Abstract
Enterolactone, a major metabolite of plant-based lignans, has been shown to inhibit prostate cancer growth and development, but the mechanistic basis for its anticancer activity remains largely unknown. Activation of insulin-like growth factor-1 (IGF-1) receptor (IGF-1R) signaling is critical for prostate cancer cell growth and progression. This study examined whether the growth inhibitory effect of enterolactone was related to changes in the IGF-1/IGF-1R system in PC-3 prostate cancer cells. At nutritionally relevant concentrations (20–60 μmol/L), enterolactone inhibited IGF-1–induced activation of IGF-1R and its downstream AKT and mitogen-activated protein kinase/extracellular-signal regulated kinase signaling pathways. Inhibition of AKT by enterolactone resulted in decreased phosphorylation of its downstream targets, including p70S6K1 and glycogen synthase kinase-3 β. Enterolactone also inhibited cyclin D1 expression. As a result, enterolactone inhibited proliferation and migration of PC-3 cells. Knockdown of IGF-1R by plasmids with siRNA (si) against IGF-1R mRNA resulted in inhibition of proliferation of PC-3 cells and cell numbers did not differ when the si-IGF-1R groups (cells transfected with plasmids containing siRNA against IGF-1R mRNA) were treated or untreated with enterolactone. These results suggest that enterolactone suppresses proliferation and migration of prostate cancer cells, at least partially, through inhibition of IGF-1/IGF-1R signaling. The finding of this study provides new insights into the molecular mechanisms that enterolactone exerts against prostate cancer.
Introduction
Prostatic carcinoma is the second most frequently diagnosed cancer worldwide and is one of the major life-threatening diseases among men (1). Geographical differences in prostate cancer incidence and mortality, and the shift in risk with migration from low to high incidence countries suggest that diet plays a primary role in the development and progression of this disease (1–4). The consumption of diets high in lignans is thought to protect against prostate cancer (5), because the incidence and mortality rates are much lower in Asia with the traditional plant-based diet rich in lignans, than in the US and Northern Europe with diets higher in animal products (1,2,6). Furthermore, accumulating evidence from clinical trials, animal models, and cell culture studies allow us to underline specific anticancer mechanism(s) of lignans on growth and development of prostate cancer (7–13).
Dietary lignans have phytoestrogenic properties (14) and are broadly available in cereals, legumes, fruits, vegetables, and grains, although the richest sources include flaxseed and sesame seeds (15,16). Dietary lignans, such as secoisolariciresinol, matairesinol, and sesamin, are converted by the intestinal microflora to mammalian lignans enterodiol and enterolactone, 2 major forms in the biological fluids of humans and animals (17). Previously, we demonstrated that enterodiol and enterolactone significantly decreased cell viability in 3 human prostate cancer cell lines (LNCaP [hormone sensitive] and PC-3 and DU-145 [hormone insensitive]); enterolactone proved more potent than enterodiol (10). Furthermore, in our recent study, enterolactone was found to induce apoptosis in LNCaP cells via a mitochondrial-mediated, caspase-dependent pathway (11). However, the molecular mechanisms by which enterolactone acts to inhibit human hormone-insensitive prostate cancer cells are not well understood.
LNCaP cells provide a model for early androgen-sensitive prostate cancer, whereas PC-3 cells display hormone-insensitive properties representing a later stage of the disease (18–20). However, it is unclear etiologically how hormone-sensitive prostate carcinoma progresses to hormone-insensitive status. Upregulated growth and survival signaling via the insulin-like growth factor-1 (IGF-1)5/IGF-1 receptor (IGF-1R) system has been suggested to play a key role in promoting survival and malignant transformation of prostate cancer cells (21). IGF-1 binds to the tyrosine kinase membrane receptor, IGF-1R, which is composed of 2 α subunits (responsible for ligand binding) and 2 β subunits (responsible for tyrosine kinase activity) (22). Upon binding, the tyrosine kinase activity is activated by autophosphorylation of specific tyrosine residues. Subsequently, the activated tyrosine kinase recruits and phosphorylates insulin receptor substrate proteins and activates intracellular signaling pathways including Ras/mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3′-kinase (PI3K)/AKT, which takes part in a variety of cellular activities, including survival, mitogenesis, and migration (23,24). Case-control studies have linked elevated serum levels of IGF-1 with increased risk of prostate cancer (25,26). Overexpression of IGF-1 in transgenic mice also causes spontaneous tumorigenesis in the prostate epithelium (27). Furthermore, introducing antisense RNA to IGF-1R in mice suppresses tumor growth and prevents invasion of prostate cancer cells both in vitro and in vivo (28,29). Additionally, the expression of IGF-1R and the phosphorylation levels of IGF-1R are enhanced and downstream events such as AKT and MAPK/extracellular-signal regulated kinase (ERK) signaling pathways are activated when androgen-dependent prostate cancer cells (30) or prostate cancer xenografts (31) progress to an androgen-independent state. These findings illustrate the importance of IGF-1/IGF-1R signaling in the progression of prostate cancer. Thus, in the present study, we sought to determine whether enterolactone could inhibit IGF-1/IGF-1R signaling and affect the proliferation, apoptosis, and migration of PC-3 cells.
Materials and Methods
Reagents.
We used antibodies to phospho-AKT [Ser473], AKT, phospho-glycogen synthase kinase-3 β (GSK-3 β), phospho-p70S6K1, and cyclinD1 (Cell Signaling), IGF-1R β [C-20], phospho-ERK1/2 [E4], and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Santa Cruz), and phospho-tyrosine kinase [4G10] (Upstate). Enterolactone, 3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide (MTT), and all other reagents were purchased from Sigma.
Cell culture and treatment.
The androgen-independent human prostate carcinoma PC-3 cells from American Type Culture Collection (Manassas, VA) were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 10 mmol/L HEPES, 1% penicillin, and streptomycin (GIBCO BRL). The cells were maintained at 37°C in a 5% CO2 humidified incubator. Enterolactone was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 100 mmol/L and stored at −20°C. Serial dilutions of enterolactone were made from stock solutions with cell culture medium. DMSO was employed as negative control; the final concentration of DMSO was 0.1% or lower (v:v) in cell culture experiments.
Cell proliferation assay.
Cell proliferation was determined using the MTT assay. For the MTT assay, 5 × 103 cells/well were cultured in 96-well plates and incubated in standard culture medium overnight and then transferred to serum-free medium. In 24 h, the old medium was replaced with fresh serum-free medium containing IGF-1 (40 μg/L) in the presence or absence of enterolactone. After incubation for 24 h, the MTT assay was performed as previously described (11). Data are presented from 3 separate experiments and the percentage of enterolactone-induced cell growth inhibition was determined using DMSO-treated cells (control) as the denominator.
Cell cycle analysis.
To analyze the cell cycle of PC-3 cells arrested by enterolactone, propidium iodide staining was performed by flow cytometry. The cells at 40–50% confluence were allowed to grow in the standard culture medium overnight and then transferred to serum-free medium. In 24 h, the old medium was replaced with fresh serum-free medium containing IGF-1 (40 μg/L) in the presence or absence of enterolactone. After incubation for 24 h, the cells were collected and prepared for flow cytometric analysis as described previously (11). The cell cycle distribution was analyzed using the ModFit LT for Mac V1.01 software.
Transient transfection of siRNA against IGF-1R.
The plasmid containing siRNA (si) against IGF-1R mRNA (designated si-IGF-1R) and the inverted control (designated si-con) were gifts from Dr. Jing Fang (Institute for Nutritional Sciences, Shanghai, China) (32). PC-3 cells at 60–70% confluence were transfected with siRNA plasmids using the Cell Line Nucleofector kit V (catalog no. VCA-1003, for PC-3 cells) from Amaxa according to the manufacturer's instructions.
Immunoblotting and immunoprecipitation.
Isolation of cellular proteins and immunoblotting were performed as described previously (11). For immunoprecipitation, 500 μg of cellular proteins were incubated with 1 μg of anti-IGF-1R β antibody at 4°C for 4 h. A total of 30 μL of protein A/G agarose beads (Santa Cruz Biotechnology) were added and incubated at 4°C overnight. The beads were spun down and washed 4 times with lysis buffer at 4°C. SDS sample buffer (36 μL) was added and boiled for 3 min. After centrifugation at 3000 × g; 0.5 min, the supernatant was loaded unto SDS-PAGE gel (8%). The phosphorylated IGF-1R was detected using the antibody against phospho-tyrosine kinase. GAPDH was used as a loading control.
Migration assays.
The effects of enterolactone on IGF-1–induced migration of prostate cancer cells were examined using Boyden Chamber assays. The PC-3 cells (1 × 105/well) were cultured in 50 μL serum-free medium with 60 μmol/L enterolactone in the upper wells of a 12-well chamber system (Neuroprobe). IGF-1 in 163 μL serum-free medium, with a final concentration 40 μg/L, was added to the lower wells. The cells in upper wells were separated from the lower wells by an 8-μm-pore size polycarbonate filter. Migrated cells through the filters were determined after 20 h of incubation at 37°C by fixing and staining with hematoxylin and eosin. Finally, cells on the filters were photographed and counted at 10× magnification. Images were obtained from 3 representative experiments.
Statistical analysis.
All experiments were performed at least 3 times and the results are expressed as the means ± SEM. Data were analyzed by 1-way ANOVA followed by Tukey's multiple comparison tests. Differences were considered significant when P < 0.05.
Results
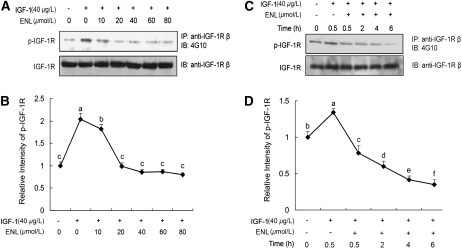
Enterolactone inhibits IGF-1–induced tyrosine phosphorylation of IGF-1R.
IGF-1 induced tyrosine phosphorylation of IGF-1R, whereas enterolactone inhibited IGF-1–induced phospho-IGF-1R in a dose-dependent (Fig. 1A,B; P < 0.05) and time-related manner (Fig. 1C,D; P < 0.05).
FIGURE 1 .
Enterolactone inhibits IGF-1-induced tyrosine phosphorylation of IGF-1R in a dose- (A,B) and time-related (C,D) manner. (A) PC-3 cells were starved in serum-free medium for 24 h and pretreated with indicated doses of enterolactone for 0.5 h followed by stimulation with IGF-1 for 15 min. After treatment, cellular proteins were isolated and immunoprecipitation and immunoblotting were performed. (B) Quantified data of A. The intensity of phospho IGF-1R and total IGF-1R protein signals obtained in A was quantified using Chem Doc densitometry software (Quantity One, version 4.5.0; Bio-Rad). The densitometry data were normalized to IGF-1R levels and those of the control (lane 1). (C) PC-3 cells were starved for 24 h, followed by treatment with enterolactone (60 μmol/L) for 0.5 h, then IGF-1 was added, cells were incubated and harvested at various time points, and levels of phospho-IGF-1R and IGF-1R were determined. (D) Quantified data of C. Values are means ± SEM, n = 4. Means without a common letter differ, P < 0.05. ENL, Enterolactone.
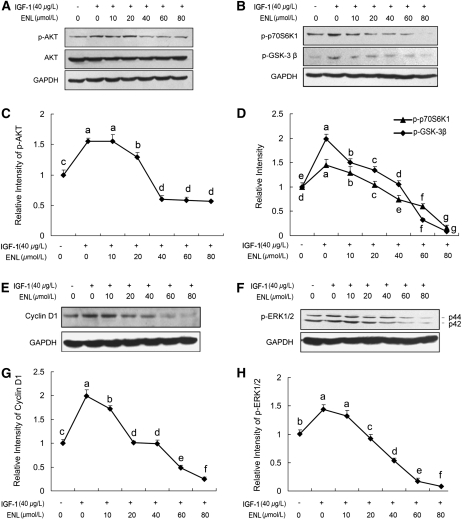
Enterolactone inhibits downstream signaling of IGF-1R.
IGF-1 enhanced phospho-AKT levels in PC-3 cells; however, this effect was reduced by enterolactone (Fig. 2A,C). Evidence of the inhibitory effect of enterolactone on AKT phosphorylation was further supported by the results of Western blotting in which the levels of phosphorylated GSK-3 β and phosphorylated p70S6K1, key downstream elements of the AKT signaling pathway (33,34), were decreased with increased doses of enterolactone, concomitantly with reduced levels of phosphorylated AKT (Fig. 2B,D). Cyclin D1 levels also decreased with enterolactone treatment (Fig. 2E,G). Moreover, enterolactone inhibited IGF-1–induced ERK phosphorylation (Fig. 2F,H). Together, these data suggest that enterolactone inhibits the IGF-1/IGF-1R system and its downstream signaling.
FIGURE 2 .
Enterolactone inhibits phosphorylation of AKT (A,C), its downstream targets (B,D,E,G), and phosphorylation of ERK1/2 (F,H). PC-3 cells were starved in serum-free medium for 24 h. Then, cells were pretreated with indicated doses of enterolactone for 0.5 h. IGF-1 was added to stimulate cells for 15 min (A,B,F) and 6 h (E). After treatment, cellular proteins were isolated and immunoblotting was performed. (C,D,G,H) Quantified data of immunoblotting. The densitometry data of phospho-AKT, phospho-p70S6K1, phospho-GSK-3β, cyclin D1, and p-ERK1/2 obtained in A,B,E, and F were normalized to GAPDH levels and those of the control (lane 1). Values are means ± SEM, n = 4. Means without a common letter differ, P < 0.05. ENL, Enterolactone.
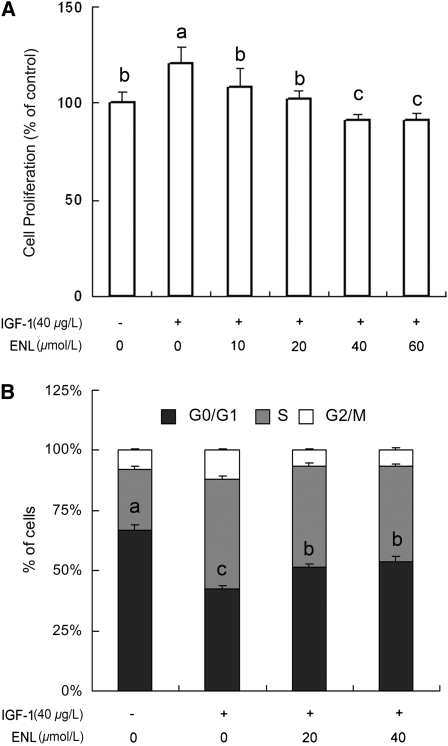
Enterolactone inhibits IGF-1–stimulated proliferation and migration of PC-3 cells.
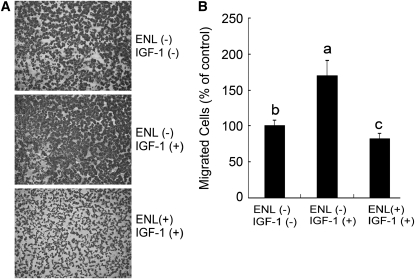
Data from the MTT assay showed that IGF-1 significantly induced cell growth (120% of control) and enterolactone inhibited IGF-1–induced cell proliferation with concentrations ≥20 μmol/L (Fig. 3A; P < 0.05). Findings of flow cytometry indicated that 66.5% of PC-3 cells were blocked in the G0/G1 phase under the serum-free state. When the cells were stimulated with IGF-1, the percentage of cells in the G0/G1 phase decreased to 42.1%. Treatment of the cells with enterolactone (40 μmol/L) for 24 h in the presence of IGF-1 made the cells arrest repeatedly in the G0/G1 phase (53.7%; Fig. 3B; P < 0.05). The sub-G0/G1 (apoptotic cells) contents were not detectable even after an extended treatment time (72 h in the standard medium) and the cells were still blocked in the G0/G1 phase (data not shown). Furthermore, data from a Boyden Chamber assay demonstrated that enterolactone counteracted the effect of IGF-1 on the increase of migrated cell numbers (Fig. 4A,B; P < 0.05).
FIGURE 3 .
Enterolactone inhibits IGF-1–induced cell proliferation of PC-3 cells (A) and blocks the cell cycle in the G0/G1 phase (B). (A) MTT assay. (B) Flow cytometry assay. The untreated controls were set to 100%. Values are means ± SEM, n = 6. Means without a common letter differ, P < 0.05. ENL, Enterolactone.
FIGURE 4 .
Enterolactone inhibits IGF-1–induced cell migration (A,B). (A) Representative photomicrographs (10× magnification) of the Boyden Chamber assay on PC-3 cells after exposure of cells to 60 μmol/L enterolactone treated or untreated with IGF-1 (40 μg/L) for 20 h. (B) Statistic analysis of the results of A. The untreated controls were set to 100%. Values are means ± SEM, n = 10. Means without a common letter differ, P < 0.05. ENL, Enterolactone.
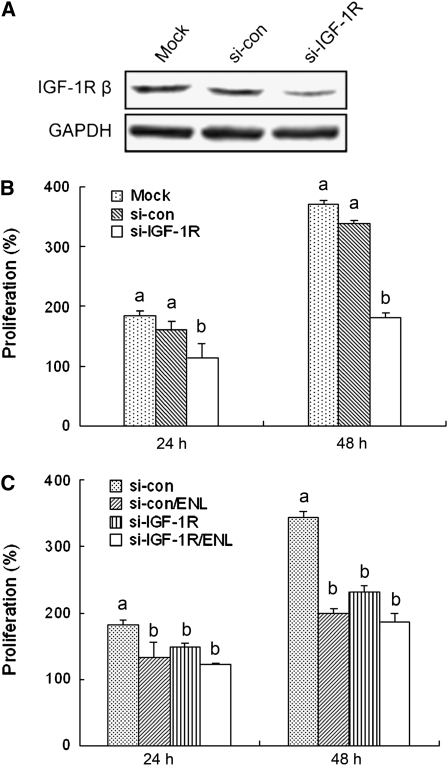
IGF-1R participates in the inhibitory effects of enterolactone on PC-3 cells.
Results from si-RNA technology further elucidated the role of IGF-1R in the anticancer activity of enterolactone. The introduction of si-IGF-1R to the cells decreased IGF-1R protein levels, whereas the inverted control si-con did not affect IGF-1R expression (Fig. 5A). Furthermore, the knockdown of IGF-1R suppressed proliferation of PC-3 cells (Fig. 5B; P < 0.05).
FIGURE 5 .
Protein expression levels of IGF-1R (A) and cell proliferation after knockdown of IGF-1R (B,C). (A) After transfection, the cells were kept for incubation for 24 h followed by immunoblotting. (B) After transfection, the cells were incubated overnight and then distributed on a 12-well plate at 5 × 104/well (0 h). Cell numbers were determined at 24 and 48 h using a hemocytometer. (C) The transfected cells were incubated overnight and distributed on 12-well plates at 5 × 104/well (0 h). Enterolactone (60 μmol/L) was supplemented and the incubation continued. Cell numbers were determined the same as B. Data were expressed as percentages of cell numbers of 0 h. Values are means ± SEM, n = 9. Means at a time without a common letter differ, P < 0.05. ENL, Enterolactone.
Finally, when we treated cells transfected with si-IGF-1R or si-con with enterolactone to examine the role of IGF-1R in the inhibitory effects of enterolactone on PC-3 cells, the results showed that the cell numbers did not differ in the si-IGF-1R groups treated or untreated with enterolactone (Fig. 5C). These data support the critical role of IGF-1R in the antiproliferative effects of enterolactone.
Discussion
To the best of our knowledge, this is the first report to document that enterolactone inhibits IGF-1/IGF-1R signaling in human hormone-insensitive prostate cancer PC-3 cell lines. At nutritionally relevant concentrations (20–60 μmol/L), enterolactone inhibited IGF-1–induced tyrosine phosphorylation of IGF-1R and activation of AKT and ERK and resulted in the inhibition of proliferation and migration of prostate cancer cells.
Despite intensive efforts made to identify new and more effective therapeutics, prostate cancer still remains the second leading cause of cancer mortality in males in western countries. Although androgen ablation therapy is effective in its initial stages, after a period of remission, prostate carcinoma often progresses to an androgen-independent state characterized by a high proliferation rate and strong propensity to metastases (35). Prostate cancer preferentially metastasizes to bone, which is rich in IGF (28,29). Studies have shown that IGF binds to IGF-1R in prostate cancer cells to stimulate cancer cell growth and facilitate the development of bone metastasis (36). Once IGF-1R is activated, the tyrosine kinase activates the downstream PI3K/AKT signaling pathway, which is crucial in regulating cell survival and proliferation. Thus, the IGF-1/IGF-1R axis is regarded as a key target for dietary chemoprevention (37). In the present study, we employed a human androgen-independent prostate carcinoma PC-3 cell line to investigate the molecular mechanisms of enterolactone against prostate cancer, a cell line that was established by Kaighn et al. (38) from the bone marrow aspirates of a patient with confirmed metastatic disease. We found that enterolactone inhibited IGF-1–induced tyrosine phosphorylation of IGF-1R. Inhibition of phospho-IGF-1R by enterolactone blocked IGF-1–induced activation of AKT and its downstream targets, such as phospho-p70S6K1 and phospho-GSK-3 β. The p70S6K1 protein, a serine/threonine kinase, mediates several effects of the PI3K/AKT pathway, including cell cycle progression (34) and cellular transformation (39). Dephosphorylation of p70S6K1 was reported to block the G1 cell cycle progression (34). Concomitantly with decreased levels of phospho-GSK-3 β after enterolactone treatment, the protein levels of cyclin D1 also declined, which is a substrate of phospho-GSK-3 β and required for G1 cell cycle progression. Another known pathway regulated by IGF-1R is MAPK/ERK signaling, which is a key mediator of the mitogenic potential of growth factors. In our study, IGF-1 activated ERK, whereas enterolactone inhibited IGF-1–induced phospho-ERK. Although no extant study has evaluated the role of enterolactone on phospho-IGF-1R, studies have found that genistein, which exerts phytoestrogenic activities and is the counterpart of lignan, inhibits the tyrosine phosphorylation of IGF-1R induced by IGF-1 (40). Some compounds, such as cyclolignan and picropodophyllin that share a similar structure to enterolactone, have been shown to inhibit tyrosine kinase IGF-1R in melanoma, breast carcinoma, and sarcoma cell lines (41).
The physiological actions of the inhibition of IGF-1/IGF-1R signaling by enterolactone were further determined in the present study and enterolactone was found to suppress IGF-1–induced cell proliferation and arrested cell cycling in the G0/G1 phase. However, no apparent apoptosis was detected, even in cells treated for 3 d. In a previous study under similar conditions, we discovered that enterolactone significantly induced apoptosis in human hormone-dependent LNCaP prostate cancer cells (11). It was reported that IGF-1R expressed in PC-3 cells is roughly 10-fold greater than that observed in LNCaP cells (42). The differential in expression status of IGF-1R in these 2 cell lines may explain why human hormone-independent PC-3 cells were less sensitive to enterolactone treatment than LNCaP cells. Indeed, PC-3 cells are more resistant to anticancer drugs and produce a malignant phenotype that metastasizes to bone (43,44). Besides inhibition of cell proliferation, enterolactone also inhibited IGF-1–induced migration of PC-3 cells. This effect is consistent with the observation from our previous animal study in which a 5% flaxseed (richest in lignans) supplemented diet reduced the incidence of lung and lymph node metastasis in male transgenic adenocarcinoma mouse prostate mice (9). These results suggest that the inhibition of the IGF-1/IGF-1R network may be one of the molecular mechanisms by which enterolactone hinders prostate cancer.
To further address the salience of IGF-1R in the anticancer effects of enterolactone, we introduced siRNA against IGF-1R to knockdown IGF-1R expression in PC-3 cells. Similar to other findings (32), we found that proliferation of prostate cancer cells was significantly suppressed by the knockdown of IGF-1R. The result that decreased cell numbers were similar in si-IGF-1R groups treated or untreated with enterolactone suggested that IGF-1/IGF-1R signaling plays a critical role in enterolactone-induced inhibition of prostate cancer cell proliferation. However, we cannot exclude the possibility that enterolactone has other targets besides IGF-1R.
Progression of prostate cancer from its nonmetastatic androgen-dependent phenotype to a more malignant metastatic androgen-independent phenotype is a slow, multi-step process that provides an extended and opportune window for cancer prevention with nontoxic dietary compounds. Recently, Zhang et al. (45) reported that supplementation of 600 mg/d of secoisolariciresinol diglucoside (a major plant lignan in flaxseed) for 4 mo significantly reduced International Prostate Symptom Scores and improved the quality of life in men with benign prostatic hyperplasia. This level of supplementation used in this study was not associated with any adverse events and resulted in plasma enterolactone levels of 436 nmol/L (45). Although the circulating level of enterolactone is usually low, Morton et al. (46) previously reported that this compound could accumulate in the human prostatic fluid from several times to several dozen times higher than that in the plasma. Thus, the effective dose of enterolactone (20–60 μmol/L) within this investigation may be achievable in the prostate by supplementation with 600 mg/d of secoisolariciresinol diglucoside. In addition, in our previous paper, we observed that enterolactone (50 μmol/L) selectively decreased cancer cell viability, as demonstrated by its impact on LNCaP cells, but did not affect normal tissue, as demonstrated by null effects on nontumorigenic prostate epithelial CRL2221 cells, normal human ovarian epithelial IOSE80 cells, and hepatocyte HL-7702 cell lines (11). Thus, the effective doses of enterolactone indicated by this study appear nontoxic to normal cells.
Although this study provides new information regarding the potential molecular mechanisms involved with high lignan foods, there are some limitations that may reduce the generalizability of our findings. The greatest limitation stems from the fact that enterolactone usually circulates in conjugated rather than in free form in both human serum and urine (14); furthermore, enterolactone also is converted into a variety of aliphatic and aromatic hydroxylated derivatives by the microsomes in rat, pig, and human livers (47). Although the metabolites and derivatives of enterolactone are not commercially available at present, such compounds have obvious relevance and need to be developed and further investigated. In addition, another limitation of our study is the use of an in vitro system. Certainly, the growth of prostate cancer cells is much more complex in vivo compared with in vitro and could be affected by many factors, such as androgens, estrogens, growth factors, and the interactions between the stroma and epithelium. Thus, further in vivo studies are needed. Taken together, the results of the present study indicate that enterolactone (metabolized from dietary lignans) inhibits IGF-1/IGF-1R signaling in human prostate cancer cells and also inhibits cell proliferation and migration. Our current study, in tandem with previous studies, also suggests that lignan or a lignan-rich diet could suppress various stages of prostate cancer, from hormone-sensitive to hormone-insensitive conditions (9–11). This work will enhance our knowledge regarding the mechanisms of dietary phytochemicals against prostate carcinogenesis and ultimately expands the scope of alternative approaches in prostate cancer prevention.
Supported by National Natural Science Foundation of China (grant no. 30371209), the Innovation Direction Projects of the Chinese Academy of Sciences (KSCX2-2-25), the Chief Scientist Program of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (SIBS2008006), the Ministry of Science and Technology of China (Grant 2008DFA31960), and the National Cancer Institute (R01 CA85740).
Author disclosures: L-H. Chen, J. F. Zhijian Sun, H. Li, Y. Wu, W. Demark-Wahnefried, and X. Lin, no conflicts of interest.
Abbreviations used: DMSO, dimethyl sulfoxide; ERK, extracellular-signal regulated kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GSK-3 β, glycogen synthase kinase-3 β; IGF-1, insulin-like growth factor-1; IGF-1R, insulin-like growth factor 1 receptor; MAPK, mitogen-activated protein kinase; MTT, 3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide; PI3K, phosphatidylinositol 3′-kinase; si, siRNA; si-con, plasmids with inverted sequences of siRNA against IGF-1R mRNA; si-IGF-1R, plasmids with siRNA against IGF-1R mRNA.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 2.Adlercreutz H, Mazur W, Bartels P, Elomaa V, Watanabe S, Wahala K, Landstrom M, Lundin E, Bergh A, et al. Phytoestrogens and prostate disease. J Nutr. 2000;130:S658–9. [DOI] [PubMed] [Google Scholar]
- 3.Kolonel LN, Hankin JH, Lee J, Chu SY, Nomura AM, Hinds MW. Nutrient intakes in relation to cancer incidence in Hawaii. Br J Cancer. 1981;44:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angwafo FF. Migration and prostate cancer: an international perspective. J Natl Med Assoc. 1998;90:s720–3. [PMC free article] [PubMed] [Google Scholar]
- 5.McCullough ML, Giovannucci EL. Diet and cancer prevention. Oncogene. 2004;23:6349–64. [DOI] [PubMed] [Google Scholar]
- 6.Breslow N, Chan CW, Dhom G, Drury RA, Franks LM, Gellei B, Lee YS, Lundberg S, Sparke B, et al. Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer, Lyons, France. Int J Cancer. 1977;20:680–8. [DOI] [PubMed] [Google Scholar]
- 7.Hedelin M, Klint A, Chang ET, Bellocco R, Johansson JE, Andersson SO, Heinonen SM, Adlercreutz H, Adami HO, et al. Dietary phytoestrogen, serum enterolactone and risk of prostate cancer: the cancer prostate Sweden study (Sweden). Cancer Causes Control. 2006;17:169–80. [DOI] [PubMed] [Google Scholar]
- 8.Bylund A, Saarinen N, Zhang JX, Bergh A, Widmark A, Johansson A, Lundin E, Adlercreutz H, Hallmans G, et al. Anticancer effects of a plant lignan 7-hydroxymatairesinol on a prostate cancer model in vivo. Exp Biol Med (Maywood). 2005;230:217–23. [DOI] [PubMed] [Google Scholar]
- 9.Lin X, Gingrich JR, Bao W, Li J, Haroon ZA, Demark-Wahnefried W. Effect of flaxseed supplementation on prostatic carcinoma in transgenic mice. Urology. 2002;60:919–24. [DOI] [PubMed] [Google Scholar]
- 10.Lin X, Switzer BR, Demark-Wahnefried W. Effect of mammalian lignans on the growth of prostate cancer cell lines. Anticancer Res. 2001;21:3995–9. [PubMed] [Google Scholar]
- 11.Chen LH, Fang J, Li H, Demark-Wahnefried W, Lin X. Enterolactone induces apoptosis in human prostate carcinoma LNCaP cells via a mitochondrial-mediated, caspase-dependent pathway. Mol Cancer Ther. 2007;6:2581–90. [DOI] [PubMed] [Google Scholar]
- 12.Demark-Wahnefried W, Price DT, Polascik TJ, Robertson CN, Anderson EE, Paulson DF, Walther PJ, Gannon M, Vollmer RT. Pilot study of dietary fat restriction and flaxseed supplementation in men with prostate cancer before surgery: exploring the effects on hormonal levels, prostate-specific antigen, and histopathologic features. Urology. 2001;58:47–52. [DOI] [PubMed] [Google Scholar]
- 13.Demark-Wahnefried W, Polascik TJ, George SL, Switzer BR, Madden JF, Ruffin MT, Snyder DC, Owzar K, Hars V, et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol Biomarkers Prev. 2008;17:3577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adlercreutz H, van der WJ, Kinzel J, Attalla H, Wahala K, Makela T, Hase T, Fotsis T. Lignan and isoflavonoid conjugates in human urine. J Steroid Biochem Mol Biol. 1995;52:97–103. [DOI] [PubMed] [Google Scholar]
- 15.Thompson LU, Robb P, Serraino M, Cheung F. Mammalian lignan production from various foods. Nutr Cancer. 1991;16:43–52. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Saarinen NM, Thompson LU. Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats. J Nutr. 2006;136:906–12. [DOI] [PubMed] [Google Scholar]
- 17.Borriello SP, Setchell KD, Axelson M, Lawson AM. Production and metabolism of lignans by the human faecal flora. J Appl Bacteriol. 1985;58:37–43. [DOI] [PubMed] [Google Scholar]
- 18.McCann MJ, Gill CI, Linton T, Berrar D, McGlynn H, Rowland IR. Enterolactone restricts the proliferation of the LNCaP human prostate cancer cell line in vitro. Mol Nutr Food Res. 2008;52:567–80. [DOI] [PubMed] [Google Scholar]
- 19.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–90. [DOI] [PubMed] [Google Scholar]
- 20.Rinker-Schaeffer CW, Partin AW, Isaacs WB, Coffey DS, Isaacs JT. Molecular and cellular changes associated with the acquisition of metastatic ability by prostatic cancer cells. Prostate. 1994;25:249–65. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds AR, Kyprianou N. Growth factor signalling in prostatic growth: significance in tumour development and therapeutic targeting. Br J Pharmacol. 2006;147:S144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollak M. Insulin-like growth factors and prostate cancer. Epidemiol Rev. 2001;23:59–66. [DOI] [PubMed] [Google Scholar]
- 23.Papatsoris AG, Karamouzis MV, Papavassiliou AG. Novel insights into the implication of the IGF-1 network in prostate cancer. Trends Mol Med. 2005;11:52–5. [DOI] [PubMed] [Google Scholar]
- 24.Baserga R. Insulin-like growth factor I receptor signalling in prostate cancer cells. Growth Horm IGF Res. 2000;10:S43–4. [DOI] [PubMed] [Google Scholar]
- 25.Stattin P, Bylund A, Rinaldi S, Biessy C, Dechaud H, Stenman UH, Egevad L, Riboli E, Hallmans G, et al. Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst. 2000;92:1910–7. [DOI] [PubMed] [Google Scholar]
- 26.Chan JM, Stampfer MJ, Ma J, Gann P, Gaziano JM, Pollak M, Giovannucci E. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst. 2002;94:1099–106. [DOI] [PubMed] [Google Scholar]
- 27.DiGiovanni J, Kiguchi K, Frijhoff A, Wilker E, Bol DK, Beltran L, Moats S, Ramirez A, Jorcano J, et al. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci USA. 2000;97:3455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burfeind P, Chernicky CL, Rininsland F, Ilan J, Ilan J. Antisense RNA to the type I insulin-like growth factor receptor suppresses tumor growth and prevents invasion by rat prostate cancer cells in vivo. Proc Natl Acad Sci USA. 1996;93:7263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grzmil M, Hemmerlein B, Thelen P, Schweyer S, Burfeind P. Blockade of the type I IGF receptor expression in human prostate cancer cells inhibits proliferation and invasion, up-regulates IGF binding protein-3, and suppresses MMP-2 expression. J Pathol. 2004;202:50–9. [DOI] [PubMed] [Google Scholar]
- 30.Krueckl SL, Sikes RA, Edlund NM, Bell RH, Hurtado-Coll A, Fazli L, Gleave ME, Cox ME. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res. 2004;64:8620–9. [DOI] [PubMed] [Google Scholar]
- 31.Nickerson T, Chang F, Lorimer D, Smeekens SP, Sawyers CL, Pollak M. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR). Cancer Res. 2001;61:6276–80. [PubMed] [Google Scholar]
- 32.Fang J, Zhou Q, Shi XL, Jiang BH. Luteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cells. Carcinogenesis. 2007;28:713–23. [DOI] [PubMed] [Google Scholar]
- 33.Cross DAE, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. [DOI] [PubMed] [Google Scholar]
- 34.Gao N, Flynn DC, Zhang Z, Zhong XS, Walker V, Liu KJ, Shi X, Jiang BH. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol. 2004;287:C281–91. [DOI] [PubMed] [Google Scholar]
- 35.Marelli MM, Moretti RM, Procacci P, Motta M, Limonta P. Insulin-like growth factor-I promotes migration in human androgen-independent prostate cancer cells via the alphavbeta3 integrin and PI3-K/Akt signaling. Int J Oncol. 2006;28:723–30. [PubMed] [Google Scholar]
- 36.Gennigens C, Menetrier-Caux C, Droz JP. Insulin-like growth factor (IGF) family and prostate cancer. Crit Rev Oncol Hematol. 2006;58:124–45. [DOI] [PubMed] [Google Scholar]
- 37.Macaulay VM. The IGF receptor as anticancer treatment target. Novartis Found Symp. 2004;262:235–43. [PubMed] [Google Scholar]
- 38.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 39.Ferrari S, Thomas G. S6 phosphorylation and the p70s6k/p85s6k. Crit Rev Biochem Mol Biol. 1994;29:385–413. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, DeGroff VL, Clinton SK. Tomato and soy polyphenols reduce insulin-like growth factor-I-stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase. J Nutr. 2003;133:2367–76. [DOI] [PubMed] [Google Scholar]
- 41.Girnita A, Girnita L, Prete FD, Bartolazzi A, Larsson O, Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004;64:236–42. [DOI] [PubMed] [Google Scholar]
- 42.Pietrzkowski Z, Mulholland G, Gomella L, Jameson BA, Wernicke D, Baserga R. Inhibition of growth of prostatic cancer cell lines by peptide analogues of insulin-like growth factor 1. Cancer Res. 1993;53:1102–6. [PubMed] [Google Scholar]
- 43.Grunwald V, DeGraffenried L, Russel D, Friedrichs WE, Ray RB, Hidalgo M. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 2002;62:6141–5. [PubMed] [Google Scholar]
- 44.Bagi CM. Targeting of therapeutic agents to bone to treat metastatic cancer. Adv Drug Deliv Rev. 2005;57:995–1010. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Wang X, Liu Y, Tian H, Flickinger B, Empie MW, Sun SZ. Effects of dietary flaxseed lignan extract on symptoms of benign prostatic hyperplasia. J Med Food. 2008;11:207–14. [DOI] [PubMed] [Google Scholar]
- 46.Morton MS, Chan PS, Cheng C, Blacklock N, Matos-Ferreira A, branches-Monteiro L, Correia R, Lloyd S, Griffiths K. Lignans and isoflavonoids in plasma and prostatic fluid in men: samples from Portugal, Hong Kong, and the United Kingdom. Prostate. 1997;32:122–8. [DOI] [PubMed] [Google Scholar]
- 47.Jacobs E, Metzler M. Oxidative metabolism of the mammalian lignans enterolactone and enterodiol by rat, pig, and human liver microsomes. J Agric Food Chem. 1999;47:1071–7. [DOI] [PubMed] [Google Scholar]