Abstract
The effects of nitric oxide (NO) are mediated by cyclic guanosine monophosphate (cGMP)-dependent and cGMP-independent processes. Most cGMP-independent effects are mediated by the actions of S-nitrosothiols (SNOs). SNOs have been shown to play a role in health and in disease. In studies performed in the mouse and rat, the ventilatory response to hypoxia is regulated in the nucleus tractus solitarius by SNOs exported from red blood cells. This may affect the treatment of respiratory distress in newborns and sleep apnea in adults. Likewise, SNOs have been shown to alter the stability and abundance of the transcription factor hypoxia inducible factor-1, altering the expression of hypoxia-regulated genes. Identification of the proteins involved in these signaling events will lead to new therapeutic approaches in the treatment of diseases characterized by limited oxygen availability.
Keywords: γ glutamyl transpeptidase, hypoxia inducible factor-1, S-nitrosothiols
S-nitrosothiols (SNOs) are products of S-nitrosylation that can be processed, transported, or degraded (1, 2). The formation of SNOs is coupled to the stimulation of nitric oxide synthase (NOS) isoforms (3–5) and requires the presence of an electron acceptor (6). Red blood cells (RBCs) can be a vehicle for O2-regulated delivery of NO signals in the form of SNOs. Hemoglobin binds NO at cysteine β93 and converts it to bioactive SNO as a function of blood O2 concentration (7, 8). The binding of O2 changes the conformation of hemoglobin from the deoxygenated state to the oxygenated state (7, 8). This permits the transfer of nitrosonium (NO+) equivalents from the heme to the thiol. Upon deoxygenation, NO+ equivalents are transferred from the cysteine β93 to a receptor cysteine in the cytosolic amino terminus of the anion exchange protein 1 (9, 10). The NO+ equivalents located on anion exchange protein 1 are exported from the RBCs by a mechanism that is not well defined. This transfer is mediated directly through cell–cell transfer or indirectly through the formation of S-nitrosoglutathione (GSNO) or S-nitrosocysteine (10). Entry of GSNO into cells can be mediated by γ glutamyl transpeptidase (γGT) or other transporters (11). This transfer of NO can alter protein function and abundance resulting in physiologic and pathologic changes.
Respiration is an autonomic response regulated by a network of neurons in the hindbrain. Respiratory control centers are responsible for modulating ventilation according to oxygen availability and metabolic demand. Exposure to hypoxia increases ventilation. Yet, failure to increase ventilation in response to hypoxia can be fatal. This can lead to respiratory distress in newborns and unfortunate consequences in adults with obstructive sleep apnea.
Several observations suggest that SNOs play a role in regulating respiration. First, V̇e increases linearly with decreased oxyhemoglobin saturation. Second, afferents from peripheral hypoxia chemoreceptors project to NOS-rich areas of the nucleus tractus solitarius (NTS) in the brain (12). Third, activation of NOS isoforms has been linked to the production of SNOs (3, 4). Last, GSNO, an endogenously produced SNO, has been shown to be 7 μM in the rat brainstem (13). These observations have lead Lipton and coworkers (14) to examine the ability of SNO to modulate V̇e.
Using conscious rats and whole-body plethysmography, Lipton and coworkers (14) examined the effects of endogenously produced SNOs in the NTS on V̇e in conscious rats. The results obtained were compared with changes in V̇e on short-term exposure to hypoxia. Rapid increases in V̇e followed by recovery to baseline were seen on injection of 1 nmol S-nitrosocysteinyl glycine (CGSNO), the bioactive bioproduct of GSNO (Figure 1A). A similar pattern of response was seen after a brief exposure to hypoxia (Figure 1B), suggesting that SNOs could mimic the ventilatory response to hypoxia. Moreover, the effects are stereospecific because only L—not D—isomers were able to elicit this response.
Figure 1.
S-nitrosothiols mimic the effects of hypoxia. (A) S-nitrosocysteinyl glycine (CGSNO; 1 nmol) was injected into the nucleus tractus solitarius of conscious rats, or (B) rats were exposed to a brief period of hypoxia. Changes in V̇e were measured by whole-body plethysmography. The effects of CGSNO on V̇e were similar to those seen in hypoxia. Reprinted by permission from Reference 14.
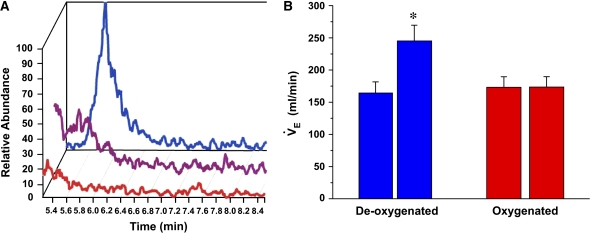
GSNO is an endogenously produced SNO formed during oxyhemoglobin desaturation. Having determined that SNOs can mimic the ventilatory effects of hypoxia, Lipton and colleagues (14) wanted to determine if SNOs, specifically GSNO, formed from deoxygenated blood could similarly increase V̇e. Mass spectrometry was used to demonstrate that GSNO is more efficiently generated in deoxygenated blood than in oxygenated blood and that the formation of GSNO is disrupted by ultraviolet light photolysis, which destroys the SNO by releasing NO (Figure 2A). Injection of GSNO isolated from the deoxygenated blood into the NTS reproduced the effects seen with endogenously produced SNO (Figure 2B). No effects were seen with SNOs produced in oxygenated blood. Thus, the ventilatory response to hypoxia is regulated in the nucleus tractus solitarius by SNO, specifically GSNO, exported from RBCs.
Figure 2.
S-nitrosoglutathione (GSNO) formed from blood deoxygenation mimics the effects of hypoxia. The formation of GSNO from deoxygenated (blue), oxygenated (red), and deoxygenated blood + ultraviolet light photolysis (purple) was determined by mass spectroscopy and reductive chemiluminescence. S-nitrosothiols isolated and purified from the peak fractions were injected into the nucleus tractus solitarius, and changes in V̇e were determined as described in Figure 1. GSNO derived from deoxygenated blood altered V̇e. Reprinted by permission from Reference 14.
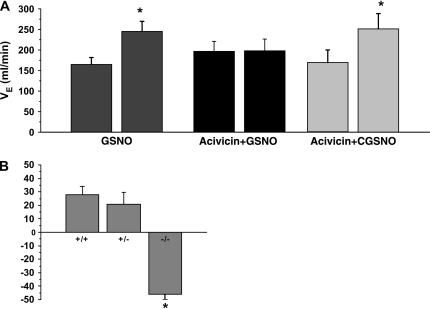
γGT is required for bioactivation of GSNO to cell-permeable CGSNO (11). To determine if this enzyme was required for the effect of SNOs on V̇e, Lipton and colleagues (14) used pharmacologic and physiologic approaches. Pretreatment of the NTS with acivicin, an inhibitor of γGT, before injection of GSNO was found to block the effects of GSNO but not CGSNO (Figure 3A). Moreover, γGT-deficient mice were found to have an altered response to hypoxia (Figure 3B). Taken together, these findings suggest that SNOs require the activity of γGT to duplicate the physiologic response to the exposure to and recovery from hypoxia.
Figure 3.
The effect of S-nitrosothiols on V̇e requires γ glutamyl transpeptidase (γGT). (A) Changes in V̇e were determined after treatment with GSNO, GSNO after pretreatment with acivicin (an inhibitor of γGT), and CGSNO (the bioactive component of GSNO after pretreatment with acivicin). GSNO-induced increases in V̇e were abolished by acivicin. Acivicin had no effect on the ability of CGSNO to induce an increase in V̇e. (B) Changes in V̇e in γGT-deficient mice (+/+, wild type; +/− heterozygous; −/−, null) after exposure to hypoxia. Data are expressed as percent changes from prehypoxia baseline. Reprinted by permission from Reference 14.
In summary, the research by Lipton and colleagues (14) defines one mechanism by which hypoxia can alter V̇e. The data demonstrate that (1) SNOs can mimic the effects of hypoxia on V̇e at the NTS, (2) the effects are stereospecific, and (3) the effects are related to O2 saturation and the RBCs rather than Po2. In addition, this effect of SNOs (particularly GSNO) is inhibited by γGT. The identification of the role of SNOs in regulating V̇e provides a starting point to identify and characterize components in this signaling pathway, which may lead to the identification of protein targets that may modulate the effects of SNOs on the hypoxic response and thus provide treatments for sleep apnea.
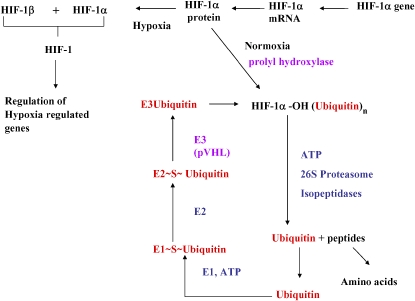
Hypoxia can cause pulmonary hypertension in humans and in animals. In addition, it has been shown to induce the expression of the transcription factor hypoxia inducible factor-1 (HIF-1). This transcription factor is a heterodimer composed of α and β subunits. The α subunit is constitutively transcribed and translated but is rapidly degraded, whereas the β subunit is constitutively expressed. In response to hypoxia, HIF-1α is stabilized (16). Normoxia is sensed by proline hydroxylation at residues Pro564 and Pro402 of HIF-1α, permitting interaction with von Hippel Lindau protein and subsequent ubiquitination and degradation of HIF-1α by the proteasome (Figure 4) (17–20). However, in hypoxia, hydroxylation does not occur, and HIF-1 is stabilized, dimerizes with a β subunit, and alters the transcription of hypoxia-regulated genes.
Figure 4.
Regulation of hypoxia inducible factor-1 (HIF-1). Schematic representation of the pathways regulating the expression of the transcription factor HIF-1. The HIF-1α gene generates HIF-1α mRNA, which is transcribed into HIF-1α protein. In normoxia, HIF-1α protein is hydroxylated and is targeted for ubiquitination and destruction. In hypoxia, HIF-1α protein is no longer hydroxylated and can combine with HIF-1β to regulate the activation of hypoxia-regulated genes. Activities altered by S-nitrosothiols are indicated in purple. E1 = ubiquitin-activating enzyme; E2 = ubiquitin-activating enzyme 2; E3 = ubiquitin protein ligase; VHL = von Hipple Lindau protein.
SNOs have been shown to stabilize HIF-1α in normoxia in cultured cells (Figure 5) (15). The activation of HIF-1 exhibits classical S-nitrosylation pharmacology: (1) It is not mimicked by 8-bromo cGMP; (2) it is not inhibited by oxyhemoglobin; (3) it is reversed by dithiothreitol; and (4) for GSNO, it is inhibited by the γGT inhibitor acivicin (15). Two potential mechanisms have been described for HIF-1 stabilization by GSNO. GSNO has been shown to impair prolyl hydroxylase activity (21) and/or directly impair S-nitrosylate HIF-1 at Cys 800 (22), with the former resulting in a decrease in HIF-1α ubiquitination and the latter increasing the recruitment of the p300 coactivator proteins.
Figure 5.
Stabilization of HIF-1α by GSNO. Proteins present in nuclear extracts made from bovine pulmonary artery endothelial cells treated with GSNO were separated by Western blot analysis. Stabilization of HIF-1α is seen to occur with time of exposure. Reproduced by permission from Reference 15.
Hypoxia-induced pulmonary hypertension has been shown to be impaired in mice heterozygous for HIF-1α (23) and is eliminated in mice heterozygous for HIF-2α (24). Thus, aberrant expression of HIF-1 in vivo could lead to the development of pulmonary hypertension. The Po2 values used to stabilize HIF in vitro are much less than the Po2 values normally seen in the pulmonary vasculature (postnatal 40 mm Hg, prenatal 30 mm Hg). Because SNO formation has been found to be associated with hemoglobin deoxygenation, one could speculate that signaling through SNOs rather than changes in Po2 could alter HIF-1α expression and thus provide an alternative mechanism for the development of this disease.
In summary, many effects of NO are mediated by the actions of SNOs. SNOs have been found to duplicate the ventilatory response to the exposure to and recovery from hypoxia and to change the stability and DNA-binding activity of the transcription factor HIF-1. Identification of the physiologic processes that are altered by S-nitrosylation and the proteins involved in SNO signaling could lead to the development of new therapeutic approaches for the treatment of disease, especially in cases where limited supplies of oxygen are involved.
Supported by National Institutes of Health grant HL068173.
Conflict of Interest Statement: L.A.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Gaston BM, Carver J, Doctor A, Palmer LA. S-nitrosylation signaling in cell biology. Mol Interv 2003;3:11–21. [DOI] [PubMed] [Google Scholar]
- 2.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 2005;67:99–145. [DOI] [PubMed] [Google Scholar]
- 3.Gow AJ, Chen Q, Hess DT, Day BJ, Ischiropoulos J, Stamler JS. Basal and stimulated protein S-nitrosylation in multiple cell types and tissues. J Biol Chem 2002;277:9637–9640. [DOI] [PubMed] [Google Scholar]
- 4.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 2001;3:193–197. [DOI] [PubMed] [Google Scholar]
- 5.Eu JP, Liu L, Zeng M, Stamler JA. An apoptotic model for ntirosative stress. Biochemistry 2000;39:1040–1047. [DOI] [PubMed] [Google Scholar]
- 6.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol Med 2003;9:160–168. [DOI] [PubMed] [Google Scholar]
- 7.Stamler JS, Jia L, Eu JP, McMahon TJ, Denchendo IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 1997;276:2034–2037. [DOI] [PubMed] [Google Scholar]
- 8.Luchsinger BP, Rich EN, Gow AJ, Williams EM, Stamler Singel DJ. NO interaction with oxidized hemes in human hemoglobin: routes to SNO-hemoglobin formation with preferential reactivity within the β subunits. Proc Natl Acad Sci USA 2003;100:461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawloski JR, Hess DT, Stamler JS. Export of red blood cells of nitric oxide bioactivity. Nature 2001;409:622–626. [DOI] [PubMed] [Google Scholar]
- 10.Scharfstein JS, Keaney JF, Slivka A, Welch GN, Vita JA, Stamler JS, Loscalzo J. In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. J Clin Invest 1994;94:1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci USA 2004;101:7891–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krowicki ZK, Sharkey KA, Serron SC, Nathan NA, Hornby PJ. Distribution of nitric oxide synthase in rat dorsal vagal complex and effect of microinjection of nitric oxide compound upon gastric motor function. J Com Neurology 1997;377:49–69. [DOI] [PubMed] [Google Scholar]
- 13.Kluge I, Gutteck-Amsler U, Zollinger M, Don KQ. S-nitrosoglutathione in rat cerebellum: identification and quantification by liquid chromatography-mass spectrometry. J Neurochem 1997;69:2599–2607. [DOI] [PubMed] [Google Scholar]
- 14.Lipton A, Johnson M, McDonald T, Leiberman M, Gozal D, Gaston BM. S-nitrosothiols signal the ventilatory response to hypoxia. Nature 2001;413:171–174. [DOI] [PubMed] [Google Scholar]
- 15.Palmer LA, Gaston B, Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox dependent effect of nitrogen oxides. Mol Pharmacol 2000;58:1197–1203. [DOI] [PubMed] [Google Scholar]
- 16.Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev 1996;76:839–885. [DOI] [PubMed] [Google Scholar]
- 17.Semenza G. HIF-1 and the mechanism of hypoxia sensing. Curr Opin Cell Biol 2001;13:167–171. [DOI] [PubMed] [Google Scholar]
- 18.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylase. Nat Rev Mol Cell Biol 2004;5:343–354. [DOI] [PubMed] [Google Scholar]
- 19.Semenza G. Hydroxylation of HIF-1: oxygen sensing at the molecular levels. Physiology 2004;19:176–182. [DOI] [PubMed] [Google Scholar]
- 20.Wenger RH. Cellular adaptation to hypoxia: O2 sensing protein hydroxylases, hypoxia inducible transcription factors, and O2 regulated gene expression. FASEB J 2002;16:1151–1162. [DOI] [PubMed] [Google Scholar]
- 21.Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B. Nitric oxide impairs normoxic degradation of HIF-1 by inhibition of prolyl hydroxylases. Mol Biol Cell 2003;14:2470–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasinska IM, Sumbayev VV. S-Nitrosation of Cys 800 on HIF-1α protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett 2003;549:105–109. [DOI] [PubMed] [Google Scholar]
- 23.Yu AY, Shimoda LA, Iyer NV, Juso DL, Sun X, McWilliams RM, Beaty T, Sham JSK, Wiener CM, Sylvester JT, et al. Impaired physiological response to chronic hypoxia in mice partially deficient for hypoxia inducible factor 1α. J Clin Invest 1999;103:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, Carmeliet P. Heterozygous deficiency of hypoxia-inducible factor 2α protect mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest 2003;11:1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]