Abstract
OBJECTIVES
To determine whether higher levels of inflammatory blood markers, D-dimer, and homocysteine were associated with greater impairment in lower extremity functioning in persons with peripheral arterial disease (PAD).
DESIGN
Cross-sectional.
SETTING
Three Chicago-area medical centers.
PARTICIPANTS
Four hundred twenty-three persons with PAD (ankle-brachial index (ABI) <0.90).
MEASUREMENTS
Lower extremity performance was assessed using the 6-minute walk and with usual- and fast-paced 4-m walking speed. Blood markers were D-dimer, C-reactive protein (CRP), interleukin-6 (IL-6), soluble vascular cellular adhesion molecule-1 (sVCAM-1), soluble intracellular adhesion molecule-1 (sICAM-1), and homocysteine. Calf muscle area was measured using computed tomography.
RESULTS
Adjusting for confounders, higher levels of D-dimer (P<.001), IL-6 (P<.001), sVCAM-1 (P=.006), CRP (P=.01), homocysteine (P=.004), and sICAM-1 (P=.046) were associated with poorer 6-minute walk performance. Higher levels of D-dimer (P<.001), IL-6 (P=.003), sVCAM-1 (P=.001), and homocysteine (P=.005) were associated with slower usual-paced 4-m walking speed. Higher levels of D-dimer, sVCAM-1, sICAM-1, IL-6, and homocysteine were associated with slower fast-paced walking speed. Results were attenuated after additional adjustment for calf muscle area.
CONCLUSION
Higher levels of inflammation and D-dimer were associated with poorer lower extremity performance in participants with PAD, independent of confounders including ABI.
Keywords: intermittent claudication, peripheral vascular disease, physical functioning, inflammation
Persons with lower extremity peripheral arterial disease (PAD) have higher inflammatory biomarker levels, greater functional impairment, and faster functional decline than persons without PAD.1-3 Higher levels of inflammation are associated with greater functional impairment and faster functional decline in persons with PAD.4,5 Reasons for these associations are unclear, but inflammation plays a key role in the initiation and progression of atherosclerosis.6 Additionally, inflammation may contribute to sarcopenia, an age-related reduction in muscle strength and mass.7,8 Lower muscle mass has been associated with greater functional impairment in persons with PAD.9
It was previously reported that, in persons with PAD, higher levels of D-dimer and the inflammatory marker C-reactive protein (CRP) were associated with poorer lower extremity functional performance.4 In persons with PAD, higher levels of serum amyloid A and fibrinogen were not associated with greater functional impairment.4 Associations between other blood markers and functional performance in persons with PAD have not been tested to the authors’ knowledge. This study determined whether, in patients with PAD, higher levels of emerging inflammatory biomarkers, D-dimer, and homocysteine were associated with greater functional impairment. Homocysteine was studied, because it promotes processes that contribute to atherosclerosis, including inflammation.10 Homocysteine causes oxidative damage to endothelial cells and proteins and affects the ability of cells to regenerate, thereby potentially contributing to weakness and atrophy of skeletal muscle.11,12 Inflammatory biomarkers studied were interleukin-6 (IL-6), soluble vascular cellular adhesion molecule (sVCAM-1), and soluble intracellular adhesion molecule (sICAM-1). sVCAM-1 and sICAM-1 were studied, because these leukocyte adhesion molecules promote inflammatory-mediated acceleration of atherosclerosis by enabling adherence and transendothelial migration of leukocytes to endothelial cells.13 Associations between CRP and functional impairment and between D-dimer and functional impairment were also studied. High levels of D-dimer indicate activation of the coagulation system. D-dimer can also increase inflammation by promoting activation of monocytes and neutrophils and by inducing secretion of IL-6.14,15 Prior work was built on by determining whether associations between higher biomarker levels and greater functional impairment were independent of physical activity levels and calf muscle area in persons with PAD.
METHODS
Participant and Identification
The institutional review boards of Northwestern University Feinberg School of Medicine and Catholic Health Partners Hospitals approved the protocol. Participants gave informed consent. Participants included persons with PAD attending their fourth annual follow-up visit in the Walking and Leg Circulation Study (WALCS)2,3 and participants newly identified with PAD for the present study (WALCS II).9 In WALCS and WALCS II, participants with PAD were identified consecutively from among patients diagnosed with PAD in three Chicago-area noninvasive vascular laboratories.2,3,9 Because participants in the original WALCS cohort were aged 59 and older at the time of this data collection, an inclusion criterion for newly identified participants was aged 59 and older.
Of 238 PAD participants returning for their fourth annual follow-up visit for WALCS, 202 (84.7%) had blood drawn and were included in the present analyses. An additional 240 participants with PAD were newly identified for WALCS II. Of these, 221 (92.1%) had blood drawn at their visit and were eligible for the present study.
Exclusion Criteria
PAD was defined as an ankle-brachial index (ABI) less than 0.90.3-5 The following exclusion criteria were applied at the time of enrollment for WALCS and for those newly identified for WALCS II. Patients with dementia were excluded because of their inability to answer questions accurately. Nursing home residents, wheelchair-bound patients, and patients with foot or leg amputations were excluded, because they have severely impaired functioning. Non-English-speaking patients were excluded, because investigators were not fluent in non-English languages. Patients with recent major surgery were excluded.
ABI Measurement
The ABI was measured using established methods.2,3,16 After participants rested supine for 5 minutes, a handheld Doppler probe (Nicolet Vascular Pocket Dop II, Golden, CO) was used to measure systolic pressures in the right brachial artery, right dorsalis pedis and posterior tibial arteries, left dorsalis pedis and posterior tibial arteries, and left brachial artery. Each pressure was measured twice: in the order listed and then in reverse order. The ABI was calculated in each leg by dividing average pressures in each leg by the average of the four brachial pressures.2,3,9,16 Average brachial pressures in the arm with highest pressure were used when one brachial pressure was higher than the other in both measurement sets and the two brachial pressures differed by 10 mmHg or more in at least one measurement set, because in such cases, subclavian stenosis was possible.17 The lowest leg ABI was used in analyses.
Six-Minute Walk
Following a standardized protocol,2-5 participants walk up and down a 100-foot hallway for 6 minutes after instructions to cover as much distance as possible.
Four-Meter Walking Velocity
Walking velocity was measured during a 4-m walk performed at “usual” and “fastest” pace. For the usual-paced walk, participants were instructed to walk at their usual pace, “as if going down the street to the store.” Each walk was performed twice. The faster walk in each pair was used in analyses.18
Objective Physical Activity Measures
Physical activity levels were measured objectively over 7 days using a vertical accelerometer (Caltrac, Muscle Dynamics Fitness Network, Inc., Torrence, CA).19,20 The Caltrac accelerometers are designed to calculate the number of kilocalories expended based on activity (vertical movement), age, weight, height, and sex. To compare activity between participants irrespective of individual variation in age, weight, height, and sex, accelerometers were programmed using identical weight, height, age, and sex for each participant.19,20 Thus, the accelerometers measured “activity units.”19,20 After wearing activity monitors continuously for 7 days, participants reported the number of activity units displayed on the accelerometer over the telephone and mailed their activity monitors back to the investigators.
Sixty percent of participants wore Caltrac activity monitors. Systematic data on reasons that some participants did not wear monitors were not collected. Some refused to wear monitors, others wore monitors but did not return them and could not be reached at 7-day follow-up, some participants’ monitors malfunctioned, and in some instances no monitors were available.
Blood Collection and Storage
Blood was collected into ethylenediaminetetraacetic acid and sodium citrate Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) and immediately iced. Tubes were spun at 3,000 revolutions per minute for 20 minutes at 4°C in a refrigerated centrifuge. Blood was stored at -70°C until analyses were completed. Most, but not all, blood collection occurred in the morning.
Blood Factor Levels
IL-6 was measured using an ultrasensitive enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN). The assay has a sensitivity of 0.094 pg/mL. The percentage coefficient of variation (CV%) in a randomly selected subsample from the cohort was 4.2%. sICAM-1 and sVCAM-1 were measured using ELISAs (R&D Systems). CV% values were 4.3% for sICAM-1 and 5.6% for sVCAM-1. An Asserachrom D-Di kit (Diagnostica Stago, Parsippany, NJ) was used to measure fibrin D-dimer, using an ELISA procedure. The Asserachrom D-Di kit has a lower detection limit of 5 ng/mL. The CV% for D-dimer was 7.2%. Concentrations of high-sensitivity CRP were determined using an immunoturbidimetric assay on the Hitachi 911 analyzer (Roche Diagnostics, Indianapolis, IN), using reagents and calibrators from Denka Seiken (Niigata, Japan). This assay has a sensitivity of 0.003 mg/dL. The CV% for CRP was 2.1%. The concentration of homocysteine was determined using an enzymatic assay on the Hitachi 917 analyzer (Roche Diagnostics), using reagents and calibrators from Catch Inc. (Seattle, WA). The CV% for homocysteine was 6.9%.
Measuring Calf Muscle Characteristics
Using a computed tomography scanner (LightSpeed, General Electric Medical Systems, Waukesha, WI), a 2.5-mm cross-sectional image of the calves was obtained at 66.7% of the distance from the distal to the proximal tibia.9 BonAlyse software (BonAlyse LTD, Jyvaskyla, Finland) was used to measure the muscle area of the calf. The muscle outline was traced manually and excluded subcutaneous fat and bone. When measuring muscle area, the BonAlyse software quantifies voxels within a range corresponding to muscle density (9-271 mg/cm3) and excludes voxels corresponding to fat density (-270 to 8 mg/cm3). Previous cadaver studies have demonstrated that these methods provide an estimate of muscle area that is highly correlated with direct anatomic measures.21
Leg Symptoms
Leg symptoms were classified into one of five groups using the San Diego Claudication Questionnaire as follows:22 intermittent claudication; leg pain on exertion and rest; atypical exertional leg pain, carry on; atypical exertional leg pain, stop; asymptomatic.
Comorbidities
Algorithms developed for the Women’s Health and Aging Study and the Cardiovascular Health Study were used to document comorbidities, combining data from patient report, physical examination, medical record review, medications, laboratory values, and a primary care physician questionnaire.23 Comorbidities assessed were angina pectoris, diabetes mellitus, myocardial infarction, stroke, heart failure, spinal stenosis, and disk disease. American College of Rheumatology criteria were used to adjudicate knee and hip osteoarthritis.24,25
Other Measures
Height and weight were measured at the study visit. Body mass index was calculated as weight (kg)/(height (m))2. Cigarette smoking history was based on self-report. Creatinine clearance was calculated using serum creatinine levels and the Modification of Diet in Renal Disease equation.26
Statistical Analyses
The associations between quartiles of each blood marker and lower extremity performance were evaluated using analyses of covariance, adjusting for age, sex, race, cigarette smoking, body mass index (BMI), leg symptoms, recruitment cohort (original WALCS vs newly identified), and comorbidities. Comorbidities adjusted for were knee arthritis, hip arthritis, spinal stenosis, disk disease, diabetes mellitus, pulmonary disease, cancer, and other cardiovascular diseases (myocardial infarction, angina pectoris, heart failure, and stroke). Associations of D-dimer and homocysteine were additionally adjusted for creatinine clearance, because impaired renal function can increase levels of D-dimer and homocysteine. The fully adjusted analysis of covariance was repeated, adding all blood markers into one model to determine whether any biomarkers were significantly associated with functional performance after adjustment for all biomarkers simultaneously. Associations between each biomarker and lower extremity performance were repeated after additional adjustment for calf muscle area and physical activity level. The purpose of these latter analyses was to determine whether significant associations between biomarker levels and lower extremity functioning were independent of calf muscle area and physical activity. Statistical analyses were performed using SAS Statistical Software version 9.0 (SAS Institute, Inc., Cary, NC).
RESULTS
The average age of the 423 participants with PAD was 74.9 ± 8.2. Forty-six percent were women, 16.6% were African American, 32.4% had diabetes mellitus, 16.3% were current smokers, and 58.6% had other clinically evident cardiovascular disease. The average ABI was 0.63 ± 0.01. Twenty-five percent had symptoms of intermittent claudication, and 23.52% were asymptomatic.
Correlation coefficients between age and biomarker levels were -0.041 for CRP, 0.121 for D-dimer, 0.297 for sVCAM-1, -0.016 for sICAM-1, 0.100 for IL-6, and 0.132 for homocysteine.
Figure 1 shows associations between quartiles of each biomarker and 6-minute walk performance, adjusting for age, sex, race, smoking, BMI, recruitment cohort, leg symptoms, ABI, comorbidities, and creatinine clearance (D-dimer and homocysteine only). Higher levels of D-dimer, CRP, IL-6, sVCAM-1, sICAM-1, and homocysteine were associated with poorer 6-minute walk performance, adjusting for confounders (Figure 1).
Figure 1.
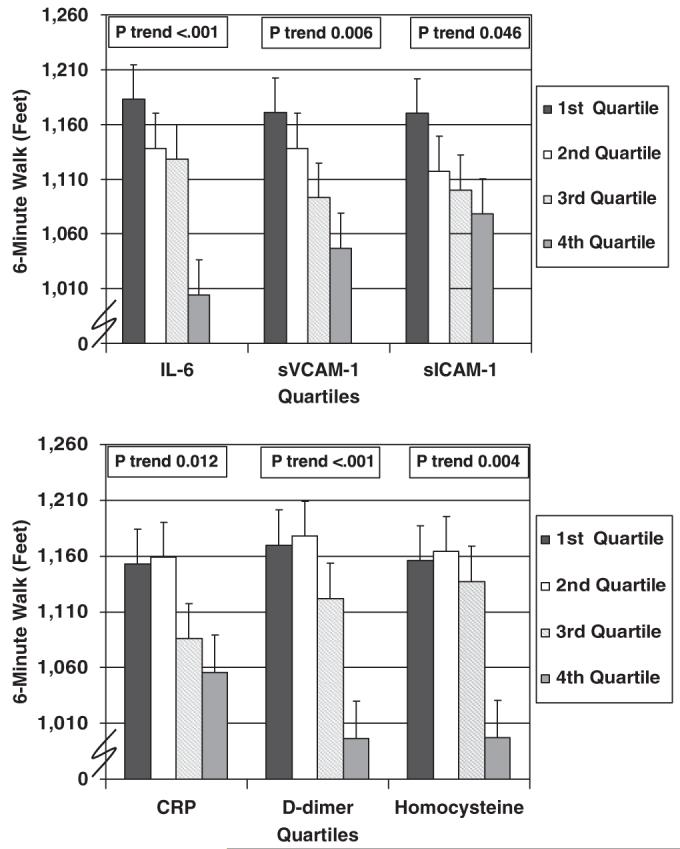
Adjusted associations between blood marker quartiles and 6-minute walk performance in participants with lower extremity peripheral arterial disease (n=423). Data are adjusted for age, sex, race, cigarette smoking, body mass index, ankle-brachial index, leg symptoms, recruitment cohort, comorbidities, and creatinine clearance (for D-dimer and homocysteine only). Comorbidities adjusted for were knee arthritis, hip arthritis, spinal stenosis, disk disease, diabetes mellitus, cancer, pulmonary disease, and other cardiovascular diseases (myocardial infarction, stroke, angina pectoris, and heart failure). IL-6=interleukin 6; CRP=C-reactive protein; sVCAM-1=soluble vascular cellular adhesion molecule-1; sICAM-1=soluble intracellular adhesion molecule-1.
Figure 2 shows associations between biomarker quartiles and usual-paced 4-m walking velocity, adjusting for known and potential confounders. Higher levels of IL-6, D-dimer, sVCAM-1, sICAM-1, and homocysteine were associated significantly and independently with slower usual-paced 4-m walking velocity, adjusting for confounders. Findings for associations between blood markers and fast-paced 4-m walking speed were similar to those shown for usual-paced walking speed. Higher levels of IL-6 (P=.002), D-dimer (P<.001), sVCAM-1 (P<.001), sICAM-1 (P=.03), and homocysteine (P=.002) were associated significantly and independently with slower fast-paced 4-m walking speed (data not shown).
Figure 2.
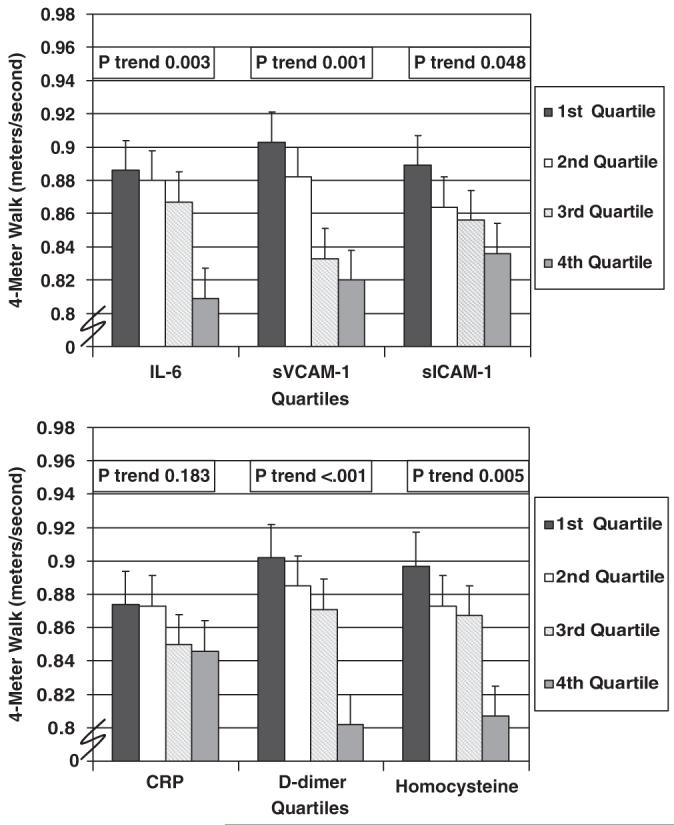
Adjusted associations between blood factor quartiles and 4-m walking speed (usual pace) in participants with lower extremity peripheral arterial disease (n=423). Data are adjusted for age, sex, race, cigarette smoking, body mass index, leg symptoms, ankle-brachial index, recruitment cohort, comorbidities, and creatinine clearance (for D-dimer and homocysteine only). Comorbidities adjusted for were knee arthritis, hip arthritis, spinal stenosis, disk disease, diabetes mellitus, cancer, pulmonary disease, and other cardiovascular diseases (myocardial infarction, stroke, angina pectoris, and heart failure). IL-6=interleukin 6; CRP=C-reactive protein; sVCAM-1=soluble vascular cellular adhesion molecule-1; sICAM-1=soluble intracellular adhesion molecule-1.
Within the subset of participants who wore Caltrac accelerometers, associations between higher biomarker levels and poorer functional performance were not substantially changed after additional adjustment for physical activity levels (data not shown).
To determine whether associations between higher biomarker levels and greater functional impairment were independent of associations between higher biomarker levels and smaller calf muscle area, comparisons between biomarker levels and functional performance were repeated with additional adjustment for calf muscle area. Table 1 presents results of these associations. After additional adjustment for calf muscle area, associations between higher levels of CRP and sICAM-1 and poorer 6-minute walk performance were no longer statistically significant (Table 1). Levels of statistical significance for associations between sVCAM-1, IL-6, homocysteine, and D-dimer and 6-minute walk performance (Figure 1) were somewhat attenuated but remained statistically significant after additional adjustment for calf muscle area (Table 1). Significant associations between IL-6 and sICAM-1 and normal-paced 4-m walking speed (Figure 2) were no longer statistically significant after additional adjustment for calf muscle area (Table 1). The significant associations between D-dimer and sVCAM-1 and normal-paced 4-m walking speed (Figure 2) were attenuated after additional adjustment for calf muscle area (Table 1). Independent associations between D-dimer and sICAM-1 and fast-paced 4-m walking speed were no longer statistically significant after additional adjustment for calf muscle area. The association between IL-6 and fast-paced 4-m walking speed was attenuated after additional adjustment for calf muscle area (Table 1).
Table 1. Associations Between Blood Factors and Lower Extremity Functioning in Patients with Peripheral Arterial Disease, Including Additional Adjustment for Calf Muscle Area (N=423)*.
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
|---|---|---|---|---|---|
| Lower Extremity Functioning | Mean (Standard Error) | Trend P Value | |||
| C-reactive protein, gm/L | <0.96 | 0.96-1.87 | 1.88-3.97 | ≥3.98 | |
| Six-minute walk, feet | 1,151 (30.7) | 1,144 (31.0) | 1,086 (31.7) | 1,088 (31.4) | .08 |
| Four-meter walk, usual pace, m/s | 0.87 (0.02) | 0.85 (0.02) | 0.84 (0.02) | 0.87 (0.02) | .83 |
| Four-meter walk, fast pace, m/s | 1.16 (0.02) | 1.15 (0.02) | 1.14 (0.02) | 1.17 (0.02) | .92 |
| Soluble vascular cellular adhesion molecule-1, ng/mL | <858 | 858-1,073 | 1,074-1,361 | ≥1,362 | |
| Six-minute walk, feet | 1,157 (32.1) | 1,145 (30.1) | 1,095 (31.7) | 1,063 (33.9) | .03 |
| Four-meter walk, usual pace, m/s | 0.89 (0.02) | 0.89 (0.02) | 0.83 (0.02) | 0.83 (0.02) | .004 |
| Four-meter walk, fast pace, m/s | 1.22 (0.02) | 1.17 (0.02) | 1.12 (0.02) | 1.10 (0.03) | <.001 |
| Interleukin-6, pg/mL | <1.81 | 1.81-2.80 | 2.81-4.68 | ≥4.69 | |
| Six-minute walk | 1,173 (30.9) | 1,131 (31.1) | 1,126 (31.4) | 1,035 (31.7) | .003 |
| Four-meter walk, usual pace, m/s | 0.87 (0.02) | 0.87 (0.02) | 0.87 (0.02) | 0.83 (0.02) | .07 |
| Four-meter walk, fast pace, m/s | 1.17 (0.02) | 1.18 (0.02) | 1.19 (0.02) | 1.09 (0.02) | .04 |
| Homocysteine, μmol/L | <8.50 | 8.50-10.76 | 10.77-13.62 | ≥13.63 | |
| Six-minute walk, feet | 1,144 (34.1) | 1,173 (30.7) | 1,122 (31.2) | 1,028 (36.0) | .03 |
| Four-meter walk, usual pace, m/s | 0.89 (0.02) | 0.88 (0.02) | 0.85 (0.02) | 0.81 (0.02) | .006 |
| Four-meter walk, fast pace, m/s | 1.20 (0.03) | 1.19 (0.02) | 1.14 (0.02) | 1.10 (0.03) | .006 |
| D-dimer, μg/mL | <0.49 | 0.49-0.67 | 0.68-1.04 | ≥1.05 | |
| Six-minute walk, feet | 1,141 (31.7) | 1,179 (31.2) | 1,118 (31.2) | 1,034 (33.4) | .01 |
| Four-meter walk, usual pace, m/s | 0.88 (0.02) | 0.88 (0.02) | 0.87 (0.02) | 0.82 (0.02) | .03 |
| Four-meter walk, fast pace, m/s | 1.18 (0.02) | 1.18 (0.02) | 1.16 (0.02) | 1.12 (0.03) | .07 |
| Soluble intracellular adhesion molecule-1 | <252 | 252-294 | 295-349 | ≥350 | |
| Six-minute walk, feet | 1,159 (31.6) | 1,122 (31.6) | 1,103 (31.3) | 1,086 (31.7) | .10 |
| Four-meter walk, usual pace, m/s | 0.88 (0.02) | 0.86 (0.02) | 0.85 (0.02) | 0.84 (0.02) | .17 |
| Four-meter walk, fast pace, m/s | 1.19 (0.02) | 1.15 (0.02) | 1.14 (0.02) | 1.13 (0.02) | .10 |
Data are adjusted for age, sex, race, cigarette smoking, body mass index, leg symptoms, recruitment cohort (original Walking and Leg Circulation Study vs newly identified), comorbidities, ankle-brachial index, creatinine clearance (homocysteine and D-dimer only), tibia length, and calf muscle area. Comorbidities adjusted for were knee arthritis, hip arthritis, spinal stenosis, disk disease, diabetes mellitus, pulmonary disease, cancer, and other cardiovascular disease (myocardial infarction, angina pectoris, heart failure, and stroke).
Comparisons between biomarkers and lower extremity functioning were repeated, in which all biomarkers were entered into one model. Adjusting for age, sex, race, smoking, BMI, recruitment cohort, leg symptoms, ABI, comorbidities, creatinine clearance, and all blood markers simultaneously, homocysteine remained significantly and independently associated with 6-minute walk performance (P trend=.001), usual-paced 4-m walking speed (P trend<.001), and fast-paced 4-m walking speed (P trend=.001). In these analyses, sVCAM-1 was associated significantly with 6-minute walk performance (P trend=.047) and fast-paced 4-m walking speed (P trend=.001).
DISCUSSION
In 423 men and women with PAD, results reported here show that higher levels of sVCAM-1, IL-6, homocysteine, sICAM-1, and D-dimer were associated with poorer performance on the 6-minute walk and usual- and fast-paced 4-m walks, after adjusting for confounders, including ABI. In contrast, higher levels of CRP were associated only with poorer 6-minute walk performance in participants with PAD. In analyses adjusting for all blood markers simultaneously, homocysteine and sVCAM-1 remained significantly and independently associated with the degree of functional impairment on multiple outcome assessments. Thus, associations between homocysteine and sVCAM-1 and lower extremity performance are more robust than the other markers studied in persons with PAD.
Previously published data from 423 participants with PAD in the WALCS II cohort demonstrated that higher levels of IL-6, D-dimer, CRP, and sVCAM-1 are associated with smaller calf muscle area, as measured using computed tomography, even after adjusting for confounders including level of physical activity.27 A previous study also demonstrated that smaller calf muscle area is associated with poorer lower extremity performance in persons with PAD.9 In the present study, after additional adjustment for calf muscle area, most significant associations between higher biomarker levels and greater functional impairment were no longer statistically significant or were attenuated. Therefore, a potential causal pathway for associations between high biomarkers and greater functional impairment is that high biomarker levels may lead to smaller calf muscle area, which in turn is associated with greater functional impairment in persons with PAD.9 Consistent with this hypothesis, prior studies have suggested that inflammatory cytokines alter muscle homeostasis by inhibiting repair after tissue injury and by promoting muscle proteolysis.7,8 Alternatively, higher biomarker levels may be more precise measures of the extent of systemic atherosclerosis than other measures, such as ABI. The extent of systemic atherosclerosis may be an important determinant of the degree of functional impairment in PAD. This latter mechanism of association may be most pertinent to associations between D-dimer and the degree of functional impairment, because D-dimer may be a systemic measure of the degree of atherosclerotic burden.
Despite the findings that high levels of CRP were associated independently with poorer performance on only one measure of lower extremity functioning, a prior study showed that higher CRP levels at baseline were associated with greater risk for developing PAD in community-dwelling persons in the Physician’s Health Study.28 In addition, of 1,572 community dwelling men and women in the Edinburgh Artery Study, higher levels of CRP, sICAM-1, and IL-6, but not higher levels of sVCAM-1, were associated with greater decline in ABI at 10-year follow-up.29 Together, these data suggest that some inflammatory markers (e.g., CRP) may be more important for the development of PAD in individuals without PAD, whereas other biomarkers (e.g., sVCAM-1) may be involved in adverse outcomes in persons with established PAD.
This study has limitations. First, the study design was cross-sectional. Associations reported here might not be causal. Second, biomarkers were measured just once, and not all blood samples were collected in the morning. Diurnal variation, analytical factors, and other biological properties can influence biomarker levels. Third, findings may not be generalizable to the individuals who met the exclusion criteria. Fourth, only 60% of participants had Caltrac accelerometer data. Finally, most study participants were Caucasian.
CONCLUSION
This study suggests that high levels of IL-6, sVCAM-1, D-dimer, sICAM-1, and homocysteine are associated with greater functional impairment in persons with PAD. Lower calf muscle mass in persons with PAD with higher levels of these blood markers may contribute to associations between higher blood marker levels and greater functional impairment in persons with PAD. Further longitudinal study is needed to determine the causal pathways for the findings reported here.
ACKNOWLEDGMENTS
Conflict of Interest: Mary McDermott receives salary support from the National Heart, Lung, and Blood Institute and honoraria for educational activities from Bristol Meyers Squibb Sanofi Aventis. Kiang Liu, Lu Tian, David Green, Jin Tan, Yihua Liao, Kimberly McCue, William H. Pearce, Paul M. Ridker, Nader Rifai, and Michael H. Criqui receive salary support from the National Heart, Lung, and Blood Institute. Paul Ridker is a co-inventor of the assay used for analyzing C-reactive protein. Supported by grants R01-HL58099, R01-HL64739, R01-HL71223, and R01-HL076298 from the National Heart, Lung, and Blood Institute and by grant #RR-00048 from the National Center for Research Resources, National Institutes of Health (NIH). Supported in part by the Intramural Research Program, National Institute on Aging, NIH.
Footnotes
Sponsor’s Role: The funding agency played a role in the study design, methods, subject recruitment, data collection, data analyses, or paper preparation.
REFERENCES
- 1.McDermott MM, Guralnik JM, Corsi A, et al. Patterns of inflammation associated with peripheral arterial disease: The InCHIANTI study. Am Heart J. 2005;150:276–281. doi: 10.1016/j.ahj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: The Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: Associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM, Greenland P, Green D, et al. D-dimer, inflammatory markers, and lower extremity functioning in patients with and without peripheral arterial disease. Circulation. 2003;107:191–198. doi: 10.1161/01.CIR.0000074227.53616.CC. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Ferrucci L, Liu K, et al. D-dimer and inflammatory markers as predictors of functional decline in men and women with and without peripheral arterial disease. J Am Geriatr Soc. 2005;53:1688–1696. doi: 10.1111/j.1532-5415.2005.53510.x. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Ridker PM. Inflammation and atherothrombosis. From population biology and bench research to clinical practice. J Am Coll Cardiol. 2006;48:A33–A46. [Google Scholar]
- 7.Mitch WE, Goldberg AL. Mechanism of muscle wasting: The role of ubiquitin-proteasome pathway. N Engl J Med. 1996;335:1897–1905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 8.Goodman MN. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med. 1994;205:182–185. doi: 10.3181/00379727-205-43695. [DOI] [PubMed] [Google Scholar]
- 9.McDermott MM, Hoff F, Ferrucci L, et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55:400–406. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338:1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 11.Xu D, Neville R, Finkel T. Homocysteine accelerates endothelial cell senescence. FEBS Lett. 2000;470:20–24. doi: 10.1016/s0014-5793(00)01278-3. [DOI] [PubMed] [Google Scholar]
- 12.Dudman NP. An alternative view of homocysteine. Lancet. 1999;354:2072–2074. doi: 10.1016/S0140-6736(99)03383-8. [DOI] [PubMed] [Google Scholar]
- 13.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 14.Robson SC, Shephard EG, Kirsch RE. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1 beta, IL-6 and plasminogen activator inhibitors from monocytes in vitro. Br J Haematol. 1994;86:322–326. doi: 10.1111/j.1365-2141.1994.tb04733.x. [DOI] [PubMed] [Google Scholar]
- 15.Shorr AF, Thomas SJ, Alkins SA, et al. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest. 2002;121:1262–1268. doi: 10.1378/chest.121.4.1262. [DOI] [PubMed] [Google Scholar]
- 16.McDermott MM, Criqui MH, Guralnik JM, et al. Lower ankle brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and associations with functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 17.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: Prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower extremity function in persons over 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDermott MM, Ohlmiller SM, Liu K, et al. Gait alterations associated with walking impairment in people with peripheral arterial disease with and without intermittent claudication. J Am Geriatr Soc. 2001;49:747–754. doi: 10.1046/j.1532-5415.2001.49151.x. [DOI] [PubMed] [Google Scholar]
- 20.Garg PK, Tian L, Criqui MH, et al. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–248. doi: 10.1161/CIRCULATIONAHA.105.605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1999;86:1097–1098. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 22.Criqui MH, Denenberg JO, Bird CE, et al. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Fried LP, Simonsick EM, et al. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. National Institute on Aging; Bethesda, MD: 1995. NIH publication No. 95-4009. [Google Scholar]
- 24.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 25.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 27.McDermott MM, Ferrucci L, Guralnik JM, et al. Elevated levels of inflammation, D-dimer, and homocysteine are associated with adverse calf muscle characteristics and reduced calf muscle strength in peripheral arterial disease. J Am Coll Cardiol. 2007;50:897–905. doi: 10.1016/j.jacc.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis. A comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein A, and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 29.Tzoulaki I, Murray GD, Lee AJ, et al. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population. Edinburgh Artery Study. Circulation. 2005;112:976–983. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]


