Abstract
The requirement for precise geometric organization of endothelial cells and epithelial cells makes the gas-exchange region of the lung especially vulnerable to the adverse consequences of toxic products released from inflammatory cells. However, as a filter for large volumes of atmospheric gas, the lung is continually exposed to microorganisms and other toxic insults that require robust inflammatory defense. Enhanced production of extracellular matrix proteins is one important mechanism for restricting tissue damage, but excessive matrix production also has serious adverse effects on gas exchange. The amazing ability of the lung to recover from a barrage of environmental insults depends on precisely regulating both inflammation and extracellular matrix production in space and time. Below I review some of the evidence implicating members of the transforming growth factor β family as critical mediators of this delicate dance and describe examples of how disruption of this balance by alterations in the magnitude of spatially restricted transforming growth factor β activation can contribute to pathologic consequences of alveolar and airway injury and inflammation.
Keywords: emphysema, integrins, lung inflammation, pulmonary fibrosis, transforming growth factor β
In response to tissue injury or infection, initial host defense depends on rapid and robust induction of inflammation. However, the balance between host defense and inappropriate tissue damage requires pathways to wall off injured or infected areas and to rapidly turn off inflammatory responses. Members of the transforming growth factor β (TGF-β) family play important roles in both walling off injured areas and in negatively regulating tissue inflammation (1). In this way, TGF-β functions as a central regulator of tissue repair.
FUNCTIONS OF TGF-β
In mammals, there are three isoforms of TGF-β, TGF-β1, 2, and 3, which are each the product of a separate gene. In vitro, all three isoforms bind to and activate the same TGF-β receptors, activate a similar canonical signaling pathway, and produce largely similar effects on cell behavior. However, knockouts of the genes encoding each isoform in mice result in widely divergent phenotypes (2–4). These different phenotypes might be largely explained by differences in the pattern of expression of each isoform, but differential effects on noncanonical signaling pathways remain a possibility.
TGF-β family members exert pleiotropic effects on cell behavior (1). Major effects described more than a decade ago include inhibition of epithelial proliferation, induction of expression of genes encoding components of the extracellular matrix, and inhibition of expression of metalloprotease genes. The phenotype of mice expressing a null mutation of the TGF-β1 gene, lethal widespread tissue inflammation, and autoimmunity (3), strongly suggested an effect of TGF-β isoforms in negatively regulating both innate and acquired immunity.
Just as exaggerated or prolonged inflammation can lead to tissue destruction and loss of function, increased TGF-β activity can lead to exaggerated scar formation and loss of function (5). Negative regulation of inflammation can produce its own adverse effects, preventing the necessary actions of inflammatory cells in killing invading microorganisms and removing potentially toxic debris. The location and concentration of TGF-β activity therefore needs to be tightly regulated. One mechanism for such regulation is precise control of the amount of TGF-β protein that is synthesized and secreted by cells in injured organs. However, this mechanism is too slow to respond to the rapidly changing environmental circumstances and does not allow for precise subcellular localization of TGF-β action. So, like many potent cytokines and growth factors, TGF-β isoforms are made in excess in most organs and are stored in inactive form (6). Activation of latent TGF-β is thus an important mechanism for regulating TGF-β function.
MECHANISMS OF TGF-β ACTIVATION
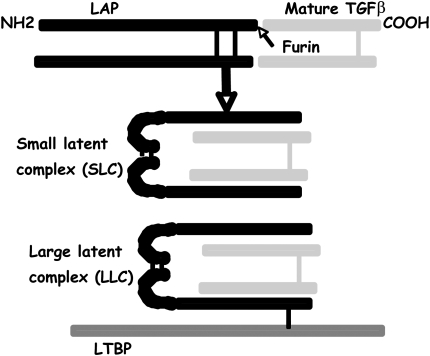
TGF-β isoforms are synthesized as gene products that include both the active cytokine and an endogenous inhibitor of TGF-β activity called the latency associated peptide (LAP). This product is cleaved in the endoplasmic reticulum by the endoprotease furin and assembled into a complex that contains a disulfide-linked homodimer of the shorter carboxy-terminal fragment that serves as active, mature TGF-β and a disulfide-linked homodimer of the larger amino-terminal fragment (LAP). These homodimers associate noncovalently, and in this form TGF-β is unable to bind to its receptors or induce most TGF-β-mediated effects. In virtually all cells, this complex is further modified by disulfide linkage of LAP to members of another protein family termed latent TGF-β binding proteins to form what are called large latent complexes. After secretion, latent TGF-β binding proteins are directly cross-linked to fibronectin in the extracellular matrix through the action of the extracellular enzyme tissue transglutaminase. Latent TGF-β is thus stored at high concentration in the extracellular matrix of most organs of healthy adult mammals with little evidence of TGF-β effects (Figure 1).
Figure 1.
Synthesis and organization of latent complexes of transforming growth factor β (TGF-β). All three mammalian isoforms of TGF-β are initially synthesized as a precursor protein that is cleaved in the endoplasmic reticulum into the amino-terminal fragment (called latency associated protein [LAP]) and the shorter carboxy-terminal fragment that is the mature cytokine. These fragments are assembled as a double homodimer called the small latent complex. In virtually all cells this complex is further modified by disulfide linkage to one of a family of proteins called latent TGF-β binding proteins (LTBPs), to form the large latent complex.
Because of the key role played by activation of preformed TGF-β, the mechanisms that regulate this activation process have been intensely studied. In vitro, latent complexes can be activated by numerous stimuli that induce mild protein denaturation, including extremes of temperature or pH and exposure to oxidants or ionizing radiation (6). In addition, cleavage of LAP by a variety of proteases can release free, active TGF-β. However, the significance of these activation mechanisms in vivo remains controversial. Two mechanisms of TGF-β activation have been definitively shown to be important in vivo: interaction with the secreted protein thrombospondin 1 (7) and activation by the integrin αvβ6 (8). However, because mice lacking both thrombospodin 1 and αvβ6 (9) are born alive and manifest phenotypes considerably more mild than the phenotypes of mice homozygous for null mutations of TGF-β1, 2, or 3, it is clear that additional mechanisms of TGF-β activation must play important roles in vivo.
Evidence supporting an important role for the αvβ6 integrin in TGF-β activation came from observation of the phenotype of mice homozygous for a null mutation of the β6 subunit. These mice develop exaggerated inflammation of the skin and lungs (10). In response to the anticancer drug bleomycin, a widely used inducer of pulmonary fibrosis, these mice develop exaggerated inflammation but are dramatically protected from subsequent pulmonary fibrosis (8), phenotypic features that suggested a role for TGF-β. The αvβ6 integrin binds directly to the LAP of TGF-β1 and TGF-β3 (11) and cells expressing the integrin generate TGF-β activity in vitro that can be completely inhibited by blocking antibodies to the integrin. Microarray analysis of the lungs of wild-type or β6 knockout mice treated with intratracheal bleomycin identified a large group of TGF-β-inducible genes that were induced at substantially lower levels in β6 knockout mice (12).
ROLE OF TGF-β ACTIVATION IN MAINTENANCE OF ALVEOLAR HOMEOSTASIS
Integrin β6 subunit knockout mice are normal at birth, but develop low-grade inflammation of the lungs and skin. Cutaneous inflammation mostly consists of infiltration of the dermis with macrophages, an effect that is principally seen at sites of low-grade skin trauma. Pulmonary inflammation in these animals is characterized by an increase in the number of alveolar macrophages and the presence of small numbers of air space neutrophils, lymphocytes, and eosinophils (10, 13), cells that are not normally present in the air spaces of healthy mice maintained in barrier facilities. In addition, macrophage morphology is grossly abnormal, with increases in macrophage size and a foamy appearance of the cytoplasm that is often associated with macrophage activation. Microarray analysis of the lungs of 2 mo old β6 knockout mice demonstrated a more than 40-fold increase in expression of the metalloprotease MMP12, which is generally restricted in its expression to macrophages (12).
At 2 mo of age, alveolar size in β6 knockout mice is normal, but there is a progressive increase in alveolar size with age, so that by 6 mo alveoli are significantly larger in β6 knockout mice than in littermate controls, and by 14 mo of age, the presence of emphysema is easily visually apparent on stained lung sections (14). Several lines of evidence suggest that the emphysema in these animals is a consequence of unrestrained macrophage protease production as a consequence of loss of integrin-mediated TGF-β activation. First, crossing the β6 knockout mice with mice lacking MMP12 completely rescues these animals from the development of age-related emphysema. Second, emphysema can also be rescued by crossing β6 knockout mice with mice expressing a transgenic form of the human β6 subunit under the control of the human surfactant C promoter (expressed in alveolar type II cells and a subset of bronchiolar epithelial cells). Furthermore, a mutant form of this transgene that can activate TGF-β, but is incapable of other β6-dependent effects (e.g., enhanced epithelial cell proliferation [15]) is also completely protective, while a mutant transgene that cannot activate TGF-β has no effect. In addition, expression of a double transgenic system that allows inducible expression of active TGF-β1 in airway epithelial cells in response to doxycycline feeding nearly completely reversed the MMP12 induction and the change in alveolar macrophage morphology in β6 knockout mice.
Importantly, whereas low concentrations of active TGF-β are required to maintain alveolar homeostasis and prevent the development of emphysema, transgenic expression of high concentrations of TGF-β can actually induce emphysema, apparently through a pathway that involves apoptosis of alveolar epithelial cells (16). These observations underscore the importance of precisely regulating the magnitude and location of this potent cytokine during the process of lung repair.
ROLE OF TGF-β IN ACUTE LUNG INJURY
The availability of β6 subunit knockout mice has led to identification of TGF-β as an important effector molecule in the development of pulmonary edema after acute lung injury. These mice are dramatically protected from the increase in lung permeability that occurs within a few days of administration of intratracheal bleomycin (17). This effect also appears to be due to a loss of activation of TGF-β, because administration of a TGF-β blocking reagent, a chimeric fusion protein of the high affinity TGF-β II receptor and the immunoglobulin Fc domain, completely protects wild-type mice from bleomycin-induced pulmonary edema. Blocking TGF-β also prevents pulmonary edema in response to intratracheal endotoxin. This protection is explained by three parallel effects of TGF-β on the cells that form the alveolar–capillary barrier. TGF-β directly increases the permeability of pulmonary endothelial monolayers and also increases the permeability of alveolar epithelial monolayers (17). Furthermore, TGF-β leads to a dramatic reduction in expression of the sodium channel ENaC on the apical surface of alveolar epithelial cells (18), thereby impairing the removal of salt and water from the alveolar lumen.
It thus appears that activation of TGF-β by the αvβ6 integrin plays an essential role in adjusting the set point for the amount of active TGF-β at specific sites in the lung parenchyma. In response to lung injury, integrin-mediated activation of TGF-β on the apical surface of alveolar epithelial cells inhibits the increase in macrophage protease expression that is a common consequence of lung injury or inflammation. At the same time, integrin-mediated activation on the basolateral surface of the epithelium induces production of collagen and other extracellular matrix proteins from alveolar fibroblasts, and probably contributes to induction of myofibroblasts, for example by effects on resting lung fibroblasts or fibrocytes recruited from the blood stream or by inducing epithelial to mesenchymal transformation of adjacent alveolar epithelial cells. Furthermore, TGF-β activated on the basal surface increases the permeability of adjacent endothelial cells and thus contributes to the influx of clotting factors and other proteins from the serum. Together, all these responses probably contribute to walling off of the injured (or infected) region, limiting the damage caused by unrestricted inflammation and initiating the process of alveolar repair.
As noted above, recent evidence from mice expressing a high concentration of inducible transgenic active TGF-β in the airways suggests that TGF-β could induce a significant increase of epithelial cell apotosis (16). This effect was maximal within a few days of induction of TGF-β expression and returned to basal levels within a few days. Despite the transient nature of the increase in apoptosis, prevention of apoptosis with pharmacologic caspase inhibitors also prevented the subsequent development of pulmonary and airway fibrosis in these animals, suggesting that such a mechanism could be biologically important in vivo, However, the relevance of these findings to physiologic concentrations of spatially restricted, active TGF-β remain to be determined.
Importantly, αvβ6-mediated TGF-β activation appears to be absolutely dependent on direct cell–cell contact and does not release any diffusible free TGF-β (8). Such a pathway is ideally suited to the alveolar space where all of the relevant cell types are likely to make direct contacts with one another through a thin and discontinuous basement membrane. Tight spatial and temporal control of this process is likely important in preventing the potentially damaging consequences of active TGF-β on adjacent uninjured regions of the lung, allowing the optimal maintenance of normal gas exchange.
ROLES OF TGF-β IN THE CONDUCTING AIRWAYS
In addition to their roles in diseases of the gas-exchanging regions of the lungs, TGF-β isoforms have also been implicated in the development of chronic airway diseases, including cystic fibrosis (19), chronic bronchitis, and asthma (20–22). For example, chronic airway diseases are commonly associated with subepithelial fibrosis, and the subepithelial fibrosis that occurs in at least some transgenic mouse models of chronic airway disease is inhibited by inhibition of TGF-β (23). Furthermore, marked overexpression of active TGF-β in the conducting airways causes severe subepithelial fibrosis. However, in contrast to parenchymal pulmonary fibrosis, the subepithelial fibrosis induced by overexpression of the cytokines interleukin-13 or interleukin-11 is not prevented in mice lacking the integrin β6 subunit (unpublished observations). Furthermore, these mice develop the same degree of subepithelial fibrosis in response to chronic allergen challenge as that seen in wild-type mice. It thus appears that the mechanisms underlying pathologic TGF-β activation in the conducting airways are different from those relevant to most of the pathologic consequences of TGF-β in the alveoli. This makes good sense if one considers the central role for direct contact with αvβ6-expressing epithelial cells in αvβ6-dependent TGF-β activation. In the conducting airways it seems likely that the fibroblasts responsible for subepithelial fibrosis are not in direct contact with the overlying epithelial cells. TGF-β activation on the surface of nonepithelial cells or by mechanisms that allow for release of free TGF-β therefore seem likely to be important in this process. As noted above, these could include activation by oxidation (24, 25), by exposure to thrombospondin 1, or by proteolytic degradation of TGF-β LAP. Support for a role for proteases comes from studies in mice that develop subepithelial fibrosis as a consequence of transgenic overexpression of IL-13. Crossing these mice into a line lacking the metalloproteinase MMP-9 or treatment of the mice with a pharmacologic MMP inhibitor substantially reduced (but did not completely prevent) subepithelial airway fibrosis (23). However, it is still formally possible that these effects could be explained by secondary actions of MMP9 on one or more other mechanism of TGF-β activation.
ACTIVATION OF TGF-β BY THE INTEGRIN αvβ8
One additional mechanism of TGF-β activation that could be relevant in the conducting airways is activation by the integrin αvβ8. This integrin binds to the same arginine–glycine–aspartic acid site in TGF-β1 and 3 LAP as does αvβ6 (26). However, the mechanisms of αvβ6- and αvβ8-mediated activation appear to be entirely different. Whereas αvβ6 appears to actively change the conformation of latent complexes of TGF-β without causing release of free TGF-β, αvβ8 appears to present the latent complex to the transmembrane protease MMP-14, which in turn cleaves LAP and releases free TGF-β. This mechanism therefore does not depend on direct cell–cell contact and could release free TGF-β, which could diffuse away and affect cells at a distance from the αvβ8-expressing cell. Like αvβ6, αvβ8 is highly expressed on airway epithelial cells and its expression there could therefore contribute to subepithelial fibrosis. αvβ8 mRNA is also expressed on a variety of leukocytes, including dendritic cells and macrophages (27).
Mice homozygous for a null mutation of the integrin β8 subunit die during embryonic development or soon after birth (28). The major pathologic abnormality in these mice is a defect in cerebrovascular development that leads to fatal intracranial hemorrhage. Mice lost during earlier stages of development also demonstrate vascular development abnormalities, also a prominent feature of TGF-β1 knockout mice. A substantial fraction of β8 knockout animals also develop cleft palate, a feature of TGF-β3 knockout mice (2). Furthermore, in vitro evidence suggests that αvβ8 expressed on glial cells can inhibit the migration of cerebrovascular endothelial cells, and that this effect is mediated by TGF-β (29). Together, these results are at least consistent with an important in vivo role for αvβ8-mediated activation of TGF-β1 and 3.
ADDITIONAL POTENTIAL ROLES OF TGF-β IN AIRWAY DISEASES
It is likely that the role of TGF-β in the conducting airways is considerably more complex than simply inducing subepithelial fibrosis. TGF-β can induce dramatically different effects on virtually every cell type present in the normal or diseased airway wall. Depending on which cell type is affected and on the local concentration of TGF-β activity, TGF-β could have either disease-promoting or disease-modifying effects. For example, TGF-β has been shown to induce the expression of a variety of contractile genes in mesenchymal cells, an effect that could contribute to the expansion of airway smooth muscle mass that is a feature of chronic asthma. TGF-β has been reported to be a potent chemotactic factor for macrophages and mast cells (30) and is a potent inducer of expression of the integrin αEβ7 on mast cells and lymphocytes (31). This integrin binds to the epithelial adhesion protein E cadherin (32), providing a mechanism for TGF-β to enhance retention of mast cells and lymphocytes within the airway epithelium. A similar process has been studied in detail in the gastrointestinal mucosa of mice infected with the parasite Nippostrongylus brasiliensis, which is thought, like allergic asthma, to be a model of Th2-mediated immune response. In response to Nippo infection there is a massive increase in the number of intraepithelial mast cells, and this effect is nearly completely abrogated in mice unable to activate TGF-β as a consequence of a null mutation of the β6 integrin subunit (33). These mice have also been shown to have a profound defect in induction of αEβ7 expression on intraepithelial mast cells (34). It is thus conceivable that active TGF-β contributes to the development or severity of asthma by enhancing the recruitment and/or retention of masts cells and lymphocytes to the airway epithelium.
However, as noted above, TGF-β is also a potent suppressor of both innate and adaptive immunity. Because innate immunity is clearly central to the development and progression of allergic asthma, it has been suggested that TGF-β might play an important role in ameliorating this disease. There are multiple mechanisms by which TGF-β can suppress inflammation and immunity. In vitro, TGF-β potently suppresses the differentiation of naive T cells into effector memory cells and also inhibits the proliferation of T cells. Furthermore, TGF-β plays an important role in the induction and/or function of regulatory T cells, a subset of T cells that has recently received renewed interest as important suppressors of excess immune responses and autoimmunity (35). Some of the most convincing evidence suggesting a critical role for TGF-β in the generation of regulatory T cells comes from studies overexpressing a transgenic dominant negative TGF-β receptor exclusively in T cells using the CD4 promoter. Mice expressing this transgene, which have T cells that cannot respond to TGF-β, have a marked defect in the development of regulatory T cells and evidence of excessive tissue inflammation and age-dependent autoimmunity (36). Further evidence that TGF-β can suppress allergic inflammation came from studies in which T cells expressing a transgenic T-cell receptor were transduced ex vivo to express TGF-β. Administration of these cells potently suppressed the consequences of airway antigen challenge (37).
CONCLUSIONS
TGF-β isoforms are central regulators of the balance that must be established to allow appropriate inflammatory responses to occur and be walled off without inducing either excessive matrix production (fibrosis) or excessive tissue destruction (emphysema or bronchiectasis). In both the alveoli and the conducting airways, TGF-β isoforms play this central role, but the nature of the target cells and the functional consequences of inappropriately large or small amounts of TGF-β activity are different at each site. Because large amounts of TGF-β are present in both the alveoli and airways of healthy adults, much of the regulation of TGF-β occurs at the level of activation of stored latent complexes. In the alveoli, much of this activation appears to be controlled through spatially restricted activation by the integrin αvβ6 (Figure 2), but the mechanisms regulating TGF-β activity in the conducting airways remain to be determined. Although the important pathologic consequences of excessive TGF-β activity make these proteins attractive targets for intervention in lung and airway diseases, careful attention to the differential effects of different amounts of TGF-β activity will need to be taken into consideration for the optimal use of such a strategy.
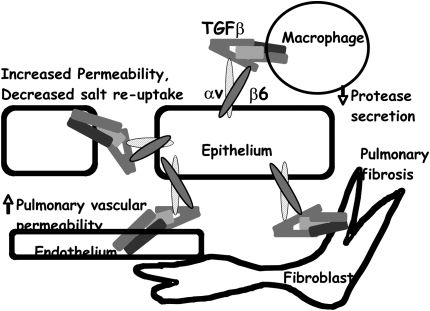
Figure 2.
Model of the consequences of αvβ6 integrin-mediated activation of TGF-β by alveolar epithelial cells. Integrin expressed on the luminal surface can present active TGF-β to luminal macrophages, thereby inhibiting protease secretion and maintaining alveolar homeostasis. Integrin on the basal surface can present active TGF-β to fibroblasts, which in excess contributes to the development of pulmonary fibrosis, and to endothelial cells, which regulate pulmonary vascular permeability. Integrin on the lateral surface can present active TGF-β to adjacent epithelial cells, increasing epithelial permeability and decreasing the reabsorption of salt, and therefore water, from the alveolar space.
Supported by grants HL56385, HL64353, HL53949 and HL66600 from the National Heart, Lung, and Blood Institute.
Conflict of Interest Statement: D.S. has had a sponsored research agreement from BiogenIdec for $99,000 annually from January 2004 to December 2007. He holds patents on the use of antibodies against the integrin αvβ6 for treatment of pulmonary fibrosis and acute lung injury.
References
- 1.Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta). Growth Factors 1993;8:1–9. [DOI] [PubMed] [Google Scholar]
- 2.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet 1995;11:415–421. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 1993;90:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGF-beta2 knockout mice have multiple developmental defects that are non-overlapping with other TGF-beta knockout phenotypes. Development 1997;124:2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts A, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 1986;83:4167–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int 1997;51:1376–1382. [DOI] [PubMed] [Google Scholar]
- 7.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 1998;93:1159–1170. [DOI] [PubMed] [Google Scholar]
- 8.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha v beta 6 binds and activates latent TGF-beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–328. [DOI] [PubMed] [Google Scholar]
- 9.Ludlow A, Yee KO, Lipman R, Bronson R, Weinreb P, Huang X, Sheppard D, Lawler J. Characterization of integrin beta6 and thrombospondin-1 double-null mice. J Cell Mol Med 2005;9:421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV Jr, Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 1996;133:921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annes JP, Rifkin DB, Munger JS. The integrin alphaVbeta6 binds and activates latent TGF-beta3. FEBS Lett 2002;511:65–68. [DOI] [PubMed] [Google Scholar]
- 12.Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, Morris D, Huang X, Sheppard D, Heller RA. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci USA 2000;97:1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang XZ, Wu JF, Zhu W, Pytela R, Sheppard D. Expression of the human integrin beta6 subunit in alveolar type II cells and bronchiolar epithelial cells reverses lung inflammation in beta6 knockout mice. Am J Respir Cell Mol Biol 1998;19:636–642. [DOI] [PubMed] [Google Scholar]
- 14.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature 2003;422:169–173. [DOI] [PubMed] [Google Scholar]
- 15.Agrez M, Chen A, Cone R, Pytela R, Sheppard D. The alpha v beta 6 integrin promotes proliferation of colon carcinoma cells through a unique region of the beta 6 cytoplasmic domain. J Cell Biol 1994;127:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med 2004;200:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, et al. TGF-beta is a critical mediator of acute lung injury. J Clin Invest 2001;107:1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank J, Roux J, Kawakatsu H, Su G, Dagenais A, Berthiaume Y, Howard M, Canessa CM, Fang X, Sheppard D, et al. Transforming growth factor-beta1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem 2003;278:43939–43950. [DOI] [PubMed] [Google Scholar]
- 19.Drumm ML, Konstan MW, Schluchter MD, Handler A, Pace R, Zou F, Zariwala M, Fargo D, Xu A, Dunn JM, et al. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med 2005;353:1443–1453. [DOI] [PubMed] [Google Scholar]
- 20.Nagpal K, Sharma S, Nahid BRC, Niphadkar PV, Sharma SK, Ghosh B. TGF-beta1 haplotypes and asthma in Indian populations. J Allergy Clin Immunol 2005;115:527–533. [DOI] [PubMed] [Google Scholar]
- 21.Silverman ES, Palmer LJ, Subramaniam V, Hallock A, Mathew S, Vallone J, Faffe DS, Shikanai T, Raby BA, Weiss ST, et al. Transforming growth factor-beta1 promoter polymorphism C-509T is associated with asthma. Am J Respir Crit Care Med 2004;169:214–219. [DOI] [PubMed] [Google Scholar]
- 22.Pulleyn LJ, Newton R, Adcock IM, Barnes PJ. TGF-beta1 allele association with asthma severity. Hum Genet 2001;109:623–627. [DOI] [PubMed] [Google Scholar]
- 23.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med 2001;194:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 1996;10:1077–1083. [DOI] [PubMed] [Google Scholar]
- 25.Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest 1994;93:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol 2002;157:493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbas AR, Baldwin D, Ma Y, Ouyang W, Gurney A, Martin F, Fong S, van Lookeren Campagne M, Godowski P, Williams PM, et al. Immune response in silico (IRIS): immune-specific genes identified from a compendium of microarray expression data. Genes Immun 2005;6:319–331. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. Beta8 integrins are required for vascular morphogenesis in mouse embryos. Development 2002;129:2891–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin αvβ8-mediated activation of transforming growth factor-β by perivascular astrocytes: an angiogenic control switch. Am J Pathol 2005;166:1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Boer WI, van Schadewijk A, Sont JK, Sharma HS, Stolk J, Hiemstra PS, van Krieken JH. Transforming growth factor beta1 and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:1951–1957. [DOI] [PubMed] [Google Scholar]
- 31.Miller HR, Wright SH, Knight PA, Thornton EM. A novel function for transforming growth factor-beta1: upregulation of the expression and the IgE-independent extracellular release of a mucosal mast cell granule-specific beta-chymase, mouse mast cell protease-1. Blood 1999;93:3473–3486. [PubMed] [Google Scholar]
- 32.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature 1994;372:190–193. [DOI] [PubMed] [Google Scholar]
- 33.Knight PA, Wright SH, Brown JK, Huang X, Sheppard D, Miller HR. Enteric expression of the integrin alpha(v)beta(6) is essential for nematode-induced mucosal mast cell hyperplasia and expression of the granule chymase, mouse mast cell protease-1. Am J Pathol 2002;161:771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown JK, Knight PA, Pemberton AD, Wright SH, Pate JA, Thornton EM, Miller HR. Expression of integrin-alphaE by mucosal mast cells in the intestinal epithelium and its absence in nematode-infected mice lacking the transforming growth factor-beta1-activating integrin alphavbeta6. Am J Pathol 2004;165:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med 2002;195:1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorelik L, Flavell RA. Abrogation of TGF-beta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 2000;12:171–181. [DOI] [PubMed] [Google Scholar]
- 37.Hansen G, McIntire JJ, Yeung VP, Berry G, Thorbecke GJ, Chen L, DeKruyff RH, Umetsu DT. CD4(+) T helper cells engineered to produce latent TGF-beta1 reverse allergen-induced airway hyperreactivity and inflammation. J Clin Invest 2000;105:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]