The topic of my talk, the aquaporins, is something that we have been studying in our lab for the last 15 years. It is not something that I anticipated when starting my career. In fact, I am a hematologist, but in science, sometimes what we find is not what we are looking for. A typical middle-aged man, I weigh 75 kg, of which 50 are water. This is something that we all share. Water is the major component of all our cells in all of our tissues. This is also shared with all other life forms, other vertebrates, invertebrates, microorganisms, and plants. Water can be described as the “solvent of life”—without water, there is no life. Multicellular life comes with potential problems. One of these problems is the movement of fluids across biological barriers, and the principal barrier for most of our tissues is the plasma membrane. The aquaporins, which I will describe, are an answer to how water crosses biological membranes, but specific questions still need be defined. I am very pleased to be speaking to the American Thoracic Society because I believe that many of the questions related to fluid movements in lung are still unsettled.
When we began our studies some years back, the problem of membrane water permeability had already been looked at by a generation of biophysicists and physiologists. With the discovery of the lipid bilayer in the 1920s, it was speculated that a finite degree of water permeability would occur by simple diffusion through the plasma membrane. However, the work of very vigorous biophysicists and physiologists indicated that in certain tissues—for example, renal tubule, secretory glands, and red cells—the water permeability is much larger than could be explained through simple diffusion through the plasma membrane. And these investigators predicted correctly that there must be specialized water-selective channels in these membranes. The current view is that both mechanisms occur—diffusion through all membranes and flow through aquaporins that are present in certain special membranes. The biophysical differences are quite significant; diffusion is a low-capacity bidirectional movement of water, whereas water channels have a high capacity and great selectivity for water. The channels are so selective that water passes through them, and acid does not. As we all know, protons exist in fluid as the hydronium ion. This distinction is really quite important. Every day, our kidneys filter and reabsorb about 180 L of water. If we do not reabsorb that water, we would die of dehydration. If we reabsorb the water, along with protons, we would become systemically acidotic. The movement of water through the aquaporins is driven by osmotic gradients. For example, red cells dropped into seawater will shrink because water leaves the cells; red cells dropped into fresh water will swell and explode since water enters the cell. The process of osmosis that we all learned about as children is known to occur very rapidly in membranes in which aquaporins are present.
An experiment by Bob Macy at the University of California, Berkeley, in 1970 made a very important observation. It was known that diffusion of water through membranes is not inhibited by any known pharmacologic agents. Macy discovered that mercuric chloride reduces the water permeability of red cells, and when the membranes have been treated with reducing agents, the water permeability was restored. Macy concluded that the water channel protein must contain a free sulfhydryl somewhere within the pore. This provided very strong evidence that water channels must exist; however, most physiologists and other scientists remained very skeptical. They could not identify water channel molecules, much less purify them, clone them, express them, or reconstitute them.
Our laboratory got into the water channel field by accident. We discovered a polypeptide in red cells that we didn't expect to see. Interestingly, it was identical to a polypeptide in the kidney. So, on a great leap of faith, we decided to clone the complementary DNA (cDNA). This was in the early days before the genome was sequenced, so it was a lot of hard work. We obtained a cDNA encoding a 269–amino acid polypeptide. Identical transcripts were obtained from red cell and kidney libraries. The new protein was found to have six bilayer-spanning domains. The N-terminus of the protein resides within the cell, and then two interesting repeats appear, each corresponding to three bilayer-spanning domains. They are not a perfect sequence repeats but are genetically similar sequences. Most curiously, the two repeats appear to be oriented at 180° to each other. We looked at the DNA database in 1991. We found a number of similar sequences had been reported, but none of the proteins had a function that had been defined. Thus, we obtained no obvious clue to the function of our protein. The related sequences corresponded to a protein from the lens of eye from beef cattle, a protein from brain of Drosophila fruit flies, a protein that permits Escherichia coli to use glycerol as a carbon source, and a series of related proteins from various plant tissues. It was really the observation that red cells and kidney tubules are highly water permeable, and the existence of plant homologs, that caused us to pursue this further.
We established the water transport function of our protein in collaboration with our colleague Bill Guggino in the Physiology Department at Johns Hopkins. To do this, we injected the complementary RNA into Xenopus laevis oocytes. These amphibian eggs are about a millimeter in diameter and are known to be quite water impermeable (Figure 1). On the left is a controlled oocyte injected only with buffer alone and on the right is an oocyte injected with the complementary RNA for our new protein. After culture, the oocytes looked about the same. But when they were then dropped into distilled water, an amazing difference was observed. The controlled oocyte had failed to swell, the test oocyte had swollen and exploded. This produced much jubilation in the laboratory! Shown in Figure 1 is Gregory Preston, the Postdoctoral Fellow who did these studies. I took this photograph of Greg three years after the discovery, and he was still celebrating. So, I think that young scientists need to know that gratification is often delayed in science, and it is even permissible to cheer for yourself.
Figure 1.


Discovery of aquaporin-1 (AQP1). Functional demonstration of water transport in X. laevis oocytes. Top panel: Control oocyte not expressing AQP1 (left) and test oocyte expressing AQP1 (right) 15 s after transfer to hypoosmotic culture medium. Middle panel: Same oocytes 3 min after transfer to hypoosmotic culture medium. The control oocyte failed to swell. The test oocyte swelled rapidly by osmosis and ruptured. Reprinted by permission from Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992;256:385–387. Bottom panel: Jubilation expressed by Postdoctoral Fellow Gregory Preston 3 years after original report.
We spent a considerable amount of effort studying the structure of our new water channel protein. The internal repeats contain two loops B and E, which join the second and third, then the fifth and sixth bilayer-spanning domains and contain a degree of hydrophobicity. We thought that loops B and E may actually fold together and make a central aqueous pore. There is a special motif (Asn-Pro-Ala [NPA]) in each loop that overlaps in the center of the pore. The original hourglass model was just a pencil-and-paper sketch. But in fact it has been confirmed by high-resolution protein studies that we undertook with Professor Yoshinori Fujiyoshi from Kyoto, Japan, and Professor Andreas Engel from the Biocenter in Basel, Switzerland.
Together with these scientists, we solved the structure of the highly purified AQP1 protein from our lab. The reconstituted protein was 100% water permeable, indicating that the structure that we would deduce would be the biologically relevant structure. By a combination of cryo-electron microscopy and atomic force microscopy, a three-dimensional electron density map at 3.8-Å resolution was calculated, and models were built. The protein is tetromeric, but the functional unit is the monomer. A single aqueous pore passes down through the center of the protein. The protein fold has been further defined by X-ray photographic studies from Bob Stroud from the University of California, San Francisco, and Bing Jap from Lawrence Berkeley Laboratory. Molecular dynamic simulations were undertaken by deGroot and Grubmüller in Göttingen and Schulten and colleagues at the University of Illinois at Urbana-Champaign.
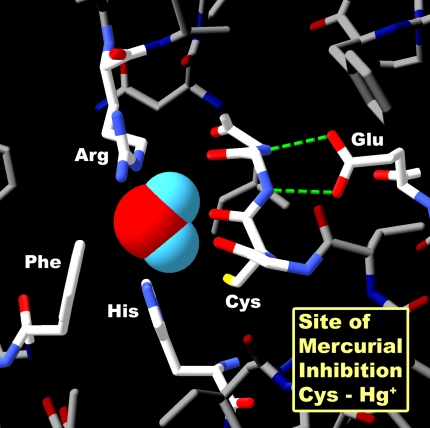
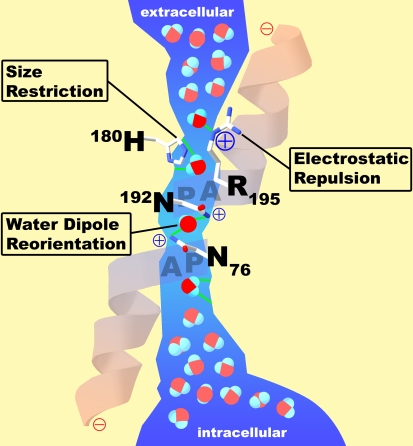
We now have a clear understanding of how the aquaporin proteins work. Aquaporins have a narrow pathway that is a very tight fit for water, the smallest biological molecule (Figure 2). Shown in Figure 2 is a space-filling representation of the single water molecule in the very center of the pore of the cross-section, and you can see that the adjacent charged residues, arginine and histidine, are very close. One can see here that there is a perfectly conserved residue, a cysteine, which is the site of mercurial inhibition. Now, how did this water pass through the membrane, but fail to carry protons? My father was a chemistry professor back in Minnesota, and he liked to tell his first-year students about the chemistry of water—H2O. Because it is 18 Da in mass, the molecule would be predicted to be a gas, which of course water is in the atmosphere. But in solution, the hydrogen bonds between water molecules cause water to be a liquid. In bulk solution, the water molecules are close together and hydrogen bonding occurs. This allows free movement of protons hopping between the molecules. In the extracellular vestibule of the hourglass and in the intracellular vestibule, water exists in bulk solution (Figure 3). But the center of the aquaporin has a 20-Å trim span where water transits the pore in single file. The narrowest diameter of the pores is 2.8 Å—just big enough for a single water molecule. A fixed positive charge on the adjacent arginine side chain will repel protons. The water molecules then are spaced within the pore at intervals so that hydrogen bonding cannot occur between them. A second barrier exists in the center of the pore, where an isolated water molecule will transiently form hydrogen bonds to the side chains of two highly conserved asparagines residues. This provides a very interesting mechanism—one that allows water to move with no resistance.
Figure 2.
Structure of AQP1–Hg++ inhibitory site. Space-filling representation of a single water molecule in the narrowest point in the pore. Surrounding residues arginine-195 (Arg) and histidine-180 (His) provide positive charges that repel protons. Cysteine-189 (Cys) is the site of Hg++ inhibition. Phenylalanine-56 (Phe) and glutamic acid-142 (Glu) are other important residues in the pore region. Reprinted by permission from Kozono D, Yasui M, King LS, Agre P. Aquaporin water channels: atomic structure molecular dynamics meet clinical medicine. J Clin Invest 2002;109:1395–1399.
Figure 3.
Functional schematic for water passage through AQP1. The extracellular vestibule and the intracellular vestibule of the channel contain water in bulk solution. They are connected by a 20-Å span where water molecules pass in single file. Barriers to the passage of protons are visible. Arginine-195 (R195) and histidine-180 (H180) provide fixed positive charges to repel proton passage. A single water molecule forms hydrogen bonds with the side chains of highly conserved asparagines-76 and -192 (N76 and N192). Partial positive charges are provided by the orientation of the two α helices that enter but do not entirely span the bilayer. Reprinted by permission from Kozono D, Yasui M, King LS, Agre P. Aquaporin water channels: atomic structure molecular dynamics meet clinical medicine. J Clin Invest 2002;109:1395–1399.
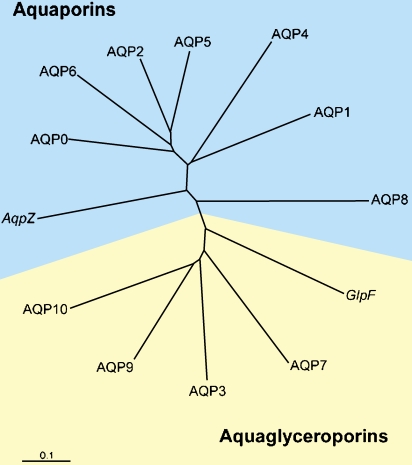
We and others sought by homology cloning to identify other members of the aquaporin protein family. A gene pileup represents a partial listing of human aquaporins (Figure 4). We now know of 12 human sequences that are closely related to AQP1. They are classed into two subsets: those permeated by water alone, the classical aquaporins, and those permeated by water plus glycerol, the aquaglyceroporins. Knowing the biophysical functions of the proteins and the sites where they are expressed permits us to predict their physiologic and pathophysiologic functions. I will briefly discuss some of each subset. AQP1 in the kidney is responsible for the countercurrent concentrating mechanism in the proximal tubules. AQP2 is the vasopressin-regulated water channel in the collecting duct. Mutations in the gene encoding AQP1 cause mild nephrogenic diabetes insipidus, but mutations in the gene encoding AQP2 are much more severe. AQP4 is expressed in brain, and is involved in both the pathogenesis and the recovery from brain edema. AQP0 is expressed in lens, and mutations in this protein cause cataracts. The aquaglyceroporin AQP7 is expressed in fat tissue where it is responsible for glycerol release during fasting and starvation, while AQP9 in liver permits glycerol uptake by liver for gluconeogenesis.
Figure 4.
Human aquaporin repertoire. Two subsets of members exist: those permeated only by water (aquaporins) and those permeated by water plus glycerol (aquaglyceroporins). Included are the two members from Escherichia coli: AqpZ, a water channel, and GlpF, a glycerol transporter. Reprinted by permission from Kozono D, Yasui M, King LS, Agre P. Aquaporin water channels: atomic structure molecular dynamics meet clinical medicine. J Clin Invest 2002;109:1395–1399.
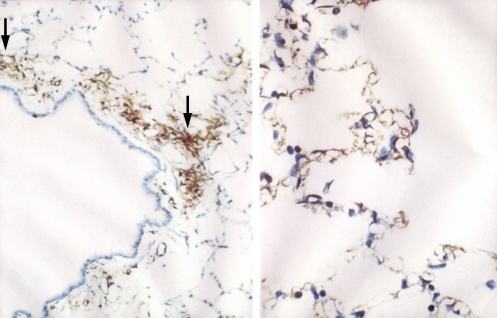
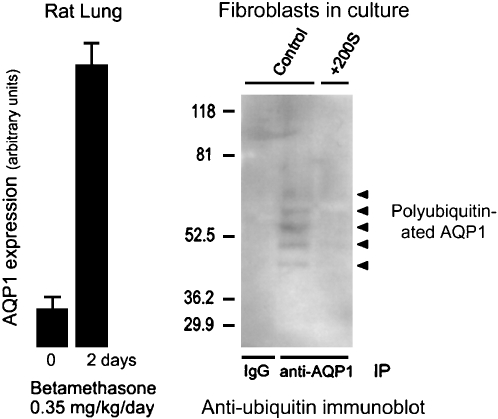
To define the physiologic functions of AQP1, we collaborated with Søren Nielsen from the University of Aarhus in Denmark who is a world-class microscopist. Søren localized the AQP1 in lung and other tissues as well, but lung is the topic of the talk today. AQP1 is present in the capillary endothelium in a peribronchiolar distribution. In the alveolar section, a lower degree of specific staining is visible (Figure 5). Immunogold electromicroscopy of a single capillary endothelial cell from human lung exhibits immunogold labeling of both the lumenal membrane and the ablumenal membrane of the capillary (Figure 6). Studies by our colleague Landon King in the Division of Respiratory and Critical Care Medicine at Johns Hopkins demonstrated that the level of AQP1 expression in lung microvasculature is not constant. The amount of AQP1 rises dramatically in lung at the time of birth. In addition, AQP1 expression is exquisitely sensitive to corticosteroids—about an eightfold increase in expression in adult rat lung (Figure 7, left panel). Like many others, I suffer from a mild form of asthma; I use a corticosteroid inhaler, and the beneficial effects may be partly explained by the induction of AQP1 expression.
Figure 5.
AQP1 in rat lung capillary endothelium. Left panel: Immunoperoxidase staining reveals AQP1 in capillary endothelium (arrows) in peribronchiolar distribution. Right panel: Immunoperoxidase staining reveals AQP1 in capillary endothelium in alveolar distribution. Reprinted by permission from Nielsen S, Smith BL, Christensen EI, Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci 1993;90: 7275–7279.
Figure 6.
AQP1 in human lung capillary endothelium. Immunogold electron microscopic analysis reveals labeling of plasma membranes on lumenal surface (top) and ablumenal surface (bottom). Reprinted by permission from King LS, Nielsen S, Agre P, Brown RH. Decreased pulmonary vascular permeability in aquaporin-1-null humans. Proc Natl Acad Sci 2002;99: 1059–1063.
Figure 7.
Dynamics of AQP1 expression. Left panel: Expression of AQP1 in rat lung is induced by corticosteroid exposure for 2 d. Reprinted by permission from King LS, Nielsen S, Agre P. Aquaporin-1 water channel protein in lung: ontogeny, steroid-induced expression, and distribution in rat. J Clin Invest 1996;97:2183–2191. Right panel: Level of AQP1 expression in cultured fibroblasts induced by hypertonic shock is regulated by degradation through the ubiquitin–proteosome pathway. Controls (left lane and center lane) denote fibroblasts grown in isotonic culture media producing extremely low levels of AQP1 accumulation. +200S denotes fibroblasts grown in culture medium made hypertonic by addition of 200 mOsm sorbitol. Anti-AQP1 immunoprecipitation (IP) was followed by antiubiquitin immunoblot. The left lane shows no signal in the control lacking immunoprecipitation control. The center lane shows polyubiquitination of AQP1 (ladder of reactive bands) in fibroblasts grown in isotonic culture media, thereby keeping AQP1 expression levels low. The right lane shows no polyubiquitination of AQP1 in fibroblasts grown in hypertonic culture medium, allowing AQP1 expression levels to rise to high levels. Reprinted by permission from Leitch V, Agre P, King LS. Altered ubiquitination and stability of aquaporin-1 in hypertonic stress. Proc Natl Acad Sci 2001;98:2894–2898.
AQP1 protein expression can be induced, but expression levels can also be reduced, because the protein is degraded by the ubiquitin pathway. Here I should add that my talk is going to lead up to the one by Aaron Ciechanover, discoverer of the ubiquitin pathway (see page 21). In another study, Landon King looked at the degradation of AQP1 by the ubiquitin proteosome in cultured fibroblasts (Figure 7, right panel). Fibroblasts normally only express very low levels of AQP1 when cultured in isotonic medium. Immunoprecipitations analyzed by antiubiquitin immunoblotting show that the AQP1 is polyubiquitinated—a low level of AQP1 is expressed and rapidly ubiquitinated targeting the protein for degradation. When fibroblasts are grown in culture medium made hypertonic by the addition of 200 mOsm sorbitol, the protein accumulated in abundance. Immunoprecipitations analyzed by antiubiquitin immunoblotting show that when fibroblasts are grown in hypertonic media, AQP1 is not ubiquitinated, and the level of protein rises dramatically. This provides an efficient mechanism for effectively accumulating AQP1 protein when it is needed and rapidly reducing AQP1 protein when no longer needed by the cell.
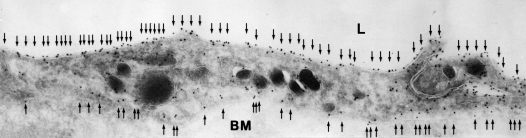
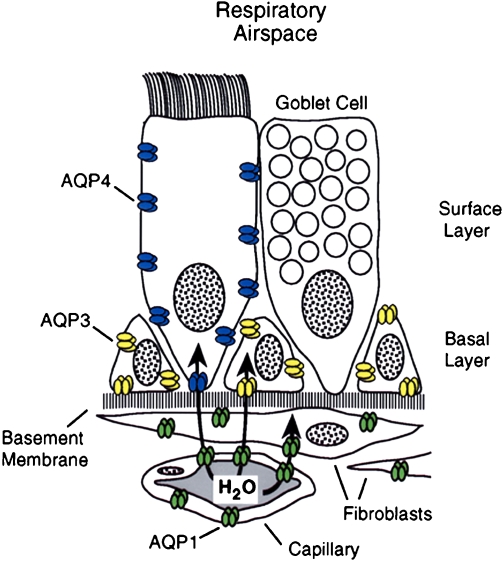
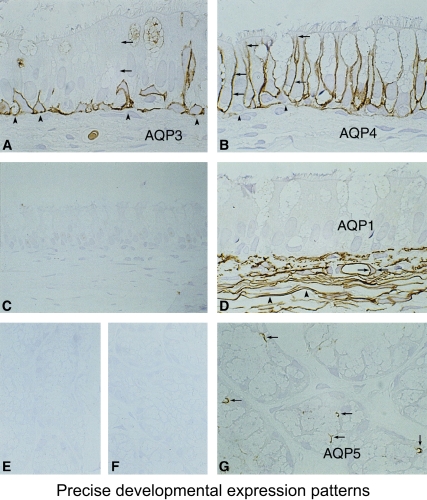
The lung is complicated, and Søren Nielsen and Landon King have defined the presence of multiple aquaporins at different levels of the respiratory tree (Figure 8). As described, AQP1 is present in capillaries and fibroblasts below the basement membrane. A different expression pattern is seen in the surface epithelium that lies above the basement membrane. Surface columnar cells contain AQP4 along the basolateral membranes. The adjacent goblet cells apparently do not contain aquaporins. The basal cells that reside along the basement membrane do not reach the surface and express high levels of AQP3. Thus, a clear distinction exists at the sites where AQP1, AQP3, and AQP4 are expressed. Still more complexity is found in the submucosal glands where AQP5 is expressed.
Figure 8.
Aquaporins in respiratory epithelia. Upper panel: Schematic demonstrating absence of aquaporin expression in goblet cells, AQP4 in surface columnar cells, AQP3 in basal cells, and AQP1 in underlying fibroblasts and capillaries. Lower panels: Immunoperoxidase, brown staining, reveals aquaporins in rat tissue sections corresponding to nasopharyngeal surface epithelium. (A) AQP3, (B) AQP4, (C) AQP5 (no staining). and (D) AQP1. Immunoperoxidase, brown staining, reveals aquaporins in rat tissue sections corresponding to subepithelial glands. (E) AQP3 (no staining), (F) control (no staining,) and (G) AQP5 (staining of apical surface). Reprinted by permission from Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol Cell Physiol 1997; 273:C1549–C1561.
How does one distinguish the importance of multiple aquaporin proteins that exist alongside each other in the complex airway epithelia? The level of expression is tightly regulated during fetal development. AQP1 and AQP3 are expressed before birth, but AQP4 is only expressed after birth. So here is an example of where clinical medicine provides basic scientific insight. Mice with gene knockouts have been prepared and have provided important insight. But as physicians, we are really interested in the human biology.
The structural gene for AQP1 is colocalized with a specific blood antigen on human chromosome 7. As a hematologist, I am interested in blood group antigens. Although there are hundreds of different antigens, they comprise about 25 families—each family being a genetic locus. As it turns out, the Colton blood antigen (Co) is a simple polymorphism on the surface of the AQP1 protein. Most of us have the same Co blood types, so they are usually not important clinically. Nevertheless, our colleagues at the International Blood Group Referencing Laboratory in Bristol, England, have only identified a handful of individuals lacking Co. So of course these are the very people that we became interested in. The Co-null individuals we were able to study were all women who apparently became sensitized during pregnancy. We determined that each was homozygous for different disrupting mutations in the gene encoding AQP1—a frameshift, an exon deletion, or a structurally important missense mutation—and were AQP1-null. Our Johns Hopkins colleague Landon King studied two unrelated AQP1-null individuals. A longstanding and distinguished member of the ATS, Landon was an ideal scientist to evaluate AQP1 in lung. The AQP1- null individuals have circulating anti-Co antibodies and are unable to tolerate a blood transfusion from other blood donors. Nevertheless, the AQP1-null individuals look and feel well. But clinical medicine provides us with a very interesting and powerful approach to identifying the importance of proteins. Presumably, many of us bear mutations in different genes. While these mutations usually do not cause symptoms, if we're properly stressed, they can provoke a disease phenotype.
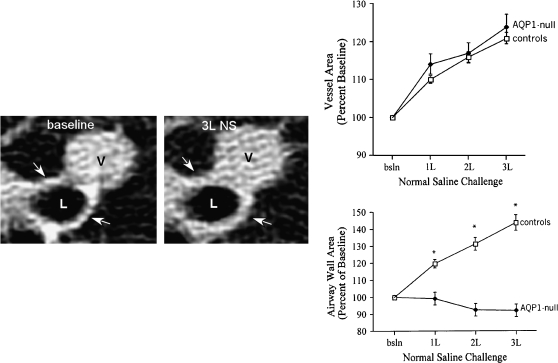
Landon King, in collaboration with Robert Brown at Johns Hopkins, used high-resolution computed tomography scans of the lung to evaluate lung capillary water permeability of these individuals before and after intravenous infusion of saline. Shown in Figure 9 are images from normal individuals: a 2-mm bronchiole and an adjacent venule at baseline and the same structures after infusion of 3 L of physiologic saline. It is obvious that the venule has become distended after intravenous fluid caused engorgement of the blood microvasculature. Similar increases are found in all normal individuals and all of the AQP1-null individuals. After infusion of saline, the wall thickness of the bronchioles in all normal individuals has increased due to water released from the blood vessels into the surrounding soft tissues. Thus, the bronchiolar wall thickness has increased as water collects in the perivascular soft tissues, forming incipient pulmonary edema. Surprisingly, the AQP1-null individuals had no such release of fluid to the surrounding soft tissues. Their bronchioles remained unchanged throughout the study—strong evidence that they have clear and measurable decrease in the vascular water permeability. At this point, we can only speculate about the clinical significance of this finding. With the first breath of life, the infant's lung absorbs amniotic fluid through the airway epithelium, and this is taken into the surrounding blood vessels. Thus, AQP1 may be important in perinatal fluid absorption by lung. If this is a reason for the low prevalence of the AQP1-null state, then presumably those who survive are somehow compensating for the lack of AQP1 in the microvasculature.
Figure 9.
AQP1-null humans—pulmonary capillary defect. Left panels: High-resolution computed tomography scan of lung before (left) and after (right) infusion of 3 L of physiologic saline. The lumen of a 2-mm bronchiole is denoted (L) as well as an adjacent venule (V). After infusion of saline, the venule becomes engorged and the wall of the bronchiole becomes thickened due to accumulation of fluid (see arrows). Upper right panel: This was reproduced in multiple normal individuals. Lower right panel: When AQP1-null individuals were investigated, they did not accumulate fluid surrounding the bronchiole, since the vascular water permeability was reduced *p<0.001 between groups. Reprinted by permission from King LS, Nielsen S, Agre P, Brown RH. Decreased pulmonary vascular permeability in aquaporin-1-null humans. Proc Natl Acad Sci 2002;99:1059–1063.
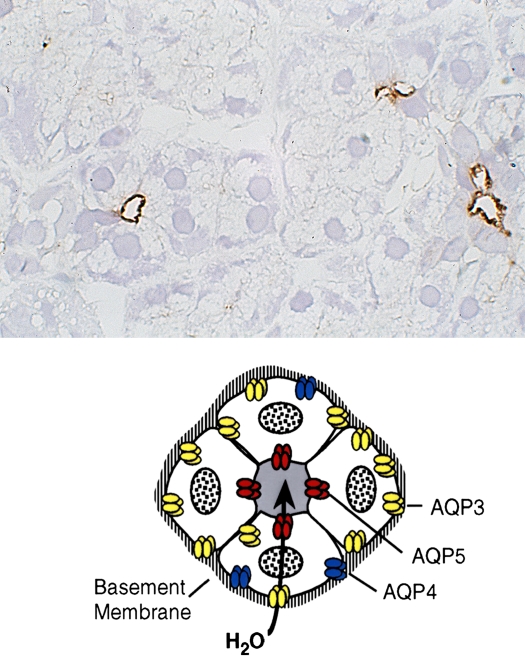
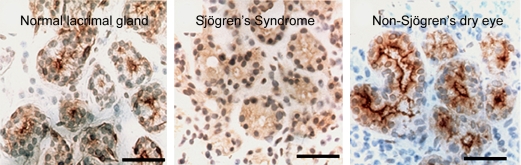
Now, I will talk about another aquaporin from the lungs: AQP5 is expressed in secretory glands, including airway submucosal glands, lacrimal glands, salivary glands, and sweat glands. AQP5 is expressed in the apical membrane of the secretory glands (Figure 10). The apical membrane where AQP5 resides is the last membrane water passes through during the secretion of airway fluids, tears, saliva, and sweat. Evidence suggests that this is important in clinical medicine. One study undertaken in collaboration with Chris Delporte in Brussels and a second study undertaken in collaboration with Kazuo Tsubota in Tokyo analyzed biopsies from a small number of patients with Sjögren's syndrome. This well-recognized disorder leads to dry eye and dry mouth problems, and pulmonary disorders as well. While Sjögren's syndrome is known to be an autoimmune problem, loss of glandular function often occurs in glandular elements where infiltration of lymphocytes is not seen. Normal biopsies have intense anti-AQP5 staining at the apical surface (Figure 11, left panel). In contrast, diffuse presence of AQP5 protein is notable throughout the glandular epithelium (Figure 11, center panel). This indicates that a mistake in trafficking of AQP5 protein has occurred, presumably as a secondary defect. An independent study of a larger number of Sjögren's syndrome biopsies was undertaken by Roland Jonsson, from the University of Bergen in Norway, and his collaborators. Interestingly, these investigators found normal AQP5 distribution, but they determined that AQP1 expression was reduced in the surrounding myoepithelial cells surrounding the glandular acini. So while there appears to be heterogeneity, all three groups found some aquaporin defects in this important clinical disorder, Sjögren's syndrome, and we can as yet only speculate what is happening in submucosal glands of lung.
Figure 10.
AQP5 in secretory glands. Drawings of secretory glands reveal AQP5, colored red, at the apical membrane (lower panel). The immunohistochemically stained tissue section of rat salivary gland reveals AQP5, stained brown, at the apical surface of three acini but not basolateral membranes (upper panel). Reprinted by permission from Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol Cell Physiol 1997;273:C1549–C1561.
Figure 11.
AQP5 in lacrimal glands—anti-AQP5 immunohistochemical staining of normal versus Sjögren's syndrome biopsies. Paraffin sections of human biopsies show AQP5, brown staining, in the apical membrane of normal (left panel) and non-Sjögren's dry eye (right panel). Center panel: The biopsy from a patient with Sjögren's syndrome reveals diffuse staining over the cell body indicating that a mistake in membrane trafficking had occurred. Studies by other investigators (Beroukas and colleagues) did not reveal abnormal AQP5 distribution but demonstrated AQP1 is reduced in myoepithelium (not shown). Reprinted by permission from Tsubota K, Hirai S, King LS, Agre P, Ishida N. Defective cellular trafficking of lacrimal gland aquaporin-5 is Sjögren's syndrome. Lancet 2001;357:688–689.
Another important role of AQP5 is in sweat glands. In collaboration with Lene Nejsum and Søren Nielsen, analysis of sweat glands was undertaken comparing normal mice with AQP5 gene knockout mice engineered by Anil Menon and Carissa Krane from the University of Cincinnati. Although both mice have normal-appearing sweat glands, the AQP5-null mice exhibit diminished function of sweat glands after pharmacologic stimulation. We do not yet have direct evidence for what is happening in airway submucosal glands; it is our belief an important function of AQP5 will be identified.
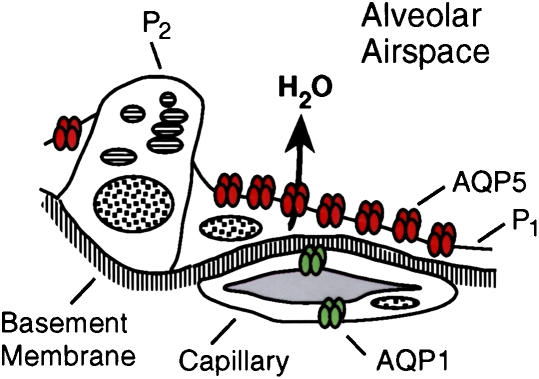
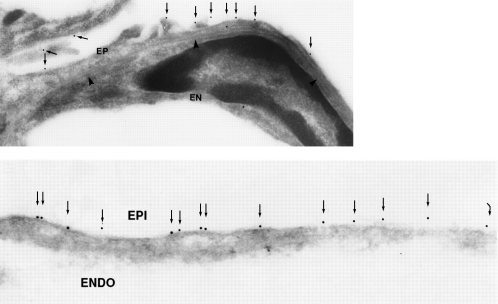
Another place where AQP5 is expressed in lung is in the type 1 pneumocytes in the alveoli. A simple model of the alveolar level of lung is shown (Figure 12, upper panel). The type 2 cells, where a number of transport functions are known to occur, lack AQP5, but the flattened type 1 cells contain an abundance of AQP5. Immunogold decoration reveals an abundance of AQP5 in the apical membranes but not in the basolateral membranes (Figure 12, lower panel). Leland Dobbs at the University of California in San Francisco has undertaken a classic study that demonstrated that these type 1 pneumocytes have the highest water transport capacity of any cell type in the body. While the physiologic significance is still uncertain, I would like to leave this challenge to the young scientists here. In contrast to kidney and eye, for which advanced understanding of the role of aquaporins has emerged, I think the importance of aquaporins in airways is still in need of a lot of research.
Figure 12.
AQP5 in alveolar type 1 pneumocytes. Upper panel: Drawing of alveolar space reveals AQP5, colored red, in apical membrane of type 1 pneumocytes (P1) but not type 2 pneumocytes (P2). Middle and lower panels: Immunogold labeling of AQP5 at apical membranes denoted with arrows. ENDO = endothelium; EPI = epithelium. Reprinted by permission from Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol Cell Physiol 1997;273:C1549–C1561 (upper and middle panels); King LS, Nielsen S, Agre P. Respiratory aquaporins in lung inflammation: the night is young. Am J Respir Cell Mol Biol 2000;22:8–10 (lower panel).
So in closing, I will talk about a completely irrelevant system in terms of human disease, but an interesting illustration of the ubiquity of the aquaporins. We now know that aquaporins are expressed in all life forms. Arabidopsis thaliana is a small mustard plant that is often the subject of investigation by plant physiologists. An interesting study executed by Ralf Kaldenhoff in Germany showed that parental strains of A. thaliana need only a slender group of rootlets to support the necessary water uptake of the plant. Kaldenhoff then engineered a genetically modified A. thaliana in which rootlet expression of the Pip1b group of aquaporins is only 20% of the normal level. While the plants looked fine from the surface up, the genetically modified plant has compensated for the reduced rootlet aquaporin expression by sending out many more rootlets. Plants are known to express dozens of aquaporins, and their tissue distribution is well preserved. Microorganisms also contain aquaporins, and we hope that some of these may prove to be useful targets for new antibiotic development.
Finally, to summarize my lecture, I talked about aquaporins and how they are selectively permeated by water but not acid. Certain homologs are permeated by other substrates, such as glycerol. The structural model explains the functions beautifully, and AQP1 is regarded as the first atomic structure of a human channel protein. Aquaporins have been implicated in a number of clinical disorders, but the roles of aquaporins in respiratory diseases remain poorly defined. It is easy to imagine what lung aquaporins might be doing in terms of osmotic water movements—freshwater drownings, for example. But how do lung aquaporins participate in asthma, cystic fibrosis, and wound healing, or in response to infection? I think these issues are quite relevant to the mission of the ATS, and I sincerely hope that some of the young scientists attending this meeting will take up this challenge.
Now, let me spend just a moment to tell you what it is like to win a Nobel Prize, since people often ask me. Well, it is a lot of fun, but it starts out a little puzzling. The phone rang in my bedroom at 5:30 in the morning on October 8, 2003, and a very distinguished Swedish gentleman notified me that I would share the Nobel Prize in chemistry. I was obviously delighted to hear the message, but my second thought was “chemistry, what in hell do I know about chemistry?” Our next speaker knows a lot about chemistry, since Aaron Ciechanover is a real biochemist. When I came to work a few hours after the phone call, I noticed there was a big party underway in my laboratory, organized by the young people in our group. Several ATS members were in the thick of it, including Landon and other members of his team, Virginia, Ramana, and Kelly, and were celebrating enthusiastically. Our university president even came over to see me—I had no idea that we were such good friends! My telephone voicemail was filled with requests for interviews, and then there was the voice of a very dear colleague from Norway. And I will never forget his voice; he said, “Peter, Peter, we just heard the news—it's unbelievable.” So that shows the confidence that your colleagues may have for you. But then the voice came back on and said, “No, no, it's very believable—it's wonderful.” And if the day wasn't crazy enough, while driving home that evening, the large sign in front of the discount liquor store at our neighborhood shopping center read, “Congrats Dr. Agre” in large letters, indicating that I already had become a local celebrity. And I'd just like to point out the implication that I am their best customer is really a great exaggeration. When I got home, our youngest daughter was home from high school, and she's a real shy kid. I told her I hoped that others would not bother her at school about this, and she said the most amazing thing that a teenager could tell her dad, “No, Daddy, my friends tell me this is so cool, but you should know that really famous people are on Simpsons and you're not.”
So, with that, let me thank you again for the invitation to be here at the ATS Centennial—THANK YOU!
Supported by grants from the National Heart, Lung, and Blood Institute, and a grant from the National Eye Institute.
Conflict of Interest Statement: P.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.