Summary
Human Papillomaviruses (HPV) 16 is a DNA virus encoding three oncogenes – E5, E6, and E7. The E6 and E7 proteins have well-established roles as inhibitors of tumor suppression, but the contribution of E5 to malignant transformation is controversial. Using spontaneously immortalized human keratinocytes (HaCaT cells), we demonstrate that expression of HPV16 E5 is necessary and sufficient for the formation of bi-nucleated cells, a common characteristic of precancerous cervical lesions. Expression of E5 from non-carcinogenic HPV6b does not produce bi-nucleate cells. Video microscopy and biochemical analyses reveal that bi-nucleates arise through cell-cell fusion. Although most E5-induced bi-nucleates fail to propagate, co-expression of HPV16 E6/E7 enhances the proliferation of these cells. Expression of HPV16 E6/E7 also increases bi-nucleated cell colony formation. These findings identify a new role for HPV16 E5 and support a model in which complementary roles of the HPV16 oncogenes lead to the induction of carcinogenesis.
Keywords: cell fusion, cervical cancer, HPV
Introduction
Prior infection with human papillomavirus (HPV), a small, non-enveloped DNA virus, is strongly associated with the development of cervical cancer, anogenital cancer, and certain proliferative disorders (Bosch et al., 2002). Of the approximately 120 types of HPV, thirteen (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66) have been designated as “carcinogenic” by the International Agency for Research on Cancer (IARC) (Cogliano et al., 2005). Of these carcinogenic HPVs, HPV16 is the most prevalent and is detected in 57.6% of cervical cancers (Munoz et al., 2004). Despite the clear association between cervical cancer and HPV, the sequence of molecular events that follow HPV infection and lead to cervical cell transformation remain to be defined.
The ∼8000 base pair HPV genome is comprised of 8 genes. Three of these, early genes, E5, E6, and E7 have been shown to be oncogenic. E6 and E7 inhibit the function of the tumor suppressors p53 and Rb, respectively (Jones, Thompson, and Munger, 1997; Werness, Levine, and Howley, 1990). Expression of E6 or E7 enables cells to bypass normal cell cycle checkpoints and allows uncontrolled replication of cervical cells persistently infected with HPV. This process enables cells to accumulate additional genomic aberrations, but is not thought to be directly responsible (Incassati, Patel, and McCance, 2006; Plug-DeMaggio et al., 2004). It has been suggested that the development of genomic instability is secondary to inhibition of cell cycle checkpoint regulators (Duensing and Munger, 2002).
The third oncogenic protein of the HPV genome, E5, is sufficient to transform mouse fibroblasts and keratinocytes in culture, as assessed by anchorage independent growth and colony formation assays (Leechanachai et al., 1992; Pim, Collins, and Banks, 1992; Straight et al., 1993). Co-expression of E5 with either E6 or E7 potentiates the transforming properties of either protein alone (Bouvard et al., 1994; Stoppler et al., 1996; Valle and Banks, 1995). However, the mechanism by which E5 participates in transformation is unclear. The diverse functions proposed for E5 include protecting the cell against apoptosis (Kabsch and Alonso, 2002; Zhang, Spandau, and Roman, 2002), interfering with cell-cell communication (Oelze et al., 1995), and inhibition of antigen presentation in infected cells (Zhang et al., 2003). However, the most commonly accepted model is that the E5 gene product potentiates the signaling of the epidermal growth factor receptor (EGFR) by slowing EGFR endocytic trafficking and degradation (Straight, Herman, and McCance, 1995; Straight et al., 1993; Zhang et al., 2005). Identifying a molecular role for E5 in infected tissues has been difficult due to its low level of protein expression, rare integration of the E5 gene into the host chromosome, and a lack of reagents, antibodies, and animal models (Conrad et al., 1994; Disbrow, Hanover, and Schlegel, 2005; Oelze et al., 1995; Oetke et al., 2000; Straight, Herman, and McCance, 1995). The scarcity of data due to these technical difficulties has impeded the generation of a definitive model for E5 function.
In this report, we examine the effect of HPV16 E5 expression in human keratinocytes, the cell type transformed in vivo by HPV16. Expression of HPV16 E5 causes the formation of bi-nucleated cells by inducing cell-cell fusion. Normally, intrinsic cell cycle checkpoints prevent propagation of bi-nucleated cells. However, expression of HPV16 E6/E7 inhibits p53 and Rb, and thereby facilitates cell proliferation and transformation. These data support a model in which E5 plays a critical initiating role in the early stages of HPV-induced cellular transformation.
Results
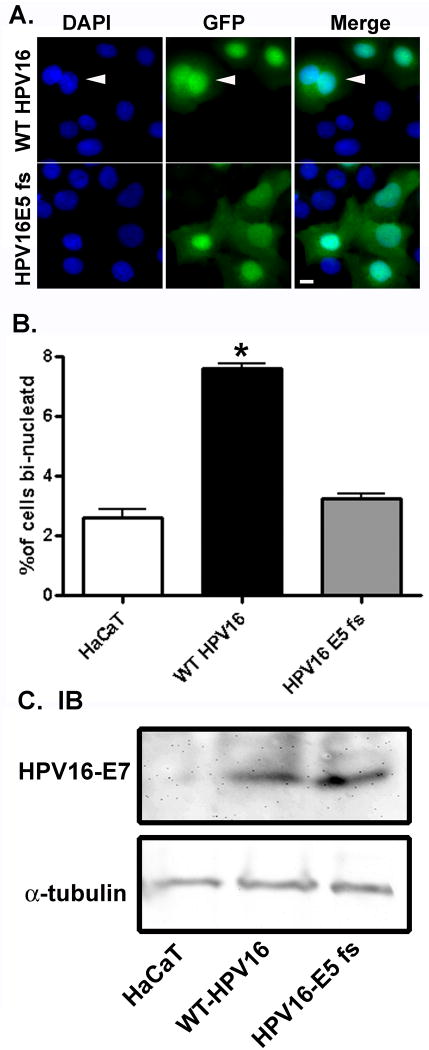
There are conflicting reports of HPV16 E5 function in the literature. To better understand the role of HPV16 E5 in the context of the whole virus, we expressed either the genome of wild type HPV16 (WT HPV16) or HPV16 with a frame shift mutation in the E5 gene (HPV16 E5 fs) in HaCaT cells, a spontaneously immortalized human keratinocyte cell line (Boukamp et al., 1988). This frameshift mutant only expresses the first 11 amino acids of the protein, and this E5 fragment is not a functional protein (Genther et al., 2003). There were striking differences in the cell morphology following expression of WT HPV16 and HPV16 E5 fs (Figure 1A). Cells expressing WT HPV16 had more than three times the number of bi-nucleated cells as those expressing HPV16 E5 fs mutation (Figure 1B) despite similar levels of the HPV16 genome being expressed (Figure 1C).
Figure 1. Expression of the wild type HPV16 genome, but not HPV16 with an E5 frameshift mutation, causes the formation of bi-nucleated cells.
A) HaCaT cells were co-transfected with 2 μg of pEGFP-C1 and 2 μg of linearized WT HPV16 or HPV16 E5 fs by nucleofection. Following recovery of the cells (72 hours), cells were fixed, stained with DAPI, and examined by fluorescent microscopy. Shown are representative images of DAPI stained nuclei, GFP-positive (transfected) cells, and the merger of the two images. Arrow indicates a bi-nucleated cell. Size bar = 10 μm. B) Quantification of the percentage of bi-nucleated cells (n=3). Data are plotted as the average ± S.E.M. of percentage of bi-nucleated cells following transfection with nothing, wild type HPV16, or HPV16 with an E5 frameshift mutation. * indicates p-value < 0.01 (two tailed student's t-test) C). Cell lysates from the experiment described in A were collected, resolved by 16% Tris-tricine gel, and immunoblotted for HPV16 E7 (Zymed).
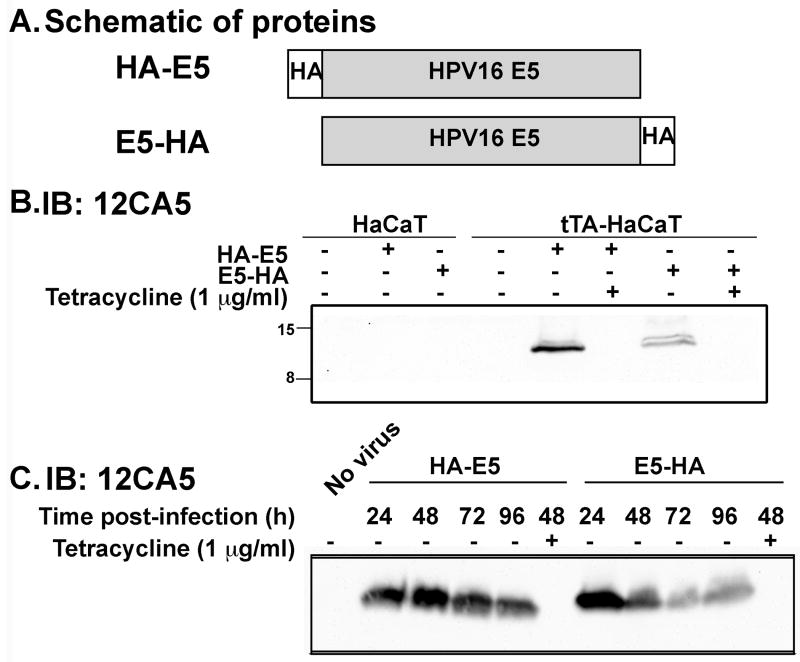
To better study the function of HPV16 E5, we generated tetracycline-regulatable adenoviruses that express hemagglutinin (HA) tagged HPV16 E5. The cDNA encoding the HA epitope allows detection of the exogenous HPV16 E5 since antibodies to HPV16 E5 itself are not available. To enhance protein expression, codons of the HPV16 E5 cDNA were optimized to the tRNAs that are prevalent in mammalian cells (Disbrow et al., 2003). The HA epitope was engineered onto either the 5′ or 3′ end of the HPV16 E5 cDNA and the resulting proteins were designated as HA-E5 and E5-HA, respectively (Figure 2A). HaCaT cells were stably transfected with the tetracycline transactivator (tTA-HaCaT) to produce a “tet-off” system (Gossen and Bujard, 1992). tTA-HaCaT cells were infected separately with each E5-encoding adenovirus. Infection with either adenovirus resulted in E5 protein expression in ∼90% of cells by 24 hours post-infection. The addition of tetracycline to infected cells abrogated expression of the E5 protein (Figure 2B) and served as a control for any potentially deleterious effects of the adenovirus. This model system allows the examination of the functional consequence of E5 expression for up to 96 hours (Figure 2C).
Figure 2. Expression of Hemaggluttinin-tagged HPV16 E5 by adenovirus is tetracycline regulatable.
A) Hemaggluttinin (HA) tagged HPV16 E5 proteins used in this study. B) Parental HaCaT and tTA-HaCaT cells were uninfected or infected with the indicated adenoviruses (20 plaque forming units/cell) in the absence (-) or presence (+) of tetracycline (1 μg/ml). Twenty-fours hours after infection, cell lysates (30 μg) were resolved on a 16% Tris-tricine gel and immunoblotted with the 12CA5 (anti-HA) mouse monoclonal antibody. C) tTA-HaCaT cells were infected with nothing, HA-E5, or E5-HA in the absence (-) or presence (+) of tetracycline (1 μg/ml). Cells lysates were collected at the indicated times post infection (24, 48, 72, 96 hours) and processed as in (B).
Expression of HPV16 E5 increases the percentage of bi-nucleated cells
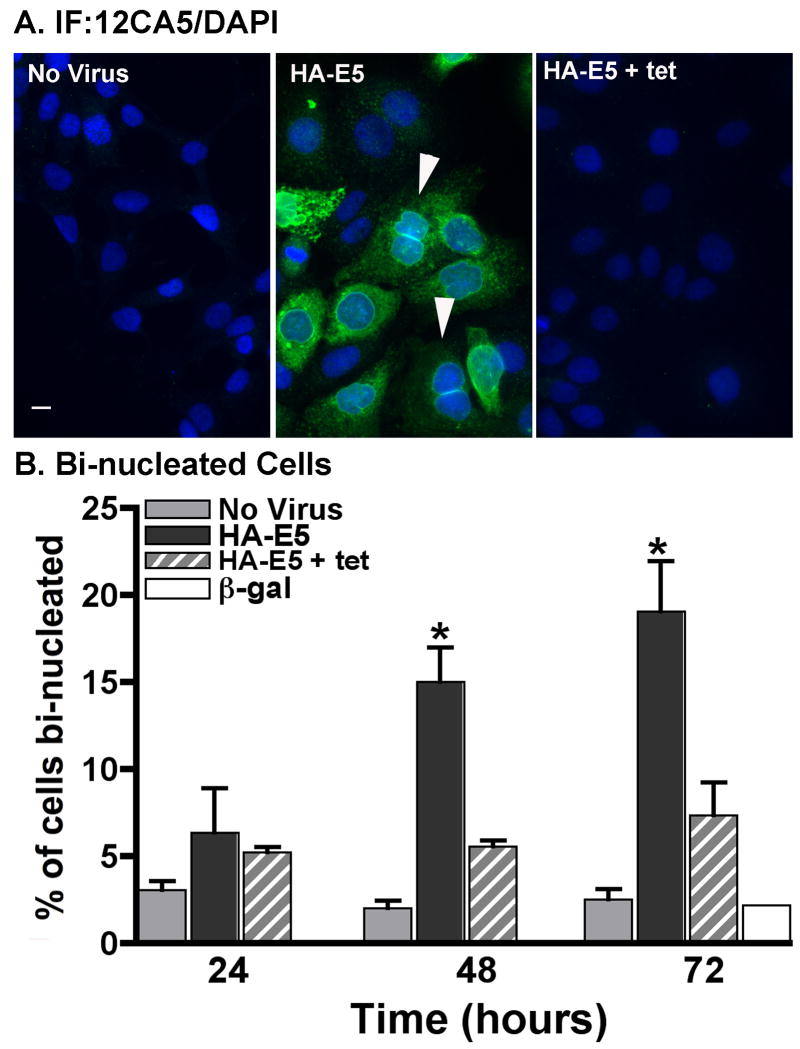
Consistent with our observation following expression of the WT HPV16 genome, expression of HPV16 E5 alone caused an increase in bi-nucleated cells. The number of bi-nucleate cells increased 3-fold within 72 hours of E5 expression as compared to time-matched controls (Figure 3). Cells infected with adenovirus in the presence of tetracycline to inhibit HA-E5 expression did not give rise to bi-nucleated cell, nor did cells infected with a β-galactosidase encoding control adenovirus. Similar increases in bi-nucleation were seen with expression of HPV16 E5-HA (data not shown).
Figure 3. Expression of HPV16 E5 increases the percentage of bi-nucleate cells.
A) tTA-HaCaT cells were infected with nothing or HA-E5 in the absence or presence of tetracycline (tet) as indicated. Cells were fixed and stained by indirect immunofluorescence with the 12CA5 mouse monoclonal antibody and nuclei were stained with DAPI. Arrow indicates a bi-nucleated cell. Images were collected using Olympus AX70 epifluorescent microscope with Q-Capture software. Size bar is 10 μm. B) The percentage of bi-nucleate cells treated as in (A). Shown is the average percentage ± S.E.M. of bi-nucleate cells from three independent experiments (≥500 cells/experiment). * indicates a p-value of <0.05 (two-tailed, unpaired t-test) as compared to time-matched cells infected in the presence of tetracycline.
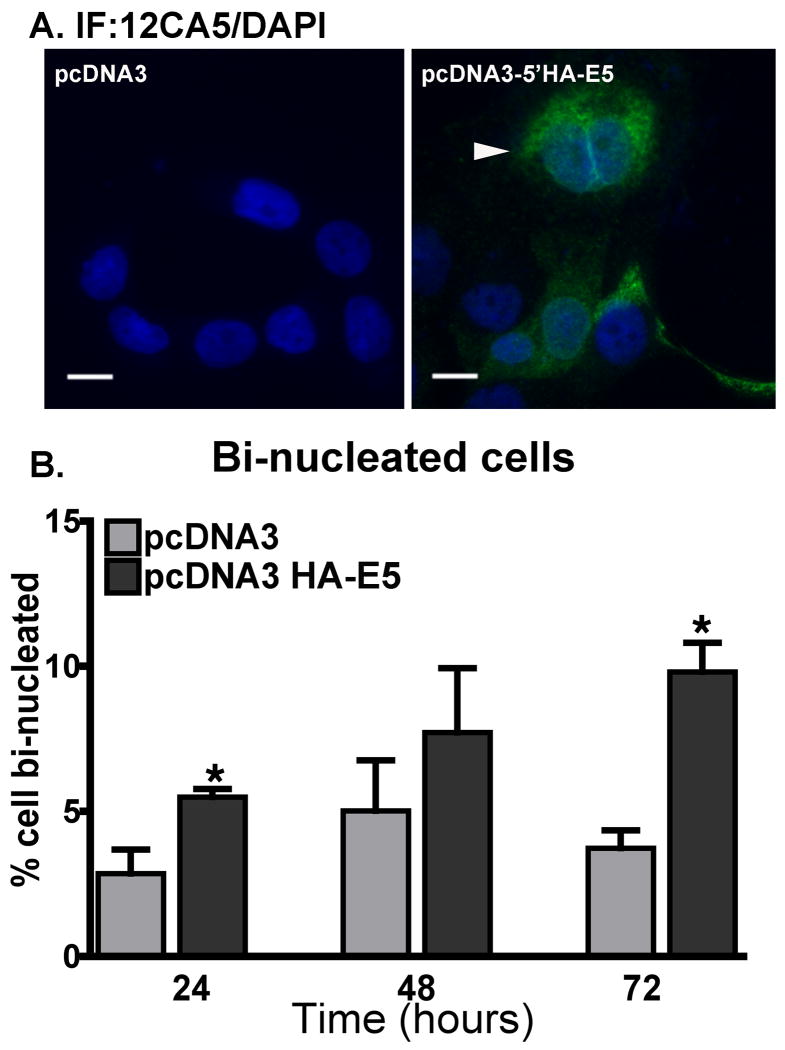
To ensure that the increases in bi-nucleated cells were not a by-product of adenovirus infection, we transfected cells with a plasmid encoding HPV16 HA-E5. Bi-nucleate cells were formed in HaCaT cells following transient transfection with plasmids encoding HA-E5 (pcDNA3- HA-E5), but not with the parental plasmid alone (Figure 4).
Figure 4. Transfection of cells with HA-E5 causes an increase in the percentage of bi-nucleate cells.
A plasmid encoding HA-E5 under the direction of a CMV promoter (pcDNA3-HA-E5) or empty vector (pcDNA3) was introduced into cells by nucleofection (Amaxa Biosystems, Gaithersburg, MD). A) Transfected cells were fixed and stained by indirect immunofluorescence using the 12CA5 antibodies and an Alexa488-labeled goat anti-mouse secondary antibody. Cell nuclei are labeled with DAPI. Arrow indicates a bi-nucleated cell. Size bar = 10 μm B). The percentage of HA-E5 transfected cells that are bi-nucleate. Transfected cells that were fixed and stained as in (A) were scored for bi-nucleation. Data are plotted as the average ± S.E.M. from 3 experiments from at least 500 cells per experiment. * p-value <0.05 (two tailed, unpaired t-test) as compared to cells transfected with empty vector.
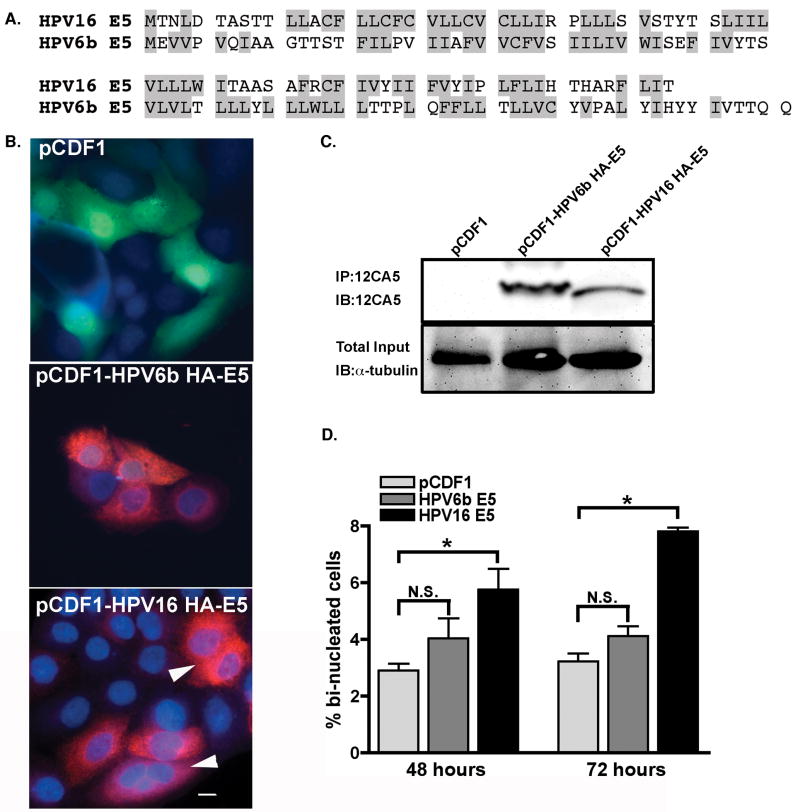
To determine whether the increase in the percentage of bi-nucleated cells was specific for HPV16 E5 or could arise following the expression of E5 from any HPV, we examined the cell morphology of HaCaT cells following expression of HPV6b E5. HPV6b is a non-carcinogenic HPV, but its E5 protein contains a similar percentage of hydrophobic amino acids as the E5 from HPV16 (61% and 58%, respectively) (Figure 5A). The HPV6b E5, like the HPV16 E5, was codon-optimized to enhance protein expression in mammalian cells. When HaCaT cells were transfected with a plasmid encoding HPV6b E5 (pCDF1-HPV6b-HA-E5), the percentage of bi-nucleated cells was comparable to cells transfected with empty plasmid (Figure 5B, and 5D), in contrast to HaCaT cells transfected with a plasmid encoding HPV16 HA-E5 (pCDF1-HPV16 HA-E5) (Figure 5B and 5D).
Figure 5. Expression of the E5 protein from a non-carcinogenic HPV does not cause an increase in the percentage of bi-nucleate cells.
A) Single letter amino acid code representation of the sequences of the E5 proteins from HPV16 (Seedorf et al., 1985) and HPV6b (Schwartz et al., 1983). Hydrophobic amino acids are highlighted in grey. B) Shown are representative micrographs from HaCaT cells transfected with empty vector (pCDF1-MCS2-EF1-copGFP – subsequently referred to as pCDF1), the HA-tagged HPV6b E5, or HA-tagged HPV16 E5. After transfection (48 hours), the cells were fixed, subjected to indirect immunofluorescence using the 12CA5 (anti-HA) antibody and Alexa568-goat anti-mouse secondary antibody (red) to indicate the presence of the HA-tagged E5 protein. Nuclei were stained with DAPI (blue). Cells transfected with empty vector are green, indicating the expression of the pCDF1 associated green fluorescent protein (GFP). Arrow indicates a bi-nucleated cell. Size bar = 10 μm. C) Cells were transfected as in (B). Cell lysates were prepared and quantitatively immunoprecipitated using the 12CA5 antibody. Immunoprecipitates were resolved by SDS-PAGE and immunoblotted with the 12CA5. Total starting materials was probed with an anti-α-tubulin antibody (Sigma) to indicate amounts of starting material. D) The percentage of bi-nucleated cells was calculated from cells transfected as in (B). The average percentage of bi-nucleate cells ± S.E.M. from >200 cells/experiment is plotted (n =3). N.S. indicates the data are not significantly different compared to vector control. * indicates a p value <0.01 compared to vector control (two-tailed, unpaired t-test).
HPV16 E5 causes bi-nucleated cells by inducing cell-cell fusion
Cells can become bi-nucleated if they fail cytokinesis or by cell-cell fusion. To determine whether E5 caused aberrant cytokinesis, we used video microscopy to monitor cells as they progressed through mitosis. More than 40 cells expressing HPV16 E5 were filmed and all underwent normal cytokinesis (data not shown). Thus, we concluded that the bi-nucleate cells induced by E5 do not appear to be a consequence of abortive cytokinesis.
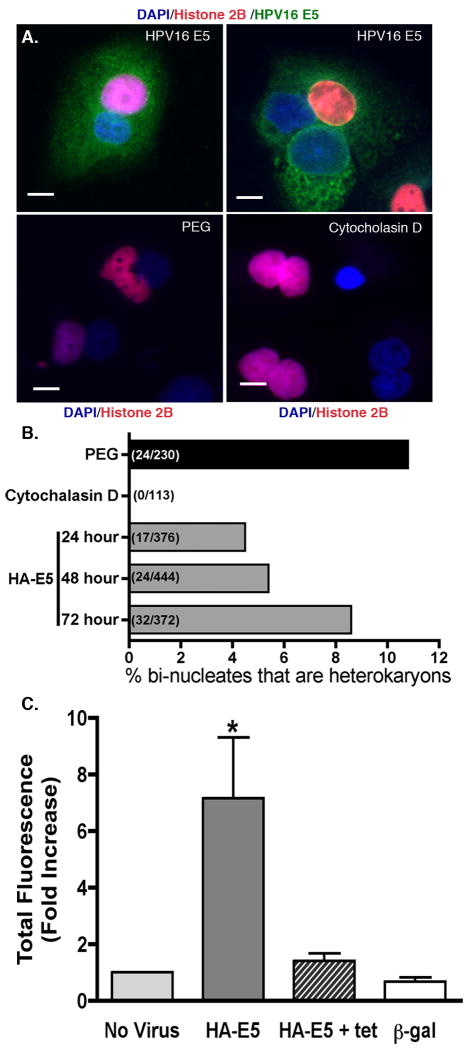
However, during the live cell analysis, we observed HPV16 E5-positive cells undergoing cell fusion (Supplemental Figure 1). To quantify the percentage of cells that fused, tTA-HaCaT cells were transfected with a plasmid encoding Red Fluorescent Protein-tagged histone H2B (pRK7-H2B-RFP). These cells were mixed with untransfected cells in a 1:1 ratio. The mixed culture was infected with an adenovirus expressing HPV16 E5, and incubated for 24-72 hours. At 24-hour intervals, the cells were fixed, permeabilized, and DAPI stained. Bi-nucleate cells were analyzed by epifluorescent microscopy for the presence of RFP-H2B labeled and unlabeled nuclei. Bi-nucleate cells that arose due to failed cytokinesis would contain two labeled or two unlabeled nuclei as shown with cells whose cytokinesis is inhibited by treatment with cytochalasin D (Figure 6A) (or latrunculin B – data not shown). The presence of heterokaryons, cells containing multiple, genetically different nuclei, would indicate cell fusion. As a positive control for cell fusion, we treated labeled and unlabeled cells with polyethylene glycol (PEG 8000) (Figure 6A). As seen in our video microscopy studies, cells expressing HPV16 E5 gave rise to heterokaryons, indicating cell fusion had occurred (Figure 6A). At 72 hours, 8.6% of all bi-nucleate cells were heterokaryons (Figure 6B).
Figure 6. Expression of HPV16 E5 causes cell fusion.
A) Equal numbers of tTA-HaCaT cells were transfected by nucleofection (Amaxa Biosystems) with either nothing or pRK7-histone2B-RFP and mixed together at a 1:1 ratio. After recovery (24 hours), cells were either infected with HA-E5 adenovirus or incubated with 50% polyethylene glycol (PEG 8000) or 0.6 μg/ml cytochalasin D. After incubation (5 minutes for PEG treatment, 48 hours for HA-E5 and cytochalasin D), cells were fixed, and stained with DAPI. Bi-nucleated cells were analyzed by fluorescent microscopy for the presence of heterokaryons. Shown are representative images of bi-nucleate cells indicating the fusion of two different cell populations in HA-E5 expressing cells and PEG treated cells. A failure of cytokinesis was induced by treatment with cytochalasin D. All resulting bi-nucleated cells had similarly labeled nuclei. Size bar = 10 μm. B) Graphical representation of the percentage of hetrokaryons in the population of bi-nucleate cells (n=2-3). The number of heterokaryons per total bi-nucleated cells is indicated parenthetically in each bar. C) tTA-HeLa cells were transfected with either pRK7-T7 RNA polymerase or YFP under the control of the T7 promoter (pT7-YFP), co-plated, infected with HPV16 HA-E5 adenovirus, incubated for 72 hours, and analysed for the presence of YFP by FACS (see Materials and Methods for details). Data indicate the fold increase in fluorescence intensity of the YFP-positive cells (calculated from the percentage of YFP positive cells multiplied by the mean fluorescent intensity) as compared to the ‘No virus’ control. Shown are the mean ± S.E.M. (n=3-5). * = p < 0.05 (two-tailed, unpaired t-test). Representative FACS data are shown in Supplemental Figure 2.
The observed percentage of heterokaryons is significantly less than the predicted 1:2:1 ratio of unlabeled bi-nucleate cells:heterokaryons:labeled bi-nucleate cells. However, data from cells that were fused by PEG indicated that other factors are likely affecting cell fusion. One explanation may be the transfection efficency of H2B-RFP. Alternatively, after a cell divides, the close proximity of the recently formed daughter cells makes them more likely to fuse with one another. Either event would skew the prediction of a 1:2:1 ratio that is based on the assumption that each cell has an equal number of neighboring labeled and unlabeled cells. Importantly, the inhibitors of cytokinesis, latrunculin B (not shown) and cytochalasin D (Figure 6B) produced no heterokaryons.
As a third independent method of assessing whether cell fusion occurred, we developed a quantifiable cell fusion assay. Using tTA-HeLa cells, separate populations of cells were transfected with either a plasmid encoding T7 RNA polymerase (pRK7-T7 RNA polymerase) or a plasmid encoding yellow fluorescence protein (YFP) driven by a T7 promoter (pT7-YFP). Only when the cells fuse together is the YFP produced. Using this assay, we are able to monitor the formation of fused cells over time as well as rapidly quantify the number of YFP-positive cells by FACS.
In cells that express HPV16 HA-E5, there is an increase in the percentage of YFP positive cells as well as an increase in the mean fluorescent intensity of those cells (See Supplemental Figure 2). Shown in Figure 6C, there is a 7-fold increase in total YFP production (percentage of YFP positive cells multiplied by mean fluorescent intensity) as compared to cells that were uninfected, infected with HPV16 HA-E5 in the presence of tetracycline, or infected with a control β-galactosidase adenovirus. These experiments corroborate the video microscopy findings (Supplemental Figure 1) and confirms the observation that HPV16 E5 is a fusogenic agent. Furthermore, these data establish that the ability of HPV16 E5 to induce cell-cell fusion is not restricted to HaCaT cells, as HPV16 E5 also induces the fusion of HeLa cells.
Most bi-nucleated cells fail to propagate
Cell-cell fusion has been proposed as an initiator of carcinogenesis, and is a feature of many oncogenic viruses (Duelli and Lazebnik, 2007). Models implicating a role for cell-cell fusion in carcinogenesis suggest that the vast majority of the bi-nucleated cells that arise from cell-cell fusion become quiescent or apoptotic. Those that escape cell cycle arrest or cell death and replicate, have an increased propensity to become aneuploid, presumably because the cell is not equipped for processing twice the normal number of chromosomes and over time segregation errors occur. The likelihood of these errors to go undetected is enhanced when cell cycle checkpoints are disrupted.
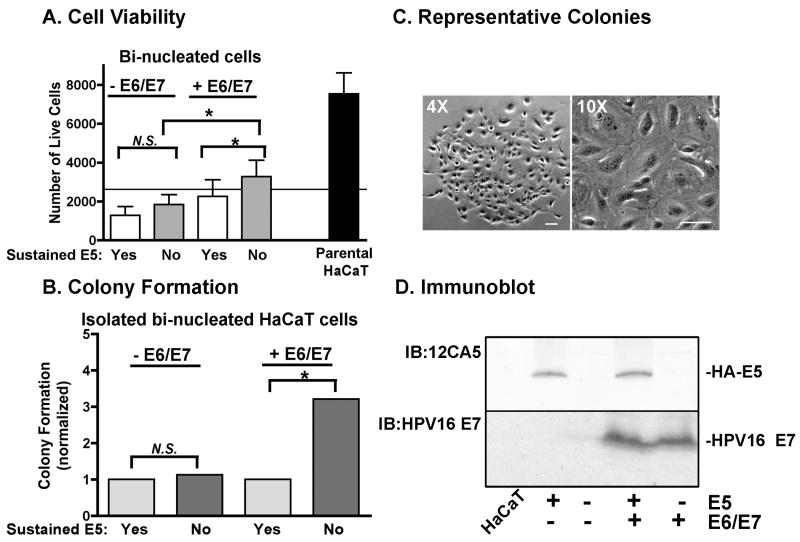
To determine if the bi-nucleated cells that formed in response to HPV16 E5 expression acted in accordance with these models, we monitored cell viability and transformation. Using our YFP-reporter assay, cells that had undergone cell-cell fusion were collected by FACS, re-plated in growth media, and were assayed for cell viability by an MTT assay (Figure 7A). In contrast to the parental, mono-nucleated HaCaT cells that grew 3-fold over 72 hours, there was a reduced viability of bi-nucleated cells. Bi-nucleated cell viability increased slightly if expression of HPV16 HA-E5 was ablated after cell-cell fusion by the addition of tetracycline. We inhibited expression of HPV16 E5 because there are numerous reports in the literature that its expression is transient (Pater and Pater, 1985; Schwartz et al., 1985; Stoler et al., 1992). To assess the contribution of other HPV16 oncogenes, we co-transfected the cells with p1321 HPV-16 E6/E7, a plasmid that expresses HPV16 E6/E7 from the human β-actin promoter (Munger et al., 1989). HPV16 E6/E7 impede cell cycle checkpoint inhibitors (p53 and Rb) and permit propagation of cells that would otherwise be growth arrested. Bi-nucleated cells that no longer express HPV16 HA-E5, but do express HPV16 E6/E7 had more viable cells than the original plating density, but this cell growth was not statistically significant.
Figure 7. Bi-nucleated cells have increased colony formation in the presence of HPV16 E6/E7.
tTA-HaCaT cells were transfected with pRK7-T7 RNA polymerase or pT7-YFP, mixed in a 1:1 ratio, and infected with HPV16 HA-E5 adenovirus. Some cells were co-transfected with p1321 HPV-16 E6/E7 (+E6/E7). After infection (72 hours), YFP-positive, bi-nucleated cells were collected by FACS and plated for MTT assay, colony formation assay, or cultured to collect cell lysate. Parental, mononucleated tTA-HaCaT cells were plated in parallel. To sustain HPV16 HA-E5 expression (indicated by “yes”), cells were growth without tetracycline; to abrogate HPV16 HA-E5 expression (indicated by “no”), cells were grown with tetracycline. A) Cells were plated at 2500 cells/well of a 96-well dish (plating density is indicated with solid line) and grown for 72 hours in growth media. Cell viability was measured by MTT assay. Data are plotted as the average number of cells/well (n=4). * indicates p <0.05 in paired student's t-test. B) Cells were plated at 500 cells/well of 6 well dish (6 wells/condition in each assay). Cells were grown for 21 days with media changes every 3 days. Colonies were stained with methyl violet. Only colonies visible to the naked eye were counted (colonies contained >100 cells/colony). Data are normalized to growth without tetracycline for each transfection condition. (n=6). * indicates p<0.01 using chi square analysis. C) Representative images of a colony at 4× and 10× magnification (size bar = 100μm). D) Parallel dishes of cells were grown with and without tetracycline for 72 hours. Cell lysates were prepared, resolved on a 16% Tris-tricine gel, and immunoblotted for either HA-E5 (12CA5 antibody) or HPV16 E7. Since HPV16 E6 and E7 are driven by the same promoter on the same plasmid, expression of HPV16 E7 was interpreted as expression of both HPV16 E6 and E7.
To determine whether fused cells had an increased propensity to be transformed, we monitored the ability of cells to form colonies on plastic, a measure of cell transformation (Liu et al., 2000). Ablation of HPV16 HA-E5 in concert with continual expression of HPV16 E6/E7 produced three times the number of colonies observed with constitutive HPV16 HA-E5 expression (Figure 7B). In the absence of HPV16 E6/E7, colony growth was not affected by the continual presence of HPV16 HA-E5. Colonies that formed were comprised primarily, although not exclusively, of mono-nucleated cells (Figure 7C). This indicates that the bi-nucleated phenotype can be lost with cell division.
Together, these data provide evidence that while most bi-nucleated cells formed as the result of HPV16 E5 expression cannot proliferate, the coordinated expression of HPV16 E6/E7 promotes some cells to overcome the barriers of quiescence and/or apoptosis and lead to cell transformation.
Discussion
Although it is widely held that particular types of HPV are the causative agents of cervical carcinoma, a major unresolved issue in the molecular etiology of this disease has been the role and contribution of the virally-encoded E5 protein. In the work reported here, we have identified a novel function for HPV16 E5 as an inducer of cell fusion. Importantly, we provide corroborating evidence for how this fusogenic property of E5 cooperates with HPV16-encoded E6 and E7 to promote the initiation of cell transformation. In addition, we provide a cellular rationale for how the transient and limited E5 expression observed in patient samples could promote carcinogenesis.
HPV16 E5 forms of bi-nucleated cells that do not form with expression of E5 from the non-oncogenic, HPV6b E5. Three independent lines of evidence establish that HPV16 E5 causes bi-nucleate cell formation by inducing cell-cell fusion. First, live-cell video microscopy reveals HPV16 E5 expressing cells can fuse and bi-nucleate cell formation did not result from abortive cytokinesis (Supplementary Figure 1). Second, using a heterokaryon fusion assay in which a population of cells expressing RFP-H2B was mixed with a population of unlabeled cells, we observed the presence of bi-nucleate cells containing differentially-labeled nuclei (Figure 6A and 6B). Third, using a fluorescent reporter assay in which the expression of YFP could only be detected if a cell transfected with a plasmid encoding T7 RNA polymerase fused with a cell transfected with a plasmid encoding YFP under the control of the T7 promoter. Only mixed populations of cells expressing HPV16 E5 produced YFP (Figure 6C). Consistent with all of this cell fusion data, we also found that bi-nucleated cells were produced with greater than a 3-fold frequency upon introduction of the intact HPV16 genome into HaCaT cells as compared to cells transfected with an HPV16 genome harboring a mutant E5 gene encoding a truncated, nonfunctional E5 protein.
Cell-cell Fusion in Biology and Pathology
Cell-cell fusion has been reported to occur naturally during a number of developmental processes (i.e. skeletal and cardiac muscles) (Chen and Olson, 2005). Recently, there has been increased interest in the role of cell-cell fusion in oncogenesis, particularly fusogenic events mediated by viruses. Many oncogenic viruses, such as Hepatitis B virus, Hepatitis C virus, Karposi sarcoma virus, and Epstein-Barr virus, mediate cell-cell fusion and this fusogenic activity has been posited to be a key initiating event in the development of cancer (Duelli and Lazebnik, 2007). The fact that the carcinogenic HPV16 encodes a protein that causes cell-cell fusion is not entirely surprising, although the assignment of this role to E5 is novel. Further, this finding correlates well with the presence of bi-nucleated cells in pre-cancerous cervical lesions (Mittal, Chan, and Demopoulos, 1990; Prasad et al., 1993).
HPV16 E5 has many of the features common to fusogenic proteins. It is a class I integral membrane proteins with a high degree of hydrophobicity, and can undergo oligermerization (Gieswein, Sharom, and Wildeman, 2003; Martin and Ryuysschaert, 2000). Hydropathy profiles of the E5 gene products from all HPVs reveal that the proteins can be phylogenetically and biochemically divided into four major groups (E5α, β, δ, and γ) (Bravo and Alonso, 2004). The E5 proteins from all 13 carcinogenic HPVs belong to the E5α group that shares the common structure of three hydrophobic domains. HPV16 E5 is comprised of three hydrophobic stretches of 15-22 amino acids, although only the first is predicted to be long enough to span the plasma membrane. In contrast, HPV6b E5 is a member of the E5γ group, and has two longer hydrophobic stretches (30 and 40 amino acids each). For HPV16 E5, the carboxyl and amino termini are hydrophilic, and thought not to be associated with the plasma membrane, although the exact orientation is not known (Nath et al., 2006). Immunofluorescence analysis of non-permeabilized cells revealed that HPV16 E5 localizes to the plasma membrane1. Given the data indicating that HPV16 E5 can mediate cell-cell fusion, the three hydrophobic domains may be a necessary structural feature that confers fusogenic properties to E5s. Consistent with the idea that there is a structural component to HPV16 E5 mediated cell fusion is the observation that the comparably hydrophobic, but structurally distinct, HPV6b E5 does not cause cell-cell fusion.
Role for cell Fusion in HPV-mediated Carcinogenesis
E5 expression is believed to be transient and limited following the infection of cervical epithelium by HPV. This notion stems from the kinetics of E5 mRNA levels in tissue samples and the observation that E5 expression is not observed in malignant cervical tumors (Schwartz et al., 1985; Stoler et al., 1992). The failure of the HPV16 E5 gene to integrate into the host cell's genome (Schwartz et al., 1985) suggests sustained expression of E5 is not necessary. Finally, numerous HPV-positive cell lines fail to generate E5 mRNA at detectable levels (Pater and Pater, 1985; Schwartz et al., 1985). Of note, E5 mRNA is the best estimate of E5 expression given the absence of antibodies to detect the protein. These observations coupled to our novel findings that E5 is a fusogen (Figures 6 and 7) and that sustained E5 expression compromises cell viability (Figure 7), have led to our model that transient E5 expression favors cellular transformation whereas sustained E5 expression likely leads to multiple cell fusion events and cell death (Figure 7). Thus, our data provide the first experimental rationale for transient expression of E5 that apparently occurs in HPV-infected patients.
Experimental evidence for cell fusion contributing to carcinogenesis comes from a recent paper by Duelli et al. in which they demonstrate that cell fusion is sufficient to induce chromosomal instability (Duelli et al., 2007). The authors show that if cell-cell fusion occurs concomitantly with oncogene expression and cell cycle deregulation, tumor formation can result. The authors speculate that if such a fusogenic agent were expressed in the presence of HPV16 E6 and E7, that could explain how cervical cancer is induced.
Progression of an HPV-infected cell to a tumor is a rare event that occurs over a long timeframe. Neoplastic progression is associated with cells containing an abnormal complement of DNA and a dysregulated cell cycle which enables them to divide despite their detachment from basement membrane. Progression to fully evolved cancer would require the accumulation of additional key abnormalities; events that likely occur infrequently. Based on our data and reports in the literature, we have developed the “hit and run” model for the contribution of E5 to the initiation of tumorigenesis in the context of an HPV infection. The salient features of this model are: (1) cervical epithelial cells are infected with HPV and the HPV genome is episomally maintained. (2) The “hit” - E5 mRNA levels peak and expression of the E5 protein leads to cervical cell-cell fusion and the accumulation of tetraploid and polyploid cells. (3) The resulting genomic insult triggers the stabilization and activation of p53. (4) The expression of E5 is downregulated – the “run”. (5) The expression of E6 promotes the ubiquitin-dependent degradation of p53, thus enabling the cells to overcome the gatekeeper function of p53 which functions to arrest genomically-compromised cells. (6) Expression of E7 blocks Rb thereby preventing terminal differentiation of the infected epithelial cells. Collectively then, the expression of E5, E6, and E7, produces cells that (i) have an abnormal amount of DNA due to cell-cell fusion, (ii) are defective in cell cycle regulation, and (iii) are no longer properly programmed to terminally differentiate.
Data presented in Figure 7 support this model. By specifically examining bi-nucleated cells derived following E5 expression, we were able to assess the interplay of HPV16 E5, E6, and E7 on cell viability and transformation. Under all conditions, bi-nucleated cells fail to propagate over the course of 72 hours (Figure 7A), although cell viability was increased by attenuating expression of HPV16 E5 or increasing expression of HPV16 E6/E7. Although few bi-nucleated cells form colonies, the likelihood for colony formation increases 3-fold when expression of HPV16 HA-E5 is temporally regulated and the E6/E7 oncogenes are expressed. Further, the colonies that arise from these bi-nucleated cells consist mainly of mono-nucleated cells indicating the bi-nucleated cells can divide when HPV16 E6/E7 are present.
Unlike the other two HPV16 oncogenes, E6 and E7, which cause unregulated cell proliferation, our findings indicate that E5 induces bi-nucleated cell formation, a characteristic of cervical cancer. These results support a role for HPV16 E5 inducing bi-nucleated cells that may promote genomic instability in subsequent rounds of cell division. These data provide the groundwork for a plausible mechanism by which E5, E6 and E7 work in concert to increase the likelihood of cellular transformation.
Materials and Methods
Generation of tTA-HaCaT cells
HaCaT cells were retrovirally infected with the tetracycline transactivator (tTA) (Clontech Retroviral GeneTransfer and Expression System). Neomycin resistant colonies were grown in 400 μg/ml G418. Individual colonies were isolated, amplified, and screened based on their ability to regulate expression of tetracycline-regulatable adenovirus. tTA-HeLa cells were generously obtained from Dr. Sandra Schmid (The Scripps Research Institute).
Parental HaCaT and tTA-HaCaT cells were maintained in Dulbecco's Minimal Essential Media (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, 100 units/ml streptomycin, and 2 mM glutamine. tTA-HeLa cells were maintained in the same media but with 5% FBS. Cells stably transfected with tTA were maintained in 400 μg/ml G418. All three cell lines were maintained at 37°C in 5% CO2.
Adenovirus generation/expression
Codon optimized HPV16 E5 was generated as previously described (Disbrow et al., 2003). A hemagglutinin (HA) epitope (MEYDVPDYAH) was engineered to be expressed on either the amino (HA-E5) or carboxyl (E5-HA) terminus of the protein by PCR, making the appropriate modification of stop codons. Adenoviruses were generated using Clontech Adeno-X™ Tet-off expression system. All adenoviruses were sequenced at the DNA sequencing facility at the Oklahoma Medical Research Foundation.
Plasmids and transfection
HA-tagged, codon optimized HPV6 and HPV16 E5 were generated as described above and subcloned into either the pcDNA3 (Invitrogen) or pCDF1-MCS2-EF2-copGFP (System Biosciences).
The wild type HPV16 genome and E5 frameshift mutation were cloned into a pUC-18 plasmid for the replication and antibiotic selection for purification (Genther et al., 2003). Prior to transfection, CsCl purified cDNA (10 μg) was digested with BamH1 to isolate the HPV16 genome from the backbone plasmid and the result ∼8000 bp DNA fragment was isolated by gel purification. The HPV16 genome (2 μg) along with GFP-neomycin (pEGFP-C1; Clontech) was introduced into 2×106 HaCaT cells by nucleofection (Amaxa).
pRK7-T7 RNA polymerase was generated by using PCR primers to clone T7 RNA polymerase from BL21 E. coli (primer pairs 5′ TTA CAT TCT AGA ATG AAC ACG ATT AAC ATC GCT AAG- 3′ and 5′ATG TAA CTC GAG TTA CGC GAA CGC GAA CGC GAA GTC CGA – 3′). The primers incorporated XbaI and XhoI restriction enzyme sites. The resulting PCR product was digested with XbaI and XhoI, and ligated into the same sites in pRK7 (with a multiple cloning site that has been altered to include an XhoI site) such that the gene was driven by the plasmid's CMV promoter. The T7 promoter driving the expression of His6-S-tagged Yellow Fluorescent Protein (YFP) in pET30 (Novagen) was amplified using PCR primers that added Spe1 sites upstream of the T7 promoter and downstream of the t7 terminator. The PCR product was digested with SpeI, and subcloned into the pRK7 that was digested with SpeI and XbaI to remove the CMV promoter. The resulting plasmid is referred to as pT7-YFP.
Cell lysates and Immunoblotting
Cell lysates were prepared as previously described (Dinneen and Ceresa, 2004). Proteins were resolved on 16% Tris-Tricine gels and transferred to nitrocellulose. Antibodies were obtained from the indicated sources: anti-HA (12CA5 antibody, Roche), α-tubulin (Sigma), HPV16 E7 (Zymed). Proteins were visualized with enhanced chemiluminescence and documented using a UV Products Imaging system.
Indirect immunofluorescence
Indirect immunofluorescence was performed as previously described (Dinneen and Ceresa, 2004). The 12CA5 antibody was used at a dilution of 1:1000 and Alexa 488- or Alexa 568-conjugated goat anti-mouse (Molecular Probes) was used at a dilution of 1:250. Cells were also stained with 10 ng/ml DAPI (Sigma). Images were captured using Olympus AX70 epifluorescent microscope with Q-Capture software. Bi-nucleated cells were calculated as the number of cells with two nuclei divided by the total cells.
Heterokaryon formation assay
tTA-HaCaT cells (2 × 106 cells) were transfected with pRK7-histone 2B-RFP (1.5 μg plasmid) by nucleofection using the Amaxa Nucleofector I (transfection efficiency ≥50%). After 24 hours recovery, cells were mixed in a 1:1 ratio with untransfected cells and then infected with HA-E5 adenovirus (20 pfu/cell) or treated with cytocholasin D or latrunculin B. At 24-hour intervals, cells were fixed and observed by fluorescence microscopy for bi-nucleated cells. As a positive control, heterokaryons were induced by treatments with polyethylene glycol for 5 minutes (Madan and DeFranco, 1993).
FACS analysis
Cells were fixed in 70% ethanol, and incubated with 20 μg/ml propidium iodide (Sigma) for one hour at 37°C. Cells were analyzed using a FACS Calibur (Becton Dickinson). The data from 10,000 cells/experiment were analyzed using Modfit LT 2.0 software (Verity).
Video Microscopy
Time-lapse phase-contrast images were collected using a Zeiss Axiovert 200M microscope equipped with a Hamanatsu ORCA camera and processed with Metamorph Software (Molecular Devices) as previously described (Potapova et al., 2006).
Cell Fusion assay
tTA-HeLa cells were transfected by calcium phosphate with either a plasmid expressing T7 RNA polymerase (pRK7-T7 RNA polymerase) or yellow fluorescent protein (YFP) driven by a T7 promoter (pT7-YFP). Following 16 hours of recovery, cells were washed, and replated with 200,000 cells of each population (1:1 ratio) into a well of a 12-well dish. Cells were infected with adenovirus as indicated in the figure legend using the method described above. Seventy-two hours post-infection, cells were collected, washed in PBS, and analyzed using a FACS Calibur (Benton Dickson) at the OUHSC Flow and Imaging Cytometry Laboratory. The data from >10,000 cells/experiment were analyzed using Modfit LT 2.0 software (Verity). Data were plotted as the relative increase in the total percentage of YFP (percentage of positive cells multiplied by their fluorescent mean intensity) as compared to cells without virus infection.
Cell viability and colon formation assays
Cells were transfected as described for the cell fusion assay or co-transfected with p1321 HPV-16 E6/E7 plasmid (Addgene plasmid 8641) (Munger et al., 1989). Bi-nucleated, fused cells were isolated by an Influx Cell Sorter at the OUHSC Flow and Imaging Cytometry Laboratory. For cell viability assays, cells were plated at a density of 2500 cells/well and grown in growth media with and without tetracycline for 72 hours. As a control, the parental tTA-HaCaT cells were plated at the same density. Cell viability was monitored by MTT assay as previously described (Hansen, Nielsen, and Berg, 1989). Colony formation was measured by plating cells at a density of 500 bi-nucleated cells/35 mm dish. Cells were maintained in growth media with and without tetracycline for 21 days (media changed every three days), fixed and stained with methyl violet (Liu et al., 2000). Only cell clusters of greater than 100 cells were considered colonies.
Supplementary Material
tTA-HaCaT cells expressing HA-E5 were monitored 24 hours after adenoviral infection. Shown are two separate cells that fuse together. Size bar = 10 μm. Time is indicated as Hour:minutes.
tTA-HeLa cells were transfected with either pRK7-T7 RNA polymerase or pT7-YFP and mixed in a 1:1 ratio. Cells were infected as indicated. Shown are representative FACS analyses from a single experiment that was repeated 3-5 times. The X-axis (FL1-H) is a measure of the forward light scatter (green fluorescence) and Y-axis (FL2-H) is a measure of the side scatter (auto-fluorescence).
Acknowledgments
This work was funded by American Cancer Society Research Scholar's Grant 03-021-01, OCAST grant HR03-014, and The Milheim Foundation for Cancer Research (B.P.C.). We thank Jim Henthorn (OUHSC Flow and Imaging Cytometry Laboratory) and Dr. David Thompson (OUHSC Biostatistics and Epidemology). We thank Paul F. Lambert (UW-Madison) for critical evaluation of this manuscript.
Abbreviations
- HPV
Human Papillomavirus
- HA
hemagglutinin
- tTA
tetracycline transactivator
- tTA-HaCaT
HaCaT cells stably transfected with tTA
- FACS
Fluorescence activated cell sorting
- FISH
Fluorescence In Situ Hybridization
Footnotes
L. Hu and B. Ceresa, data not shown.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bosch FX, Lorincz A, Munoz N, Meijer CJLM, Shah KV. The Causal Relation between Human Papillomavirus and Cervical Cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal Keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard V, Matlashewski G, Gu ZM, Storey A, Banks L. The Human Papillomavirus type 16 E5 gene cooperates with the E7 gene to stimulate proliferation of primary cells and increases viral gene expression. Virol. 1994;203:73–80. doi: 10.1006/viro.1994.1456. [DOI] [PubMed] [Google Scholar]
- Bravo IG, Alonso A. Mucosal Human Papillomaviruses Encode Four Different E5 Proteins Whose Chemistry and Phylogeny Correlate with Malignant or Benign Growth. J Virol. 2004;78(24):13613–13626. doi: 10.1128/JVI.78.24.13613-13626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EH, Olson EN. Unveiling the Mechanisms of Cell-Cell Fusion. Science. 2005;308:369–373. doi: 10.1126/science.1104799. [DOI] [PubMed] [Google Scholar]
- Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Chissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncology. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- Conrad M, Goldstein D, Andresson T, Schlegel R. The E5 Protein of HPV-6, but not HPV-16, Associates Efficiently with Cellular Growth Factor Receptors. Virol. 1994;200:796–800. doi: 10.1006/viro.1994.1244. [DOI] [PubMed] [Google Scholar]
- Dinneen JL, Ceresa BP. Constitutive activation of rab5 results in a ligand independent redistribution of the EGFR and attenuates its ability to signal. Traffic. 2004;5(8):606–615. doi: 10.1111/j.1398-9219.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Disbrow GL, Hanover J, Schlegel R. Endoplasmic Reticulum-Localized Human Papillomavirus Type 16 E5 Protein Alters Endosomal pH but Not trans-Golgi pH. J Virol. 2005;79(9):5839–5846. doi: 10.1128/JVI.79.9.5839-5846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow GL, Sunitha I, Baker CC, Hanover J, Schlegel R. Codon Optimization of HPV-16 E5 Gene Enhances Protein Expression. Virol. 2003;311:105–114. doi: 10.1016/s0042-6822(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Duelli D, Lazebnik Y. Cell-to-cell fusion as a link between viruses and cancer. Nature Reviews Cancer. 2007;7:968–976. doi: 10.1038/nrc2272. [DOI] [PubMed] [Google Scholar]
- Duelli DM, Padilla-Nash HM, Berman D, Murphy KM, Ried T, Lazebnik Y. A Virus Causes Cancer by Inducing Massive Chromosomal Instability through Cell Fusion. Curr Biol. 2007;17:1–7. doi: 10.1016/j.cub.2007.01.049. [DOI] [PubMed] [Google Scholar]
- Duensing S, Munger K. The Human Papillomavirus Type 16 E6 and E7 Oncoproteins Independently Induce Numerical and Structural Chromosome Instability. Cancer Res. 2002;62:7075–7082. [PubMed] [Google Scholar]
- Genther SM, Sterling S, Duensing S, Munger K, Sattler C, Lambert PF. Quantitative Role of the Human Paillomavirus Type 16 E5 Gene during the Productive Stage of the Viral Life Cycle. J Virol. 2003;77(5):2832–2842. doi: 10.1128/JVI.77.5.2832-2842.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieswein CE, Sharom FJ, Wildeman AG. Oligomerization of the E5 protein of human papillomavirus type 16 occurs through multiple hydrophobic regions. Virol. 2003;313:415–426. doi: 10.1016/s0042-6822(03)00296-4. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. PNAS. 1992;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MB, Nielsen SE, Berg K. Re-examination and further developoment of a precise method for measuring cell growth/cell kill. J Immunol Methods. 1989;119(2):203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Incassati A, Patel D, McCance DJ. Induction of tetraploidy through loss of p53 and upregulation of Plk1 by human papillomavirus type-16 E6. Oncogene. 2006;25:2444–2451. doi: 10.1038/sj.onc.1209276. [DOI] [PubMed] [Google Scholar]
- Jones DL, Thompson DA, Munger K. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virol. 1997;56:4620–4624. doi: 10.1006/viro.1997.8851. [DOI] [PubMed] [Google Scholar]
- Kabsch K, Alonso A. The human papillomavirus type 16 E5 protein impairs TRAIL- and FasL-mediated apoptosis in HaCaT cells by different mechanisms. J Virol. 2002;76:12162–12172. doi: 10.1128/JVI.76.23.12162-12172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leechanachai P, Banks L, Moreau F, Matlashewski G. The E5 gene from human papillomavirus type 16 is an oncogene which enhances growth factor-mediated signal transduction to the nucleus. Oncogene. 1992;7(1):19–25. [PubMed] [Google Scholar]
- Liu B, Fang M, Schmidt M, Lu Y, Mendelsohn J, Fan Z. Induction of apopotosis and activation of the caspase cascade by anti-EGF receptor monoclonal antibodies in DiFi human colon cancer cells do not involve the c-jun N-terminal Kinase activity. Brit J of Cancer. 2000;82(12):1991–1999. doi: 10.1054/bjoc.2000.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan AP, DeFranco DB. Bidirectional transport of glucocorticoid receptors across the nuclear envelope. Proc Natl Acad Sci U S A. 1993;90(8):3588–92. doi: 10.1073/pnas.90.8.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Ryuysschaert JM. Common Properties of Fusion Peptides from Diverse Systems. Biosci Reports. 2000;20(6):483–500. doi: 10.1023/A:1010454803579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal KR, Chan W, Demopoulos RL. Sensitivity and specificity of various morphological features of cervical condylomas. Arch Pathol Lab Med. 1990;114:1038. [PubMed] [Google Scholar]
- Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillmoavirus as necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, Shah KV, Meijer CJLM. Against which human papillomavirus types shall we vaccinate and screen? The International perspective. Int J Cancer. 2004;111(2):278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- Nath R, Mant C, Kell B, Cason J, Bible J. Analyses of variant human papillomavirus type -16 E5 proteins for their ability to induce mitogenesis of murine fibroblasts. Cancer Cell International. 2006;6(1):19. doi: 10.1186/1475-2867-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze I, Kartenbeck J, Crusius K, Alonso A. Human papillomavirus type 16 E5 protein affects cell-cell communication in an epithelial cell line. J Virol. 1995;69:4489–4494. doi: 10.1128/jvi.69.7.4489-4494.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetke CE, Auvinen E, Pawlita M, Alonso A. Human Papillomavirus type 16 E5 protein localizes to the Golgi apparatus but does not grossly affect cellular glycosylation. Arch Virol. 2000;145:2183–2191. doi: 10.1007/s007050070048. [DOI] [PubMed] [Google Scholar]
- Pater MM, Pater A. Human Papillomavirus types 16 and 18 sequences in carcinoma cell lines of the cervix. Virol. 1985;145:313–318. doi: 10.1016/0042-6822(85)90164-3. [DOI] [PubMed] [Google Scholar]
- Pim D, Collins M, Banks L. Human Papillomavirus type 16 E5 gene stimulates the transforming activity of the epidermal growth factor receptor. Oncogene. 1992;7(1):27–32. [PubMed] [Google Scholar]
- Plug-DeMaggio AW, Sundsvold T, Wurscher MA, Koop JI, Klingelhutz AJ, McDougall JK. Telomere erosion and chromosomal instability in cells expressing the HPV oncogene 16E6. Oncogene. 2004;23:3561–3571. doi: 10.1038/sj.onc.1207388. [DOI] [PubMed] [Google Scholar]
- Potapova TA, Daum JR, Pittman BD, Hudson JR, Jones TN, Satinover DL, Stukenberg PT, Gorbsky GJ. The reversibility of mitotic exit in vertebrate cells. Nature. 2006;440(7086):954–958. doi: 10.1038/nature04652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad CJ, Sheets E, Selig AM, McArthur MC, Crum CP. The bi-nucleated Squamous Cell: Histologic Spectrum and Relationship to Low-grade Squamous Intraepithelial Lesions. Mod Pathol. 1993;6(3):313–317. [PubMed] [Google Scholar]
- Schwartz E, Durst M, Demankowski C, Lattermann O, Zech R, Wolfsperger E, Shuai S, zur Hausen H. DNA sequence and genome organization of genital human papillomavirus type 6b. EMBO J. 1983;12:2341–2348. doi: 10.1002/j.1460-2075.1983.tb01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314:111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Seedorf K, Krammer G, Durst M, Suhai S, Rowekamp WG. Human papillomavirus type 16 DNA sequence. Virol. 1985;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Stoler MH, Rhodes CR, Whitbeck A, Wolinsky SM, Chow LT, Broker TR. Human Papillomavirus Type 16 and 18 Gene Expression in Cervical Neoplasias. Human Pathology. 1992;23(2):117–128. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- Stoppler MC, Straight SW, Tsao G, Schlegel R, McCance DJ. The E5 Gene of HPV-16 Enhances Keratinocyte Immortalization by Full-Length DNA. Virol. 1996;223:251–254. doi: 10.1006/viro.1996.0475. [DOI] [PubMed] [Google Scholar]
- Straight SW, Herman B, McCance DJ. The E5 Oncoprotien of Human Paillomavirus Type 16 Inhibits the Acidification of Endosomes in Human Keratinocytes. J Virol. 1995;69(5):3185–3192. doi: 10.1128/jvi.69.5.3185-3192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight SW, Hinkle PM, Jewers RJ, McCance DJ. The E5 Oncoprotein of Human Papillomavirus Type 16 Transforms Fibroblasts and Effects the Downregulation of the Epidermal Growth Factor Receptor in Keratinocytes. J Virol. 1993;67(8):4521–4532. doi: 10.1128/jvi.67.8.4521-4532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle GF, Banks L. The human papillomavirus (HPV)-6 and HPV-16 E5 proteins co-operate with HPV-16 E7 in the transformation of primary rodent cells. J Gen Virol. 1995;76:1239–1245. doi: 10.1099/0022-1317-76-5-1239. [DOI] [PubMed] [Google Scholar]
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Zhang B, Li P, Wang E, Brahmi Z, Dunn KW, Blum JS, Roman A. The E5 protein of human papillomavirus type 16 perturbs MHC class II antigen maturation in human foreskin keratinocytes treated with interferon-gamma. Virol. 2003;310:100–108. doi: 10.1016/s0042-6822(03)00103-x. [DOI] [PubMed] [Google Scholar]
- Zhang B, Spandau DF, Roman A. E5 Protein of Human Papillomavirus Type 16 Protects Human Foreskin Keratinocytes from UV B-Irradiation-Induced Apoptosis. J Virol. 2002;76(1):220–231. doi: 10.1128/JVI.76.1.220-231.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Srirangam A, Potter DA, Roman A. HPV16 E5 protein disrupts the c-Cbl-EGFR interation and EGFR ubiquitination in human foreskin keratinocytes. Oncogene. 2005:1–4. doi: 10.1038/sj.onc.1208453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
tTA-HaCaT cells expressing HA-E5 were monitored 24 hours after adenoviral infection. Shown are two separate cells that fuse together. Size bar = 10 μm. Time is indicated as Hour:minutes.
tTA-HeLa cells were transfected with either pRK7-T7 RNA polymerase or pT7-YFP and mixed in a 1:1 ratio. Cells were infected as indicated. Shown are representative FACS analyses from a single experiment that was repeated 3-5 times. The X-axis (FL1-H) is a measure of the forward light scatter (green fluorescence) and Y-axis (FL2-H) is a measure of the side scatter (auto-fluorescence).