Mucous secretions are frequently implicated in the morbidity and mortality associated with respiratory illness and airway disease, but we still do not understand precisely how these secretions develop or how to control them. A likely possibility is that the increased mucus is a consequence of overproduction and subsequent secretion in the setting of mucous cell metaplasia. In that regard, airway inflammatory diseases are invariably characterized by excessive mucous cell metaplasia, but precisely how inflammatory stimuli might influence mucous cell levels remains uncertain. We have used mouse models of viral bronchiolitis in concert with studies of patients with hypersecretory airway disease to define the cellular and molecular mechanisms for mucous cell metaplasia. Here we review our recent work that defines upstream immune events and downstream epithelial events that drive persistent mucous cell metaplasia. To date, we now recognize that upstream events include a new immune axis for growth factor and cytokine production and downstream events that include ciliated epithelial cell survival and transdifferentiation to mucous cells as well as expression of chloride channel calcium-activated (Clca) genes. To the extent that we can monitor these events in humans, it appears that similar alterations are found in patients with asthma and patients with chronic obstructive pulmonary disease (COPD). Together, the studies achieve more precise definition of just how viruses reprogram airway behavior and thereby provide a more rational basis for restoring epithelial architecture to normal.
An excess of airway mucous secretions is likely one of the most common maladies of humanity. The condition is not only an invariable feature of acute respiratory illnesses but is also a major feature of chronic lung diseases (Figure 1). In fact, the association with complex airway diseases such as asthma and chronic obstructive pulmonary disease (COPD) is likely responsible for much of the morbidity and mortality associated with these conditions. In the case of asthma, reports of mucus plugging and inspissation are often portrayed as characteristic of autopsies of patients with asthma. Similarly, much of the distress of patients with COPD may depend on disease of small airways that are grossly overpopulated with mucous cells (Figure 1). Indeed, some of these patients manifest only little of the other classical airway disease trait, namely, airway hyperreactivity, and yet still exhibit marked airway obstruction as well as functional compromise. Indeed, our impressions are that the degree of mucous cell metaplasia in chronic bronchitis/COPD is greater than the degree of airway hyperreactivity, whereas the opposite trend may hold for these traits in asthma. Nonetheless, in both of these diseases, as well as other hypersecretory conditions and diseases, mucous cell metaplasia is likely a major cause of respiratory symptoms, including cough and shortness of breath.
Figure 1.
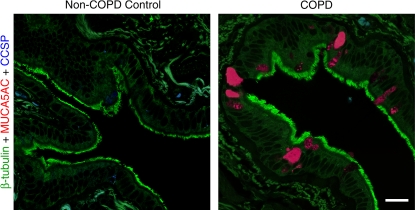
Histologic evidence of mucous cell metaplasia in COPD. Representative photomicrographs of airway sections from patients with COPD and from control patients without COPD using whole lung explants obtained at the time of lung transplantation. Sections were immunostained for β-tubulin (green), MUC5AC (red), and CCSP (blue) and imaged by laser scanning confocal microscopy as described previously (29, 84). Bar = 20 μm.
So, how do we explain the development of mucous cell metaplasia, especially in the setting of chronic airway disease? We have reasoned that two basic issues must be resolved: first, what are the upstream regulatory events that drive an airway epithelial cell to become a mucous cell; and second, what are the corresponding events that occur downstream at the level of the airway epithelial cell? In the first case, we have taken primarily an immunologic approach to define the inflammatory process that impacts airway epithelial behavior. In the second case, we have taken a combined genetic and genomic approach to better understand intrinsic epithelial cell biology. The present review contains two major sections to summarize progress on each of these questions. Perhaps as expected, there is significant overlap in the two issues, so we also attempt to integrate these concepts into a scheme for the development of mucous cell metaplasia and to point out the gaps for future studies of this problem.
IMMUNE PROGRAMS FOR MUCOUS CELL METAPLASIA
In defining the upstream regulatory events that might modify airway epithelial cell behavior, it was natural to consider the influence of the immune system. Thus, the concept has gradually evolved that chronic airway diseases, typified by asthma and COPD, are driven by a detrimental inflammatory response, and further that this mechanism represents an aberration or extension of the normal immune response. Despite the complexity of the immune response (or perhaps because of it), a relatively simple scheme was developed for asthma pathogenesis that rests on the classification of the adaptive immune system, and especially the T cell responses to allergic and nonallergic stimuli that enter the airway. This scheme rests on a relative increase in Th2 in combination with a decrease in Th1 cellular responses. Mouse models indicate that Th1 cells may still be necessary for the development of a Th2 response (1, 2) and that CD8+ T cells, NKT cells, and regulatory T cells (Treg) may also contribute to the allergic response (3–6). However, these variations can still be integrated into the hypothesis that asthma pathogenesis depends critically if not solely on the overproduction of Th2 cytokines (7–12). Among Th2 cytokines, perhaps the strongest case exists for IL-13 and perhaps IL-9 in driving the special problem of mucous cell metaplasia (13–15).
Some have argued that asthma may overlap with COPD pathogenesis (16). Shared mechanisms are highlighted by the association of abnormal airway inflammation with progression of COPD (17). Immune cell infiltration in asthma (typically by eosinophils, mast cells, and CD4+ Th2 cells) is often contrasted with inflammation in COPD (often characterized by neutrophils, macrophages, and CD8+ T cells). However, CD4+ T cells appear prominently in COPD and CD8+ T cells may contribute to asthma (17, 18), and activated macrophages may be found in both conditions (19). In either case, the same mediator profile may be produced by more than one cellular source, so even with a distinct inflammatory cell profile, it is still possible that asthma and COPD share similar molecular mechanisms and consequent overlap in endorgan dysfunction. In that regard, genetic analysis indicates that candidate genes such as IL-13 yield similar susceptibility profiles in cohorts of subjects with asthma and COPD (20), and overexpression of IL-13 in mice may lead to disease traits common to asthma and COPD (21). In the absence of allergy, it is therefore possible that another component of the immune response could lead to Th2 cytokine production and consequent airway disease. As developed further below, viral induction of chronic IL-13 gene expression is a candidate for such a shared mechanism among airway diseases.
An Alternative Immune Model: Airway Responses to Virus
To address the issue of asthma and COPD pathogenesis, we turned our attention to the host response to respiratory viruses. The approach was a natural consequence of our interest in developing a model that included airway epithelial cell and macrophage activation, since we considered these to be essential features of chronic airway disease (19, 22). For species, we chose a mouse model, since it is particularly suited to defining immunologic and genetic determinants that can then be tested for mechanistic relevance in patients with asthma or COPD. For viruses, we focused on common respiratory viruses that target the airways and have been linked to development or at least exacerbation of chronic airway disease. This raised the possibility of using a paramyxovirus, and within this family of negative-strand RNA viruses, there are several common human and mouse pathogens available for laboratory use (23). Among these viruses, RSV would have been a useful choice, as it is the most common cause of serious respiratory illness in early childhood and is most often associated with the development of childhood asthma. Unfortunately, mice are relatively resistant to infection with RSV, so a high threshold inoculum of virus must be given and the resulting all-or-none pattern of illness manifests primarily as alveolitis with viral localization to type I alveolar epithelial cells (24). Thus, although RSV was particularly useful in studies of isolated human cells, its utility was limited for experimental studies done in vivo. We also considered using a newly identified human pathogen, human metapneumovirus (hMPV), that appears to be associated with asthma, but the native virus is also weakly pathogenic in immunocompetent mice (E. Agapov and M. J. Holtzman, unpublished observations). In immunocompromised animals and humans, hMPV causes more severe infection (25, 26), but implications for airway disease are still being defined. Recently, another hMPV isolate has been reported to cause a persistent infection and Th2 cytokine response in mice (27). This type of variability has also been found for RSV and pneumonia virus of mice (PVM), so in each case it will be of interest to define the genetic differences between viral isolates (or with viral passage) that account for a change in host range and response.
While we continue to study these issues, we initiated our in vivo studies by using a related paramyxovirus, namely, mouse parainfluenza type I or Sendai virus (SeV) in the mouse model. SeV was isolated initially from humans and exhibits natural pathogenicity in immunocompetent mice. Delivery of intranasal SeV in the proper inoculum (e.g., 105 pfu) and mouse strain (e.g., C57BL/6J) allows for the development of viral bronchiolitis that maintains high fidelity with what we observe in humans. In particular, there is reversible illness with infection limited to the airway mucosa and inflammation largely restricted to peribronchial and bronchiolar tissues (28, 29). At higher inoculum, there is propagation of infection to distal airspaces with bronchopneumonia that if severe enough can lead to respiratory death (19, 30). At either inoculum, viral replication is localized primarily to airway epithelial cells (although detectable in airspace macrophages as well), thereby resembling the pattern that is found in children with severe paramyxoviral infection due to RSV (30, 31). Moreover, the infected epithelial cell population expresses a profile of immune-response gene expression similar to the one found in cultured airway epithelial cells infected with RSV or SeV (32). Subsequent work has indicated that both the epithelial cell (via IFN-β–IFNAR signaling) and the macrophage (via chemokine CCL5-CCR5 signaling) are necessary for antiviral defense and host survival (30, 33, and unpublished observations, L. P. Shornick and M. J. Holtzman). It also appears that more severe infection will drive a more prominent chronic response as developed in the next section.
Chronic Airway Responses to Virus
Our initial studies of paramyxoviral infection provided a useful framework for new observations on innate antiviral immunity, and the particular contributions of airway epithelial cells and macrophages, but the work focused largely on the acute response to viral infection. Because asthma and chronic bronchitis are often lifelong diseases and are strongly influenced by genetic background, we next questioned whether our experimental approach could be further developed to address the critical issues of chronicity and susceptibility. We therefore next focused on whether we could detect a long-term effect of respiratory viral infection on airway behavior, and if so, whether we could segregate this chronic change from the acute events that surround viral infection. We reasoned that inhibition of the acute inflammatory response could be achieved by targeted disruption of airway epithelial immune-response genes and so influence acute but not chronic inflammatory disease phenotypes. As noted above, among candidate genes that might mediate immune cell traffic, intercellular adhesion molecule (ICAM)-1 is the predominant determinant for adhesion of immune cells to epithelial cells in vitro (34–36). Thus, loss of ICAM-1 function would likely lead to decreased airway inflammation. Indeed, we found that ICAM-1 expression is induced primarily on host airway epithelial cells by viral infection and is necessary for the full development of acute inflammation and concomitant postviral airway hyperreactivity (28). These findings finally established a cause-and-effect relationship between acute airway inflammation and hyperreactivity that had been proposed in our initial report (37).
While these findings linking inflammation and hyperreactivity were perhaps expected, the next results were surprising. Thus, as we monitored host response over time, we discovered that a single, primary viral infection also caused airway hyperreactivity and mucous (goblet) cell metaplasia that lasted for at least a year after complete clearance of virus (28). This long-term (essentially permanent) phenotype developed regardless of ICAM-1 deficiency and ICAM-1–dependent alteration of the acute inflammatory response, thereby indicating distinct determinants for acute and chronic airway responses. Furthermore, the long-term virus-induced abnormalities were uninfluenced by IFN-γ deficiency, since each trait developed in IFN-γ–null mice as well. Each of these features is distinct from allergen-induced airway hyperreactivity and mucous cell metaplasia in the same mouse system. Thus, airway hyperreactivity and mucous cell metaplasia are also inducible by allergen challenge in this genetic background, but in the case of allergy, the phenotypes resolve spontaneously with time (in the absence of treatment or additional challenges) and are sensitive to the levels of IFN-γ. Indeed, the IFN-independent nature of virus-induced disease traits in this experimental setting is reminiscent of the low levels of IFN-γ or IFN-γ–producing cells found in subjects with asthma (22). Whether IFN production is similarly low in COPD, and whether other features of this inflammatory response are similar to the pattern in subjects with asthma and those with COPD, is still under study. While we define the mechanism for development of disease traits in this mouse model, we have therefore designated the phenotype as “asthmitis,” recognizing that the type of inflammation is distinct from the allergic response and (as developed below) likely shares cellular and molecular features found in both subjects with asthma and in those with chronic bronchitis.
Before concentrating further on the host response, it is also useful to consider the viral determinants for how respiratory viral infections can cause permanent abnormalities in airway behavior in the experimental setting and perhaps in humans. We recognized at least three possibilities to explain long-term actions of paramyxoviruses: (1) persistent infection (similar to hepatitis C virus), (2) mutant quasi-species (similar to coronaviruses), or (3) a hit-and-run phenomenon (similar to adenoviruses). Experiments aimed at monitoring tissue (especially lung) levels of virus with immunoblotting, immunostaining, plaque-forming assay, and real-time PCR all indicated that SeV was completely cleared from the organism by 2 wk after inoculation. This finding indicated that the actions of SeV to cause a chronic airway disease phenotype are based on a hit-and-run strategy since viral effects persist after clearance. Indeed, the results provide evidence of the capacity for nononcogenic RNA viruses to irreversibly reprogram host cell behavior in a manner previously restricted to oncogenic DNA viruses (38, 39). Further proof and understanding of this part of the process will depend on identifying specific viral gene products responsible for altering host gene expression and consequent phenotype. We have not found the same degree of chronic response after infection with influenza virus or hMPV. This preliminary finding raises the possibility of a subtractive genomic approach to define viral determinants for induction of chronic airway disease traits.
Determinants of the Chronic Response
Our initial work indicated that a single paramyxoviral infection could cause both acute and chronic responses in the host. These findings therefore raised the possibility that asthma not only resembles a persistent antiviral response (19, 22) but may also be caused by such a response, and so provided the experimental link between paramyxoviral infection in early life with subsequent asthma in childhood and perhaps adulthood. The findings also provide a substrate for segregating a complex disease into individual traits that can then be linked to the expression of specific host genes. Thus, ICAM-1 expression appears capable of regulating the acute response, whereas additional determinants may confer individual traits (i.e., airway hyperreactivity and mucous cell metaplasia) that constitute the chronic phenotype. This process can be modeled using a time-dependent scheme for acute and chronic responses to viral infection (Figure 2). Having uncovered several determinants of the acute response, our next experiments aimed at identifying candidates to mediate the chronic response to virus.
Figure 2.
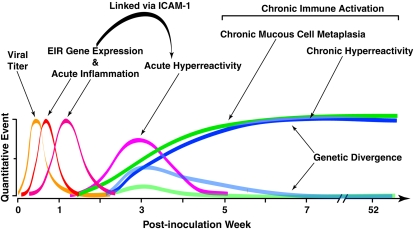
Model for acute and chronic airway responses to respiratory viruses. The model is based on the time course of quantitative traits that develop after primary paramyxoviral infection in mice. Events begin with viral replication (which peaks at 3–5 d after inoculation) that is later cleared from the lung (by 2 wk after inoculation). This initial infection is followed by induction of epithelial immune-response gene expression (which peaks at 5 d after inoculation) and is followed by immune cell infiltration (which peaks at various times depending on cell type, e.g., 3 d for neutrophils, 8 d for macrophages, and 12 d for lymphocytes). Each of these events is linked to the subsequent development of acute airway hyperreactivity that depends on ICAM-1 gene expression and peaks at ∼ 21 d after inoculation. After this time, there is progressive and chronic mucous cell metaplasia and hyperreactivity that persist indefinitely after infection and are driven by ongoing pressure a reprogrammed innate and adaptive immune system. These chronic disease traits can be genetically segregated by choice of inbred mouse strain or breeding for susceptible and resistant offspring. A similar set of events may follow respiratory viral infection in children that develop a persistent or recurrent asthma phenotype. Modified from Ref. 115.
In searching for candidates to mediate the virus-induced chronic response, we recognized that recent work on mucous cell metaplasia often focused on signaling pathways initiated by activation of the IL-13 receptor (IL-13R) and the epidermal growth factor receptor (EGFR, also designated ErbB1 or HER1). The experimental role of IL-13R was established when a decoy receptor for IL-13 (soluble IL-13Rα2-Fc) was found to inhibit allergen-induced mucous cell formation in mice (13, 14). These reports have been followed by evidence that IL-13 can directly drive mucin gene expression in airway epithelial cells cultured under physiologic culture conditions and in vivo (40–43). Moreover, IL-13 is often overexpressed in the setting of mucous cell metaplasia in asthma and COPD (8, 44, 45). The downstream events connecting IL-13R activation to mucin gene expression are incompletely defined, but preliminary work indicates requirements for Stat6, MEK/ERK, p38 MAPK, and PI3K activation in vitro but not always in vivo (42, 46). These effects may develop in concert with calcium-activated chloride conductance to promote fluid secretion and consequent mucociliary clearance (47). As developed below, this function may be connected to expression of a calcium-activated chloride channel (CLCA) that is specific for mucous cells (48). Thus, IL-13 appeared to directly stimulate epithelial mucin formation, but the type of epithelial cell that was targeted and the cellular process for mucous cell differentiation remained less certain.
Similar to the case for IL-13, the pathway for EGFR activation leading to mucous cell metaplasia was not well defined. Altered EGFR expression was found in asthma and in animal models of asthma, but expression was variably found on mucous cells as well as other types of airway epithelial cells (e.g., squamous, basal, ciliated, and Clara cells) (49–58). This variability was further complicated by uncertainty over the specificity of anti-EGFR antibodies and their capacity to define EGFR activation status. In addition, similar to the case for IL-13, animal models often relied on allergen challenge that appeared to drive mucin gene expression predominantly in cells that resemble Clara cells by morphology (59–61). These cells expressed Clara cell secretory protein (CCSP), but tracking cell lineage was complicated by EGFR and IL-13–dependent stimulation of CCSP expression, perhaps in multiple cell types (62). Furthermore, extensions of these studies to signaling mechanisms was often performed in transformed cell lines (54, 56, 63–68), and even when primary airway epithelial cells were used, cultures were not fully differentiated under physiologic conditions to a respiratory epithelium (55, 69–71). Thus, one scheme from this work suggested that IL-13 stimulation of EGFR signaling leads to mucin gene expression (58), but studies of airway epithelial cells under physiologic conditions show that IL-13 fully stimulated mucous cell metaplasia despite EGFR blockade (42). Thus, there was likely a fundamental requirement for EGFR activation in mucociliary differentiation (42, 72), but how this requirement may be linked to the modification of epithelial cell growth or differentiation during cytokine stimulation, inflammation, or infection still needed to be defined. Indeed, the whole subject of EGFR regulation of cell survival, which appears critical for neoplasia, had not yet been taken into account in the process of epithelial cell metaplasia during inflammatory disease. Moreover, previous approaches concentrated predominantly on the acute phase of epithelial remodeling without addressing chronic mucous cell metaplasia in hypersecretory airway diseases.
We addressed these issues in our mouse model of airway epithelial remodeling that features a delayed but permanent virus-inducible switch to mucous cell metaplasia (28). When we examined the behavior of EGFR signaling in this model, we detected acute activation of EGFR during the epithelial repair phase that was localized to basal cells and immune cells, but this was replaced by chronic activation of EGFR localized to ciliated epithelial cells during the development of mucous cell metaplasia. This chronic activation coincided with ciliated cell hyperplasia without a requirement for ongoing epithelial proliferation. Both ciliated cell hyperplasia and mucous cell metaplasia could be prevented by treatment with a new irreversible inhibitor of EGFR tyrosine kinase. These findings suggested a role for EGFR-dependent signaling pathways in ciliated cell survival, and we subsequently detected and defined such a mechanism that proceeds via PI3K/Akt signaling to selectively protect ciliated epithelial cells from apoptosis.
However, this ciliated cell mechanism did not readily explain a requirement for EGFR signaling in mucous cell metaplasia until we next detected ciliated cells that appeared to transdifferentiate to mucous cells under pressure from IL-13 stimulation at least in vitro. Consistent with this finding, inhibition of IL-13 signaling blocked mucous cell formation but also, by preventing transdifferentiation, further increased the level of ciliated cell hyperplasia in vivo. The results thereby provided a new paradigm for chronic mucous cell metaplasia that depends on persistent activation of two complementary pathways: EGFR-PI3K signals that protect against ciliated cell apoptosis and IL-13 signals that promote ciliated to mucous cell transdifferentiation. This scheme is consistent with EGFR and IL-13 effects on airway epithelial cells in vitro as well as ciliated cell EGFR activation, IL-13 production, ciliated-to-mucous cell transdifferentiation, and mucous cell metaplasia in the epithelium of patients with asthma and of those with COPD. Therefore, treatment to fully restore normal epithelial behavior in asthma and related hypersecretory conditions such as COPD may need to be directed at combined correction of EGFR- and IL-13–dependent abnormalities in airway epithelial cell survival and differentiation. In that regard, we established the efficacy of an orally active and irreversible EGFR inhibitor and a soluble IL-13 decoy to prevent these abnormalities in epithelial architecture at least under experimental conditions in mice.
Our study provided initial evidence that transient viral bronchiolitis causes a long-term switch to ciliated cell hyperplasia as well as mucous cell metaplasia, and that the hyperplastic ciliated cell population exhibits persistent EGFR activation without proliferation. This finding came in some contrast to the fate of ciliated cells in other lung injury models in which this cell population is also primarily responsible for airway repair in concert with EGFR expression but depends on a marked proliferative response (51, 73). The findings therefore raised the unexpected possibility that prolonged EGFR-dependent cell survival (not proliferation) is critical for remodeling of epithelial structure toward a chronic asthma/bronchitis phenotype. Support for this possibility was obtained when we showed that EGFR blockade prevented ciliated cell hyperplasia in vivo and caused ciliated epithelial cell apoptosis in vitro. Additional study of airway epithelial cells cultured under physiologic conditions indicated that ciliated epithelial cell survival depends on uninterrupted EGFR signaling to PI3K. Otherwise, the ciliated cells proceed toward programmed cell death (via caspase activation) in a manner that appears analogous to virus-inducible apoptosis (30). The fidelity of our model to human disease is supported by initial experiments that detect ciliated epithelial cells with activated EGFR in airway sections from subjects with asthma, but further studies will be needed to verify this finding and extend it to other chronic airway diseases. Nonetheless, the findings indicate that the plasticity and responsiveness of ciliated cells in the setting of airway damage and inflammation is an underappreciated but seminal feature of airway epithelial remodeling.
Another major set of findings from our work was focused on IL-13 signaling and the capacity of ciliated cells to transdifferentiate to mucous cells under IL-13 stimulation. This finding was also unexpected, since previous work had suggested that IL-13 may cause a decrease in ciliated cells and an increase in mucous cells, but no apparent connection was drawn between the two events (40, 42). We were able to capture snapshots of epithelial cells in vitro and in vivo that appear to be transitioning from a ciliated to a mucous cell phenotype under pressure from IL-13. In addition, we detected reciprocal increases in ciliated cell levels during IL-13 blockade in vivo. Additional cell lineage studies of this process are needed to fully define the mechanism of transdifferentiation, but the evidence points to a program that carefully coordinates ciliated and mucous cell formation to achieve proper mucosal immunity. Indeed, epithelial EGFR activation, ciliated cell hyperplasia, IL-13 production, and mucous cell metaplasia appear to develop together in concert. This type of coordination is likely required for efficient mucociliary function. Our results suggest that abnormally prolonged IL-13 production may lead to mucous cell metaplasia beyond the initial repair phase, and so explain how mucous cell metaplasia may develop in this setting. Thus, genetic susceptibility to the development of persistent EGFR activation as well as IL-13 production after viral infection may allow for the consequent development of mucous cell metaplasia. Whether a similar process occurs in response to other asthmagenic stimuli (e.g., allergen exposure) will need further study in models that mimic the human condition. Nonetheless, the fidelity of the present model to human disease is again supported by experiments that detect ciliated-to-mucous cell transdifferentiation in airway sections and cultured cells from patients with COPD likely under the influence of IL-13.
Together, our results on EGFR- and IL-13–dependent signaling provide a new paradigm for epithelial host defense and remodeling (summarized in Figure 3) that should be useful for developing a rational basis for therapies aimed at down-regulating hypersecretory conditions. Tyrosine kinase inhibitors in general and EGFR tyrosine kinase inhibitors in particular are being broadly developed for use in conditions exhibiting abnormal secretion, including asthma and COPD, but the cellular signaling context for their application to airway disease was uncertain. Our initial strategy used an irreversible inhibitor of EGFR tyrosine kinase activity with efficacy in preventing epithelial hyperplasia in a model of intestinal neoplasia (74). In that setting, the pharmacologic strategy was aimed at inhibiting epithelial proliferation, but our findings indicate that interrupting anti-apoptotic signals may be the primary target in inflammatory airway disease. Further development of approaches for targeting EGFR (as well as those directed at IL-13–dependent events) will also benefit from further defining the signaling events that regulate airway epithelial cell apoptosis and transdifferentiation. In that regard, we provide evidence that downstream signaling to prevent epithelial cell apoptosis proceeds through EGFR-dependent PI3K but not MEK/ERK activation based on selective inhibition. This finding implies that the targeted pathway extends from EGFR homo- or hetero-dimerization and activation of the receptor tyrosine kinase cytosolic domain to autophosphorylation of tyrosine resides within the cytoplasmic domain, docking of Gab2/PI3K, and subsequent activation of Akt signaling (75). EGFR survival signals may be mediated by Akt-dependent phosphorylation and inactivation of the pro-apoptotic factor BAD (76, 77). Defining these downstream signals that preserve mitochondrial integrity may also help to develop therapeutic strategies aimed at blocking mucous cell metaplasia.
Figure 3.
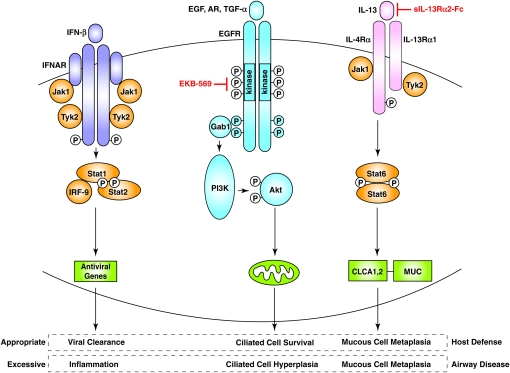
Scheme for virus-inducible IFN, EGF, and IL-13 receptor–dependent signaling pathways in the airway epithelium. IFN signaling (shown here for IFN-β) proceeds via IFNAR activation to form the Stat1–Stat2–IRF-9 complex that upregulates expression of a series of antiviral genes. Production of EGF (or amphiregulin or TGF-α) causes EGFR activation followed by Gab1 recruitment and PI3K activation that causes activation of Akt that inactivates pro-apoptotic factors at the level of the mitochondria and promotes ciliated cell survival. IL-13 activates IL-13R and then Stat6 that drives expression of CLCA and MUC genes that promote cilia to mucous cell transdifferentiation. Under appropriate physiologic conditions, these pathways may lead to protection from viral infection, but if there is persistent or inappropriate activation in a susceptible genetic background, the same pathways may lead to airway inflammation, ciliated cell hyperplasia, and mucous cell metaplasia. Rational use of specific inhibitors (e.g., EGFR and IL-13 receptor blockers) may help restore balance and a normal epithelial architecture. Modified from Ref. 29.
Our results for chronic EGFR-PI3K survival signaling stand in some contrast to reports of EGFR and other signals to ERK1/2 to promote cell survival under other circumstances (78). For example, respiratory syncytial virus (RSV) may trigger EGFR and ERK1/2 activation in cultured epithelial cells during the acute infection (79). This study was performed using transformed cells with necessarily altered death pathways or in submerged cell cultures that do not differentiate into ciliated epithelial cells. Nonetheless, we recently showed that infection with RSV (as well as SeV and influenza virus) may promote epithelial cell survival in the acute setting based on chemokine-dependent activation of either PI3K/Akt or MEK/ERK signaling both in vitro (using well-differentiated mouse and human cells) and in vivo (using a mouse model of acute infection) (30). This signaling mechanism is therefore distinct from the situation in the chronic setting. We also found evidence of EGFR activation in vivo during the repair phase after viral infection, but whether this signal is necessary for epithelial repair still needs to be determined. Here again, this issue will need to be addressed for successful use of EGFR inhibitors.
Our results complement previous ones suggesting that Clara cells also give rise to mucous cells. Thus, allergen-induced mucous cell metaplasia is accompanied by morphologic and biochemical evidence of CCSP-positive Clara cells containing mucous granules in mice (59–61). Inhibitor effects were not reported in those studies, but at least one report showed a decrease in Clara cell level concomitant with an increase in mucous cell level consistent with Clara-to-mucous cell transdifferentiation (59). Similarly, others used the rat CCSP promoter to delete endogenous IL-13 receptor signaling and downregulate mucous cell metaplasia in allergen-challenged mice (43). Given the present results, residual metaplasia in that model may have been derived from ciliated cells. However, comparison to the present results is complicated by the change in stimulus (from allergen to virus) and genetic background (from Balb/cJ to C57BL/6J). Moreover, IL-13 may regulate CCSP expression (Y. You and S. Brody, unpublished observations, and Ref. 62) and the rat CCSP promoter system may not be specific for mouse Clara cell expression. In fact, significant levels of CCSP-driven recombination can be found in ciliated airway epithelial cells (80). Thus, it may be necessary to track other markers of Clara cell lineage to fully define the relative contribution of Clara versus ciliated cell populations to mucous cell metaplasia. We found virus-induced decreases in Clara cell levels that were reversed by EGFR blockade but unchanged after IL-13 inhibition, so the significance of any Clara cell contribution to mucous cell metaplasia after viral infection remains uncertain. Nonetheless, our observations of CCSP and β-tubulin co-localization with MUC5AC in mice and in humans suggests that ciliated and Clara cells may each demonstrate sufficient plasticity to contribute to mucous cell metaplasia.
Our work extends the line of reasoning that airway epithelial cells may be specially programmed for normal immune defense (especially against respiratory viruses) and abnormally programmed in airway disease. Previous work focused on other epithelial immune responses (notably interferon signaling) and how the epithelium cleared infection in the short term (33, 81), whereas the subsequent work focused on EGFR and IL-13 and how the epithelium responds in the long term. Similar to other epithelial responses, the EGFR and IL-13 signals were likely developed to protect host epithelial cells from the lethal effect of viruses and to optimize mucosal immunity (see again Figure 3). As noted above, the epithelial EGFR anti-apoptotic system may protect the host cell (often the ciliated cell) from cytopathic effects that allow spread of infection. In parallel, the IL-13–dependent mucous cell system may direct increases in mucus formation to aid mucociliary clearance of cellular and microbial debris from the airway. Both of these strategies are likely achieved in concert with epithelial interferon signaling that also protects the host cell by inhibiting viral replication. The present work adds the critical piece that demonstrates how, in some genetic settings, this normally protective response may be skewed toward a persistent response that results in a chronic asthma/bronchitis phenotype. Further study is needed to determine whether this genetic susceptibility is linked to EGFR mutations that confer anti-apoptotic signals and inhibitor sensitivity in lung cancer cells (82) or whether persistent EGFR activation is connected to IFN signaling and chronic Stat1 activation found in asthma (22) as may be the case in other epithelial barriers (83). Nonetheless, our results demonstrate that the abnormality in epithelial immune-response programming can be corrected by targeted inhibition of critical signaling steps.
EPITHELIAL PROGRAMS FOR MUCOUS CELL METAPLASIA
In addition to defining upstream events that drive mucous cell metaplasia, we have also studied events at the level of the airway epithelial cell that regulate this process. We were particularly interested in events that occur in vivo. Thus, in contrast to earlier studies by our group and others of isolated airway epithelial cells, we explored this question in the mouse model of viral bronchiolitis and persistent mucous cell metaplasia. In that regard, the mouse model is particularly suited to defining genetic determinants that can then be tested for mechanistic relevance in subjects with asthma or COPD. Critical questions include how to segregate individual traits that comprise the chronic phenotype, and how host genetics determine susceptibility to developing the phenotype.
Accordingly, in another study, we aimed to fully segregate airway disease traits and thereby identify a pathway that might be associated with a single trait. We especially focused on defining the role of genes responsible for chronic mucous cell metaplasia versus airway hyperreactivity. As noted above, we recognized that both of these traits are inducible on a long-term basis after viral bronchiolitis in the C57BL/6J strain of inbred mice (28). In contrast, we next realized that the Balb/cJ strain responded similarly during the acute infection but then failed to develop any chronic alteration in airway behavior (84). We took advantage of this difference in genetic susceptibility to develop an F2 intercross population with phenotypic extremes that exhibited one or the other disease trait, and we analyzed these extremes by differences in gene expression using oligonucleotide microarrays. This combined genetic and genomic strategy provided evidence of a selective association between expression of a member of the calcium-activated chloride channel (Clca) gene family (i.e., mClca3) with the development of mucous cell metaplasia but not airway hyperreactivity. When mucous cell metaplasia persisted despite loss of mClca3 function in newly developed mClca3−/− mice, we then defined the role of another Clca family member (i.e., mClca5) as being capable of shared biologic function. The findings thereby provided a useful model for segregating and defining complex disease traits in general and airway disease traits in particular. Moreover, the results help to clarify the specific role of the CLCA gene family in the development of mucous cell metaplasia in airway disease and thereby point to homologies in this family as a target for selective therapeutic intervention.
This new work provided several new insights into the pathogenesis of complex airway diseases. In particular, we developed an experimental model for segregating airway disease traits and thereby defined independent genetic susceptibilities for developing the individual traits of mucous cell metaplasia versus airway hyperreactivity. We also took advantage of segregation into phenotypic extremes and genomic analysis to identify mClca3 gene expression as being linked to one trait (mucous cell metaplasia) but not the other (airway hyperreactivity). In addition, we developed an mClca3−/− mouse and Clca gene-transfer system to show that mClca3 was sufficient but not necessary for mucous cell metaplasia. This finding in turn led to the identification of mClca5 as a functional homolog and thereby capable of substituting for mClca3 in the development of this disease trait. The study thereby establishes the principles of genetic segregation and redundancy in the development of airway disease. Because of the clinical overlap and variability among individual patients with inflammatory airway diseases such as asthma and COPD, this principle is critical for understanding how these conditions develop and for developing more specific biomarkers and therapies for them.
Our study comes in the context of others that aim to define the genetic influence on the development of complex airway diseases. In that regard, inbred mouse strains have been used to define genetic differences in native airway reactivity as well as increases in reactivity after exposure to inhaled irritants (85–89). Others have examined differences between mouse strains or among populations of hyperreactive to hyporeactive crosses to establish determinants of airway hyperreactivity after allergen challenge (90–93). In the usual case, these previous studies were directed at defining a genetic locus for a single quantitative trait rather than segregating one trait from another in a complex phenotype. Our findings may serve to encourage a reductionist approach that isolates one trait within a complex phenotype and thereby allows for more precise definition of molecular mechanism for the trait under study.
Our results should also help to clarify the findings from previous studies of CLCA function in mice and humans. In an initial study, mClca3 was found to be sufficient to cause airway hyperreactivity and mucous cell metaplasia when expressed in the mouse airway using adenoviral gene transfer (94). Furthermore, expression of an antisense construct for mClca3 suppressed hyperreactivity and mucus production induced by allergen challenge in mice. Based on this information, mClca3 was proposed as both necessary and sufficient for allergen-induced airway hyperreactivity and mucous cell metaplasia. However, others recently reported that allergen-induced mucous cell metaplasia is no different in mClca3−/− mice (developed in a 129/C57BL/6J chimeric background) compared with wild-type control mice, but these investigators did not examine airway reactivity (95). In addition, they analyzed only mClca1, mClca2, and mClca4 gene expression, and when these genes showed no allergen induction, they concluded that other Clca family members could not compensate for the loss of mClca3 in the development of mucous cell metaplasia. In fact, our work indicates that mClca5 appears to be a suitable candidate for compensatory function.
In that context, we note that the airway system may be designed for redundancy in the pathways leading to mucous cell metaplasia and hyperreactivity. There are currently at least 20 mucin genes and 6 CLCA family members, and both types of mucus regulatory genes appear to be expressed in airway tissue (96–100). The genes for CLCA family members are clustered on the short arm of chromosome 1 in human (syntenic for chromosome 3 in mouse) suggesting the presence of a large family derived from gene duplication. Airway mucin genes (including MUC5AC) likely arose from a single ancestral gene on chromosome 11 as well (101). Moreover, mucous cell mucins and CLCA proteins may regulate cell adhesion as well as apoptosis in addition to other possible roles in host defense, repair, and ion movement (102). Thus, redundant pathways for preserving the CLCA-MUC axis and the consequent mucous cell lineage would likely confer a useful advantage to the host. An obvious corollary of this circumstance is that hCLCA1/mClca3 inhibition achieved by niflumic acid (a chloride-channel blocker) may be effective by producing a more widespread inhibition of CLCA activities than a targeted gene knockout (103–106). Similarly, for antisense strategies, it is possible that the inhibitory effect on mucous cell metaplasia found in previous studies was due to cross-reactivity with other closely related and homologous targets, including other calcium-activated chloride channels (97, 98). Thus, the loss of either mClca3 or mClca5 alone would not be sufficient to block mucous cell metaplasia in vivo.
Precisely how mClca3 or mClca5 cause mucous cell metaplasia is uncertain. Structure–function studies indicate that expression of CLCA proteins results in a chloride current that is induced by the calcium ionophore ionomycin and inhibited by niflumic acid (107), but a connection between ion channel function and mucin gene expression remains uncertain. Possibilities include regulation of ATP-mediated mucin exocytosis as well as adenosine induction of mucin synthesis via EGFR transactivation, since these processes are also inhibited by niflumic acid (108, 109). However, other studies suggest that CLCA proteins may function not as ion channels but instead as signaling molecules (110). For example, all CLCA family members contain a von Willebrand factor (VWF) domain with signaling capabilities. Indeed, mClca1 binding to β4-integrin activates focal adhesion kinase (FAK) and downstream ERK with the potential to regulate mucin synthesis (111). This work and most other previous studies were performed in cell lines that may exhibit differences from primary-culture cells in the regulation of mucin gene expression (Y. Alevy, A. C. Patel, and M. J. Holtzman, unpublished observations). Thus, additional work is needed to determine the role of ion channel activity or protein–protein interactions in regulating mucin synthesis, mucin secretion/exocytosis, and mucous cell metaplasia. The present results suggest that mClca3 and mClca5 may share structural determinants that regulate mucous cell metaplasia and thereby provide a basis for further definition of structure–function relationships for this family of proteins (summarized in Figure 4).
Figure 4.
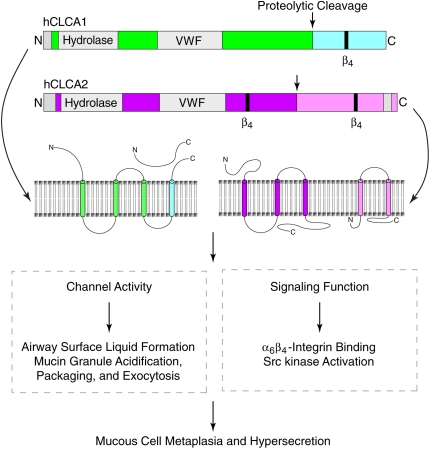
Scheme for CLCA structure and function. Proposed domain structure for hCLCA1 and hCLCA2 includes a hydrolase domain that may regulate proteolytic cleavage of the protein (116) and a von Willebrand factor (VWF) domain that may regulate interactions with other proteins, including ion channels. Following glycosylation and then proteolytic cleavage at the indicated sites, both hCLCA1 and hCLCA2 are internalized in the cell surface or mucin granule membrane and secreted as shown. In other schemes, all fragments of the mature protein are secreted. In either case, after processing, the mature proteins may participate in calcium-activated chloride channel activity (lower left box) or in signal transduction (lower right box) to regulate the indicated biological events and consequently contribute to mucous cell metaplasia and mucus hypersecretion.
Similarly, we have not yet determined the upstream events that regulate CLCA expression or the downstream events that mediate CLCA function. However, we note that the 5′-regulatory region of the mClca3 gene contains a putative binding site for the Stat6 transcription factor that mediates IL-13R signal transduction and that IL-13 stimulates mClca3 (and mClca5) gene expression in cultured airway epithelial cells (A. C. Patel, J. D. Morton, and M. J. Holtzman, unpublished observations). Moreover, IL-13 expression is inducible in the mouse model of viral infection as well as the one for allergen challenge (112). Thus, it appears likely that mClca3 and mClca5 expression is driven at least in part by IL-13 in these settings. We are currently using a cell line that stably expresses hCLCA1 or hCLCA2 in an inducible promoter system to help define the capacity of CLCA proteins to regulate mucin gene expression.
To date, our work provides the first model for genetic segregation of airway disease traits of mucous cell metaplasia and airway hyperreactivity and the first evidence that mClca3 gene expression is associated with one trait but not the other. The results further indicate that other Clca family members (e.g., mClca5) may also mediate mucous cell metaplasia in the setting of an isolated deficiency in the mClca3-dependent pathway responsible for mucous cell formation. The mouse system appears to be directly relevant to human airway disease because the human homologs for mClca3 and mClca5, hCLCA1 and hCLCA2, are both overexpressed in airway tissue of subjects with asthma and subjects with cystic fibrosis and are again co-localized to mucous cells (48, 113, 114). The results thereby highlight the complex nature of chronic airway disease as well as individual asthma traits, and provide a rational basis to specifically and effectively inhibit mucous cell metaplasia by globally inhibiting CLCA family members that may mediate this trait. Further understanding of how CLCA gene expression is regulated and how CLCA proteins act to regulate mucus production and secretion downstream signaling should provide critical insights into therapeutic strategies for hypersecretory diseases.
CONCLUSION AND FUTURE DIRECTIONS
Traditional proposals for mucous cell metaplasia in the setting of respiratory illness and disease have focused on the adaptive immune system and overproduction of Th2 cytokines. We have questioned whether additional immune mechanisms might be identified if a high-fidelity model of the chronic disease process could be developed and analyzed. In that regard, we have used a mouse model of viral bronchiolitis in concert with studies of patients with hypersecretory airway disease to define upstream immune events and downstream epithelial events that drive persistent mucous cell metaplasia. Critical questions include: how specific viral genes confer the chronic disease phenotype; how memory for the phenotype is preserved; and how host genetics determine susceptibility to developing individual disease traits? Initial results provide only partial answers to these questions. Thus, immunologic and genetic approaches have now defined upstream events that include a new immune axis for growth factor and cytokine production and downstream events that include ciliated epithelial cell survival and transdifferentiation to mucous cells as well as expression of chloride channel calcium-activated (Clca) genes. Similar events appear to develop in patients with asthma and those with COPD. Together, the studies achieve more precise definition of just how viruses reprogram airway behavior and thereby provide a more rational basis for restoring epithelial architecture to normal. Additional work is needed to fully answer these questions, including better definition of the interface between upstream and downstream events. Nonetheless, it is encouraging that several therapeutic targets have already been identified in an area where no selective and effective therapy yet is available. In that regard, we recognize that these abnormalities represent changes in systems that are normally required for host defense. Thus, a particular challenge for therapeutic strategies will be to restore proper balance to the immune system without compromising host defense.
Acknowledgments
The authors gratefully acknowledge their laboratory colleagues for valuable assistance and advice in the course of this work.
Supported by grants from the National Institutes of Health (Heart, Lung, and Blood Institute), Martin Schaeffer Fund, and Alan A. and Edith L. Wolff Charitable Trust.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0092SF on March 16, 2006
Conflict of Interest Statement: M.J.H. was the Principal Investigator for a $37,000 research grant from Roche Palo Alto in 2003–2004 and 2004–2005 to study the genetic basis for chronic airway disease. J.T.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.C.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Randolph DA, Stephens R, Carruthers CJL, Chaplin DD. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J Clin Invest 1999;104:1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen G, Berry G, DeKruyff R, Umetsu D. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airay hyperreactivity but cause severe airway inflammation. J Clin Invest 1999;103:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, Dakhama A, Ott VL, Gelfand EW. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med 2004;10:865–869. [DOI] [PubMed] [Google Scholar]
- 4.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, Dekruyff RH, Umetsu DH. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyper-reactivity. Nat Med 2003;9:582–588. [DOI] [PubMed] [Google Scholar]
- 5.Stock P, Akbari O, Berry G, Freeman GJ, Dekruyff RH, Umetsu DH. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat Immunol 2004;5:1149–1156. [DOI] [PubMed] [Google Scholar]
- 6.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, De Kruyff RH, Umetsu DH. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induced airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci USA 2006;103:2782–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kay AB. Allergy and allergic diseases. N Engl J Med 2001;344:30–37. [DOI] [PubMed] [Google Scholar]
- 8.Berry MA, Parker D, Neale N, Woodman L, Morgan A, Monk P, Bradding P, Wardlaw AJ, Pavord ID, Brightling CE. Sputum and bronchial submucosal IL-13 expression in asthma and eosiniophililc bronchitis. J Allergy Clin Immunol 2004;114:1106–1109. [DOI] [PubMed] [Google Scholar]
- 9.Ober C, Moffatt MF. Contributing factors to the pathobiology: the genetics of asthma. Clin Chest Med 2000;21:245–261. [DOI] [PubMed] [Google Scholar]
- 10.Willliams CMM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med 2000;192:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandeira-Melo C, Herbst A, Weller PF. Eotaxins. Contributing to the diversity of eosinophil recruitment and activation. Am J Respir Cell Mol Biol 2001;24:653–657. [DOI] [PubMed] [Google Scholar]
- 12.Grayson MH, Holtzman MJ. Lessons from allergic rhinitis versus asthma pathogenesis and treatment. Immunol Allergy Clin North Am 2002;22:845–869. [Google Scholar]
- 13.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 15.Reader JR, Hyde DM, Schelegle ES, Aldrich MC, Stoddard AM, McLane MP, Levitt RC, Tepper JS. IL-9 induces mucous cell metaplasia independent of inflammation. Am J Respir Cell Mol Biol 2003;28:664–672. [DOI] [PubMed] [Google Scholar]
- 16.Postma DS, Boezen HM. Rationale for the Dutch hypothesis. Chest 2004;126:96S–104S. [DOI] [PubMed] [Google Scholar]
- 17.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 18.O'Sullivan S, Cormican L, Faul JL, Ichinohe S, Johnston SL, Burke CM, Poulter LW. Activated, cytotoxic CD8+ T lymphocytes contribute to the pathology of asthma death. Am J Respir Crit Care Med 2001;164:560–564. [DOI] [PubMed] [Google Scholar]
- 19.Walter MJ, Kajiwara N, Karanja P, Castro M, Holtzman MJ. IL-12 p40 production by barrier epithelial cells during airway inflammation. J Exp Med 2001;193:339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyers DA, Larj MJ, Lange L. Genetics of asthma and COPD. Chest 2004;126:105S–110S. [DOI] [PubMed] [Google Scholar]
- 21.Lanone S, Zheng T, Zhu Z, Liu W, Lee CG, Ma B, Chen Q, Homer RJ, Wang J, Rabach LA, et al. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13-induced inflammation and remodeling. J Clin Invest 2002;110:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sampath D, Castro M, Look DC, Holtzman MJ. Constitutive activation of an epithelial signal transducer and activator of transcription (Stat1) pathway in asthma. J Clin Invest 1999;103:1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holtzman MJ, Tyner JW, Kim EY, Lo MS, Patel AC, Shornick LP, Agapov E, Zhang Y. Acute and chronic airway responses to viral infection: implications for asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol 1988;26:153–162. [DOI] [PubMed] [Google Scholar]
- 25.Sumino KC, Agapov E, Pierce RA, Trulock EP, Pfeifer JD, Ritter JH, Gaudreault-Keener M, Storch GA, Holtzman MJ. Detection of severe human metapneumovirus infection by real-time polymerase chain reaction and histopathological assessment. J Infect Dis 2005;192:1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agapov E, Sumino KC, Gaudreault-Keener M, Storch GA, Holtzman MJ. Genetic variability of human metapneumovirus infection: evidence of a shift in viral genotype without a change in illness. J Infect Dis 2005;193:396–403. [DOI] [PubMed] [Google Scholar]
- 27.Alavarez R, Tripp RA. The immune response to human metapneumovirus is associated with aberrant immunity and impaired virus clearance in BALB/c mice. J Virol 2005;79:5971–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter MJ, Morton JD, Kajiwara N, Agapov E, Holtzman MJ. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J Clin Invest 2002;110:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 2006;116:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyner JW, Uchida O, Kajiwara N, Kim EY, Patel AC, O'Sullivan MP, Walter MJ, Schwendener RA, Cook DN, Danoff TM, et al. CCL5–CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat Med 2005;11:1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domachowske JB, Rosenberg HF. Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment. Clin Microbiol Rev 1999;12:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koga T, Sardina E, Tidwell RM, Pelletier MR, Look DC, Holtzman MJ. Virus-inducible expression of a host chemokine gene relies on replication-linked mRNA stabilization. Proc Natl Acad Sci USA 1999;96:5680–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo MS, Brazas RM, Holtzman MJ. Respiratory syncytial virus nonstructural proteins NS1 and NS2 mediate inhibition of Stat2 expression and type I interferon responsiveness. J Virol 2005;79:9315–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima S, Look DC, Roswit WT, Bragdon MJ, Holtzman MJ. Selective differences in vascular endothelial- vs. airway epithelial-T cell adhesion mechanisms. Am J Physiol 1994;267:L422–L432. [DOI] [PubMed] [Google Scholar]
- 35.Nakajima S, Roswit WT, Look DC, Holtzman MJ. A hierarchy for integrin expression and adhesiveness among T cell subsets that is linked to TCR gene usage and emphasizes Vδ1+ γδ T cell adherence and tissue retention. J Immunol 1995;155:1117–1131. [PubMed] [Google Scholar]
- 36.Taguchi M, Sampath D, Koga T, Castro M, Look DC, Nakajima S, Holtzman MJ. Patterns for RANTES secretion and intercellular adhesion molecule-1 expression mediate transepithelial T cell traffic based on analyses in vitro and in vivo. J Exp Med 1998;187:1927–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holtzman MJ, Fabbri LM, O'Byrne PM, Gold BD, Aizawa H, Walters EH, Alpert SE, Nadel JA. Importance of airway inflammation for hyperresponsiveness induced by ozone in dogs. Am Rev Respir Dis 1983;127:686–690. [DOI] [PubMed] [Google Scholar]
- 38.Shen Y, Zhu H, Shenk T. Human cytomegalovirus IE1 and IE2 proteins are mutagenic and mediate “hit and run” oncogenic transformation in cooperation with the adenovirus E1A proteins. Proc Natl Acad Sci USA 1997;94:3341–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nevels M, Tauber B, Spruss T, Wolf H, Dobner T. “Hit and run” transformation by adenovirus oncogenes. J Virol 2001;75:3089–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laoukili J, Perret E, Willems T, Minty A, Parthoens E, Houcine O, Coste A, Jorissen M, Marano F, Caput D, et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest 2001;108:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kondo M, Tamaoki J, Takeyama K, Nakata J, Nagai J. Interleukin-13 induces goblet cell differentiation in primary cell culture from guinea pig tracheal epithelium. Am J Respir Cell Mol Biol 2002;27:536–541. [DOI] [PubMed] [Google Scholar]
- 42.Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithleial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol 2003;285:L730–L739. [DOI] [PubMed] [Google Scholar]
- 43.Kuperman DA, Huang X, Nguyenvu L, Holscher C, Brombacher F, Erle DJ. IL-4 receptor signaling in Clara cells is required for allergen-induced mucus production. J Immunol 2005;175:3746–3752. [DOI] [PubMed] [Google Scholar]
- 44.Schmid-Grendelmeier P, Altznauer F, Fischer B, Bizer C, Strauman A, Menz G, Blaser K, Wuthrich B, Simon H-U. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J Immunol 2002;169:1021–1027. [DOI] [PubMed] [Google Scholar]
- 45.Miotto D, Ruggieri MP, Boschetto P, Cavallesco G, Papi A, Bononi I, Piola C, Murer B, Fabbri LM, Mapp CE. Interleukin-13 and -4 expression in the central airways of smokers with chronic bronchitis. Eur Respir J 2003;22:602–608. [DOI] [PubMed] [Google Scholar]
- 46.Lee PJ, Zhang X, Shan P, Ma B, Lee CG, Homer RJ, Zhu Z, Rincon M, Mossman BT, Elias JA. ERK1/2 mitogen-activated protein kinase selectively mediates IL-13-induced lung inflammation and remodeling in vivo. J Clin Invest 2006;116:163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danahay H, Atherton HC, Jones G, Bridges RJ, Poll CT. Interleukin-13 induces a hyper-secretory ion transport phenotype in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2002;282:L226–L236. [DOI] [PubMed] [Google Scholar]
- 48.Hoshino M, Morita S, Iwashita H, Sagiya Y, Nagi T, Nakanishi A, Ashida Y, Nishimura O, Fujisawa Y, Fujino M. Increased expression of the human Ca2+-activated Cl− channel 1 (CaCC1) gene in the asthmatic airway. Am J Respir Crit Care Med 2002;165:1132–1136. [DOI] [PubMed] [Google Scholar]
- 49.Aida S, Tamai S, Sekiguchi S, Shimizu N. Distribution of epidermal growth factor and epidermal growth factor receptor in human lung: immunohistochemical and immunoelectron-microscopic studies. Respiration 1994;61:161–166. [DOI] [PubMed] [Google Scholar]
- 50.Strandjord TP, Clark JG, Guralnick DE, Madtes DK. Immunolocalization of transforming growth factor-α, epidermal growth factor (EGF), and EGF-receptor in normal and injured developing human lung. Pediatr Res 1995;38:851–856. [DOI] [PubMed] [Google Scholar]
- 51.Van Winckle LS, Isaac JM, Plopper CG. Distribution of epidermal growth factor receptor and ligands during bronchiolar epithelial repair from naphthalene-induced Clara cell injury in the mouse. Am J Pathol 1997;151:443–459. [PMC free article] [PubMed] [Google Scholar]
- 52.Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, Kawakami Y. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med 1998;157:1907–1912. [DOI] [PubMed] [Google Scholar]
- 53.Hardie WD, Bejarano PA, Miller MA, Yankaskas JR, Ritter JH, Whitsett JA, Korfhagen TR. Immunolocalization of transforming growth factor alpha and epidermal growth factor receptor in lungs of patients with cystic fibrosis. Pediatr Dev Pathol 1999;2:415–423. [DOI] [PubMed] [Google Scholar]
- 54.Takeyama K, Dabbagh K, Lee H-M, Agusti C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci USA 1999;96:3081–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polosa R, Prosperini G, Leir S-H, Holgate ST, Lackie PM, Davies DE. Expression of c-erbB receptors and ligands in human bronchial mucosa. Am J Respir Cell Mol Biol 1999;20:914–923. [DOI] [PubMed] [Google Scholar]
- 56.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. The involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J 2000;14:1362–1374. [DOI] [PubMed] [Google Scholar]
- 57.Takeyama K, Fahy JV, Nadel JA. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am J Respir Crit Care Med 2001;163:511–516. [DOI] [PubMed] [Google Scholar]
- 58.Shim JJ, Dabbagh K, Ueki IF, Dao-Pick T, Burgel P-R, Takeyama K, Tam DC-W, Nadel JA. IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am J Physiol 2001;280:L134–L140. [DOI] [PubMed] [Google Scholar]
- 59.Reader JR, Tepper JS, Schelegle ES, Aldrich MC, Putney LF, Pfeiffer JW, Hyde DM. Pathogenesis of mucous cell metaplasia in a murine asthma model. Am J Pathol 2003;162:2069–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayashi T, Ishii A, Nakai S, Hasegawa K. Ultrastructure of goblet-cell metaplasia from Clara cell in the allergic asthmatic airway inflammation in a mouse model of asthma in vivo. Virchows Arch 2004;444:66–73. [DOI] [PubMed] [Google Scholar]
- 61.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, et al. Mucin is produced by Clara cells in the proximal airway of antigen-challenged mice. Am J Respir Cell Mol Biol 2004;31:382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim S, Shim JJ, Burgel P-R, Ueki IF, Dao-Pick T, Tam DC, Nadel JA. IL-13-induced Clara cell secretory protein expression in airway epithelium: role of EGFR signaling pathway. Am J Physiol Lung Cell Mol Physiol 2002;283:L67–L75. [DOI] [PubMed] [Google Scholar]
- 63.Wu W, Graves LM, Jaspers I, Devlin RB, Reed W, Samet JM. Activation of the EGF receptor signaling pathway in human airway epithelial cells exposed to metals. Am J Physiol 1999;277:L924–L931. [DOI] [PubMed] [Google Scholar]
- 64.Takeyama K, Dabbagh K, Shim JJ, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor: role of neutrophils. J Immunol 2000;164:1546–1552. [DOI] [PubMed] [Google Scholar]
- 65.Burgel P-R, Lazarus SC, Tam DC, Ueki IF, Atabai K, Birch M, Nadel JA. Human eosinophils induce mucin production in airway epithelial cells via epidermal growth factor receptor activation. J Immunol 2001;167:5948–5954. [DOI] [PubMed] [Google Scholar]
- 66.Lemjabbar H, Basbaum C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat Med 2002;8:41–46. [DOI] [PubMed] [Google Scholar]
- 67.Hamilton LM, Torres-Lozano C, Puddicombe SM, Richter A, Kimber I, Dearman RJ, Vrugt B, Aalbers R, Holgate ST, Djukanovic R, et al. The role of epidermal growth factor receptor in sustaining neutrophil inflammation in severe asthma. Clin Exp Allergy 2003;33:233–240. [DOI] [PubMed] [Google Scholar]
- 68.Shao MXG, Ueki IF, Nadel JA. Tumor necrosis factor α-converting enzyme mediates MUC5AC mucin gene expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 2003;100:11618–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moghal N, Neel BG. Integration of growth factor, extracellular matrix, and retinoid signals during bronchial epithelial cell differentiation. Mol Cell Biol 1998;18:6666–6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu W, Samet JA, Ghio AJ, Devlin RB. Activation of the EGF receptor signaling pathway in airway epithelial cells exposed to Utah Valley PM. Am J Physiol 2001;28:L483–L489. [DOI] [PubMed] [Google Scholar]
- 71.Zhang L, Rice AB, Adler K, Sannes P, Martin L, Gladwell W, Koo J-S, Gray TE, Bonner JC. Vanadium stimulates human bronchial epithelial cells to produce heparin-binding epidermal growth factor-like growth factor. Am J Respir Cell Mol Biol 2001;24:123–131. [DOI] [PubMed] [Google Scholar]
- 72.Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol 1996;14:104–112. [DOI] [PubMed] [Google Scholar]
- 73.Lawson GW, Van Winkle LS, Toskala E, Senior RM, Parks WC, Plopper CG. Mouse strain modulates the role of the ciliated cell in acute tracheobronchial airway injury-distal airways. Am J Pathol 2002;160:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torrance CJ, Jackson PE, Montgomery E, Kinzler KW, Vogelstein B, Wissner A, Nunes M, Frost P, Discafani CM. Combinatorial chemoprevention of intestinal neoplasia. Nat Med 2000;6:1024–1028. [DOI] [PubMed] [Google Scholar]
- 75.Hackel RO, Zwick E, Prenzel N, Ullrich A. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol 1999;11:177–183. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem 2000;275:14624–14631. [DOI] [PubMed] [Google Scholar]
- 77.Gilmore AP, Valentijn AJ, Wang P, Ranger AM, Bundred N, O'Hare MJ, Wakeling A, Korsmeyer SJ, Streuli CH. Activation of BAD by therapeutic inhibition of epidermal growth factor receptor and transactivation by insulin-like growth factor receptor. J Biol Chem 2002;277:27643–27650. [DOI] [PubMed] [Google Scholar]
- 78.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 2004;68:320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monick MM, Cameron K, Staber J, Powers LS, Yarovinsky TO, Koland JG, Hunninghake GW. Activation of epidermal growth factor receptor by respiratory syncytial virus results in increased inflammation and delayed apoptosis. J Biol Chem 2005;280:2147–2158. [DOI] [PubMed] [Google Scholar]
- 80.Perl A-KT, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, alveoli. Am J Respir Cell Mol Biol 2005;33:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Takami K, Lo MS, Huang G, Yu Q, Roswit WT, Holtzman MJ. Modification of the Stat1 SH2 domain broadly improves interferon efficacy in proportion to p300/CBP coactivator recruitment. J Biol Chem 2005;280:34306–34315. [DOI] [PubMed] [Google Scholar]
- 82.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 2004;305:1163–1167. [DOI] [PubMed] [Google Scholar]
- 83.Andl CD, Mizushima T, Oyama K, Bowser M, Nakagawa H, Rustgi AK. EGFR induced cell migration is mediated predominantly by the JAK-STAT pathway in primary esophageal keratinocytes. Am J Physiol Gastrointest Liver Physiol 2004;287:G1227–G1237. [DOI] [PubMed] [Google Scholar]
- 84.Patel AC, Morton JD, Kim EY, Alevy Y, Swanson S, Tucker J, Huang G, Agapov E, Phillips TE, Fuentes ME, et al. Genetic segregation of airway disease traits despite redundancy of calcium-activated chloride channel family members. Physiol Genomics 2006;25:502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiba Y, Yanagisawa R, Sagai M. Strain and route differences in airway responsiveness to acetylcholine in mice. Res Commun Mol Pathol Pharmacol 1995;90:169–172. [PubMed] [Google Scholar]
- 86.Levitt RC, Mitzner W. Autosomal recessive inheritance of airway hyperreactivity to 5-hydroxytryptamine. J Appl Physiol 1989;67:1125–1132. [DOI] [PubMed] [Google Scholar]
- 87.Zhang LY, Levitt RC, Kleeberger SR. Differential susceptibility to ozone-induced airways hyperreactivity in inbred strains of mice. Exp Lung Res 1995;21:503–518. [DOI] [PubMed] [Google Scholar]
- 88.Miyabara Y, Yanagisawa R, Shimojo N, Takano H, Lim HB, Ichinose T, Sagai M. Murine strain differences in airway inflammation caused by diesel exhaust particles. Eur Respir J 1998;11:291–298. [DOI] [PubMed] [Google Scholar]
- 89.Konno S, Adachi M, Matsuura T, Sunouchi K, Hoshino H, Okazawa A, Kobayashi H, Takahashi T. Bronchial reactivity to methacholine and serotonin in six inbred mouse strains. Arerugi 1993;42:42–47. [PubMed] [Google Scholar]
- 90.Brewer JP, Kisselgof AB, Martin TR. Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am J Respir Crit Care Med 1999;160:1150–1156. [DOI] [PubMed] [Google Scholar]
- 91.Ewart SL, Kuperman D, Schadt E, Tankersley C, Grupe A, Shubitowski DM, Peltz G, Wills-Karp M. Quantitative trait loci controlling allergen-induced airway hyperresponsiveness in inbred mice. Am J Respir Cell Mol Biol 2000;23:537–545. [DOI] [PubMed] [Google Scholar]
- 92.Van Oosterhout AJ, Jeurink PV, Groot PC, Hofman GA, Nijkamp FP, Demant P. Genetic analysis of antigen-induced airway manifestations of asthma using recombinant congenic mouse strains. Chest 2002;121:13S. [DOI] [PubMed] [Google Scholar]
- 93.De Sanctis GT, Singer JB, Jiao A, Yandava CN, Lee YH, Haynes TC, Lander ES, Beier DR, Drazen JM. Quantitative trait locus mapping of airway responsiveness to chromosomes 6 and 7 in inbred mice. Am J Physiol 1999;277:L1118–L1123. [DOI] [PubMed] [Google Scholar]
- 94.Nakanishi A, Morita S, Iwashita H, Sagiya Y, Ashida Y, Shirafuji H, Fujisawa Y, Nishimura O, Fujino M. Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma. Proc Natl Acad Sci USA 2001;98:5175–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robichaud A, Tuck S, Kargman S, Tam J, Wong E, Abramovitz M, Mortimer J, Burston H, Masson P, Hirota J, et al. Gob-5 is not essential for mucus overproduction in preclinial murine models of allergic asthma. Am J Respir Cell Mol Biol 2005;33:303–314. [DOI] [PubMed] [Google Scholar]
- 96.Chen Y, Zhao YH, Kalaslavadi TJ, Hamati E, Nehrke K, Le AD, Ann DK, Wu R. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol 2003;30:155–165. [DOI] [PubMed] [Google Scholar]
- 97.Pauli BU, Abdel-Ghany M, Cheng H-C, Gruber AD, Archibald HA, Elble RC. Molecular characteristics and functional diversity of CLCA family members. Clin Exp Pharmacol Physiol 2000;27:901–905. [DOI] [PubMed] [Google Scholar]
- 98.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev 2002;82:503–568. [DOI] [PubMed] [Google Scholar]
- 99.Gustincich S, Batalov S, Beisel KW, Bono H, Carninci P, Fletcher CF, Grimmond S, Hirokawa N, Jarvis ED, Jegla T, et al. Analysis of the mouse transcriptosome for genes involved in the function of the nervous system. Genome Res 2003;13:1395–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Higuchi T, Orita T, Nakanishi S, Katsuya K, Watanabe H, Yamasaki Y, Waga I, Nanayama T, Yamamoto Y, Munger W, et al. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J Biol Chem 2004;279:1968–1979. [DOI] [PubMed] [Google Scholar]
- 101.Buisine M-P, Desseyn J-L, Porchet N, Degand P, Laine A, Aubert J-P. Genomic organization of the 3′-region of the human MUC5AC mucin gene: additional evidence for a common ancestral gene for the 11p15.5 mucin gene family. Biochem J 1998;332:729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gruber AD, Pauli BU. Tumorigenicity of human breast cancer is associated with loss of the Ca2+-activated Cl− channel CLCA2. Cancer Res 1999;59:5488–5491. [PubMed] [Google Scholar]
- 103.Barnett J, Chow J, Ives D, Chiou M, Mackenzie R, Osen E, Nguyen B, Tsing S, Bach C, Freire J. Purification, characterization, and selective inhibition of human prostaglandin G/H synthase 1 and 2 expressed in the baculovirus system. Biochim Biophys Acta 1994;1209:130–139. [DOI] [PubMed] [Google Scholar]
- 104.Gruber AD, Elble RC, Ji HL, Schreur KD, Fuller CM, Pauli BU. Genomic cloning, molecular characterization, and functional analysis of human CLCA1, the first human member of the family of Ca2+-activated Cl− channel proteins. Genomics 1998;54:200–214. [DOI] [PubMed] [Google Scholar]
- 105.Zhou Y, Shapiro M, Dong Q, Louahed J, Weiss C, Wan S, Chen Q, Dragwa CR, Savio D, Huang M, et al. A calcium-activated chloride channel blocker inhibits goblet cell metaplasia and mucus overproduction. Novartis Foundation Symposium 248 on Mucus hypersecretion in respiratory disease. Hoboken, NJ: Wiley; 2002. pp. 150–165. [PubMed]
- 106.Scott-Ward TS, Li H, Schmidt A, Cai Z, Sheppard DN. Direct block of the cystic fibrosis transmembrane conductance regulator Cl(−) channel by niflumic acid. Mol Membr Biol 2004;21:27–38. [DOI] [PubMed] [Google Scholar]
- 107.Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol 2004;67:719–758. [DOI] [PubMed] [Google Scholar]
- 108.Bertrand CA, Danahay H, Poll CT, Laboisse C, Hopfer U, Bridges RJ. Niflumic acid inhibits ATP-stimulated exocytosis in a mucin secreting epithelial cell line. Am J Physiol Cell Physiol 2004;286:C247–C255. [DOI] [PubMed] [Google Scholar]
- 109.McNamara N, Gallup M, Khong A, Sucher A, Maltseva I, Fahy J, Basbaum C. Adenosine upregulation of the mucine gene, MUC2, in asthma. FASEB J 2004;18:1770–1772. [DOI] [PubMed] [Google Scholar]
- 110.Gibson A, Lewis AP, Affleck K, Aitken AJ, Meldrum E, Thompson N. hCLCA1 and mCLCA3 are secreted non-integral membrane proteins and therefore are not ion channels. J Biol Chem 2005;280:27205–27212. [DOI] [PubMed] [Google Scholar]
- 111.Abdel-Ghany M, Cheng HC, Elble RC, Pauli BU. Focal adhesion kinase activated by beta(4) integrin ligation to mCLCA1 mediates early metastatic growth. J Biol Chem 2002;277:34391–34400. [DOI] [PubMed] [Google Scholar]
- 112.Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 2005;116:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toda M, Tulie MK, Levitt RC, Hamid Q. A calcium-activated chloride channel (hCLCA1) is strongly related to IL-9 expression and mucus production in bronchial epithelium of patients with asthma. Am J Respir Crit Care Med 2002;109:246–250. [DOI] [PubMed] [Google Scholar]
- 114.Hauber HP, Manoukian JJ, Nguyen LH, Sobol SE, Levitt RC, Holroyd KJ, McElvaney NG, Griffin S, Hamid Q. Increased expression of interleukin-9, interleukin-9 receptor, and the calcium-activated chloride channel hCLCA1 in the upper airways of patients with cystic fibrosis. Laryngoscope 2003;113:1037–1042. [DOI] [PubMed] [Google Scholar]
- 115.Holtzman MJ, Morton JD, Shornick LP, Tyner JW, O'Sullivan MP, Antao A, Lo M, Castro M, Walter MJ. Immunity, inflammation, and remodeling in the airway epithelial barrier: epithelial-viral-allergic paradigm. Physiol Rev 2002;82:19–46. [DOI] [PubMed] [Google Scholar]
- 116.Pawlowski K, Lepisto M, Meinander N, Sivars U, Varga M, Wieslander E. Novel conserved hydrolase domain in the CLCA family of alleged calcium-activated chloride channels. Proteins 2006; (in press). [DOI] [PubMed]