Abstract
The re-establishment of large carnivores in Norway has led to increased conflicts and the adoption of regional zoning for these predators. When planning the future distribution of large carnivores, it is important to consider details of their potential habitat tolerances and strength of inter-specific differentiation. We studied differentiation in habitat and kill sites within the large-carnivore community of south-eastern Norway.
We compared habitat selection of the brown bear Ursus arctos L., Eurasian lynx Lynx lynx L., wolf Canis lupus L. and wolverine Gulo gulo L., based on radio-tracking data. Differences in kill site locations were explored using locations of documented predator-killed sheep Ovis aries L. We modelled each species’ selection for, and differentiation in, habitat and kill sites on a landscape scale using resource selection functions and multinomial logistic regression. Based on projected probability of occurrence maps, we estimated continuous patches of habitat within the study area.
Although bears, lynx, wolves and wolverines had overlapping distributions, we found a clear differentiation for all four species in both habitat and kill sites. The presence of bears, wolves and lynx was generally associated with rugged, forested areas at lower elevations, whereas wolverines selected rugged terrain at higher elevations. Some degree of sympatry was possible in over 40% of the study area, although only 1·5% could hold all four large carnivores together.
Synthesis and applications. A geographically differentiated management policy has been adopted in Norway, aimed at conserving viable populations of large carnivores while minimizing the potential for conflicts. Sympatry of all four carnivores will be most successful if regional zones are established of adequate size spanning an elevational gradient. High prey densities, low carnivore densities, low dietary overlap and scavenging opportunities have most probably led to reduced competitive exclusion. Although regional sympatry enhances the conservation of an intact guild of large carnivores, it may well increase conflict levels and resistance to carnivore conservation locally.
Keywords: brown bear, elevational gradients, Eurasian lynx, grey wolf, habitat and predation patterns, intra-guild competition, regional zoning of large carnivores, species co-existence, wolverine
Introduction
During the last century, habitat fragmentation and increased human pressure have reduced populations of large carnivores throughout the world (Woodroffe 2000; Sunquist & Sunquist 2001). Although large carnivores are able to persist in multiple-use landscapes, many mammalian carnivores possess characteristics that may make them particularly vulnerable to landscape changes (Woodroffe & Ginsberg 1998). Carnivore species may react differently to fragmentation however, due to differences in behaviour and ecology (Sunquist & Sunquist 2001; Crooks 2002).
Apart from direct competition for prey, possible sympatry of multiple carnivore species also depends on intra-guild competition and interference. Intra-guild competition is fiercer with higher dietary or spatial overlap, is often asymmetrical and may have strong effects on the population dynamics of the subordinate competitor (Creel, Spong & Creel 2001; Heithaus 2001). Linnell & Strand (2000) hypothesized that interference may reduce population growth through temporal and spatial avoidance, changes in foraging efficiency, or direct killing, irrespective of dietary and habitat overlap. Whereas intra-guild competition is thought to be density-dependent, the degree of intra-guild interference is thought to depend on body-size differences (Buskirk 1999). Intra-guild competition and interference may ultimately lead to habitat differentiation (i.e. competitive exclusion). In addition, subordinate predators may also be suppressed in the absence of scavenging opportunities from top predators (Buskirk 1999).
Four species of large carnivores are present in Scandinavia: the brown bear Ursus arctosL., grey wolf Canis lupus L., Eurasian lynx Lynx lynxL. and wolverine Gulo guloL. The conservation of large carnivores in Scandinavia is dependent upon co-existence with humans in a multiple-use landscape. The natural recovery of carnivore populations, however, has led to increased conflict. The main causes of conflict are their depredation on semi-domestic reindeer Rangifer tarandus L. throughout the year in Fennoscandia, and on free-ranging domestic sheep Ovis aries L. during summer, primarily in Norway (Swenson & Andrén 2005). Although most predation on reindeer is caused by wolverines and lynx, all large carnivores in Norway kill free-ranging sheep. This has led to the adoption of a geographically differentiated management policy (i.e. zoning of large carnivores) aimed at conserving viable populations of large carnivores while minimizing the potential for conflicts (Ministry of Environment 2003; Linnell et al. 2005). When planning the future distribution of large carnivores, it is important to consider details of their potential habitat tolerances, and strength of differentiation among the four species. The present population goals for large carnivores in Norway are specified for eight management regions (Committee on Energy and Environment 2004). The large-carnivore region of Hedmark County is the only region that has populations of all four large-carnivore species. In this region, we analysed large-carnivore habitat use based on radio-telemetry and location of sheep kill sites. Compared to kill sites, radio-telemetry locations represent a wide spectrum of habitat used by a carnivore. Radio-telemetry locations include resting places, kill sites of wild and domestic ungulates including sheep, as well as movements between kill sites and resting places. However, kill sites represent the main cause of the conflict between large carnivores and human interests. Our initial expectation was that bears, wolves and lynx would have broadly similar patterns of occurrence (forest-dwelling species). However, through the effects of intra-guild competition and interference, they were expected to show differentiation in habitat use. By contrast, we expected the wolverine to be clearly differentiated in habitat, due to the combined effects of their perceived susceptibility to fragmentation and avoidance of other carnivores. Likewise, we expected that potential avoidance of other carnivore species would affect the distribution of sheep kill sites; especially in the lynx and wolverine as subordinate predators.
Materials and methods
study area
Norway is the country in mainland Europe with the lowest human population density (c. 12 km−2) and with large continuous areas of semi-natural landscapes. Despite the low human density, wilderness areas have declined dramatically in the last century through resource extraction (i.e. livestock grazing, hunting, timber logging), infrastructure development (i.e. roads, recreational cabins and hydropower plants), and recreation. Our study area (18 374 km2) was located in south-eastern Norway. It consists of 10 municipalities in the northern parts of Hedmark County and three bordering municipalities in Oppland County (see corner Fig. 1), and was centred on lake Storsjøen (latitude 61°27′, longitude 11°18′). The river Glomma and the adjacent national highway RV3 run from north to south in the centre of the study area. The landscape consists of boreal forests interspersed with low mountain ranges. Areas above tree line, at 900–1000 m above sea level, are mainly found in the west and north of the study area. Infrastructure is mainly found in the south and west of the study area, and in the valley bottoms. All four large-carnivore species exist within the study area and were estimated by the national large-carnivore monitoring programme at 14–17 wolves (three to four packs or scent-marking pairs), 20–30 wolverines and 31–37 lynx (Brøseth & Andersen 2004; Brøseth, Odden & Linnell 2004; Wabakken et al. 2004). The total number of bears was estimated at nine to 13 for southeast Norway (Østlandet) (Swenson et al. 2003). The populations of all four species are in a natural re-colonizing stage, with the bear population in particular being dominated by males. Bears and lynx were already present before the start of this study (1988) and have expanded their range from the (north)east and from the (south)east, respectively. Wolves re-colonized the study area in 1998 from the (south)east; wolverines followed the year after from the northeast and west. At present, all four species occur throughout the study area. The average winter densities of potential large prey species are 0·9 km−2 and 0·8 km−2 for moose Alces alces L. and roe deer Capreolus capreolus L., respectively (Solberg et al. 2003). However, roe deer are distributed less evenly over the area than moose. Other potential ungulate prey species are red deer Cervus elaphusL. and wild reindeer. Moreover, semi-domestic reindeer are herded in the north-eastern two municipalities of the study area. Potential small prey are tetraonids and other bird species, medium-sized and small rodents and insectivores, as well as medium-sized and small carnivores. Throughout the study area, with disjoint distribution and at highly variable densities, free-ranging, and mostly unattended domestic sheep and cattle Bos taurus L. graze in the forests and low mountain ranges during the summer (June–September) (Zimmermann, Wabakken & Dötterer 2003).
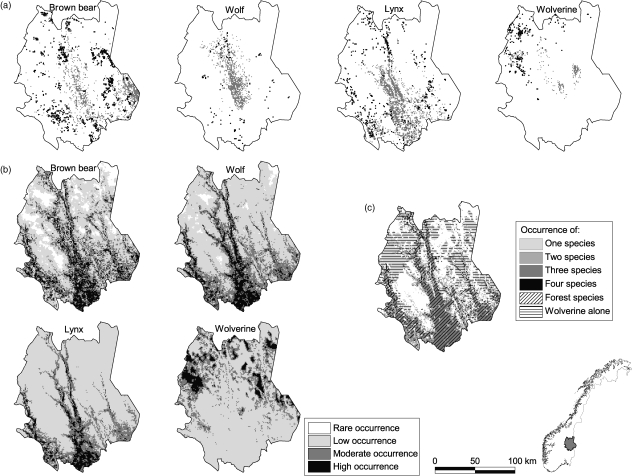
Fig. 1.
(a) Presence maps for four large-carnivore species within the study area in south-eastern Norway (see corner). Presence pixels from radio-tracking data are given in grey; locations of sheep killed by each carnivore species are given as black dots. (b) Occurrence maps for each species; probability distributions were based on species-specific resource selection function models (Fig. 2). (c) Possible sympatry based on the overlap of moderate- and high-occurrence classes for each species.
study design and spatial scale
The scale (i.e. grain/resolution and domain/extent) of investigation of the differentiation in habitat tolerances among guild members is important, as ecological processes can occur at different spatio-temporal scales, which influence the strength of habitat preferences (Boyce 2006). Our spatially, but not temporally, overlapping data sets (Table 1) on the large-carnivore guild in one specific region of Norway best fit a landscape approach. To address differentiation among wide-ranging large-carnivore species, the resolution need not be very fine; a coarser grain will reduce intra-specific spatial heterogeneity at finer resolutions leaving the inter-specific differences under study. However, the extent should be large enough to encompass the regional dynamics of the large-carnivore community in multiple-use landscapes. We therefore chose to study patterns of use on the landscape using a grain of 1 × 1 km resource units (pixels), and investigated habitat differentiation within the large-carnivore guild by comparing selection of geographical ranges among the species within the study area (first order selection, Johnson 1980).
Table 1.
Sampling statistics of the radio-tracked large carnivores and predator-killed sheep in south-eastern Norway
| Brown bear | Wolf | Lynx | Wolverine | |
|---|---|---|---|---|
| Statistics habitat | ||||
| Collection period | 1988–2004 | 2001–2005 | 1995–2002 | 2003–2004 |
| Collection methods (collar type) | VHF, GPS | GPS | VHF, GPS | GPS |
| No. of individuals | 20 | 4* | 16 | 4 |
| Adult females | 5 | 2 | 10 | 3 |
| Adult males | 15 | 2 | 6 | 1 |
| Individuals per year (± SD) | 4·9 ± 1·4 | 2·6 ± 0·9 | 7·6 ± 4·6 | 3·5 ± 0·7 |
| Total radio fixes (> 24 h apart) | 2194 | 2780 | 3681 | 453 |
| No. of radio fixes per individual (± SD) | 110 ± 139 | 498 ± 305 | 230 ± 144 | 227 ± 88 |
| No. of habitat pixels (Fig. 1) | 1169 | 874 | 1761 | 265 |
| Statistics kill sites | ||||
| No. of sheep carcasses | 1558 | 416 | 861 | 364 |
| No. of kill site pixels (Fig. 1) | 760 | 102 | 462 | 218 |
Two alpha pairs of two packs.
topographic and habitat covariates
Habitat differentiation among the four large-carnivore species was investigated using seven habitat covariates: elevation, terrain ruggedness, percentage tree cover, distance to the forest edge, and distance to the nearest public road, private road and building. Elevation was obtained from a 100 × 100 m Digital Elevation Model (DEM; Norwegian Mapping Authority). Terrain ruggedness was calculated by taking the square root of the sum of squared differences in elevation of each pixel in the 100 × 100 m DEM to its eight neighbours, thus rendering a terrain ruggedness index (Riley, DeGloria & Elliot 1999). Percentage tree cover was obtained from a MODIS map (Hansen et al. 2002). The four distance measures were obtained from digital 1:50 000 topographic maps (Norwegian Mapping Authority). Distances to the forest edge were negative inside and positive outside the forest. All maps were finally converted into overlapping 1 × 1 km pixel grids.
data sets
The study was based on radio-tracking adult individuals within research projects on large carnivores (Table 1). Only locations more than 24 h apart were used in order to reduce autocorrelation (Otis & White 1999) and standardize between GPS and VHF data (i.e. several positions per day vs. up to one position per day, respectively). As the data were collected during different time periods, this study renders insight into spatial but not necessarily temporal sympatry of the four large carnivores.
Location of kill sites was assessed using locations of documented predator-killed sheep falling within the boundaries of the study area from the period 1994–2004 (Fig. 1a). To receive compensation for losses suffered by predators, it is economically important to the owners of free-ranging sheep to intensively search for carcasses throughout the summer grazing season (~100 days yr−1). Carcasses are examined by trained personnel of the State Nature Inspectorate, who record the location and determine the species of the predator, based on well-documented species-specific kill patterns through necropsy (Landa 1999). Although the locations of sheep kills found are likely to be biased towards ease of human detection, this can be expected to be irrespective of carnivore species.
modelling and statistical analyses
All statistics were performed in r 2·5·1 (r Development Core Team 2007), the geographical analyses were performed in ArcView 3·3 and Spatial Analyst extension (ESRI Inc., Redlands, CA, USA). For each species, we transformed the set of radio-tracking locations and killed sheep into presence maps, where each 1 × 1 km pixel indicated whether or not it included one or more locations (Fig. 1). This large scale minimizes unwanted spatial autocorrelation and pseudo-replication effects. We expected a pseudo-replication effect for the members of the two wolf packs while travelling together, and for animals that were tracked over several years. Also, large carnivores, especially bears and wolves, often kill several sheep during one attack. Here we assumed that the individuals used in this study represented the resource selection of the species. Intra-specific variation was found to be insignificant compared to inter-specific variation (see Supplementary Material Appendix S1).
We modelled each species’ habitat selection relative to availability on a landscape scale, and each species’ location of kill sites relative to its habitat used (i.e. presence pixels), using logistic regression to estimate coefficients in exponential resource selection functions (Manly et al. 2002):
| eqn 1 |
with βi as the model coefficient of the ith of n habitat covariates, Xi. Availability for habitat selection was considered to be the same for all species, and was based on a ‘presence’ map generated from a data set of 2500 points randomly spread throughout the study area following the same procedure as mentioned above (rendering 2311 availability pixels). Because the focus of this study was to elucidate habitat differentiation among large carnivores, we present the full models only. To evaluate predictive success of the resource selection function models we used the k-fold cross-validation procedures as proposed by Boyce et al. (2002). Cross-validated Spearman-rank correlations were calculated between 10 resource selection function bin ranks and area-adjusted frequencies for five model ‘test-training’ sets.
The resource selection functions for habitat selection were for each cross-validation set projected spatially on each 1 × 1 km cell across the study area to generate maps of the relative probability of occurrence for each species. For ease of interpretation, we classified the relative probabilities of occurrence into four broad classes: rare- (< −1σ), low- (−1σ − µ), moderate- (µ − 1σ) and high- (> 1σ) occurrence habitats. To gain better insight into the scale of our study area vs. necessary scales for regional zoning, we calculated the degree of overlap among species for each cross-validation set based on the distribution of moderate- and high-occurrence habitats for each species (i.e. pixels with relative probability > µ). Finally, we calculated the distribution of patch sizes for each species and degree of overlap, based on occurrence maps produced from the averages over the set of five cross-validation maps. Continuous patches were identified using the Patch Analyst 2·2 extension (Rempel 2000), after smoothening the occurrence maps using a 3 × 3 moving window majority filter.
We estimated the overall strength of differentiation among species both in habitat use and location of kill sites by calculating the multivariate distance over the standardized resource selection functions coefficients. Standardized coefficients allow comparisons of the relative influence of resources on selection, regardless of the measurement scale quantifying the resource (Marzluff et al. 2004). The standardized coefficients for each resource covariate 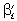 were estimated as:
were estimated as:
| eqn 2 |
where βi is the maximum-likelihood estimate of the coefficient for resource i; 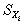 is the standard deviation of the values of resource i; and Sresp is the estimate of the standard deviation of the response values. The standardized standard errors of the coefficients
is the standard deviation of the values of resource i; and Sresp is the estimate of the standard deviation of the response values. The standardized standard errors of the coefficients 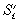 were calculated in a similar fashion. The multivariate distance between two species j and k was calculated as:
were calculated in a similar fashion. The multivariate distance between two species j and k was calculated as:
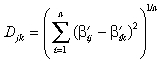 |
eqn 3 |
We incorporated the uncertainty from the resource selection functions by calculating the average multivariate distances from 1000 iterated random draws from a distribution with mean 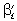 and standard error
and standard error 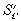 We used a linear stretch to scale the multivariate distances between –1 and +1 for totally differentiated and identical selection, respectively:
We used a linear stretch to scale the multivariate distances between –1 and +1 for totally differentiated and identical selection, respectively:
| eqn 4 |
Finally, we performed multinomial logistic regression (Hosmer & Lemeshow 2000) on the combined presence data over all species (separately for habitat and kill sites) to investigate each species’ degree of differentiation in habitat use (or location of kill sites) relative to habitat (or kill sites) used by the other species, and determine which covariates they differed, and how strongly. The species were taken as a categorical dependent variable (1 = bear, 2 = wolf, 3 = lynx, 4 = wolverine). By taking each species as a reference category in an iterative way, each unique species combination could be compared.
Results
habitat use and location of kill sites
All four habitat models had good predictive performance, given the significant Spearman-rank correlations across the five cross-validation sets (bear: 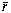 = 0·980 ± 0·010 (SD), P < 0·001; wolf:
= 0·980 ± 0·010 (SD), P < 0·001; wolf: 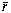 = 0·950 ± 0·026, P < 0·001; lynx:
= 0·950 ± 0·026, P < 0·001; lynx: 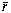 = 0·979 ± 0·014; P < 0·001; wolverine:
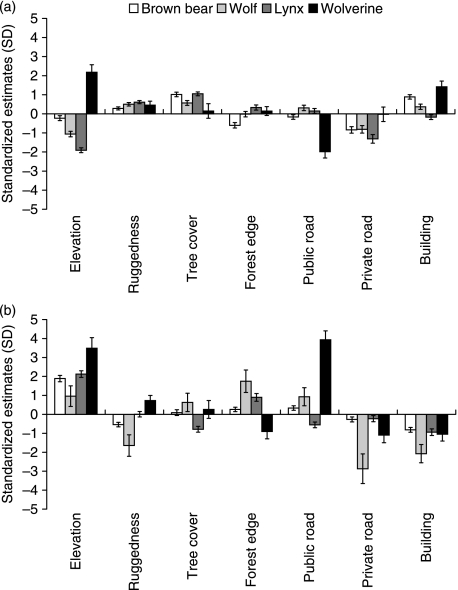
= 0·979 ± 0·014; P < 0·001; wolverine: 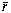 = 0·859 ± 0·097, P < 0·001). The models explained 13–14% of the deviance for bears, wolves and wolverines, and 40% for lynx (Nagelkerke's R2 of 0·139, 0·129, 0·142 and 0·402, respectively). The resource selection functions for bears, wolves and lynx indicated that the presence of these species was generally associated with rugged, forested areas at lower elevations, and relatively close to private roads (Fig. 2a, Supplementary Material Table S1). Of these species, lynx preferred the lowest elevations, the densest forests, and kept closest to infrastructure. Wolverines, on the other hand, selected rugged terrain at higher elevations and away from buildings but closer to public roads. They did not show any selection for tree cover or private roads.
= 0·859 ± 0·097, P < 0·001). The models explained 13–14% of the deviance for bears, wolves and wolverines, and 40% for lynx (Nagelkerke's R2 of 0·139, 0·129, 0·142 and 0·402, respectively). The resource selection functions for bears, wolves and lynx indicated that the presence of these species was generally associated with rugged, forested areas at lower elevations, and relatively close to private roads (Fig. 2a, Supplementary Material Table S1). Of these species, lynx preferred the lowest elevations, the densest forests, and kept closest to infrastructure. Wolverines, on the other hand, selected rugged terrain at higher elevations and away from buildings but closer to public roads. They did not show any selection for tree cover or private roads.
Fig. 2.
Standardized estimates (± SD) of the resource selection functions for (a) habitat selection relative to available habitat within the study area, and (b) location of kill sites relative to selected habitat. The last four covariates are distance measures.
The kill site models for bears, wolves and lynx had good predictive performance and explained 16–22% of the deviance (bear: 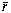 = 0·919 ± 0·048 (SD), P < 0·001, Nagelkerke's R2 = 0·201; wolf:
= 0·919 ± 0·048 (SD), P < 0·001, Nagelkerke's R2 = 0·201; wolf: 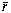 = 0·804 ± 0·081, P < 0·001, R2 = 0·163; lynx:
= 0·804 ± 0·081, P < 0·001, R2 = 0·163; lynx: 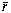 = 0·932 ± 0·018; P < 0·001, R2 = 0·215). The kill site model for wolverines had a lower, but significant, Spearman-rank correlation and explained over 50% of the deviance (wolverine:
= 0·932 ± 0·018; P < 0·001, R2 = 0·215). The kill site model for wolverines had a lower, but significant, Spearman-rank correlation and explained over 50% of the deviance (wolverine: 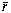 = 0·601 ± 0·159 (SD), P < 0·05, Nagelkerke's R2 = 0·570). Sheep kill sites were for all four species found at higher elevations and closer to private roads and buildings compared to their selected habitat (Fig. 2b, Supplementary Material Table S1). The three forest-dwelling species killed sheep in less rugged terrain and farther from forest edges; opposite effects were found for the wolverine. All species, except lynx, killed sheep farther from public roads.
= 0·601 ± 0·159 (SD), P < 0·05, Nagelkerke's R2 = 0·570). Sheep kill sites were for all four species found at higher elevations and closer to private roads and buildings compared to their selected habitat (Fig. 2b, Supplementary Material Table S1). The three forest-dwelling species killed sheep in less rugged terrain and farther from forest edges; opposite effects were found for the wolverine. All species, except lynx, killed sheep farther from public roads.
patterns of intra-guild distribution
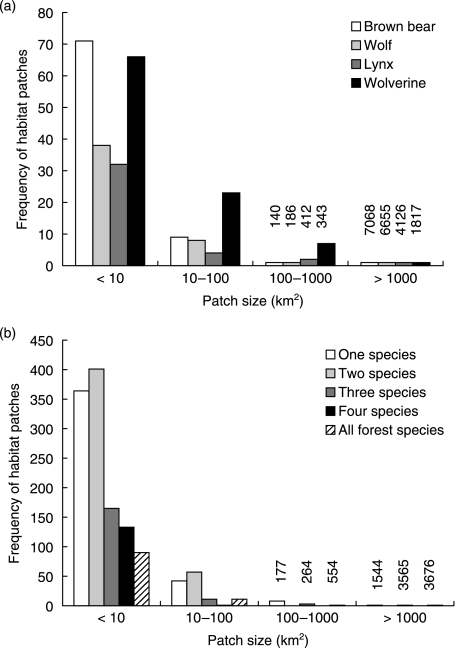
A clear distinction can be seen between the distributions of wolverines vs. the three forest-dwelling carnivore species (Fig. 1b). Whereas wolverine presence was most probable in the more mountainous northwest of the study area, the presence of the other three species was more distributed in the south and along the Glomma Valley running from north to south in the centre of the study area. Using a minimum threshold of moderate occurrence, 7490 km2 ± 87 (SD) of the study area was defined as suitable for bears, and 7126 ± 124, 5214 ± 64 and 5418 km2 ± 117 were classified as suitable for wolves, lynx and wolverines, respectively. The mean patch size for bear, wolf, lynx and wolverines were 93 ± 780 (SD), 149 ± 959, 133 ± 664 and 54 ± 210 km2, respectively. The wolverine both had a high amount of small patches (< 10 km2) and the smallest average patch size >1000 km2 (Fig. 3a).
Fig. 3.
Size distribution of habitat patches for (a) four large-carnivore species identified using resource selection functions (Fig. 1b) and (b) degree of overlap in south-eastern Norway (Fig. 1c). For the highest two categories the average patch size is given.
We calculated the degree of overlap among species and patch sizes based on the distribution of moderate- and high-occurrence habitats in the occurrence maps for each species (Fig. 1c). Using a minimum threshold of moderate occurrence, 4893 km2 ± 141 (SD) of the study area could hold only one species, whereas 2612 ± 50, 4671 ± 72 and 280 ± 38 km2 were classified as suitable for two, three and four species, respectively. In total, 4496 km2 ± 47 (SD) of the study area could hold all three forest species. Again, the clear distinction between sympatry of the three forest-dwelling species and the wolverines is clear (Fig. 1c), with a 60–99% overlap between bears, wolves and lynx and a 5–29% overlap with wolverines (Table 2). The mean patch sizes for overlap of one, two, three, four species and overlap of the three forest species were 12 ± 80 (SD), 5 ± 8, 28 ± 269, 2 ± 1 and 47 ± 365 km2, respectively. Both the overlap of three species and of the forest-dwelling species had a relatively low amount of small patches (< 10 km2) and large average patch sizes > 1000 km2 (Fig. 3b).
Table 2.
Proportional degree of overlap (± SD) in distribution between species based on the distribution of occurrence for each species (pixels with a probability higher than the mean), averaged over five cross-validation sets
| Proportional overlap with distribution of | ||||
|---|---|---|---|---|
| Species distribution | Brown bear | Wolf | Lynx | Wolverine |
| Brown bear | 0·802 ± 0·018 | 0·601 ± 0·008 | 0·209 ± 0·014 | |
| Wolf | 0·843 ± 0·017 | 0·726 ± 0·009 | 0·109 ± 0·009 | |
| Lynx | 0·863 ± 0·012 | 0·992 ± 0·010 | 0·055 ± 0·009 | |
| Wolverine | 0·289 ± 0·018 | 0·143 ± 0·011 | 0·053 ± 0·008 | |
differentiation in habitat and kill sites
Overall, wolverines differed in their habitat use compared to the three forest-dwelling carnivore species (Table 3). Bears–wolves and wolves–lynx selected similar habitat, but no differentiation was found between bears and lynx. The overall differentiation in location of kill sites showed a clear difference for wolverines compared to the three forest-dwelling species. Although no differentiation was found for bears–wolves and wolves–lynx, bears and lynx killed sheep in similar habitat (Table 3).
Table 3.
Strength of differentiation in habitat use and location of kill sites between species as measured by the multivariate distances between the standardized partial regression coefficients, given in Fig. 2. Negative mean values indicate differentiation and positive values similar use/location. When the 95% CI includes zero; neither could be determined. Significant results are given in bold
| Species pairs | Mean | SD | 95% CI | |
|---|---|---|---|---|
| Habitat use | ||||
| brown bear | wolf | 0·561 | 0·112 | 0·341–0·781 |
| brown bear | lynx | 0·114 | 0·076 | −0·034–0·263 |
| brown bear | wolverine | −0·281 | 0·117 | −0·511––0·051 |
| wolf | lynx | 0·577 | 0·116 | 0·349–0·804 |
| wolf | wolverine | −0·513 | 0·104 | −0·717––0·309 |
| lynx | wolverine | −0·735 | 0·090 | −0·911––0·559 |
| Kill sites | ||||
| brown bear | wolf | −0·181 | 0·176 | −0·526–0·163 |
| brown bear | lynx | 0·595 | 0·098 | 0·403–0·786 |
| brown bear | wolverine | −0·329 | 0·112 | −0·549––0·110 |
| wolf | lynx | −0·303 | 0·156 | −0·608–0·002 |
| wolf | wolverine | −0·627 | 0·132 | −0·886––0·368 |
| lynx | wolverine | −0·512 | 0·102 | −0·712––0·313 |
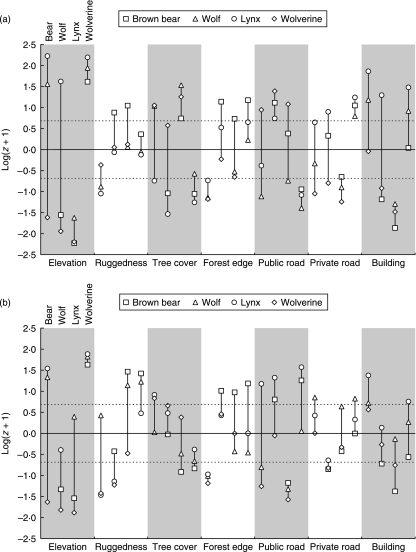
Multinomial logistic regression indicated a clear differentiation in use of habitat covariates among all four species (Nagelkerke's R2 = 0·318, Fig. 4a). The strongest differentiation in preference was found for elevation. Lynx were found at the lowest elevations, followed in rising elevation by wolves, bears and wolverines (Fig. 4a, Supplementary Material Table S1). The bear was found in less rugged terrain and closer to forest edges than the other three species. Also, a clear effect in differentiation was found for tree cover and infrastructure. The lynx preferred pixels with a higher percentage of tree cover, and closer to private roads and buildings than the bear and wolf. The wolverine was found in more open areas far from private roads and buildings. The wolf and wolverine also differentiated from bear and lynx in their proximity to public roads.
Fig. 4.
Multinomial logistic regression results for comparisons of habitat use (a) and location of kill sites (b) among four carnivore species in south-eastern Norway. The z-scores given represent the strength of differentiation between species. Each species’ degree of differentiation is shown relative to the other three species for each covariate. The sign indicates the direction of the effect. Values larger than ± 0·685 (dotted lines) indicate significant differentiation.
Multinomial logistic regression on the locations of predator-killed sheep indicated a clear differentiation in kill sites among species (Nagelkerke's R2 = 0·485, Fig. 4b, Supplementary Material Table S1). As for the differentiation in habitat, elevation of kill sites had a similar strong differentiating effect; except in the case of wolf–lynx kill sites. For these two species, ruggedness and distance to public roads at kill sites differed most. Lynx and wolverines killed sheep in more rugged terrain than bears and wolves. Wolverines killed sheep in more open areas, whereas bears chose more forested sites closer to forest edges. Lynx stayed closer to public roads and buildings than the other species. Wolves killed sheep closer to private roads than bears and wolverines.
Discussion
The results from this study indicate that the three forest-dwelling large-carnivore species, the lynx, wolf and bear, had relatively similar habitat preferences. All three species selected rugged, forested areas at lower elevations. In contrast, the wolverine selected open, rugged terrain at higher elevations and killed sheep in similar terrain, but farther from infrastructure. This result fits well with the perception that the wolverine is a carnivore of remote alpine regions (May et al. 2006; Copeland et al. 2007). Wolverines overlapped most with bears and least with lynx (cf. Carroll, Noss & Paquet 2001). Within the study area, sympatry of wolverines with the three forest-dwelling carnivores appears to depend on the availability of mountain ranges as a spatial refuge. Wolverines in our study area, however, depend highly on moose carcasses in their diet both from hunting leftovers and wolf-kills (van Dijk, Gustavsen, Mysterud, May, Flagstad, Brøseth, Andersen, Andersen, Steen & Landa 2008). The wolf is likely to be least affected by intra-guild aggression; rather it may instigate it (i.e. intra-guild predator, Palomares & Caro 1999). Additionally, wolves may facilitate wolverines with scavenging opportunities (Landa et al. 1997; Wilmers et al. 2003), which may enhance sympatry (Landa & Skogland 1995).
Despite their similar potential distribution patterns, the three forest-dwelling species had clear differences in habitat and kill sites. Bears preferred less rugged and higher elevation terrain than wolves and lynx, and chose more forested kill sites closer to forest edges. Although both wolves and bears feed on moose (Sand et al. 2005; Swenson et al. 2007), aggressive exploitative competition is not likely to be of significance because of the omnivorous diet of bears (Dahle et al. 1998) and low densities of both bears and wolves in the study area. Bears may also benefit to some extent from the presence of other predators through increased scavenging opportunities (MacNulty, Varley & Smith 2001; Wilmers et al. 2003). Our study showed that wolves and lynx differed least in habitat use. However, lynx used denser forests at lower elevations and killed sheep in more rugged terrain than wolves, which may reflect differences in hunting techniques (i.e. stalking vs. chase hunt), different habitat preference during hunting and avoidance of intra-guild predation. Also, lynx prey mainly on roe deer and small game (Odden, Linnell & Andersen 2006) in our study area.
In this study, we modelled carnivore selection for habitat and location of kill sites. Although resource selection in carnivores will also depend on local differences in wild ungulate densities and probability of encounters (Hebblewhite, Merrill & McDonald 2005), no such fine-scale prey data were available. As expected, kill sites were biased towards higher-lying, more open areas closer to private roads and buildings, indicative of sheep grazing preferences and ease of human detection. Kill sites may however be biased by specific sex or age groups of large carnivores (e.g. lynx males: Odden et al. 2006; wolverine females: Landa et al. 1997; young dispersers of all species). Also, most bear kill sites were found in the lower occurrence classes, which is likely to be due to the bears’ non-territorial behaviour. Still, understanding differentiation in kill sites among species provides important information for future management of depredation conflicts. Overall, elevation had the strongest differentiating effect both on selection of habitat and location of kill sites in all four large carnivores. The presence of guild members may well have resulted in elevational shifts in their respective distributions to avoid aggressive interactions. It is likely, however, that high prey densities, low large-carnivore densities (due to management actions) and low dietary overlap have led to a situation with reduced competitive exclusion (cf. Heithaus 2001).
In a broader regional context, our study area encompasses similar habitat/land use compositions and prey densities as that found in large stretches of southern Norway and central Sweden, and has a carnivore management regime comparable to other regions in Norway. The spatial extent of regional planning depends on the scale at which population processes are occurring. Our estimates of available patches for large carnivores inside the entire study area may render insight into the minimum area required for viable populations and scale of regional zoning (cf. Mech 1995). Large carnivores are known to be vulnerable to anthropogenic disturbance (e.g. May et al. 2006; Nelleman et al. 2007). Our modelling indicates that wolverines were most sensitive to fragmentation of habitat, given the high amount of small disjointed patches. For the three forest-dwelling species, a continuous geographical unit could be delimited in the south of the study area (see Fig. 1c).
To explain present distributions, habitat preferences and differentiation among Scandinavian large carnivores, historical management and the role of humans as a top predator in multiple-use ecosystems should not be underestimated. The main reason for the decline in large-carnivore populations in Scandinavia was human-induced mortality caused by (over)exploitation, persecution because of livestock/game conflicts, and fear (Swenson et al. 1995; Linnell et al. 2002; Linnell et al. 2005). The current forest-dominated distribution of bears in Scandinavia is based on re-colonization from remnant populations that survived in remote areas in Sweden (Swenson et al. 1995). Similarly, centuries of heavy persecution of wolverines all over Norway until 30 years ago may partly explain the habitat preferences and more remote distribution of wolverines found at present (Landa & Skogland 1995; May et al. 2006). Although the wolf was functionally extinct in the late 1960s after decades of intensive persecution, they have now re-established in south-central Scandinavia (Wabakken et al. 2001). After having been reduced to very low levels in the mid-20th century due to unregulated hunting and high bounties, changes in management have led to a recovery of lynx population in Scandinavia (Andrén et al. 2002).
Although sympatry of two or more species was possible in over 40% of the study area, only 1·5% was suitable for all four species together. Sympatry of all three forest carnivores was possible in one-quarter of the study area. Successful regional zoning of all four carnivores may therefore rely on establishing zones of adequate size spanning an elevational gradient. Zoning of all four species may enhance the conservation of an intact guild of large carnivores in the boreal forest ecosystem (Wabakken 2001). On the other hand, fostering sympatry of all four species may well increase conflict levels and resistance to carnivore conservation locally (Wabakken 2001; Linnell et al. 2005). These conflicts may be reduced by discouraging extensive sheep husbandry (Zimmermann et al. 2003; Milner et al. 2005), employing effective preventive and mitigation measures required for adequate compensation schemes, promoting different lifestyles and livelihood (e.g. ecotourism and outdoor recreation), and also allowing for limited control (Linnell et al. 2005; Swenson & Andrén 2005). However, the social context (non-material nature) of many of the large-carnivore conflicts in Norway should never be forgotten (Skogen 2003). Our study results may provide guidance to managers attempting to design regional-scale zoning to facilitate recovery of large carnivores in Scandinavia.
Acknowledgments
This was a collaborative study of the Scandinavian Brown Bear Research Project, Scandinavian Lynx Project (SCANDLYNX), Scandinavian Wolf Project (SKANDULV) and the Norwegian Wolverine Project. These projects have been financed by the Norwegian Research Council, Norwegian Directorate for Nature Management, Swedish Environmental Protection Agency, Norwegian counties, WWF-Sweden, Norwegian Institute for Nature Research, and Hedmark University College. The writing of this manuscript was made possible by a specific donation from Alertis, Fund for Bear and Nature Conservation, the Netherlands. We would furthermore like to thank the field coordinators R. Andersen, S. Brunberg and T. Strømseth, and hundreds of field trackers, volunteers and students for all the data they collected within the different carnivore projects. The comments of M. Hebblewhite and two anonymous referees greatly improved earlier versions of this manuscript.
Supplementary material
The following supplementary material is available for this article:
Appendix S1. Intra- vs. inter-specific differences among four large-carnivore species
Table S1. Statistics for the radio-telemetry data and locations of predator-killed sheep
This material is available as part of the online article from:
http://www.blackwell-synergy.com/doi/full/10.1111/j.1365-2664.2008.01527.x
(This link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Andrén H, Linnell JDC, Liberg O, Ahlqvist P, Andersen R, Danell A, Franzén R, Kvam T, Odden J, Segerström P. Estimating total lynx Lynx lynxpopulation size from censuses of family groups. Wildlife Biology. 2002;8:299–306. [Google Scholar]
- Boyce MS. Scale for resource selection functions. Diversity & Distributions. 2006;12:269–276. [Google Scholar]
- Boyce MS, Vernier PR, Nielsen SE, Schmiegelow FKA. Evaluating resource selection functions. Ecological Modelling. 2002;157:281–300. [Google Scholar]
- Brøseth H, Andersen R. Yngleregistreringer av jerv i Norge i 2004. Trondheim, Norway: Norwegian Institute for Nature Research; 2004. NINA Minirapport 016. [Google Scholar]
- Brøseth H, Odden J, Linnell JDC. Minimum antall familiegrupper, bestandsestimat og bestandsutvikling for gaupe i Norge i 2004. Trondheim, Norway: Norwegian Institute for Nature Research; 2004. NINA Minirapport 073. [Google Scholar]
- Buskirk SW. Mesocarnivores of Yellowstone. In: Clark TW, Curlee AP, Minta SC, Kareiva PM, editors. Carnivores in Ecosystems: The Yellowstone Experience. New Haven, CT: Yale University Press; 1999. pp. 167–187. [Google Scholar]
- Carroll C, Noss RF, Paquet PC. Carnivores as focal species for conservation planning in the Rocky Mountain region. Ecological Applications. 2001;11:961–980. [Google Scholar]
- Committee on Energy and Environment . Innstilling fra energi- og miljøkomiteen om rovvilt i norsk natur. Oslo, Norway: 2004. [Google Scholar]
- Copeland JP, Peek JM, Groves CR, Melquist WE, McKelvey KS, McDaniel GW, Long CD, Harris CE. Seasonal habitat associations of the wolverine in central Idaho. Journal of Wildlife Management. 2007;71:2201–2212. [Google Scholar]
- Creel S, Spong G, Creel N. Interspecific competition and the population biology of extinction-prone carnivores. In: Gittleman JL, Funk SM, Macdonald DW, Wayne RK, editors. Carnivore Conservation. Cambridge, UK: Cambridge University Press; 2001. pp. 35–60. [Google Scholar]
- Crooks KR. Relative sensitivities of mammalian carnivores to habitat fragmentation. Conservation Biology. 2002;16:488–502. [Google Scholar]
- Dahle B, Sørensen OJ, Wedul EH, Swenson JE, Sandegren F. The diet of brown bears in central Scandinavia: effects of access to free-ranging domestic sheep. Wildlife Biology. 1998;4:147–158. [Google Scholar]
- van Dijk J, Gustavsen L, Mysterud A, May R, Flagstad Ø, Brøseth H, Andersen R, Andersen R, Steen H, Landa A. Diet shift of a facultative scavenger, the wolverine, following the recolonization of wolves. Journal of Animal Ecology. 2008 doi: 10.1111/j.1365-2656.2008.01445.x. in press. [DOI] [PubMed] [Google Scholar]
- Hansen MC, DeFries RS, Townsend JRG, Sohlberg R, Dimiceli C, Carroll M. Towards an operational MODIS continuous field of percent tree cover algorithm: examples using AVHRR and MODIS data. Remote Sensing of Environment. 2002;83:303–319. [Google Scholar]
- Hebblewhite M, Merrill EH, McDonald TL. Spatial decomposition of predation risk using resource selection functions: an example in a wolf–elk predator–prey system. Oikos. 2005;111:101–111. [Google Scholar]
- Heithaus MR. Habitat selection by predators and prey in communities with asymmetrical intraguild predation. Oikos. 2001;92:542–554. [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 2000. [Google Scholar]
- Johnson DH. The comparison of usage and availability measurements for evaluating resource preference. Ecology. 1980;59:1286–1301. [Google Scholar]
- Landa A. Spor og tegn. Et hefte til hjelp i bestemmelse av store rovdyr. Trondheim, Norway: NINA·NIKU, Norwegian Institute for Nature Research; 1999. [Google Scholar]
- Landa A, Skogland T. The relationship between population density and body size of wolverines Gulo gulo in Scandinavia. Wildlife Biology. 1995;1:165–175. [Google Scholar]
- Landa A, Strand O, Swenson JE, Skogland T. Wolverines and their prey in southern Norway. Canadian Journal of Zoology. 1997;75:1292–1299. [Google Scholar]
- Linnell JDC, Strand O. Interference interactions, co-existence and conservation of mammalian carnivores. Diversity & Distributions. 2000;6:169–176. [Google Scholar]
- Linnell JDC, Løe J, Okarma H, Blanco JC, Andersone Z, Valdmann H, Balciauskas L, Promberger C, Brainerd S, Wabakken P, Kojola I, Andersen R, Liberg O, Sand H, Solberg EJ, Pedersen HC, Boitani L, Breitenmoser U. The Fear of Wolves: A Review of Wolf Attacks on Humans. Trondheim, Norway: Norwegian Institute for Nature Research; 2002. NINA Oppdragsmelding 731. [Google Scholar]
- Linnell JDC, Nilsen EB, Lande US, Herfindal I, Odden J, Skogen K, Andersen R, Breitenmoser U. Zoning as a means of mitigating conflicts with large carnivores: principles and reality. In: Woodroffe R, Thirgood S, Rabinowitz A, editors. People and Wildlife, Conflict or Co-existence? Vol. 9. New York: Cambridge University Press; 2005. pp. 162–175. [Google Scholar]
- MacNulty DR, Varley N, Smith DW. Grizzly bear, Ursus arctos, usurps bison calf, Bison bison, captured by wolves, Canis lupus, in Yellowstone National Park, Wyoming. Canadian Field Naturalist. 2001;115:495–498. [Google Scholar]
- Manly BFJ, McDonald LL, Thomas DL, McDonald TL, Erickson WP. Resource Selection by Animals: Statistical Design and Analysis for Field Studies. 2nd edn. New York: Kluwer Press; 2002. [Google Scholar]
- Marzluff JM, Millspaugh JJ, Hurvitz P, Handcock MS. Relating resources to a probabilistic measure of space use: forest fragments and Steller's jays. Ecology. 2004;85:1411–1427. [Google Scholar]
- May R, Landa A, Dijk J, van , Linnell JDC, Andersen R. Impact of infrastructure on habitat selection of wolverines Gulo gulo. Wildlife Biology. 2006;12:285–295. [Google Scholar]
- Mech LD. The challenge and opportunity of recovering wolf populations. Conservation Biology. 1995;9:270–278. [Google Scholar]
- Milner JM, Nilsen EB, Wabakken P, Storaas T. Hunting moose or keeping sheep? – Producing meat in areas with carnivores. Alces. 2005;41:49–61. [Google Scholar]
- Ministry of Environment . Rovvilt i norsk natur. Trondheim, Norway: 2003. Stortingsmelding nr. 15 (2003–2004) [Google Scholar]
- Nelleman C, Støen O-G, Kindberg J, Swenson JE, Vistnes I, Ericsson G, Katajisto J, Kaltenborn BP, Martin J, Ordiz A. Terrain use by an expanding brown bear population in relation to age, recreational resorts and human settlements. Biological Conservation. 2007;138:157–165. [Google Scholar]
- Odden J, Linnell JDC, Andersen R. Diet of Eurasian lynx, Lynx lynx, in the boreal forest of south-eastern Norway: the relative importance of livestock and hares at low roe deer density. European Journal of Wildlife Research. 2006;52:237–244. [Google Scholar]
- Otis DL, White GC. Autocorrelation of location estimates and the analysis of radiotracking data. Journal of Wildlife Management. 1999;63:1039–1044. [Google Scholar]
- Palomares F, Caro TM. Interspecific killing among mammalian carnivores. The American Naturalist. 1999;153:492–508. doi: 10.1086/303189. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . r: A Language and Environment for Statistical Computing. r Foundation for Statistical Computing. Austria: Vienna; 2007. [Google Scholar]
- Rempel R. Patch Analyst 2.2. Thunder Bay, ON, Canada: Centre for Northern Forest Ecosystem Research, Lakehead University Campus; 2000. [Google Scholar]
- Riley SJ, DeGloria SD, Elliot R. A terrain ruggedness index that quantifies topographic heterogeneity. Intermountain Journal of Sciences. 1999;5:23–27. [Google Scholar]
- Sand H, Zimmermann B, Wabakken P, Andrén H, Pedersen HC. Using GPS technology and GIS cluster analyses to estimate kill rates in wolf-ungulate ecosystems. Wildlife Society Bulletin. 2005;33:914–925. [Google Scholar]
- Skogen K. Adapting adaptive management to a cultural understanding of land use conflicts. Society and Natural Resources. 2003;16:435–450. [Google Scholar]
- Solberg EJ, Sand H, Linnell JDC, Brainerd SM, Andersen R, Odden J, Brøseth H, Swenson J, Strand O, Wabakken P. Utredninger i forbindelse med ny rovviltmelding. Store rovdyrs innvirkning på hjorteviltet i Norge: Økologiske prosesser og konsekvenser for jaktuttak og jaktutøvelse. Trondheim, Norway: Norwegian Institute for Nature Research; 2003. NINA fagrapport 63. [Google Scholar]
- Sunquist ME, Sunquist F. Changing landscapes: consequences for carnivores. In: Gittleman JL, Funk SM, Macdonald DW, Wayne RK, editors. Carnivore Conservation. Conservation Biology Series 5. Vol. 5. Cambridge, UK: The Zoological Society of London; 2001. pp. 39–418. [Google Scholar]
- Swenson JE, Andrén H. A tale of two countries: large carnivore depredation and compensation schemes in Sweden and Norway. In: Woodroffe R, Thirgood S, Rabinowitz A, editors. People and Wildlife. Conflict of Coexistence? Vol. 9. Cambridge, UK: Cambridge University Press; 2005. p. 497+xvii. [Google Scholar]
- Swenson JE, Dahle B, Arnemo JM, Brunberg S, Hustad H, Nerheim E, Sandegren F, Solberg KH, Söderberg A. Utredninger i forbindelse med ny rovvlitmelding. Status og forvaltning av brunbjørnen i Norge. Trondheim: Norwegian Institute for Nature Research; 2003. NINA fagrapport 60. [Google Scholar]
- Swenson JE, Dahle B, Busk H, Opseth O, Johansen T, Söderberg A, Wallin K, Cederlund G. Predation on moose calves by European brown bears. Journal of Wildlife Management. 2007;71:1993–1997. [Google Scholar]
- Swenson JE, Wabakken P, Sandegren F, Bjärvall A, Franzén R, Söderberg A. The near extinction and recovery of brown bears in Scandinavia in relation to the bear management policies of Norway and Sweden. Wildlife Biology. 1995;1:11–25. [Google Scholar]
- Wabakken P. Flerarts- og soneforvaltning i rovdyr-saukonflikten. In: Jaren V, Løvstad JP, editors. Utmarksbeite og store rovdyr. Delrapport 3 fra forskningsprogrammet Bruk og forvaltning av utmark. Vol. 3. Oslo, Norway: Norges forskningsråd; 2001. pp. 61–67. [Google Scholar]
- Wabakken P, Aronsson Å, Sand H, Rønning H, Kojola I. Ulv i Skandinavia. Statusrapport for vinteren 2002–2003. Hedmark, Norway: Høgskolen i Hedmark; 2004. [Google Scholar]
- Wabakken P, Sand H, Liberg O, Bjärvall A. The recovery, distribution, and population dynamics of wolves on the Scandinavian peninsula, 1978–1998. Canadian Journal of Zoology. 2001;79:710–725. [Google Scholar]
- Wilmers CC, Crabtree RL, Smith DW, Murphy KM, Getz WM. Trophic facilitation by introduced top predators: gray wolf subsidies to scavengers in Yellowstone National Park. Journal of Animal Ecology. 2003;72:909–916. [Google Scholar]
- Woodroffe R. Predators and people: using human densities to interpret declines of large carnivores. Animal Conservation. 2000;3:165–173. [Google Scholar]
- Woodroffe R, Ginsberg JR. Edge effects and the extinction of populations inside protected areas. Science. 1998;280:2126–2128. doi: 10.1126/science.280.5372.2126. [DOI] [PubMed] [Google Scholar]
- Zimmermann B, Wabakken P, Dötterer M. Brown bear–livestock conflicts in a bear conservation zone in Norway: are cattle a good alternative to sheep? Ursus. 2003;14:72–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.