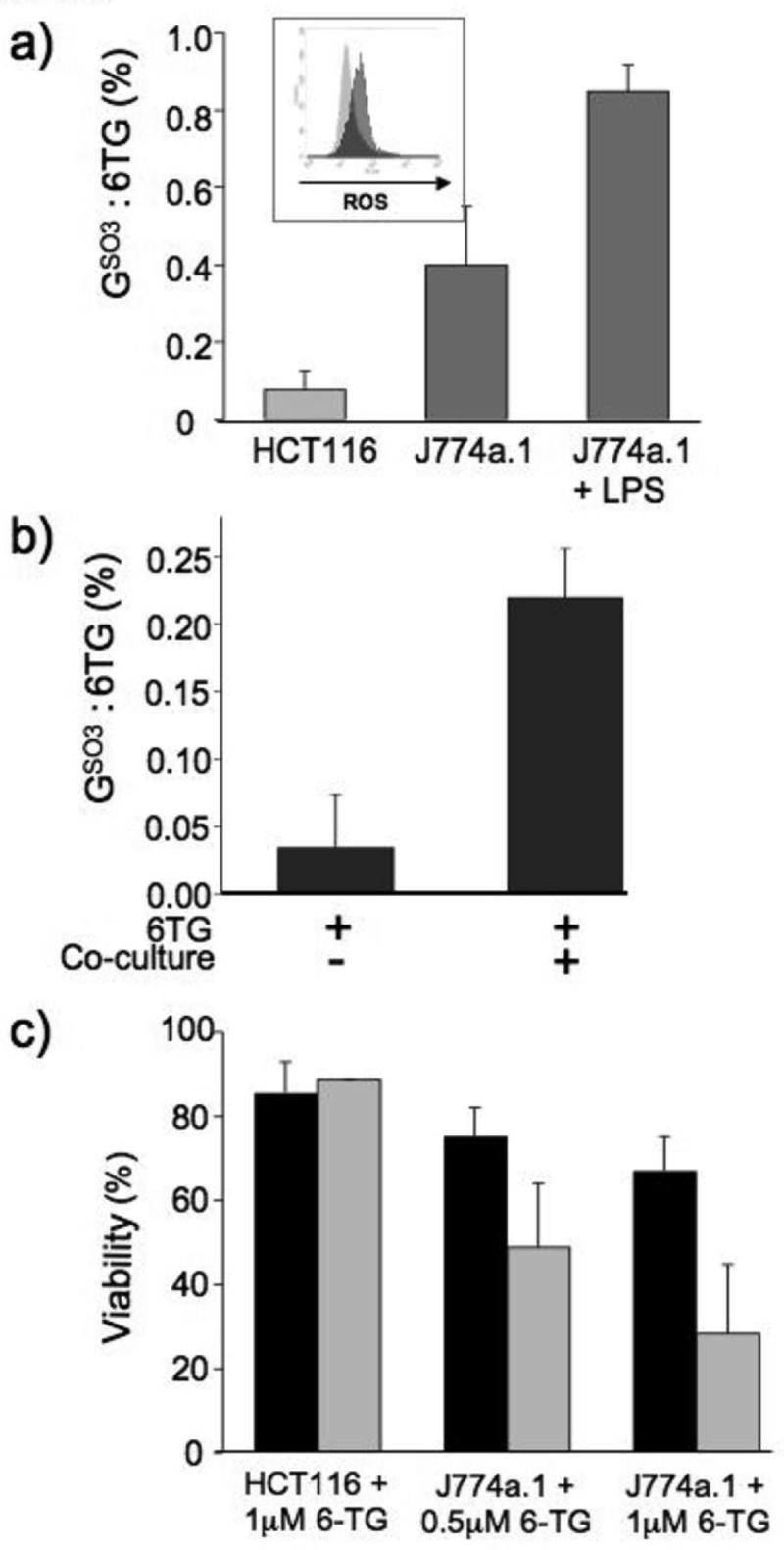
a) HCT116 or J774a.1 cells were grown for 48h in the presence or absence of LPS (100μg/ml) and 6-TG (1μM, HCT116; 0.5μM, J774a.1). DNA was extracted, digested to deoxynucleosides and separated by HPLC. 6-TGdR and GSO3dR were quantified by A342 and by fluorescence. In HCT116 cells, 6-TG replaced 0.42% DNA G. For J774a.1 cells, this value was 0.7%. Data are expressed as the percentage conversion from 6-TG to GSO3 deoxynucleoside.
Insert: ROS levels of HCT116 (light grey) or J774a.1 cells (dark grey) containing 6-TG used for the measurement of 6-TG:GSO3 described above were assessed by CM-H2DCFDA staining and FACS,
b) 6-TG treated CCRF-CEM cells (48h, 1μM) were co-incubated for further 48h with LPS-activated (100μg/ml) J774a.1 cells. DNA from non-adherent cells was extracted, digested to deoxynucleosides, separated by HPLC, and quantified as above.
c) HCT116 and J774a.1 cells were grown in the presence of 6-TG (as shown) for 48h and either left untreated (black bars) or treated with LPS (100μg/ml; grey bars) for a further 48h. Cell viability was determined by Annexin V/PI staining and FACS analysis. The data represent the mean ± SD of 3 independent experiments.
ROS produced by immune effector cells are released into the surrounding tissue. We examined whether DNA 6-TG in neighbouring cells might also be susceptible to secreted ROS. To do this, we co-cultured J774a.1 cells with CCRF-CEM leukemic cells which grow in suspension and can be easily separated from the tenaciously adherent macrophages. CCRF-CEM treated for 48h with 6-TG to replace 2-2.5% of their DNA guanine, were mixed with LPS-activated J774a.1 cells and co-cultured for a further 48h. At the end of this period, non-adherent cells were recovered and their DNA analyzed for 6-TG and GSO3. Figure 5b shows that in the absence of J774a.1 cells, 0.03% of the DNA 6-TG was present as GSO3. Following culture with activated J774a.1 cells, this value increased to 0.22%.
Cytotoxicity of ROS from immune effector cells
The accumulation of a high level of DNA GSO3 was associated with a loss of cell viability. Annexin V and propidium iodide (PI) staining and FACS analysis of J774a.1 cells that had incorporated 6-TG during 48h incubation revealed a 6-TG dose-dependent loss of viability over the subsequent 48h period (Figure 6). LPS treatment to increase the amount of DNA GSO3, significantly enhanced the cytotoxicity (Figure 5c). In contrast, the 5-fold lower level of DNA GSO3 in HCT116 was not associated with cell death and treatment of these cells with LPS did not affect cell viability. We conclude that DNA 6-TG in J774a.1 cells renders them vulnerable to their constitutively high levels of ROS and in this model macrophage, spontaneous oxidation of DNA 6-TG contributes to the cytotoxicity of 6-TG treatment. This toxic hazard is exacerbated by the increased ROS production that follows LPS activation.
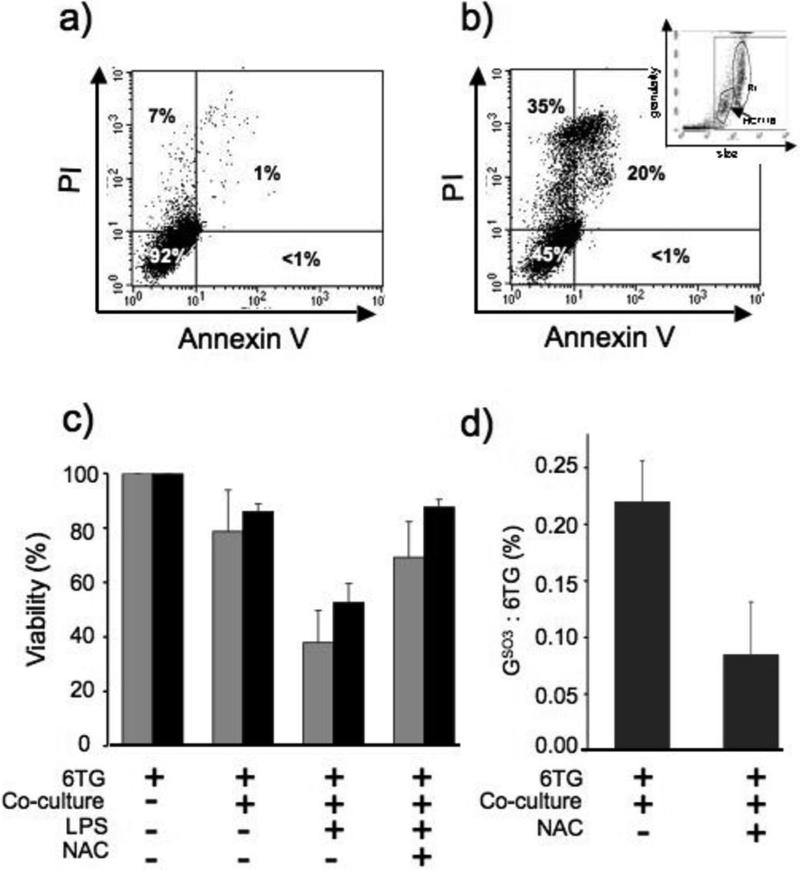
Figure 6. Cells containing 6-TG are susceptible to killing in co-culture with J774a.1.
a) Representative dot plot of 6-TG-treated HCT116 cells. Cells in the lower left quadrant (Annexin V negative/PI negative) were considered viable.
b) Co-culture. 6-TG-treated or untreated HCT116 cells were plated together with non-activated or LPS-activated J774a.1 cells. 48h later, HCT116 viability was determined by Annexin V/ PI staining and FACS. Insert: Forward (size) and side (granularity) FACS scatter plot from a mixture of HCT116 target cells and J774a. These data were used to exclude J774a.1 cells (R1) from subsequent analyses.
c) The percentage of viable 6-TG-treated HCT116 cells (grey bars) or CCRF-CEM leukemia cells (black bars) either untreated or pre-incubated with NAC (10μM, 3h) after 48h co-culture with non-activated or LPS activated J774a.1 cells. Data are from 4 independent experiments ± SD for HCT116 and 2 experiments for CCRF-CEM.
d) 6-TG treated CCRF-CEM leukemia cells were left untreated or incubated with NAC (10μM) for 3h before co-culture with LPS-activated J774a.1 cells for 48h. DNA from non-adherent cells was extracted, digested to deoxynucleosides and separated by HPLC. DNA 6-TGdR was quantified by A342 and GSO3dR by fluorescence. The data shown are the mean conversion to GSO3dR from 4 independent experiments ± SD.
To investigate the cytotoxic effects of ROS generated by immune effector cells on adjacent cells in trans, 6-TG treated HCT116 cells were co-cultured with J774a.1 macrophages. HCT116 cells with or without DNA 6-TG were plated together with J774a.1. After co-culture for 48h, all the cells were recovered and the viability of target HCT116 cells was assessed by FACS analysis with annexin V and PI staining. Figure 6a shows a representative dotplot of 6-TG-treated HCT116 cells. Negative staining for annexin V and PI confirms that the majority of these cells were viable. Co-culture with J774a.1 reduced the viability of HCT116 cells as indicated by the increased fraction of PI and/or annexin V positive cells (Figure 6b). After co-culture with J774a.1 cells, HCT116 cells were gated using forward and side scatter to exclude the larger and more granular J774a.1 cells from the analysis (Figure 6b insert). There was a small but reproducible reduction of around 20% in HCT116 viability in the presence of unstimulated J774a.1 cells and this was significantly augmented to > 60% by prior activation with LPS. Figure 6c summarizes data from four independent experiments. Qualitatively similar results were obtained with 6-TG treated CCRF-CEM cells as the target. LPS-activated J774a.1 cells reduced viability by 50% (Figure 6c).
Protection by antioxidants
Antioxidants protected DNA 6-TG containing target cells against oxidation of their DNA and against the cytotoxic effect of oxidation products. The formation of DNA GSO3 in CCRF-CEM cells co-cultured with LPS activated J774a.1 cells was largely (80%) prevented by pretreatment with N-acetylcysteine (NAC), an acknowledged ROS scavenger (Figure 6d). This protective effect was accompanied by a significant increase in cell survival and the viability of co-cultured HCT116 and CCRF-CEM cells was almost doubled by NAC treatment (Figure 6c). These observations provide confirmation of the involvement of ROS in the oxidation of DNA 6-TG and the contribution of oxidized DNA 6-TG lesions to cell death.
We conclude that cells containing DNA 6-TG are particularly vulnerable to killing by ROS produced from activated immune effector cells. This hypersensitivity affects the effector cells themselves as well as closely adjacent cells. Reduced viability is correlated with the susceptibility of DNA 6-TG to oxidation.
DISCUSSION
The cytotoxicity of 6-MP and its parent compound azathioprine, is partly mediated by metabolism into thioguanine nucleotides (TGN) (18, 19). Systemic 6-MP or azathioprine treatment causes 6-TG to accumulate in the DNA of proliferating cells and DNA 6-TG is detectable in patients' lymphocytes (4, 5) and skin (7). 6-TG is more reactive than canonical DNA bases (20) and we are interested in the implications of this reactivity for the welfare of patients undergoing long-term thiopurine therapy. DNA 6-TG is known to be cytotoxic via its interaction with DNA mismatch repair (reviewed in ref. 19). The effects of DNA 6-TG we report here were independent of mismatch repair status, however, and were similar in repair deficient HCT116 and CCRF-CEM cells and repair proficient MRC5-VA fibroblasts, two XPA fibroblast lines, and J774a.1 macrophages. Our findings reveal a new mechanism by which DNA 6-TG is hazardous to cells. Its susceptibility to ROS produced in a biological context has implications for its use as an anti-inflammatory agent.
Although DNA 6-TG levels in patients undergoing thiopurine therapy are lower that those in our cultured cells (7), they nevertheless have detectable consequences; the skin of azathioprine-treated patients is selectively hypersensitive to UVA (9). Systemic azathioprine or 6-MP treatment often continues for many months or years, entailing a concomitant risk of more subtle chronic effects. The acute UVA sensitivity of azathioprine patients' skin reflects the relatively favourable oxidation of DNA 6-TG by photochemically-generated ROS. Our present findings extend this observation and demonstrate a susceptibility to chemical oxidants H2O2 and KBrO3 both of which increase the steady-state levels of intracellular ROS. Cell death was predominantly by necrosis and both agents induced rapid replication arrest. All these observations are consistent with oxidation of cellular DNA 6-TG to GSO3 which is known to be a powerful replication block (8).
The fate of oxidized DNA 6-TG in cells is not yet established although our findings indicate that GSO3 is removed poorly from DNA and these, and other, potentially harmful oxidative DNA lesions are likely to remain in the affected cells throughout their lifetime. Oxidation products of DNA 6-TG, including DNA GSO3, are essentially iatrogenic DNA lesions of recent origin and it seems unlikely that specific DNA damage/repair responses have evolved to process them. GSO3 is bulky, causes significant destabilization of duplex DNA (8), and is therefore a potential substrate for excision by NER. DNA 6-TG produced a similar degree of sensitization to H2O2 in NER-proficient cells and XPA cells, however. Although these findings do not directly address the fate of DNA GSO3, they do indicate that NER does not efficiently excise potentially lethal oxidized 6-TG DNA adducts. Preliminary data (Feng Li, PK unpublished) confirm that DNA GSO3 formed by UVA radiation is highly persistent.
One important finding is that cells containing DNA 6-TG are also sensitive to biologically derived ROS of which immune effector cells are an acknowledged source. Sites of chronic inflammation are associated with sustained generation of ROS from the respiratory burst of infiltrating activated neutrophils and monocytes (21, 22). Macrophages are post-mitotic in vivo. Their differentiation in the presence of 6-TG would, however, lead to its incorporation during the proliferative phase of their maturation. Our findings indicate that macrophages that contain DNA 6-TG are at risk from self-inflicted DNA 6-TG oxidation and their sustained high level of endogenous ROS swiftly leads to cell death. Our co-culture experiments indicate a second potential threat of biological ROS: cells in the vicinity of activated macrophages are also at risk of DNA damage. These bystander cells also accumulated a significant burden of cytotoxic DNA GSO3 in a reaction that was prevented by free radical scavengers.
Azathioprine and 6-MP are effective immunomodulatory therapies for active IBD (23). Chronic inflammation is a predominant contributor to the development of epithelial cancers and IBD patients have an increased risk of bowel cancer (14, 24, 25). There is some evidence to suggest that azathioprine may reduce cancer risk (14) although other studies have revealed no significant effect (24-26). It is possible that the selective suicide of activated immune effector cells containing DNA 6-TG contributes to the reduction in inflammation and reduces the risk of malignancy. This protective effect may be partially offset by the consequences of accumulated irreparable DNA lesions in bystanders in the vicinity of ROS producing cells. 6-MP is used in remission maintenance regimes for leukemia. We note that because rapidly dividing tumor cells will incorporate relatively high levels of DNA 6-TG, they may have enhanced susceptibility to killing by drugs that generate ROS. The possibility that 6-TG combination therapy with this type of agent might provide a synergistic anti-cancer benefit, suggests a potential new therapeutic role for this long-established class of drugs.
In summary, cells containing DNA 6-TG are susceptible to killing by chemical oxidants. Replication blocking, potentially lethal oxidized DNA 6-TG lesions are not excised by NER and accumulate in cellular DNA. In an in vitro macrophage cell model, sustained release of H2O2 and O2− is associated with rapid and fatal accumulation of DNA GSO3. DNA 6-TG-containing target cells co-cultured with macrophages were also vulnerable to DNA oxidation and killing. In as far as this simple model mimics chronic inflammation of the gut during IBD and treatment with thiopurines, the findings suggest that the low oxidation potential of DNA 6-TG may influence the immunomodulatory effects of thiopurines and the possible long-term effects of such treatment.
REFERENCES
- 1.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease. Cancer. 2001;91:854–62. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Gaffney K, Scott DGI. Azathioprine and cyclophosphamide in the treatment of rheumatoid arthritis. Br J Rheumatol. 1998;37:824–36. doi: 10.1093/rheumatology/37.8.824. [DOI] [PubMed] [Google Scholar]
- 3.Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995;123:132–42. doi: 10.7326/0003-4819-123-2-199507150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Warren DJ, Andersen A, Slordal L. Quantitation of 6-thioguanine residues in peripheral blood leukocyte DNA obtained from patients receiving 6-mercaptopurine-based maintenance therapy. Cancer Res. 1995;55:1670–4. [PubMed] [Google Scholar]
- 5.Cuffari C, Seidmen EG, Latour S, Theoret Y. Quantitation of 6-thioguanine in preipheral blood leukocyte DNA in Crohn's disease patients on maintenance 6-mercaptopurine therapy. Can J Physiol Pharmacol. 1996;74:580–5. [PubMed] [Google Scholar]
- 6.Lehmann AR. Replication of damaged DNA by translesion synthesis in human cells. FEBS Lett. 2005;579:873–6. doi: 10.1016/j.febslet.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 7.O'Donovan P, Perrett C, Zhang X, et al. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309:1871–4. doi: 10.1126/science.1114233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Jeffs G, Ren X, et al. Novel DNA lesions generated by the interaction between therapeutic thiopurines and UVA light. DNA Repair. 2006;6:344–54. doi: 10.1016/j.dnarep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Perrett CM, Walker SL, O'Donovan P, et al. Azathioprine treatment sensitizes human skin to ultraviolet A radiation. Br J Dermatol. 2008;159:198–204. doi: 10.1111/j.1365-2133.2008.08610.x. [DOI] [PubMed] [Google Scholar]
- 10.Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–24. [PubMed] [Google Scholar]
- 11.Al-Tassan N, Chmiel NH, Maynard J, et al. Inherited variants of MYH associated with somatic G:C->T:A mutations in colorectal tumors. Nature Genetics. 2002;30:227–32. doi: 10.1038/ng828. [DOI] [PubMed] [Google Scholar]
- 12.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nature Rev Cancer. 2008;8:887–99. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 13.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–62. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 14.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Pick E. Microassays for superoxide and hydrogen peroxide production and nitroblue tetrazolium reduction using an enzyme immunoassay microplate reader. Methods Enzymol. 1986;132:407–21. doi: 10.1016/s0076-6879(86)32026-3. [DOI] [PubMed] [Google Scholar]
- 16.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. Second edition ed. ASM Press; Washington: 2006. [Google Scholar]
- 17.Vuillaume M, Calvayrac R, Best-Belpomme M, et al. Deficiency in the catalase activity of xeroderma pigmentosum cell and simian virus 40-transformed human cell extracts. Cancer Res. 1986;46:538–44. [PubMed] [Google Scholar]
- 18.Aarbakke J, Janka-Schaub G, Elion GB. Thiopurine biology and pharmacology. TIPS. 1997;18:3–8. doi: 10.1016/s0165-6147(96)01007-3. [DOI] [PubMed] [Google Scholar]
- 19.Karran P, Attard N. Thiopurines in current medical practice: molecular mehanisms and contributions to therapy-related cancer. Nat Rev Cancer. 2008;8:24–36. doi: 10.1038/nrc2292. [DOI] [PubMed] [Google Scholar]
- 20.Swann PF, Waters TR, Moulton DC, Xu Y-Z, Edwards M, Mace R. Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science. 1996;273:1109–11. doi: 10.1126/science.273.5278.1109. [DOI] [PubMed] [Google Scholar]
- 21.Dijkstra G, Moshage H, van Dullemen HM, et al. Expression of nitric oxide synthases and formation of nitrotyrosine and reactive oxygen species in inflammatory bowel disease. J Pathol. 1998;186:416–21. doi: 10.1002/(SICI)1096-9896(199812)186:4<416::AID-PATH201>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Biagioni C, Favilli F, Catarzi S, et al. Redox state and O2*- production in neutrophils of Crohn's disease patients. Exp Biol Med. 2006;231:186–95. doi: 10.1177/153537020623100209. [DOI] [PubMed] [Google Scholar]
- 23.Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50:485–9. doi: 10.1136/gut.50.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masunaga Y, Ohno K, Ogawa R, Hashiguchi M, Echizen H, Ogata H. Meta-analysis of risk of malignancy with immunosuppressive drugs in inflammatory bowel disease. Ann Pharmacother. 2007;41:21–8. doi: 10.1345/aph.1H219. [DOI] [PubMed] [Google Scholar]
- 25.Shanahan F. Relation between colitis and colon cancer. Lancet. 2001;357:246–7. doi: 10.1016/S0140-6736(00)03630-8. [DOI] [PubMed] [Google Scholar]
- 26.Matula S, Croog V, Itzkowitz S, et al. Chemoprevention of colorectal neoplasia in ulceraltive colitis: the effect of 6-mercaptopurine. Clin Gastroenterol Hepatol. 2005;3:1015–21. doi: 10.1016/s1542-3565(05)00738-x. [DOI] [PubMed] [Google Scholar]