Abstract
Lymphangioleiomyomatosis (LAM), a multisystem disease found in middle-aged women, is characterized by cystic lung destruction and abdominal tumors (e.g., angiomyolipomas, lymphangioleimyomas), resulting from proliferation of abnormal-appearing, smooth muscle–like cells (LAM cells). The LAM cells, in combination with other cells, form nodular structures within the lung interstitium and in the walls of the cysts. LAM cells contain mutations in the tuberous sclerosis complex TSC1 and/or TSC2 genes, which lead to dysregulation of the mammalian target of rapamycin, affecting cell growth and proliferation. Proliferation and migration of vascular smooth muscle cells and production of angiogenic factors are regulated, in part, by angiotensin II. To determine whether a LAM-specific renin–angiotensin system might play a role in the pathogenesis of LAM, we investigated the expression of genes and gene products of this system in LAM nodules. mRNA for angiotensinogen was present in RNA isolated by laser-captured microdissection from LAM nodules. Angiotensin I–converting enzyme and chymase-producing mast cells were present within the LAM nodules. We detected renin in LAM cells, as determined by the presence of mRNA and immunohistochemistry. Angiotensin II type 1 and type II receptors were identified in LAM cells by immunohistochemistry and immunoblotting of microdissected LAM nodules. Angiotensin II is localized in cells containing α–smooth muscle actin (LAM cells). A LAM-specific renin–angiotensin system appears to function within the LAM nodule as an autocrine system that could promote LAM cell proliferation and migration, and could represent a pharmacologic target.
Keywords: angiotensin II, lymphangioleiomyomatosis, mammalian target of rapamycin, smooth muscle cells, tuberous sclerosis complex
Lymphangioleiomyomatosis (LAM) is a rare multisystem disease found primarily in middle-aged women. Clinically, pulmonary LAM is characterized by recurrent pneumothoraces, pleural effusions, and progressive cystic destruction of the lung, leading, in some patients, to respiratory failure. These clinical and radiographic findings result from the proliferation of abnormal-appearing, smooth muscle–like cells (LAM cells) that form nodules in the pulmonary interstitium and in the walls of the cysts (1, 2). The presence of α–smooth muscle actin (SMA), smooth muscle myosin heavy chains I and II, desmin, vimentin, calponin, and h-caldesmon in LAM cells is consistent with the smooth muscle phenotype of the LAM cell. Some LAM cells, however, react with a monoclonal antibody, HMB-45, which recognizes a protein, gp100, found in melanosomal structures in melanocytes and related cells (3–5). Thus, LAM cells have a mixed smooth muscle and melanocytic phenotype.
Proliferation of the neoplastic LAM cells appears to result from mutations in the tuberous sclerosis complex (TSC) TSC1 and/or TSC2 genes (4), leading to dysregulation of the mammalian target of rapamycin (5). The mechanism(s) involved in LAM metastasis (6, 7) and formation of lymphatics (8) within the LAM nodule are less-well characterized. The renin–angiotensin system has been described as a regulator of smooth muscle cell proliferation (9, 10) and migration (11, 12), as well as a central player in vascular remodeling and angiogenesis (13). Most of these actions have been attributed to production of the peptide angiotensin II, which activates G protein–coupled, seven-transmembrane helix angiotensin II type 1 or type II receptors (AT1R and AT2R) (14). Whereas AT1R activates the Janus kinase (JAK)/signal transducers and activators of transcription (STAT) pathway, AT2R inhibits STAT activation via tyrosine and serine phosphorylation through JAK and extracellular signal–regulated kinase pathways, respectively (15).
Angiotensin II is generated through a series of enzyme-regulated events, which follows the initial cleavage of angiotensinogen by renin to form angiotensin I (16), which is converted subsequently to angiotensin II by angiotensin I–converting enzyme (ACE). Other enzymes, such as chymase, a chymostatin-inhibited serine protease, can also covert angiotensin I to angiotensin II (16, 17). In human lung, chymase is found in mast cells, which have been implicated in smooth muscle cell proliferation (18).
Given the smooth muscle phenotype of LAM cells, we questioned whether a local renin–angiotensin system is present in LAM cells. We describe here the presence of angiotensinogen, angiotensin II, ACE, renin, and receptors for angiotensin II in LAM cells, as well as the presence of degranulated mast cells within LAM nodules. These findings are consistent with participation of a renin–angiotensin system in the neoplastic phenotype of LAM cells and in LAM pathogenesis.
MATERIALS AND METHODS
Patients
The study group comprised 35 patients (mean age, 37.4 ± 7.9 yr) with a diagnosis of LAM confirmed by pulmonary histopathology, who were enrolled in a study approved by the institutional review board of the National Heart, Lung, and Blood Institute (protocol 95-H-0186). Tissues were obtained by open lung biopsy (15 untreated patients; mean age, 37.9 ± 6.25 yr), at the time of lung transplantation (19 patients treated with progesterone and/or tamoxifen; mean age, 38.14 ± 8.97), and at necropsy (1 patient). Tissues were fixed in buffered 10% formalin, embedded in paraffin, and processed as previously described (19). Frozen sections from nine patients with LAM were fixed in buffered, cold 4% paraformaldehyde, and stained as previously described (20, 21). Lung tissues from patients without pulmonary disease were obtained from the Cooperative Human Tissue Network (Philadelphia, PA).
Immunohistochemistry
For single antibody labeling, paraffin and frozen sections were immunoassayed by the peroxidase method using the indicated antibodies. The degranulation index for mast cells was determined in serial paraffin sections of LAM lung by subtracting the number of cells positively stained for chymase in one section from those positively stained for CD117 in the other. Total number of cells was determined by counting 10 random fields at 200× magnification in tissue sections, and the data were examined independently by two pathologists using an Olympus BX40 microscope (Olympus, Melville, NY). The degree of concordance between the 2 observers was ∼ 93%. This is based on the cell counts determined by 2 observers, which differed in relative numbers in 2 out of 30 patients.
Dual labeling for indirect immunofluorescence with laser-scanning confocal fluorescence microscopy was employed to evaluate the colocalization of immunoreactivity with polyclonal antibodies in paired combinations with mouse monoclonal antibodies. Frozen sections were stained by the double indirect method (using FITC- and Texas Red–conjugated secondary antibodies) (21). Nuclei were counterstained with DAPI (blue fluorescence).
Transmission Electron Microscopy
Tissues were fixed in 2% glutaraldehyde, postfixed in 1% osmium tetroxide, and embedded in PolyBed 812 (Polysciences, Warrington, PA). Sections (1 μm) were stained with toluidine blue. Ultrathin sections were stained either with uranyl acetate and lead citrate or Kajikawa stain to enhance the electron density of elastic fibers and cytoplasmic components.
Immunoblotting
Proteins were extracted from frozen tissue sections of LAM lung using both whole lung lysates and laser-capture microdissection (LCM). Whole lung lysates were processed for immunoblotting as described by Rakugi and colleagues (21). For LCM, LAM nodules were collected from the slides in a cap using a PixCell II inverted microscope (Arcturus, Mountain View, CA). LCM samples, which contained tissue from 4,000 pulses of 7.5 μm (∼ 4,000 cells) per cap, were incubated for 2 h in 25 μl of RIPA buffer (pH 7.2, containing protease inhibitor cocktail) (Boehringer-Mannheim, Indianapolis, IN) at 65°C. Protein was quantified using the BCA assay (Pierce, Rockford, IL). Proteins (30 μg/lane) were separated by electrophoresis in 10% Bis-Tris gel (NuPage gels; Novex, Carlsbad, CA), and transferred to PVDF membranes, which were incubated overnight with antibodies against AT1R (0.05 μg/ml) or AT2R (0.5 μg/ml). Donkey anti-rabbit IgG (1:10,000; Amersham, Arlington Heights, IL) and donkey anti-goat IgG (1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA) were used as secondary antibodies. Blots were developed using ChemiLuminescence reagents (Pierce). To evaluate equivalence of protein loading in each lane, stripped blots were reacted with antibodies against actin.
ELISA for ACE and Angiotensin II
ACE and angiotensin II were assayed in lysates of lungs from nine hormone-treated patients with LAM, one patient with LAM with an angiomyolipoma (AML), and three patients without lung disease. The angiotensin II assay has been published (22). For detection of ACE, a commercially available ELISA kit (Chemicon, Temecula, CA) was used according to the manufacturer's instructions. All assays were performed in duplicate; data are reported as mean ± 1/2 of the range.
Enzymatic Assays for ACE
ACE activity in the same samples studied by immunoblotting was assayed using two peptide substrates, hippuryl-His leu (Hip-His-Leu) and Abz-FRK (Dnp)-P-OH (23). With Hip-His-Leu substrate, the fluorimetric method of Friedland and Silverstein (24) was used. A sample of homogenized lung (0.5–1.5 μl) was added to the assay solution, which included 5 mM Hip-His-Leu, 100 mM potassium buffer (pH 8.3), and 300 mM NaCl, at 37°C. The product, His-Leu, was quantified using a fluorimeter (Newport Scientific, Jessup, MD) with excitation at 360 nm and emission at 500 nm. ACE activity assays with the internally quenched fluorogenic peptide Abz-FRK (Dnp)-P-OH (10 μM) substrate, were performed at 37°C, in Tris-HCl buffer (pH 7.0), 50 mM NaCl, and 10 μM ZnCl2 (total volume, 1.0 ml). Fluorescence was recorded continuously for 5 min (Hitachi F-2000 fluorimeter, 320 nm excitation and 420 nm emission; Hitachi, Tokyo, Japan), and the slope was converted to μmol of substrate hydrolyzed/min using a calibration curve. To distinguish ACE from other proteases in LAM lung, samples of human lung were incubated with lisinopril (0.5 μM) for 30 min at 37°C before addition of the substrate Hip-His-Leu or Abz-FRK(Dnp)-P-OH. The ACE activity was determined measuring the fluorescence continuously, as described above, in the absence of the inhibitor.
RNA Isolation and RT-PCR Analysis for Angiotensinogen and Renin
PolyA+ RNA was extracted from LAM tissue samples using an Oligotex direct mRNA kit (QIAGEN Inc., Valencia, CA) according to the supplied protocol. Samples (400 ng) of polyA+ RNA were treated with a combination of DNase I (Invitrogen, Carlsbad, CA) and 0.5 U of RNase inhibitor to remove contaminating DNA and block RNAses, respectively. For each reaction, we used 10 ng of RNA. The DNase I–treated RNA samples were reverse transcribed at 50°C for 30 min to generate target gene–specific cDNAs in a GeneAmp PCR system 9,700 (Applied Biosystems, Foster City, CA) in a total volume of 50 μl using the Titan One-step RT-PCR kit (Roche, Mannheim, Germany). PCR products were separated in 1.2% agarose gels in 1× Tris-acetate-EDTA buffer and visualized with ethidium bromide. Sizes of PCR products were estimated with a 100- or 250-bp DNA ladder in the same gel (Invitrogen). Sets of oligonucleotide primers to amplify the human angiotensinogen and renin genes by PCR were selected based on the published cDNA nucleotide sequence (GenBank accession numbers X15324 and GI:11125774). Oligonucleotide primers used for RT-PCR were as follows: primers for human angiotensinogen were situated over the fourth and fifth exons with the sense primer (bases 1209–1231): 5′-CTGCAAGGATCTTATGACCTGC-3′, and the antisense primer (bases 1404–1426) 5′-TACACAGCAAACAGGAATGGGC-3′ reverse; for renin, primers were forward 5′-AGGACATCATCACCGTGGGT-3′; and reverse 5′-TGGAAATTCCCTTCGTAATG-3′. All primers were purchased from Sigma Genosys (Woodlands, TX). The full-length cDNA sequence of human renin was confirmed using mRNA extracted from microdissected LAM cells, as shown in Figure 4. The sequence was identical to that in GenBank (accesion no.: NM_000537).
Figure 4.
Renin mRNA and protein in LAM cells. (A) Renin cDNA fragment synthesized from RNA samples as in legend of Figure 3. Renin mRNA was detected in four out of nine cases. (B) LAM lung nodule reacted with antibodies against renin. It was most frequently positive in the center of the nodule in spindle-shaped LAM cells. (C) Renin sequence from mRNA isolated from LAM nodules. The arrows show the primers used to generate different fragments for DNA sequencing. The renin coding sequence was compared with that of accession number NM_000537, which is codified by 10 exons. The underlined sequence shows exon 5a or exon 6, characteristic of human renin.
Statistical Analysis
All data are presented as means ± SEM unless stated otherwise. Regression and correlation analysis was performed using a paired Student's t test for the comparison of means in the correlation study. Semiquantitative data obtained from immunohistochemical studies were expressed as medians, and between-group comparisons were analyzed using the Kruskal–Wallis test. Analyses were performed using Instat3 Graphic pack (San Diego, CA). A P value < 0.05 was considered statistically significant.
RESULTS
Angiotensin-II Receptors in LAM Cells
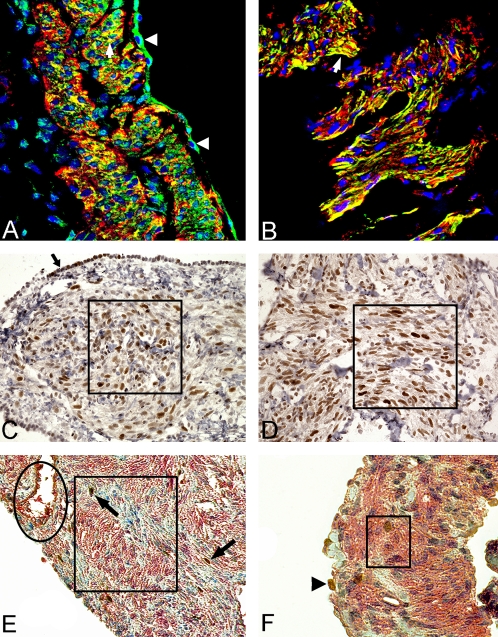
Angiotensin II action is initiated by binding to AT1R and AT2R angiotensin II receptors. Microscopically, immunoreactive AT1R was seen throughout the cytoplasm of spindle-shaped LAM cells in the center of LAM nodules in biopsy samples or explants (Figure 1A, square). AT1R reactivity was localized in the central areas of the LAM nodules within spindle-shaped LAM cells; it appears to be localized to plasma membrane and cytoplasm (Figure 1B), and AT1R is also present in type II pneumocytes (Figure 1A, arrow). LAM nodule (Figure 1B) also shows the presence of canal structures that resemble lymphatics (Figure 1B, arrows). In normal lung, vascular and bronchial smooth muscle cells showed a moderate reaction for AT1R (Figures 1C and 1D); AT1R immunoreactivity in LAM samples was not observed in type I pneumocytes located in the lung parenchyma in areas not affected by the disease. Similarly, LAM cells and type II pneumocytes were positive for AT2R (Figure 1E). Interestingly, some cells showed stronger reaction for the AT2R antibody in cells within the LAM nodule (Figure 1F). Figure 1G shows that vascular smooth muscle cells (white arrowhead) and smooth muscle cells (arrow) have similar reactivity for the AT2R antibody. The cells that showed stronger, positive staining for AT2R appeared to be in close proximity to the interstitium (Figures 1G and 1H).
Figure 1.
AT1R and AT2R in LAM cells. (A) Reaction with AT1R antibodies is seen throughout the cytoplasm in spindle-shaped LAM cells at the center of the LAM nodule; see the square showing positive staining for AT1R. However, there was variability of staining between LAM cells in nodules and cysts. Type II pneumocytes (arrow) on the surface of the nodule are also positively stained (original magnification, ×200). (B) Cytoplasmic staining for AT1R and areas of what appear to be lymphatics (empty space, arrowheads). (C) Arrows show the vascular smooth muscle cells; arrowheads point to probable lymphatic channels; LAM nodules are indicated the rectangle. (D) Epithelial cells stain weakly (arrows), and the arrowheads show staining on smooth muscle cells. Single-antibody labeling; peroxidase method and hematoxylin counterstain. (E) The reaction is localized in LAM cells in the center of the LAM nodule (arrow) and the periphery of the LAM nodule representing type II pneumocytes (arrowheads). (F) Detail of the nodule. Some LAM cells showed a strong cytoplasmic staining for AT2R inside the LAM nodule (arrow); some empty spaces suggest lymphatics. (G) The reaction in vascular smooth muscle cells (white arrowhead) are similar to that observed in LAM cells (black arrow). Note that some cells with strong cytoplasmic staining were located in the interstitium (black arrowhead). (H) Detail of AT2R-positive cells near a vascular structure and interstitium (arrows). Original magnification, ×64 (A and C) and ×100 (B and D).
By immunoblotting proteins isolated by LCM from LAM nodules, it was found that anti-AT1R antibody recognized two bands—a major band of ∼ 70 kD and a minor band of ∼ 44 kD—in all LAM lung nodules, a case of renal AML, and in a normal-lung homogenate (Figure 2A). The proteins extracted (total lung) from a single case of idiopathic pulmonary fibrosis did not show reactivity to the ∼ 70 kD protein. The antibodies against AT2R, like those against AT1R, reacted with proteins of ∼ 70 and ∼ 44 kD in samples from LAM obtained by LCM (Figure 2B). A single band of ∼ 70 kD was detected in the AML. The presence of AT1R and AT2R within the LAM nodules suggested that signaling through the renin–angiotensin pathway could regulate the neoplastic growth of LAM cells.
Figure 2.
AT1R and AT2R in LAM cells. (A) Proteins (30 μg) from LAM nodules collected by LCM from nine LAM lungs (lanes 1–9), an AML (lane 10), a lung with idiopathic pulmonary fibrosis (lane 11), and a normal lung (lane 12) were immunoblotted with antibodies against AT1R. Bands of ∼ 70 kD and ∼ 44 kD are seen in all LAM cases. Extra bands of ∼ 51 kD are seen in lanes 1 and 10–12. (B) Immunoblotting of same protein samples showed in (A) with antibodies against AT2R, gave bands of ∼ 70 kD and ∼ 44 kD in most samples, but the ratios differed from that in (A) for AT1R.
Angiotensinogen in LAM Nodules
Although it is believed that angiotensin II exerts its effects at sites distant from that of its synthesis, mRNA for its precursor, angiotensinogen, was detected in microdissected LAM nodules from six of nine LAM lung specimens (Figure 3). The levels of angiotensinogen mRNA and glyceraldehyde-3-phosphate dehydrogenase mRNA varied among patients. RNA extracted from homogenates of normal kidney and normal lung also contained angiotensinogen mRNA. Thus, the angiotensin II within the LAM nodules appears to be generated from angiotensinogen synthesized by LAM cells within the LAM nodule.
Figure 3.
Angiotensinogen mRNA in LAM cells. RNA was extracted from LCM LAM nodules, normal kidney (total tissue), and normal lung (total tissue). cDNA was used to amplify angiotensinogen, using specific primers as described in materials and methods. Angiotensinogen mRNA was detected in samples from six out of nine patients, as well as in normal kidney and normal lung (NL). The lower panel shows amplification by RT-PCR of cDNA for glyceraldehyde-3-phosphate dehydrogenase. RT, minus reverse transcriptase; T, no template.
Renin in LAM Nodules
Because angiotensin I is produced from angiotensinogen by renin-catalyzed proteolytic cleavage, we investigated whether renin is present in LAM lung nodules obtained by microdissection (Figure 4A) and by immunohistochemistry (Figure 4B). Figure 4A shows that the mRNA for renin was clearly detected in four out of nine cases. In mRNA obtained from kidney, we also observed a similar splice variant of renin (Figure 4A). To confirm the presence of renin in LAM cells, the mRNA obtained from microdissected LAM nodules was reverse transcribed, and the cDNAs were sequenced. The cDNAs from two patients encode a long form of human renin (accesion no. NM_000537), characterized by the presence of exon 5a (25) (Figure 4C).
In LAM lung, the immunoreactivity for renin was found in 27 out of 36 patients (75%) (Figure 4B). Anti-renin antibodies reacted only with LAM cells in the LAM nodules. Normal lung samples or nondisease areas in LAM lung sections showed no reactivity with anti-renin antibodies. Specificity of the renin antibody was tested in kidney, where only glomeruli were immunoreactive. We focused on the LAM nodules, where LAM cells and hyperplastic type II cells on their surface can be easily distinguished from surrounding lung.
The number of patients expressing renin in the LAM nodules was 11 out of 15 (73.3%) from the biopsy group, and 16 out of 21 (76.2%) from the transplant group. Immunohistochemical reactivity for renin was localized mainly in the periphery of the LAM nodules, and in the cytoplasm of epithelioid LAM cells more frequently than in spindle-shaped LAM cells (Figure 4B). Type II pneumocytes and vascular and bronchial smooth muscle cells were negative in LAM lung and normal lung. Thus, renin appears to be produced by LAM cells within the nodule.
Angiotensin II in LAM Cells
To evaluate a potential role of angiotensin II in LAM cells, we determined the presence of this octapeptide within LAM nodules. Angiotensin II was detected in spindle-shaped LAM cells within the nodules (Figure 5A) and in vascular smooth muscle cells, as determined by their reactivity with cells positive for α-SMA (Figure 5A). Type II pneumocytes also were reactive to the antibody against angiotensin II (Figure 5A). Similarly, the antibody against AT1R colocalized with cells positive for α-SMA (Figure 5B).
Figure 5.
Angiotensin II and AT1R are colocalized with α-SMA. (A) Angiotensin II (green) and SMA (red). Colocalization of angiotensin II with SMA is observed in yellow throughout a cystic wall in LAM area (i.e., arrow). Note that the staining for angiotensin II in the cytoplasm of type II pneumocytes (arrowheads). (B) AT1R (green) and SMA (red). A cytoplasmic staining for AT1R and SMA colocalized (yellow) in the center of a LAM nodule. Nuclear counterstain with DAPI (blue). Original magnification, ×400. (C and D) Immunoreactivity for PCNA on a nodular structure (C) or in a cystic lesion (D). Rectangles show examples of proliferative regions. Arrows outside of the rectangles show hyperplastic type II pneumocytes, which surround the LAM nodules (C). Original magnification, ×40. (E) and (F) show the staining of AT1R (red) and KI67 (brown). (E) Oval, vascular endothelial cells positive for AT1R. Strong immunoreactivity of cells is indicated with an arrow, and a higher magnification view of the cells is given (rectangle). (F) Arrowhead points to type II pneumocytes. Similar results were observed in 10 different cases. Original magnification, ×40 (E) and ×100 (F).
The same spindle-shaped cells within nodular (Figure 5C) or cystic lesions (Figure 5D) displayed immunoreactivity with antibodies to proliferating cell nuclear antigen (PCNA), a marker of cell proliferation. Some cells immunoreactive with antibodies to KI67 were also immunoreactive for AT1R (Figures 5E and 5F).
Presence of ACE in LAM Cells
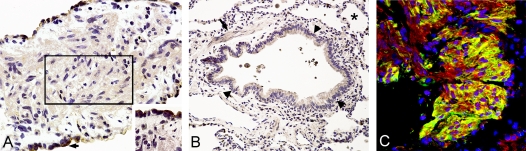
ACE cleaves angiotensin I to generate angiotensin II, which was found in LAM nodules. Immunostaining for ACE showed a weak to moderate presence on the plasma membrane and in the cytoplasm of spindle-shaped LAM cells (Figure 6A). The cytoplasm of type II pneumocytes was more strongly positive than that of LAM cells for ACE immunoreactivity (Figure 6A, inset). Moderate cytoplasmic staining for ACE in vascular and bronchial smooth muscle cells was similar in normal and LAM lungs, in both frozen and paraffin sections (Figure 6B). These cells and the endothelial cells served as internal positive controls for ACE immunoreactivity. By confocal immunofluorescence microscopy, angiotensin II and ACE were found in LAM cells in the center of the nodules (Figure 6C). Thus, ACE could generate the active octapeptide angiotensin II. By ELISA, we detected ACE and angiotensin II in homogenates of tissue from explanted LAM lungs. Homogenates of LAM lungs (n = 8) contained ACE (1.42 ± 1.40 μg/mg protein) and angiotensin II (0.22 ± 8.09 μg/mg protein). In one AML, ACE (0.54 μg/mg protein) and angiotensin II (0.14 μg/mg protein) were also detected. Thus, ACE and angiotensin II present in LAM lungs could be more locally concentrated in LAM nodules. As expected, ACE activity within LAM lung was inhibited by lisinopril. The presence of ACE in LAM nodules suggests that an active local renin–angiotensin system is present in patients with LAM.
Figure 6.
ACE and angiotensin II in LAM tissue. (A) ACE immunoreactivity is seen in spindle-shaped LAM cells (rectangle) and also in type II pneumocytes (arrow and inset). (B) Immunodetection of ACE in smooth muscle cells (arrow), bronchial epithelial cells (arrowheads), and normal tissue (asterisk). (C) Angiotensin II (red) and ACE (green). Angiotensin II is observed in the cytoplasm of cells positive for ACE within the LAM nodule. DAPI staining of blue nuclei (blue). Original magnification, ×400 (A), ×200 (B), and ×400 (C).
Mast Cells within LAM Nodules
Because angiotensin II can also be generated by different enzymes, including chymase (16, 17), we investigated whether mast cells, which produce chymase, were present in LAM nodules. For this purpose, we stained sections of LAM lung with antibody against CD117, c-Kit or Kit, which is a 145-kD transmembrane protein, is highly expressed in mast cells, and is used as a mast cell marker.
The positive CD117 cells were distributed throughout the LAM nodule and other non-LAM–related areas. The presence of CD117 staining in what appears to be a mast cell is shown in the inset to Figure 7A. Whereas all mast cells could be identified with the CD117 (c-kit) antibody, chymase is only present in a subpopulation. The number of CD117-positive mast cells (Figure 7B) was significantly higher than that of chymase-positive mast cells (n = 30; 269.0 ± 26.9 versus 130.0 ± 15.0; P < 0.01) in LAM lung (Figure 7B). The number of CD117-positive cells was significantly higher in LAM than in normal lung (269 ± 26.9 versus 124 ± 11.5; P < 0.0001). In normal lung, the difference between numbers of CD117-positive and chymase-positive mast cells was not significant (n = 8; 124.0 ± 11.5 versus 73.8 ± 16.3). The number of chymase-positive mast cells was significantly higher in LAM lung than in normal lung (130.0 ± 15.0 versus 73.8 ± 16.3; P = 0.0097). Figure 7C shows that chymase-positive cells were present through the lung and in LAM nodules. CD117-positive cells were present in close proximity to the α-SMA–positive cells (Figure 7D). Transmission electron microscopy confirmed the presence of mast cells in LAM nodules and free granules morphologically similar to those containing chymase in the extracellular space (Figures 7E and 7F). These data suggest that mast cells in LAM nodules had been activated, with resulting degranulation.
Figure 7.
Mast cells in LAM lung. (A) CD117-positive cells were randomly distributed through the lung. Note the staining in plasma membrane on what appears to be a mast cell (inset) (original magnification, ×200; ×400 for inset). (B) Number of cells positive for CD117 or chymase. Note that the number of cells positive for CD117 and chymase was larger in LAM areas than in normal regions. Chymase-positive cells were found inside LAM nodules. (C) Chymase-positive cells (green) were distributed randomly in the LAM nodule. (D) CD117-positive cells (green) were among the α-SMA–positive cells (red). (E) Electron microscopy of a mast cell containing the characteristic granules (black deposits) is clearly identified within LAM nodules. (F) Identification of secretory granules free in the interstitial tissue (arrows). BV, blood vessel using the Kajikawa stain; CF, collagen fiber; EF, elastic fiber.
DISCUSSION
The immunohistochemical and biochemical studies demonstrate that angiotensin II, ACE, renin, chymase, AT1R, and AT2R are present in LAM nodules. The data support the existence of an active renin–angiotensin system in pulmonary LAM nodules.
The renin–angiotensin system components in LAM tissue were largely localized in spindle-shaped LAM cells, but not in epithelioid LAM cells or in type II pneumocytes. Although angiotensin II can affect cell proliferation and apoptosis, we have found no evidence of large numbers of apoptotic cells within LAM nodules (data not shown). These findings are of special interest because the spindle-shaped LAM cells are thought to be more proliferative (PCNA-positive cells) than the epithelioid LAM cells and the hyperplastic type II pneumocytes at the surface of LAM nodules (2, 26). Thus, angiotensin II could be involved in the metabolism of smooth muscle–like “LAM cells,” including proliferation and cell migration.
The presence of AT1R and AT2R in LAM cells within the nodules is consistent with a role for angiotensin II signaling in these cells. Molecular sizes of human AT1R and AT2R, predicted from the cDNA sequences, are 41 kD (27) and 41.1 kD (28), respectively. Different molecular sizes for AT1R range from 43 to 116 kD (29–31), and for AT2R range from 44 to 113 kD (29–31). Paxton and colleagues (30) reported a 44-kD AT1R and/or AT2R in rat and human tissues. Our analysis of LAM nodules from nine patients revealed immunoreactive bands of ∼ 44 and ∼ 70 kD for both AT1R and AT2R, with a 51-kD AT1R band in one sample. These were obviously different from findings in AML, idiopathic pulmonary fibrosis, and normal lung. The larger size AT1R in LAM samples is similar to that reported for smooth muscle cells from human myometrium (68 kD) (32). The molecular sizes of these receptors in LAM lung are somewhat different from those reported in other tissues, which may reflect differences in glycosylation, other posttranslational modifications, and/or alternative mRNA splicing. It is of interest that homo- and heterocrosslinking of AT1R alters its effectiveness for activation of G proteins (33). The larger molecular forms of AT1R and AT2R were consistently associated with hyperactivated forms of the receptors, supporting the functional effect of angiotensin II in vivo on the LAM cells.
We propose that the presence of these receptors could be important in proliferation and migration of LAM cells. Activation of AT1R and AT2R could result in upregulation of vascular endothelial growth factor (13), leading to stimulation of smooth muscle cells. Signaling by angiotensin II results in activation of phospholipase C, mitogen-activated protein kinase, and JAK/STAT pathways. In fact, it has been shown that angiotensin II stimulates proliferation of smooth muscle cells by activation of STAT1 and STAT3, and that this effect can be blocked by specific antibodies against these proteins (34). It has been shown that there is STAT1 phosphorylation in LAM nodules (35).
All LAM lungs assayed for ACE activity were at an advanced stage of the disease, which could explain the variability of the data, as could earlier hormonal treatments. Estrogens are known effectors of renin–angiotensin system, with effects on angiotensin II production and ACE activity (36). Estrogen administration has been shown to increase the circulating levels of angiotensinogen and renin (37). In postmenopausal women, replacement therapy with 17β-estradiol reduced serum ACE activity (38) and increased serum angiotensinogen, but had inconsistent effects on renin, angiotensin II, and aldosterone (39). Thus, current treatments of patients with LAM could alter the role of the renin angiotensin system.
Angiotensin II is generated in cells targeted via the action of ACE, although the existence of other ACE-independent enzymatic pathways has been reported (16–18). Chymase-dependent formation of angiotensin II occurs after an injury or an inflammatory stimulus (18). LAM lesions, however, do not resemble regions of inflammation, but they do contain mast cells (40). The source of chymase in LAM nodules could be the mast cells, which were found to be degranulated (Figure 7). It may be relevant that mast cells produce renin (41). ACE inhibitors and AT1R antagonists were used with some success to treat radiation-induced pneumopathy and idiopathic pulmonary fibrosis (42), suggesting that they might be of benefit in the treatment of LAM. Mast cells containing cytoplasmic granules displaying different ultrastructural patterns (e.g., homogeneously dense scrolls, crystals) were identified in close proximity to LAM cells. The granules found free in the extracellular space were mostly of the scroll type, which were previously reported in lung mast cells to contain chymase (43, 44). Several factors support the conclusion that the source for chymase granules inside LAM nodules are degranulated mast cells: (1) anti-chymase antibody detected this enzyme only in mast cells inside LAM nodules; (2) the presence of mast cells immunoreactive for CD117 but not chymase constitute indirect evidence of local degranulation, considering the fact that mast cells in tissues release granules in proximity to their targets; (3) degranulation of mast cells occurs through extrusion of altered, membrane-free granules, which were identified in different morphologic states in the extracellular space in LAM nodules (Figure 7); and (4) the presence of chymase granules inside the LAM nodules and not only near vascular structures weakens the argument that they are derived from blood mast cells or other extranodular sources.
Overall, the demonstrated presence in LAM tissues of proteins required for the production and function of the renin–angiotensin system components is consistent with an autocrine role in disease pathogenesis and/or progression. Effects of steroid sex hormones on renin–angiotensin system components could also be involved in signaling and interactions among cells within a LAM nodule. The mammalian target of rapamycin pathway could be affected by pharmacologic agents that target the renin–angiotensin system, as angiotensin II causes upregulation of vascular endothelial growth factor (45), which has been correlated with the dysregulation of TSC2 (46). The renin–angiotensin system may have multiple roles in cancer (45), based on genetic data (e.g., polymorphisms in AT1R, ACE) and animal models. Similarly, based on pharmacologic studies that target the renin–angiotensin system, it may be involved in cell proliferation, angiogenesis, tissue remodeling, and metastasis. The proliferative and metastastic properties of the neoplastic LAM cells may similarly be affected by the renin–angiotensin system.
Acknowledgments
This work was initiated in the laboratory of Dr. Victor J. Ferrans. The authors are deeply indebted to him and dedicate this article to his memory. They thank Dr. Martha Vaughan for helpful discussions and critical review of the manuscript, and Michael Spencer for his help with the artwork. They thank the LAM foundation and the Tuberous Sclerosis Alliance for their assistance in recruiting patients for our studies.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Heart, Lung, and Blood Institute.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0387OC on February 10, 2006
Conflict of Interest Statement: J.C.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.P.-R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.K.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.X. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.B. receives an annual fee of €10,000 from Guerbet group (radiology contrast products company in France) for assessment of new products for IRM in experimental preclinical models of atherosclerosis. W.K.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.I. is a participant in the Senior Fellow Program from the Oak Ridge Institute for Science and Education. Z.-X.Y. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. V.J.F. is deceased. J.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Pacheco-Rodriguez G, Kristof AS, Stevens LA, Zhang Y, Crooks D, Moss J. Giles F. Filley Lecture: genetics and gene expression in lymphangioleiomyomatosis. Chest 2002;121:56S–60S. [DOI] [PubMed] [Google Scholar]
- 2.Ferrans VJ, Yu ZX, Nelson WK, Valencia JC, Tatsuguchi A, Avila NA, Riemenschn W, Matsui K, Travis WD, Moss J. Lymphangioleiomyomatosis (LAM): a review of clinical and morphological features. J Nippon Med Sch 2000;67:311–329. [DOI] [PubMed] [Google Scholar]
- 3.Dell'Angelica EC. Melanosome biogenesis: shedding light on the origin of an obscure organelle. Trends Cell Biol 2003;13:503–506. [DOI] [PubMed] [Google Scholar]
- 4.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA 2000;97:6085–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: a role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM). J Biol Chem 2002;277:30958–30967. [DOI] [PubMed] [Google Scholar]
- 6.Karbowniczek M, Astrinidis A, Balsara BR, Testa JR, Lium JH, Colby TV, McCormack FX, Henske EP. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med 2003;167:976–982. [DOI] [PubMed] [Google Scholar]
- 7.Crooks DM, Pacheco-Rodriguez G, DeCastro RM, McCoy JP Jr, Wang JA, Kumaki F, Darling T, Moss J. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci USA 2004;101:17462–17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumasaka T, Seyama K, Mitani K, Sato T, Souma S, Kondo T, Hayashi S, Minami M, Uekusa T, Fukuchi Y, et al. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol 2004;28:1007–1016. [DOI] [PubMed] [Google Scholar]
- 9.Berk BC. Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev 2001;81:999–1030. [DOI] [PubMed] [Google Scholar]
- 10.Meloche S, Pelletier S, Servant MJ. Functional cross-talk between the cyclic AMP and Jak/STAT signaling pathways in vascular smooth muscle cells. Mol Cell Biochem 2000;212:99–109. [PubMed] [Google Scholar]
- 11.Kraemer R. Regulation of cell migration in atherosclerosis. Curr Atheroscler Rep 2000;2:445–452. [DOI] [PubMed] [Google Scholar]
- 12.Mifune M, Ohtsu H, Suzuki H, Nakashima H, Brailoiu E, Dun NJ, Frank GD, Inagami T, Higashiyama S, Thomas WG, et al. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J Biol Chem 2005;280:26592–26599. [DOI] [PubMed] [Google Scholar]
- 13.Escobar E, Rodriguez-Reyna TS, Arrieta O, Sotelo J. Angiotensin II, cell proliferation and angiogenesis regulator: biologic and therapeutic implications in cancer. Curr Vasc Pharmacol 2004;2:385–399. [DOI] [PubMed] [Google Scholar]
- 14.Thomas WG, Qian H. Arresting angiotensin type 1 receptors. Trends Endocrinol Metab 2003;14:130–136. [DOI] [PubMed] [Google Scholar]
- 15.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII: the angiotensin II receptors. Pharmacol Rev 2000;52:415–472. [PubMed] [Google Scholar]
- 16.Belova LA. Angiotensin II–generating enzymes. Biochemistry (Mosc) 2000;65:1337–1345. [DOI] [PubMed] [Google Scholar]
- 17.Takai S, Jin D, Sakaguchi M, Miyazaki M. Chymase-dependent angiotensin II formation in human vascular tissue. Circulation 1999;100:654–658. [DOI] [PubMed] [Google Scholar]
- 18.Page S, Ammit AJ, Black JL, Armour CL. Human mast cell and airway smooth muscle cell interactions: implications for asthma. Am J Physiol Lung Cell Mol Physiol 2001;281:L1313–L1323. [DOI] [PubMed] [Google Scholar]
- 19.Matsui K, Takeda K, Yu ZX, Valencia J, Travis WD, Moss J, Ferrans VJ. Downregulation of estrogen and progesterone receptors in the abnormal smooth muscle cells in pulmonary lymphangioleiomyomatosis following therapy: an immunohistochemical study. Am J Respir Crit Care Med 2000;161:1002–1009. [DOI] [PubMed] [Google Scholar]
- 20.Valencia JC, Matsui K, Bondy C, Zhou J, Rasmussen A, Cullen K, Yu ZX, Moss J, Ferrans VJ. Distribution and mRNA expression of insulin-like growth factor system in pulmonary lymphangioleiomyomatosis. J Investig Med 2001;49:421–433. [DOI] [PubMed] [Google Scholar]
- 21.Rakugi H, Okamura A, Kamide K, Ohishi M, Sasamura H, Morishita R, Higaki J, Ogihara T. Recognition of tissue- and subtype-specific modulation of angiotensin II receptors using antibodies against AT1 and AT2 receptors. Hypertens Res 1997;20:51–55. [DOI] [PubMed] [Google Scholar]
- 22.Netzel-Arnett S, Mallya SK, Nagase H, Birkedal-Hansen H, Van Wart HE. Continuously recording fluorescent assays optimized for five human matrix metalloproteinases. Anal Biochem 1991;195:86–92. [DOI] [PubMed] [Google Scholar]
- 23.Araujo MC, Melo RL, Cesari MH, Juliano MA, Juliano L, Carmona AK. Peptidase specificity characterization of C- and N-terminal catalytic sites of angiotensin I–converting enzyme. Biochemistry 2000;39:8519–8525. [DOI] [PubMed] [Google Scholar]
- 24.Friedland J, Silverstein E. A sensitive fluorimetric assay for serum angiotensin-converting enzyme. Am J Clin Pathol 1976;66:416–424. [DOI] [PubMed] [Google Scholar]
- 25.Hardman JA, Hort YJ, Catanzaro DF, Tellam JT, Baxter JD, Morris BJ, Shine J. Primary structure of the human renin gene. DNA 1984;3:457–468. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto Y, Horiba K, Usuki J, Chu SC, Ferrans VJ, Moss J. Markers of cell proliferation and expression of melanosomal antigen in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol 1999;21:327–336. [DOI] [PubMed] [Google Scholar]
- 27.Furuta H, Guo DF, Inagami T. Molecular cloning and sequencing of the gene encoding human angiotensin II type 1 receptor. Biochem Biophys Res Commun 1992;183:8–13. [DOI] [PubMed] [Google Scholar]
- 28.Tsuzuki S, Ichiki T, Nakakubo H, Kitami Y, Guo DF, Shirai H, Inagami T. Molecular cloning and expression of the gene encoding human angiotensin II type 2 receptor. Biochem Biophys Res Commun 1994;200:1449–1454. [DOI] [PubMed] [Google Scholar]
- 29.Servant G, Dudley DT, Escher E, Guillemette G. The marked disparity between the sizes of angiotensin type 2 receptors from different tissues is related to different degrees of N-glycosylation. Mol Pharmacol 1994;45:1112–1118. [PubMed] [Google Scholar]
- 30.Paxton WG, Runge M, Horaist C, Cohen C, Alexander RW, Bernstein KE. Immunohistochemical localization of rat angiotensin II AT1 receptor. Am J Physiol 1993;264:F989–F995. [DOI] [PubMed] [Google Scholar]
- 31.Ciuffo GM, Johren O, Egidy G, Heemskerk FM, Saavedra JM. Heterogeneity of rat angiotensin II AT2 receptor. Adv Exp Med Biol 1996;396:189–197. [DOI] [PubMed] [Google Scholar]
- 32.Saridogan E, Djahanbakhch O, Puddefoot JR, Demetroulis C, Dawda R, Hall AJ, Vinson GP. Type 1 angiotensin II receptors in human endometrium. Mol Hum Reprod 1996;2:659–664. [DOI] [PubMed] [Google Scholar]
- 33.AbdAlla S, Lother H, Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature 2000;407:94–98. [DOI] [PubMed] [Google Scholar]
- 34.Marrero MB, Schieffer B, Li B, Sun J, Harp JB, Ling BN. Role of Janus kinase/signal transducer and activator of transcription and mitogen-activated protein kinase cascades in angiotensin II– and platelet-derived growth factor–induced vascular smooth muscle cell proliferation. J Biol Chem 1997;272:24684–24690. [DOI] [PubMed] [Google Scholar]
- 35.El-Hashemite N, Kwiatkowski DJ. Interferon-γ-Jak-Stat signaling in pulmonary lymphangioleiomyomatosis and renal angiomyolipoma: a potential therapeutic target. Am J Respir Cell Mol Biol 2005;33:227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension 1999;33:323–328. [DOI] [PubMed] [Google Scholar]
- 37.Brosnihan KB, Senanayake PS, Li P, Ferrario CM. Bi-directional actions of estrogen on the renin–angiotensin system. Braz J Med Biol Res 1999;32:373–381. [DOI] [PubMed] [Google Scholar]
- 38.Proudler AJ, Ahmed AI, Crook D, Fogelman I, Rymer JM, Stevenson JC. Hormone replacement therapy and serum angiotensin-converting-enzyme activity in postmenopausal women. Lancet 1995;346:89–90. [DOI] [PubMed] [Google Scholar]
- 39.Oelkers WK. Effects of estrogens and progestogens on the renin–aldosterone system and blood pressure. Steroids 1996;61:166–171. [DOI] [PubMed] [Google Scholar]
- 40.Inoue Y, King TE Jr, Barker E, Daniloff E, Newman LS. Basic fibroblast growth factor and its receptors in idiopathic pulmonary fibrosis and lymphangioleiomyomatosis. Am J Respir Crit Care Med 2002;166:765–773. [DOI] [PubMed] [Google Scholar]
- 41.Silver RB, Reid AC, Mackins CJ, Askwith T, Schaefer U, Herzlinger D, Levi R. Mast cells: a unique source of renin. Proc Natl Acad Sci USA 2004;101:13607–13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molteni A, Moulder JE, Cohen EF, Ward WF, Fish BL, Taylor JM, Wolfe LF, Brizio-Molteni L, Veno P. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int J Radiat Biol 2000;76:523–532. [DOI] [PubMed] [Google Scholar]
- 43.Patella V, Marino I, Lamparter B, Arbustini E, Adt M, Marone G. Human heart mast cells: isolation, purification, ultrastructure, and immunologic characterization. J Immunol 1995;154:2855–2865. [PubMed] [Google Scholar]
- 44.Beil WJ, Pammer J. In situ detection of the mast cell proteases chymase and tryptase in human lung tissue using light and electron microscopy. Histochem Cell Biol 2001;116:483–493. [DOI] [PubMed] [Google Scholar]
- 45.Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab 2005;16:293–299. [DOI] [PubMed] [Google Scholar]
- 46.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC–mTOR pathway in human disease. Nat Genet 2005;37:19–24. [DOI] [PubMed] [Google Scholar]