Abstract
Objective
Notch3, a member of the evolutionary conserved Notch receptor family, is primarily expressed in vascular smooth muscle cells. Genetic studies in human and mice revealed a critical role for Notch3 in the structural integrity of distal resistance arteries by regulating arterial differentiation and postnatal maturation.
Methods and Results
We investigated the role of Notch3 in vascular tone in small resistance vessels (tail and cerebral arteries) and large (carotid) arteries isolated from Notch3 deficient mice using arteriography. Passive diameter and compliance were unaltered in mutant arteries. Similarly, contractions to phenylephrine, KCl, angiotensin II and thromboxane A2 as well as dilation to acetylcholine or sodium nitroprusside were unaffected. However, Notch3 deficiency induced a dramatic reduction in pressure-induced myogenic tone associated with a higher flow (shear stress)-mediated dilation in tail and cerebral resistance arteries only. Furthermore, RhoA activity and myosin light chain phosphorylation, measured in pressurized tail arteries, were significantly reduced in Notch3KO mice. Additionally, myogenic tone inhibition by the Rho kinase inhibitor Y27632 was attenuated in mutant tail arteries.
Conclusions
Notch3 plays an important role in the control of vascular mechano-transduction, by modulating the RhoA/Rho kinase pathway, with opposite effects on myogenic tone and flow-mediated dilation in the resistance circulation.
Condensed abstract
Notch3 regulates arterial differentiation and postnatal maturation of smooth muscle cells. By using arteries from Notch3 knockout mice we found that Notch3 plays an important role in the control of resistance arteries mechano-transduction, by modulating the RhoA/Rho kinase pathway, which is involved in pressure-induced (myogenic) tone.
Keywords: resistance arteries, myogenic tone, Notch receptors, flow-mediated dilation, local blood flow regulation
Introduction
Arteries are specified into different calibers and types of vessels to perform different functions. Schematically, the major arteries of the trunk are elastic arteries of large diameter and low resistance. Elastic conduit arteries absorb the hemodynamic stress of cardiac systole and release this energy in the form of sustained blood pressure during diastole. Conversely, distal arteries are muscular arteries of small diameter and high resistance that are critically involved in local regulation of blood flow. Resistance arteries possess a constant basal tone which is tightly regulated by two mechanical stimuli, ie flow and pressure; basal tone provides the background tone upon which other vasoactive systems may act synergistically 1-3. Flow produces shear stress and triggers dilation, which depends in part on the production of nitric oxide and vasodilator agents, by the endothelial cells 1, 4, 5. Mechano-transduction of shear stress involves the extracellular matrix and cell structure proteins 6-8. Pressure-induced (myogenic) contraction is an inherent property of smooth muscle cells. However the robustness and nature of the response vary significantly with vascular bed and vessel caliber 1,2. The cellular structures and signaling pathways involved in the mechano-transduction of pressure into constriction have not been completely elucidated. Signaling mechanisms require calcium entry as well as calcium-sensitization of the contractile apparatus. Several lines of investigation implicate actin polymerization in myogenic tone 9. Furthermore, the RhoA-Rho kinase signaling pathway is a key regulator of the calcium sensitivity and dynamic remodeling of the actin cytoskeleton 10 and we have recently shown that RhoA activation is essential for the development of myogenic tone 11, 12
The Notch signaling pathway is an evolutionarily conserved intercellular signaling mechanism that plays a central role during vascular development and physiology in vertebrates 13. The Notch family receptors comprise 4 highly conserved members in human and rodents (Notch1 to Notch4). Among these, Notch3 is primarily expressed in vascular smooth muscle cells 14, and, recent genetic studies in human and mice have highlighted an important role for this receptor in the development and homeostasis of distal arteries 15. In human, mutations of NOTCH3 cause CADASIL, an autosomal dominant vascular dementia. Neurological symptoms arise due to a slowly progressive small-artery-disease, characterized by progressive degeneration of smooth muscle cells of small brain arteries 16. In the mouse, targeted deletion of the Notch3 gene does not affect viability nor fertility, but results in structural defects of distal arteries, particularly in the brain and the tail. Specifically, in the absence of Notch3, smooth muscle cells of distal arteries exhibit an abnormal shape and cytoskeleton because of an impaired arterial differentiation and postnatal maturation. It is noteworthy that major elastic arteries of the trunk appeared preserved at least at the histological level 17, 18.
In this study we investigated the role of Notch3 in the function of small (resistance) and large (compliance) arteries. We examined the mechanical properties and vascular reactivity to vasoactive agents or mechanical stimuli of arteries from wild-type and Notch3 null mice. We assessed the tail caudal artery and the middle cerebral artery, as distal resistance vessels, and the common carotid artery, a compliance elastic artery with minimum role in arterial resistance. Consistent with our prior observation that elastic artery did not exhibit structural alteration, we found that the mechanical properties and vascular reactivity of mutant carotid arteries were preserved. Importantly, we found that in the tail caudal and middle cerebral arteries, absence of Notch3 selectively impaired the response to pressure and flow. Furthermore, RhoA activity and myosin light chain phosphorylation were reduced in pressurized mutant tail arteries, and, myogenic tone inhibition elicited by the Rho kinase inhibitor Y-27632 was significantly attenuated in mutant tail arteries. Together these data support a specific role for Notch3 in the mechano-transduction of pressure and flow in the distal resistance arteries through a RhoA/Rho kinase pathway.
Material and methods (detailed in the online supplement: see www.ahajournals.org)
Notch3-/- mice (KO) and their wild-type littermates (WT) were obtained by crossing Notch3 heterozygous mice. Adult male mice (n= 25 per group) were anesthetized for blood pressure measurement 19 and then killed by CO2 inhalation. Common carotid, mesenteric, middle cerebral and tail caudal arteries were collected.
Histology was performed as previously described 17.
Pharmacological study was performed on 2 mm long arterial segments mounted on a wire-myograph 20. Contraction to Phenylephrine (PE), thromboxane A2 mimetic (U46619) 21 angiotensin II (AngII) and calcium was tested 22. Concentration-dependent relaxation in response to Acetylcholine (ACh) was performed with or without NO synthase blockade (L-NAME), and/or cyclooxygenase blockade (indomethacin) 23.
Pressure (myogenic) and flow-dependent tone was determined in isolated arteries cannulated in a video monitored perfusion system 24.
For Western-blotting arterial segments were dissected and snap-frozen in liquid nitrogen. Samples were analysed for eNOS, p-eNOS, caveolin-1, αV-integrin and β3-integrin, RhoA, P38, pP38, P42, pP42, P44, pP44, FAK, pFAK, MLC and pMLC. Preliminary immunoblot analysis showed that comparable results were obtained using freshly isolated arteries as compared to pressurized (75 mmHg) arterial segments (figure 1: see ahajournals.org).
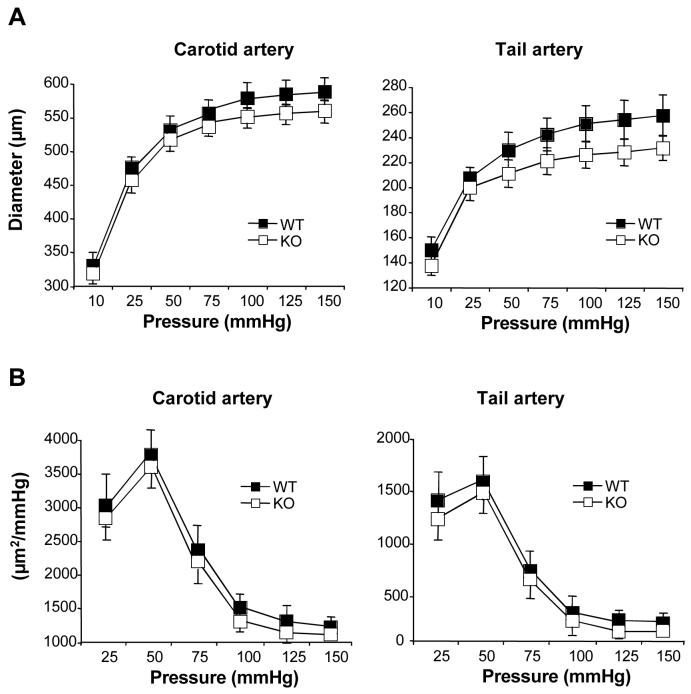
Figure 1.
Passive diameter (A) and cross-sectional compliances (B) of carotid and tail caudal arteries from wild type (WT) and Notch3-/- (KO) mice (MEAN ± SEM, n=12 WT and 9 KO); NS, KO versus WT.
RhoA activation was assessed as previously described 25 using a Rho-GTP pull-down assay kit.
Statistical analysis
Results were expressed as means ± standard error. Significance of the differences between groups was determined by analysis of variance (ANOVA for consecutive measurements for pressure-diameter curves) or one-way ANOVA followed by Bonferroni or paired t-test. P values less than 0.05 were considered to be significant.
Results
Structural and mechanical properties of KO arteries
High-resolution optic microscopy showed structural defects of the mutant caudal artery and middle cerebral artery with thinning and disorganization of the tunica media as previously reported 17. By contrast, carotid artery of Notch3 null mice appeared indistinguishable from the one of WT mice (figure 2: see ahajournals.org). To determine the effect of absence of Notch3 on the passive properties of the vascular wall, arteries were submitted to stepwise increase in intraluminal pressure. Passive arterial diameter (figure 1A, Figures 4: see ahajournals.org) and arterial cross sectional compliance (figure 1B and data not shown) were not significantly different in KO and WT mice.
Figure 2.

Contraction induced by phenylephrine (A) and calcium (B) and vasodilation induced by acetylcholine (C) in the presence of L-NAME (LN) or L-NAME plus indomethacin (LN+INDO) in tail arteries from wild-type (WT) and Notch3-/- (KO) mice (MEAN ± SEM, n=12 WT and 9 KO) NS, KO versus WT.
Figure 4.

Expression level of the MAP kinase P38, P42, P44, FAK, alphaV integrin (αV), beta 3 integrin (β3), myosin light chains (MLC), eNOS and caveolin-1 (cav-1) in tail caudal arteries from wild-type (WT) and Notch3-/- (KO) mice. The level of phosphorylated proteins was determined as well (p-P38, p-P42, p-P44, p-FAK, p-MLC and p-eNOS). Shown on the right are representative immunoblots (MEAN ± SEM, n=6 per group). *P<0.05, KO versus WT.
KCl and receptor-dependent contractions
The contraction induced by KCl (80 mmol/L) was not significantly affected by the absence of Notch3 in carotid, tail caudal and middle cerebral arteries (Figure 3 and 4: see ahajournals.org). PE, Ang II and U46619 produced a concentration-dependent contraction in carotid and tail caudal arteries. Importantly, contractile responses to these agonists were not significantly different between WT and KO mice (figure 2A, and table 1; figure 3 and 4: see ahajournals.org). Moreover, the Ca2+ dose-response curves in WT and mutant arteries were comparable (figure 2B).
Figure 3.
Response of carotid (A), tail (B) and middle cerebral arteries (C) from wild-type (WT) and Notch3-/- (KO) to stepwise increase in intraluminal pressure (Myogenic tone, right panel) or in intraluminal flow (flow-mediated dilation, left panel) (MEAN ± SEM, n=12 WT and 9 KO). *P<0.01, KO versus WT.
Table 1.
Pharmacological profile of Notch3-deficient (KO) and wild-type (WT) mice arteries. Contraction to phenylephrine, serotonin (5HT), angiotensin II and U46619 as well as dilation to sodium nitroprusside (SNP) were obtained in tail and carotid arteries
| Tail artery | Carotid artery | |||||
|---|---|---|---|---|---|---|
| WT | KO | WT | KO | units: | ||
| Phenylephrine | Emax | 7.6 ± 1.0 | 7.2 ± 0.9 | 3.2 ± 0.4 | 3.6 ± 0.4 | mN |
| EC50 | 323 ± 78 | 358 ± 65 | 45 ± 8 | 37 ± 7 | nmol/L | |
| Angiotensin II | Emax | 2.5 ± 0.3 | 2.4 ± 0.4 | 1.8 ± 0.3 | 1.6 ± 0.4 | mN |
| EC50 | 3.8 ± 0.7 | 5.1 ± 0.8 | 39 ± 6 | 35 ± 6 | nmol/L | |
| U 46619 | Emax | 6.3 ± 1.0 | 5.6 ± 0.8 | 4.8 ± 0.3 | 5.2 ± 0.5 | mN |
| EC50 | 52 ± 11 | 64 ± 15 | 79 ± 15 | 86 ± 17 | nmol/L | |
| SNP | Imax | 98 ± 2 | 96 ± 3 | 98 ± 3 | 95 ± 4 | % dilation |
| IC50 | 32 ± 7 | 34 ± 6 | 25 ± 6 | 19 ± 5 | nmol/L | |
EC50 and IC50 represent the concentration necessary to reach 50% of the maximal effect; Emax and Imax give the maximal effect of the drug (n=12 per group).
NS, KO versus WT
Endothelium-dependent and -independent dilation
Absence of Notch3 did not significantly affect ACh-induced dilation in carotid, tail, and middle cerebral arteries (figure 2C; Figure 5 and 4: see ahajournals.org). Inhibition of NO synthase by L-NAME decreased ACh-induced dilation in carotid and tail caudal arteries with the same potency in WT and KO mice in tail (figure 2C) and carotid arteries (data not shown). Indomethacin did not significantly reduce ACh-induced dilation when added after L-NAME in WT and KO tail (figure 2C) and carotid arteries (data not shown). Endothelium-independent relaxation (SNP) was similar in KO and WT mice (table 1 and data not shown).
Figure 5.
Quantification of RhoA expression level by western-blot (A) and RhoA activity by pull down assay (B) in tail and carotid arteries. The inhibitory effect of the Rho-kinase inhibitor Y27632 (0.01 to 10 μmol/L) was assessed on myogenic tone (C), phenylephrine-(D), KCl (inset in D) as well as on calcium-induced constriction (E) in the tail caudal artery (MEAN ± SEM, n=6 per group). *P<0.01, KO versus WT.
Vascular mechano-transduction of flow (shear stress) and pressure
Myogenic tone was significantly decreased by 68 and 75% (measured from the decrease in diameter induced by a pressure of 75 mmHg) in tail and cerebral arteries, respectively, from KO mice compared to WT animals. By contrast, pressure-induced contraction was not significantly different in KO and WT mice in carotid arteries (figure 3, right panel).
Flow mediated dilation (FMD) was significantly higher in mutant tail and cerebral arteries (43% and 30% increase in FMD for a flow rate of 100μl/min) as compared with WT arteries. FMD of WT and mutant carotid arteries were comparable (figure 3, left panel). The precontraction level prior to FMD was similar in WT and KO mice (figure 5: see ahajournals.org).
Biochemical analysis
To investigate the mechanisms by which absence of Notch3 affects mechanotransduction, we assessed the expression level and activation (phosphorylation) of proteins possibly involved in myogenic tone (pP38, P38, pP42, P42, pP44, P44, MLC, pMLC) 2, 9, 11, in FMD (peNOs, eNOs, Cav-1) 1 or in both (FAKs, pFAKs, αV-integrin and β3-integrin) 1, 2 in tail arteries. No difference in protein expression level between WT and mutant mice was found at the exception of pMLC, which was significantly decreased in mutant arteries (figure 4).
In order to further analyze the mechanism involved in the decrease in myogenic tone, we examined the expression level and activity of RhoA. As shown in figure 5 (A-B), Notch3 null mice exhibited a significant 46% reduction of RhoA activity, while RhoA protein level was unaltered as compared with wild-type mice.
Effect of Rho-kinase inhibition
In order to confirm the involvement of the RhoA/Rho kinase pathway in the mechano-transduction defect observed in Notch3 null mice, we measured the relaxation induced by stepwise increase in the concentration of the Rho kinase inhibitor Y-27632. Myogenic tone was concentration-dependently inhibited by Y-27632. In control mice, complete inhibition was achieved with 10 μmol/L Y-27632 whereas in Notch3 deficient mice inhibition reaches only a maximum of 49% at the same dose (figure 5C). We further assessed the relaxation induced by the Rho kinase inhibitor in tail arterial segments preconstricted with KCl (60 mmol/L), PE (0.3 μmol/L), or calcium (0.5 mmol/L). Remarkably, dose-response curves were not significantly different between WT and KO arterial segments (figure 5D,E).
Discussion
Notch3, a key regulator of vascular tone in small arteries
Recently, we demonstrated that Notch3 is critically required for the structural integrity of small distal arteries whereas it appears dispensable for the structural integrity of large conductance arteries 17. In this study, we provide the first insight into how Notch3 influences function of the arterial system. Consistent with the notion that Notch3 is dispensable for structural integrity of elastic arteries, we found that the mechanical properties and pharmacological profiles of carotid arteries were unaffected in mice completely lacking Notch3. Importantly, we found a significant decrease in myogenic tone and an enhanced flow-mediated dilation in isolated cerebral and tail caudal arteries. These alterations are unlikely to arise from a global dysfunction of vascular cells because both contraction and relaxation to pharmacological agents were unaffected. Hence, the results indicate that Notch3 deficiency selectively impairs the function of small arteries and suggest a specific role for Notch3 in the transduction of tensile and shear stress. We reported previously that in the absence of Notch3 smooth muscle cells of distal arteries lack molecular markers of arterial smooth muscle cells and exhibit histological features of venous cells 17. Myogenic tone is an inherent property of arterial smooth muscle cells. Thus the present findings indicate that in the absence of Notch3 smooth muscle cells of distal arteries lack an arterial phenotype also at the functional level and further support the concept that Notch3 is a key regulator of the arterial phenotype of smooth muscle cells.
The ability of small resistance arteries to develop myogenic tone is an important determinant of regional blood flow autoregulation as well as blood pressure 26, 27. Our prior observation of strongly compromised autoregulation of cerebral blood flow in Notch3 null mice is consistent with the present finding of an impaired myogenic response in these mice. However, it is remarkable that basal blood pressure is normal in Notch3 null mice (Supplementary results) 17. Although activation of cardiac or neurohumoral compensatory mechanisms in Notch3 null mice might solve this paradox, structural and functional analysis of additional resistance arteries from Notch3-/-mice suggests an alternative explanation. Specifically, high-resolution optic microscopy and electron microscopy of mesenteric arteries failed to detect structural defect of smooth muscle cells, although Notch3 is strongly expressed in these cells (Figure 2: see ahajournals.org and data not shown). Moreover, vasoreactivity to pharmacological agents and mechanical stimuli was similar in mutant and wild-type mesenteric arteries (Figure 7: see ahajournals.org). Given the importance of large peripheral vascular beds such as the mesenteric bed in the control of arterial blood pressure a localized vascular change in reactivity is unlikely to cause a significant change in systemic blood pressure. In addition, myogenic tone is mostly involved in the short-term control of local blood flow, whereas hormonal vasoactive systems such as the sympathetic and renin-angiotensin systems have a major role in controlling systemic blood pressure 1, 28. Thus these later findings suggest that Notch3 is critically required for vascular tone in some vascular beds including at least the brain and the tail arteries, although being dispensable in others including the mesenteric bed.
How does Notch3 influence myogenic tone?
In resistance arteries, increase in intraluminal pressure induces a rapid cell architecture distension leading to the activation of stretch-dependent ion channels and voltage-operated Ca2+ channels 29 and ultimately of calmodulin and myosin light chain Kinase 30. We 11, 12 and others 31-33 have demonstrated the key role played by the RhoA/Rho Kinase pathway in myogenic tone. Moreover, recent studies from our group 12 and others 34 support the hypothesis that activation of integrins and focal-adhesion kinase in caveolin-1 rich domains may participate in the Rho-kinase dependent sensitization of the contractile apparatus to calcium. In the present work, we provide evidence that Notch3 is an upstream modulator of the RhoA/Rho kinase pathway. First, we show that RhoA activity is significantly decreased in the tail arteries lacking Notch3. Second, Rho kinase inhibition with Y-27632, in its range of selectivity, was minimally efficient in pressurized mutant tail arteries indicating that the RhoA/Rho kinase activity was reduced in response to pressure (myogenic tone) in the absence of Notch3. Third, myosin light chain phosphorylation was significantly reduced in mutant pressurized arteries. The observation that expression levels of integrins, focal adhesion kinase (FAK), ERK1/2 and MAP kinase P38 were not affected by the absence of Notch3 suggest that Notch3 activity is unrelated or lies downstream to these kinases. The RhoA/Rho kinase pathway has been widely shown to play a key role in the sensitization of the contractile apparatus in response to many vasoconstrictors such as angiotensin II, phenylephrine or thromboxane A2 10. However, our data here suggest that only the Rho kinase pathway activated in response to blood pressure elevation is modulated by Notch3. This supports the concept that Notch3 is a key receptor in the signaling pathway translating pressure to contraction (myogenic tone). As previously mentioned, mutant tail arteries exhibit disorganized and disjunctional smooth muscle cells 17. Using specific inhibitors of gap junction, several studies 31, 35 reported the key role played by cell adhesion in the process of myogenic tone but not agonist-induced vasoconstriction. Assembly of focal adhesion contacts as well as formation of actin filaments bundles (stress fibers) has been reported to be dependent on RhoA activation 36. Indeed, RhoA participates in the formation of distinct patterns of actin organization and assembly of integrin complexes. It has been reported that, in epithelial cells, RhoA induces the establishment and maintenance of E-Cadherin mediated cell-cell adhesion. Furthermore inactivation of RhoA results in the dislocation of E-cadherin and its complex members from the adherent junction leading to loss of cell-cell adhesion 37. The reduced RhoA activity observed in the Notch3 null mice is thus certainly linked to the impaired myogenic tone and to the structural dysfunction observed in vascular smooth muscle cells. Nevertheless further studies are necessary to clarify the exact relationship between Notch3 and RhoA activation.
How does Notch3 activity influence flow-mediated dilation?
In the present study we also demonstrated that Notch3 null mice exhibited an increased FMD. In endothelial cells, transduction of shear stress into dilation involves integrin-matrix interactions 38 at focal adhesions 39. FAK activation leads to the phosphorylation of phosphatidylinositol 3-kinase (PI3K) that triggers eNOS activation via the PI3K-Akt pathway 40. Since Notch3 deficiency did not affect calcium-dependent eNOS activation (ACh), or the effects of eNOS blockade (L-NAME) on ACh-induced dilation, our results rather reflect an increase in shear stress transduction than an enhanced endothelial function.
Several reports show that the pre-existing myogenic tone regulates the vascular response to shear stress 41, 42. According to the latter authors the higher the intraluminal pressure, the higher is the myogenic tone and the less negative is the resting membrane potential. It would be expected that the open probability time of endothelial potassium channels involved in the FMD would be decreased, while the open probability time of voltage-activated calcium channels associated with constriction in vascular smooth muscle would be increased 29. We expect the opposite to be true, ie an enhanced FMD in arteries with an attenuated myogenic tone. Nevertheless, the change in myogenic tone could not directly influence the measurement of FMD because of the similar degree of preconstriction applied to arteries from WT and KO mice. It is most likely that the reduced basal tone occurring in vivo influences the sensitivity of the flow-sensing process, although the mechanism involved remains to be determined. Increased FMD in the absence of Notch3 would thus rather reflect an increased vascular smooth muscle cell capability to dilate in response to shear than an increase endothelium capability to induce dilation. We previously showed that transgenic mice expressing a mutant Notch3 protein, with the R90C mutation (TgNotchR90C), whose expression was specifically targeted in arterial smooth muscle exhibited an increase in myogenic tone associated with a decrease in FMD without endothelial dysfunction 11. The lack of endothelial dysfunction in these latter mice is one more argument in favor of a regulation of FMD by the pre-existing myogenic tone. At the present time interpretation of the finding that TgNotchR90C and Notch3 KO mice exhibit opposite vascular dysfunction remains unclear since both in vitro and in vivo analyses showed that the R90C mutation did not impair canonical Notch3 activity 43,44.
In summary, the present study provides to the best of our knowledge, the first evidence that Notch3 controls, through the RhoA/ROK signaling pathway, vascular reactivity to the mechanical factors, pressure and flow. Moreover, Notch3 null mice, because of their highly specific defects, provide an invaluable experimental model to dissect the pathways specifically involved in the modulation of myogenic tone. Finally, our work highlights Notch3 as a novel pathway for therapeutic targeting in vascular diseases where changes in myogenic responses and vascular autoregulation are thought to play a role.
Supplementary Material
Acknowledgements
Source of funding
This work was supported by the ANR (Agence Nationale de la Recherche) - Maladies Rares (Paris, France) and by the NIH (National Neurological Disorders and Stroke Institute grant R01 NS 054122). DH and AJ were supported by an “INTERFACE” grant (contrat d’interface INSERM-CHU d’Angers, DH and INSERM-AP-HP, AJ). E.J. Belin de Chantemèle was a recipient of a post-doctoral fellowship from the CNES.
Footnotes
No disclosure
Publisher's Disclaimer: This is an un-copy edited author manuscript that was accepted for publication in Arteriosclerosis, Thrombosis, and Vascular Biology, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the « Fair Use of Copyrighted Materials » (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://atvb.ahajournals.org/. The American heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Henrion D. Pressure and flow-dependent tone in resistance arteries. Role of myogenic tone. Arch Mal Coeur Vaiss. 2005;98:913–921. [PubMed] [Google Scholar]
- 2.Hill MA, Davis MJ, Meininger GA, Potocnik SJ, Murphy TV. Arteriolar myogenic signalling mechanisms: Implications for local vascular function. Clin Hemorheol Microcirc. 2006;34:67–79. [PubMed] [Google Scholar]
- 3.Dowell FJ, Henrion D, Benessiano J, Poitevin P, Levy B. Chronic infusion of low-dose angiotensin II potentiates the adrenergic response in vivo. J Hypertens. 1996;14:177–182. doi: 10.1097/00004872-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Vanhoutte PM. Endothelium-derived free radicals: for worse and for better. J Clin Invest. 2001;107:23–25. doi: 10.1172/JCI11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu HY, Henrion D, Levy BI. Alterations in flow-dependent vasomotor tone in spontaneously hypertensive rats. Hypertension. 1994;24:474–479. doi: 10.1161/01.hyp.24.4.474. [DOI] [PubMed] [Google Scholar]
- 6.Henrion D, Dechaux E, Dowell FJ, Maclour J, Samuel JL, Levy BI, Michel JB. Alteration of flow-induced dilatation in mesenteric resistance arteries of L-NAME treated rats and its partial association with induction of cyclo-oxygenase-2. Br J Pharmacol. 1997;121:83–90. doi: 10.1038/sj.bjp.0701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loufrani L, Matrougui K, Gorny D, Duriez M, Blanc I, Levy BI, Henrion D. Flow (shear stress)-induced endothelium-dependent dilation is altered in mice lacking the gene encoding for dystrophin. Circulation. 2001;103:864–870. doi: 10.1161/01.cir.103.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loufrani L, Levy BI, Henrion D. Defect in microvascular adaptation to chronic changes in blood flow in mice lacking the gene encoding for dystrophin. Circ Res. 2002;91:1183–1189. doi: 10.1161/01.res.0000047505.11002.81. [DOI] [PubMed] [Google Scholar]
- 9.Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. Faseb J. 2002;16:72–76. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- 10.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 11.Dubroca C, You D, Levy BI, Loufrani L, Henrion D. Involvement of RhoA/Rho kinase pathway in myogenic tone in the rabbit facial vein. Hypertension. 2005;45:974–979. doi: 10.1161/01.HYP.0000164582.63421.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubroca C, Loyer X, Retailleau K, Loirand G, Pacaud P, Feron O, Balligand JL, Levy BI, Heymes C, Henrion D. RhoA activation and interaction with Caveolin-1 are critical for pressure-induced myogenic tone in rat mesenteric resistance arteries. Cardiovasc Res. 2007;73:190–197. doi: 10.1016/j.cardiores.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: who is talking to whom about what? Circ Res. 2007;100:1556–1568. doi: 10.1161/01.RES.0000266408.42939.e4. [DOI] [PubMed] [Google Scholar]
- 14.Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, Piga N, Chapon F, Godfrain C, Tournier-Lasserve E. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105:597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 16.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 17.Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, Tournier-Lasserve E, Gridley T, Joutel A. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18:2730–2735. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krebs LT, Xue Y, Norton CR, Sundberg JP, Beatus P, Lendahl U, Joutel A, Gridley T. Characterization of Notch3-deficient mice: normal embryonic development and absence of genetic interactions with a Notch1 mutation. Genesis. 2003;37:139–143. doi: 10.1002/gene.10241. [DOI] [PubMed] [Google Scholar]
- 19.Baron-Menguy C, Bocquet A, Guihot AL, Chappard D, Amiot MJ, Andriantsitohaina R, Loufrani L, Henrion D. Effects of red wine polyphenols on postischemic neovascularization model in rats: low doses are proangiogenic, high doses anti-angiogenic. Faseb J. 2007;21:3511–3521. doi: 10.1096/fj.06-7782com. [DOI] [PubMed] [Google Scholar]
- 20.Loufrani L, Henrion D, Chansel D, Ardaillou R, Levy BI. Functional evidence for an angiotensin IV receptor in rat resistance arteries. J Pharmacol Exp Ther. 1999;291:583–588. [PubMed] [Google Scholar]
- 21.Bolla M, Matrougui K, Loufrani L, Maclouf J, Levy B, Levy-Toledano S, Habib A, Henrion D. p38 mitogen-activated protein kinase activation is required for thromboxane-induced contraction in perfused and pressurized rat mesenteric resistance arteries. J Vasc Res. 2002;39:353–360. doi: 10.1159/000065547. [DOI] [PubMed] [Google Scholar]
- 22.Loufrani L, Henrion D. Vasodilator treatment with hydralazine increases blood flow in mdx mice resistance arteries without vascular wall remodelling or endothelium function improvement. J Hypertens. 2005;23:1855–1860. doi: 10.1097/01.hjh.0000183944.25832.81. [DOI] [PubMed] [Google Scholar]
- 23.Ben Driss A, Devaux C, Henrion D, Duriez M, Thuillez C, Levy BI, Michel JB. Hemodynamic stresses induce endothelial dysfunction and remodeling of pulmonary artery in experimental compensated heart failure. Circulation. 2000;101:2764–2770. doi: 10.1161/01.cir.101.23.2764. [DOI] [PubMed] [Google Scholar]
- 24.Dumont O, Loufrani L, Henrion D. Key role of the NO-pathway and matrix metalloprotease-9 in high blood flow-induced remodeling of rat resistance arteries. Arterioscler Thromb Vasc Biol. 2007;27:317–324. doi: 10.1161/01.ATV.0000254684.80662.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolli-Derkinderen M, Sauzeau V, Boyer L, Lemichez E, Baron C, Henrion D, Loirand G, Pacaud P. Phosphorylation of serine 188 protects RhoA from ubiquitin/proteasome-mediated degradation in vascular smooth muscle cells. Circ Res. 2005;96:1152–1160. doi: 10.1161/01.RES.0000170084.88780.ea. [DOI] [PubMed] [Google Scholar]
- 26.Bevan JA, Laher I. Pressure and flow-dependent vascular tone. Faseb J. 1991;5:2267–2273. doi: 10.1096/fasebj.5.9.1860618. [DOI] [PubMed] [Google Scholar]
- 27.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 28.Schubert R, Mulvany MJ. The myogenic response: established facts and attractive hypotheses. Clin Sci (Lond) 1999;96:313–326. [PubMed] [Google Scholar]
- 29.Wellman GC, Bevan JA. Barium inhibits the endothelium-dependent component of flow but not acetylcholine-induced relaxation in isolated rabbit cerebral arteries. J Pharmacol Exp Ther. 1995;274:47–53. [PubMed] [Google Scholar]
- 30.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 31.Lagaud G, Gaudreault N, Moore ED, Van Breemen C, Laher I. Pressure-dependent myogenic constriction of cerebral arteries occurs independently of voltage-dependent activation. Am J Physiol Heart Circ Physiol. 2002;283:H2187–2195. doi: 10.1152/ajpheart.00554.2002. [DOI] [PubMed] [Google Scholar]
- 32.Schubert R, Kalentchuk VU, Krien U. Rho kinase inhibition partly weakens myogenic reactivity in rat small arteries by changing calcium sensitivity. Am J Physiol Heart Circ Physiol. 2002;283:H2288–2295. doi: 10.1152/ajpheart.00549.2002. [DOI] [PubMed] [Google Scholar]
- 33.Gokina NI, Park KM, McElroy-Yaggy K, Osol G. Effects of Rho kinase inhibition on cerebral artery myogenic tone and reactivity. J Appl Physiol. 2005;98:1940–1948. doi: 10.1152/japplphysiol.01104.2004. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. alphavbeta3-and alpha5beta1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;289:H322–329. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]
- 35.Earley S, Resta TC, Walker BR. Disruption of smooth muscle gap junctions attenuates myogenic vasoconstriction of mesenteric resistance arteries. Am J Physiol Heart Circ Physiol. 2004;287:H2677–2686. doi: 10.1152/ajpheart.00016.2004. [DOI] [PubMed] [Google Scholar]
- 36.Aspenstrom P, Fransson A, Saras J. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J. 2004;377:327–337. doi: 10.1042/BJ20031041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller JM, Chilian WM, Davis MJ. Integrin signaling transduces shear stress--dependent vasodilation of coronary arterioles. Circ Res. 1997;80:320–326. doi: 10.1161/01.res.80.3.320. [DOI] [PubMed] [Google Scholar]
- 39.Koshida R, Rocic P, Saito S, Kiyooka T, Zhang C, Chilian WM. Role of focal adhesion kinase in flow-induced dilation of coronary arterioles. Arterioscler Thromb Vasc Biol. 2005;25:2548–2553. doi: 10.1161/01.ATV.0000188511.84138.9b. [DOI] [PubMed] [Google Scholar]
- 40.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 41.Kuo L, Chilian WM, Davis MJ. Interaction of pressure-and flow-induced responses in porcine coronary resistance vessels. Am J Physiol. 1991;261:H1706–1715. doi: 10.1152/ajpheart.1991.261.6.H1706. [DOI] [PubMed] [Google Scholar]
- 42.Thorin-Trescases N, Bevan JA. High levels of myogenic tone antagonize the dilator response to flow of small rabbit cerebral arteries. Stroke. 1998;29:1194–1200. doi: 10.1161/01.str.29.6.1194. discussion 1200-1191. [DOI] [PubMed] [Google Scholar]
- 43.Joutel A, Monet M, Domenga V, Riant F, Tournier-Lasserve E. Pathogenic mutations associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy differently affect Jagged1 binding and Notch3 activity via the RBP/JK signaling Pathway. Am J Hum Genet. 2004;74:338–347. doi: 10.1086/381506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monet M, Domenga V, Lemaire B, Souilhol C, Langa F, Babinet C, Gridley T, Tournier-Lasserve E, Cohen-Tannoudji M, Joutel A. The archetypal R90C CADASIL-NOTCH3 mutation retains NOTCH3 function in vivo. Hum Mol Genet. 2007;16:982–992. doi: 10.1093/hmg/ddm042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.