Abstract
Recent concern about the possible secondary spread of vCJD through blood transfusion and blood products has highlighted the need for a sensitive test for the identification of PrPTSE/res in clinical specimens collected in a non-invasive way. In addition, a more accurate estimate of the prevalence of pre-clinical vCJD in the population may be possible if there were a test that could be applied to easily available material such as urine. As a step towards this goal, the detection of putative PrPTSE/res in the urine of CJD patients has been improved, based on Proteinase K digestion of samples and western blotting. The modified western blot uses concentrated urine as a starting material. After proteolytic treatment followed by electrophoresis and western blotting, membranes are incubated with an anti-PrP antibody conjugated directly with horseradish peroxidase. This study was conducted on urine samples of CJD and other neurodegenerative disease affected individuals. Proteinase K resistant high molecular weight proteins were detected, which are suggested to be a complex of urinary PrP and immunoglobulin proteins. Whether urine can be used as a diagnostic tool for the detection of PrP could not be answered in this study.
Key words: prion, transmissible spongiform encephalopathy, sporadic Creutzfeldt-Jakob disease, variant Creutzfeldt-Jakob disease, urine, PrP, outer membrane protein
Introduction
Proteinaceous infectious particles, designated prions, are understood to be the cause of transmissible spongiform encephalopathy disease (TSE) in animals and humans.1 It is thought that these particles comprise mainly of a disease-associated isoform of the cellular prion protein PrPC.2 This isoform has been designated PrPTSE/res and is detectable in several tissues including the peripheral nervous system3 and urine.4–7 The transition from normal PrPC to infectious PrPTSE/res is not fully understood.8 Human prion diseases include sporadic Creutzfeldt-Jakob disease (sCJD), familial CJD, iatrogenic CJD (iCJD) and variant CJD (vCJD).9,10–13
There is a continuing risk of a vCJD epidemic in the human population, particularly in the UK. It is possible that the infectious agent might be incubating in many individuals and transmitted silently among the population via contaminated surgical instruments, blood transfusions and other invasive procedures.14–20 Accordingly, there is an ongoing need for sensitive diagnostic tests to detect PrPTSE/res, particularly in the pre-clinical stages of infection to eliminate the secondary transmission risk and to protect the population.
To date, several diagnostic tests have been developed.5,21–40 Some of these tests have been used commercially to screen cattle and sheep before the meat from these animals enters the food chain. Nevertheless, the majority of these methods have been applied to the post mortem diagnosis of TSE using CNS tissue; very few have been evaluated for the detection of disease-associated PrP in the pre-clinical stage of disease in humans. Accordingly, sensitive diagnostic tests that can be applied ante mortem are still required. In particular, it is believed that vCJD can be predicted by the detection of disease-associated PrP in tissue obtained following tonsillectomy or appendectomy.41–43 A recent study on over twelve thousand appendectomies detected three samples containing lymphoreticular accumulation of PrPTSE/res, giving a projected prevalence of 237 vCJD per million in the UK population.43 In principle, the non-invasive detection of disease-associated PrP could involve testing urine, blood or saliva.
Several publications have reported the presence of PrPTSE/res or other aggregated proteins in urine.4,7,44–47 Initial studies appeared to indicate that protease resistant prion in urine (designated u-PrPTSE/res) was present in hamster, cattle and humans infected with the TSE agent.44 The antibodies used in all these studies were 3F4, 6H4, anti-mouse-IgG and 3O8.
Although Shiga et al. reported the detection of u-PrPTSE/res in the urine from patients with CJD and some healthy individuals,45 others have concluded that the finding is related to bacterial outer membrane proteins (OMPs)44 or aggregated Bence Jones proteins excreted in the urine.47 Infectious proteinase K resistant prions have been reported in the urine of scrapie infected animals, suggesting urine as a possible source for prion transmission.6
In this report, we describe the detection of reactive bands through the use of anti-C-terminal-PrP monoclonal antibodies by a single-antibody western blot method. These bands maybe u-PrPres, which are excreted in the urine of CJD and other neurodegenerative disorder patients.
Results
Affinity of antibodies.
The affinity measurements of three monoclonal antibodies SAF61, SAF32 and 3F4 against recombinant PrP showed that they had dissimilar affinities with PrP. 3F4 had the highest affinity to recombinant PrP, 3.17 times higher than SAF61 and 2.55 higher than SAF32. This divergence was taken into consideration for the antibody dilutions i.e., 3F4 was diluted 1:5,000, SAF61 1:1,500 and SAF32 1:2,000 in PBST for all western blot experiments.
Analysis of CJD urines.
The characteristics of CJD and disease control patients are summarized in Table 1. The PK sensitivity of urine proteins of patients affected with prion disease (n = 10), disease control patients (n = 5) and healthy controls (n = 6) was investigated. Healthy controls are numbered 1–6 and PK treatment indicated with + or − symbols. To investigate whether urine samples contain PK resistant proteins, the membranes were probed with anti-PrP-C-terminal or anti-PrP-N-terminal or anti-IgG antibodies.
Table 1.
Clinical, molecular and demographic features of CJD patients
| CJD (code #) | Codon 129 - PrPres type* | Classification | Gender | Age (years) | Lag between urine collection and death in month (m) or weeks (w) or days (d) |
| C | M/M-1 | Definite sporadic | Female | 72 | 2 m |
| D | M/M-1 | Definite sporadic | Female | 73 | 2 m |
| E | E200K | Definite sporadic | Female | 72 | 1 m |
| M/M | |||||
| F | M/M-1 | Definite sporadic | Male | 60 | < 1 m |
| G | M/M | Definite vCJD | Male | 23 | Alive |
| H | MV I | Probable sporadic | Female | 53 | 2 w |
| I | MM I | Probable sporadic | Female | 66 | 1 m |
| J | MM I | Probable sporadic | Male | 69 | 2 w |
| K | MM I | Probable sporadic | Male | 64 | 3 w |
| L | MV II | Probable sporadic | Male | 57 | 1 m |
| M | MM | Metabolic Encephalopathy | Male | 75 | Alive |
| N | N.A | Alzheimer | Male | 65 | Alive |
| O | MM | Alzheimer | Female | 74 | 2 m |
| P | N.A | Alzheimer | Male | 65 | 2 d |
| Q | N.A | Suspected Wilson disease | Male | 33 | Alive |
M, Methionine; E, Glutamic acid; K, Lysine.
For some western blot experiments, one membrane was used and, after each antibody application, stripped prior to next antibody incubation (stripping chronologically: Figs. 1A, 2A and B), (Fig. 6A and B) and (Fig. 7A and B). A control experiment to verify whether the stripping of membrane removed the transferred protein from the membrane was carried out using recombinant PrP electrophoresed and transferred via Iblot apparatus and processed as described in material and methods. Two strippings and probings did not influence the signal obtained from the imager instrument. A longer exposure time after the second stripping was required, which was taken into consideration.
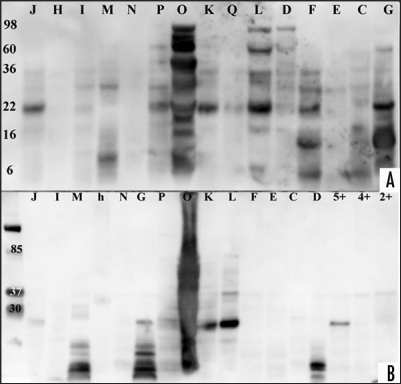
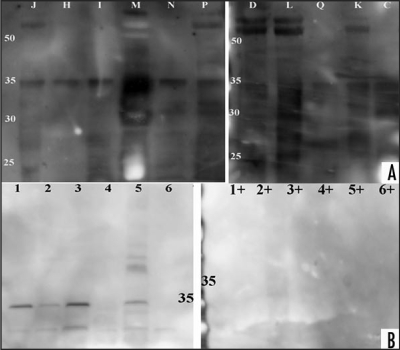
Figure 1.
Western blot analysis of CJD, disease and healthy controls. Urine samples of patient and control samples underwent proteolytic digestion with two different PK concentration and incubation time and were probed with anti-IgG-HRP anitbody. (A) Enriched urine samples from sCJD, vCJD, disease and healthy controls were treated with proteinase K (concentration 40 µg/ml for 20 min). Samples ID labelled with letters correspond to Table 1. After electrophoresis, protein was transferred to a nylon membrane using Bio-Rad instrument and probed with anti-IgG-HRP followed with ECL Plus addition. A Kodak Imager was used for photographic documentation. (B) Same or other samples were treated with proteinase K (concentration 60 µg/ml for 30 min). Samples ID labelled with letters correspond to Table 1, healthy control samples are shown with numbers. Protein was transferred via Bio-Rad and membrane probed with anti-IgG-HRP followed ECL Plus addition. Documentation was as above. (The exposure time for documentation was 5 minutes for A and B).
Figure 2.
Western blot analysis of urine proteins from CJD and disease controls. The membrane used in Figure 1A was stripped and tested with ECL Plus to verify if the previous antibody was fully removed, before processing it. (A) The membrane was probed with SAF61-HRP and documented as described before. Samples ID correspond to Table 1. (B) The membrane used in (A) was stripped and tested with ECL Plus to ensure removal of previous probed antibody, prior to probing it with SAF32-HRP. Documentation was as described before. Samples ID letters correspond to Table 1. The exposure time for documentation was 5 minutes for (A) and 45 minutes for (B). longer exposure time was necessary for (B) as it was measured with control experiment using recombinant PrP.
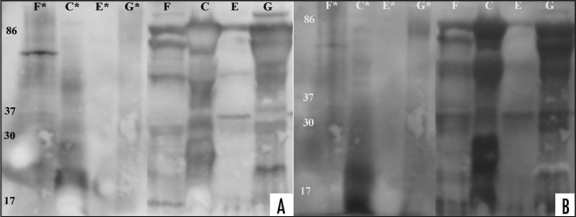
Figure 6.
Western blot analysis of sCJD and vCJD urine samples after PNGase F and acetic SDS treatment. Three sCJD and one vCJD enriched urine samples were treated over night with PNGase F, followed with PK digestion (concentration 40 µg/ml for 20 minutes). Samples ID letters correspond to Table 1. The samples were subsequently supplemented with acetic SDS or left untreated and western blotted. (*) designate acetic SDS treated samples. (A) Membrane containing acetic SDS treated or untreated samples were probed with 3F4-HRP antibody, added ECL Plus and documented using Kodak imager instrument with exposure time of 5 minutes. (B) Membrane mentioned above was stripped, tested with ECL Plus to ensure removal of previous probed antibody and subsequently probed with anti-IgG-HRP. For documentation, the procedure mentioned above was followed. Exposure time for documentation was 5 minutes.
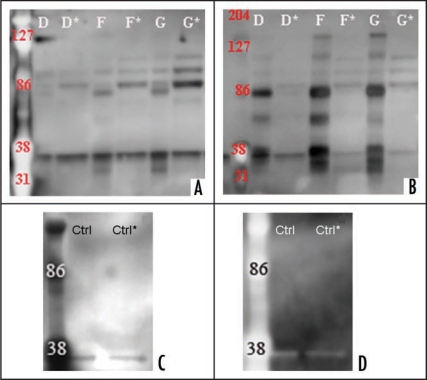
Figure 7.
Western blot analysis of CJD and control urine samples after Protein-A column chromatography. Two sCJD, one vCJD and one control urine sample were loaded on Protein-A column, eluates and flow-throughs were collected, enriched and PK digested (60 µg/ml for 30 min). Samples were subsequently electrophoresed and Western blotted. Sample IDs correspond to Table 1. Eluate is displayed with a capital letter and the flow-through with and additional (*). Healthy control is indicated with ‘Ctrl’. (A and C) Proteolytic treatment of urine samples from CJD and healthy control with proteinase K (concentration 60 µg/ml for 30 min). Proteins were transferred with a Bio-Rad instrument and the membrane probed with 3F4-HRP followed with ECL Plus. Membrane documentation was with a Kodak Imager with 5 minutes exposure time. (B and D) the membrane was stripped for 35 minutes at 37°C, verified for the removal of previously used antibody. The membrane was then probed with anti-IgG-HRP and ECL Plus was added for chemiluminescence. Membrane documentation was with a Kodak Imager with 5 minutes exposure time.
The analysis of samples treated with 40 µg/ml PK and probed with anti-IgG-HRP showed PK resistant bands in 1/1 vCJD, 3/5 disease control, 7/8 sCJD and 1/1 genetic CJD. The molecular weight (MW) of the PK resistant bands varied between the samples and was in the range 6–98 kD (Fig. 1A). The genetic CJD sample (E) showed weak PK resistant bands. After stripping the membrane and probing it with SAF61-HRP, the result showed PK resistant bands in 1/1 vCJD, 5/5 disease control, 8/8 sCJD and one genetic CJD (Fig. 2A). The MWs of the respective immunobloted bands were between 10–98 kD. All samples showed a band at 35–37 kD. Eleven out of fifteen samples showed a band at 22 kD and 6/15 at 28 kD. One of the samples gave a smear sample (O), with the amount of protein reflecting the patient's neuropathy and/or renal dysfunction. This membrane was stripped and subsequently probed with anti-N-terminal PrP antibody SAF32-HRP. The analysis showed for one sCJD sample a PK resistant band at 22 and 36 kD (Fig. 2B).
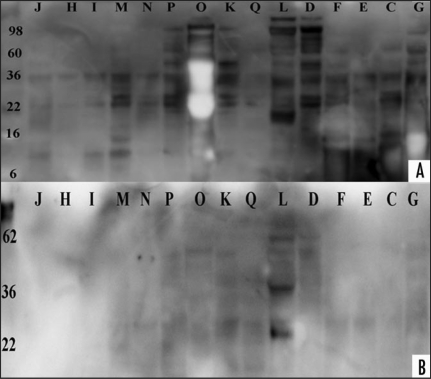
We examined the effect of another anti-C-terminal antibody, 3F4-HRP, on the PK treated urine samples. Western blot analysis of samples showed for 8/11 samples 35–37 kD, and for 6/11 samples >50 kD bands (Fig. 3A). Seven of the eleven tested samples were sCJD and four were disease controls (Fig. 3A). PK treated healthy control samples, probed with 3F4-HRP showed no PK resistant bands (Fig. 3B).
Figure 3.
Western blot analysis of urines from CJD, disease and healthy controls. PK treatment of patient and healthy controls and western blot analysis with 3F4-HRP antibody. (A) Proteinase K (concentration 40 µg/ml for 20 min) treatment of CJD and disease control samples. Samples ID letters correspond to Table 1. Proteins were transferred with Iblot apparatus and the membrane probed with 3F4-HRP followed with ECL Plus addition. Membrane documentation was with Kodak Imager with 5 minutes exposure time. (B) Proteolytic digestion of six healthy control urines in presence (+) or absence (−) of proteinase K (concentration 40 µg/ml for 20 minutes). Healthy control samples are shown with numbers 1–6 and are from different gender and age. Protein transfer was with the Iblot apparatus and the membrane probed with 3F4-HRP, followed with ECL Plus addition. Documentation was as described above.
To examine whether the PK resistant bands observed by anti-IgG or anti-PrP antibody probing varies under altered proteolytic conditions, the PK concentration and incubation time was increased. Increasing the PK concentration to 60 µg/ml, the incubation time to 30 min and subsequent western blot analysis using anti-IgG-HRP showed PK resistant bands for 1/1 vCJD, 3/8 sCJD, 0/1 genetic CJD and 1/3 disease control patients (Fig. 1B). The molecular weights of the observed bands were 37 kD, 28 kD and smaller. One disease control sample mentioned earlier showed a smear on the western blot (Fig. 1B).
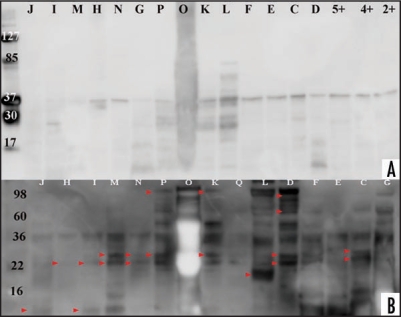
Western blot analysis of samples treated with PK (60 µg/ml for 30 min) and immunobloted with SAF61-HRP showed for 13/16 samples including 3 healthy controls a PK resistant band of 37 kD. Except for two samples, the higher molecular weight bands disappeared and 7/16 samples showed bands smaller than 37 kD (Fig. 4A). As 13 of the tested samples including healthy controls revealed bands at 37 kD, it was interpreted as nonspecific binding of probing antibody with PK protein.
Figure 4.
Western blot analysis of CJD, disease and healthy control urines. Proteinase K treatment of enriched urine samples and comparison between anti-IgG-HRP and anti-PrP-HRP probed membranes. Samples ID correspond to Table 1. Healthy control samples are shown with numbers (Proteinase K treated healthy control samples are designated with +). (A) Proteolytic treatment of urine samples from CJD, disease control and healthy control with proteinase K (concentration 60 µg/ml for 30 min). Protein transferred with Bio-Rad instrument and membrane probed with SAF61-HRP followed with ECL Plus addition. Membrane documentation was with Kodak Imager with 5 minutes exposure time. (B) This figure is a display of Figure 2A, highlighting majority of additional bands detected only with anti-PrP antibody, shown with arrows when the figure is compared to Figure 1A. The arrows point to bands detected only when SAF61-HRP was applied, the rest of the bands were detected with anti-IgG-HRP.
As the majority of visualized bands observed on the blot probed with anti-IgG co-localized with bands on the anti-PrP probed membrane, the possibility of interaction between immunoglobulin proteins and PrP was considered. To examine any possible interaction, three CJD and one vCJD urine sample were treated with PNGase F, followed with PK digestion and acetic saline supplementation. After gel electrophoresis and immunobloting with anti-PrP antibodies, the higher MW bands disappeared (Fig. 6). Sample F showed one high MW band and sample C showed a diffuse 18 kD band. The remaining samples did not show distinct high MW bands (Fig. 6A). After stripping the membrane and probing it with an anti-IgG antibody, the observed single high MW band of sample F was faint, and the 18 kD band of sample C appeared as a smear (Fig. 6B).
To examine possible interaction between prion protein and immunoglobulin proteins, and to exclude possible artefacts resulting from the concentration process, two sCJD, one vCJD and one control enriched urine samples were examined by Protein-A column chromatography. Eluates and flow throughs were collected and subsequently enriched to concentrate the collected material. They were treated with PK (60 µg/ml, 30 min) followed by electrophoresis and western blot analysis using 3F4-HRP or anti-IgG-HRP. The analysis showed for all four samples, a faint or prominent 37 kD band (Fig. 7A–D). Eluates and flow-throughs of one sCJD and vCJD sample showed three prominent bands of 86 kD and higher on membranes immunobloted with either 3F4-HRP or anti-IgG-HRP (Fig. 7A and B, samples F and G). Sample D (sporadic CJD) showed faint bands of 86 kD and higher.
Immunobloting with anti-IgG-HRP of eluates of two sCJD and one vCJD samples, showed bands by 38 and 86 kD, between 38 and 86 kD and higher than 86 kD (Fig. 7B). The flow-throughs of samples F (sporadic CJD) and G (vCJD) showed three bands of 86 kD and higher on the membrane probed with 3F4-HRP (Fig. 7B). These higher molecular weight bands did not appear in control urine samples examined under the same conditions (Fig. 7C and D).
Control experiment with OMP.
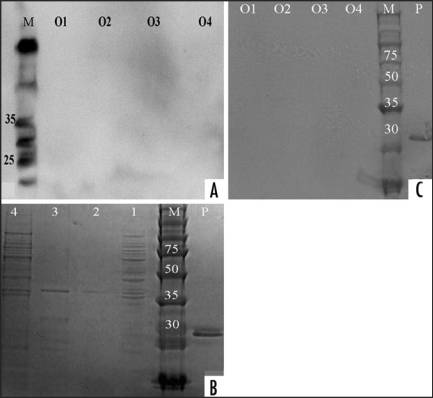
The PK digested extract and the total membrane protein extract from Klebsiella pneumonia were electrophoresed, transferred via Iblot and probed with 3F4-HRP and SAF61-HRP. There were no bands before or after PK digestion on the western blot (Fig. 5A and C). Analysis of the western blot using SAF32-HRP did not show any reaction with OMPs (data not shown). Commasie blue staining of the OMP samples showed a 35–40 kD PK resistant band (Fig. 5B).
Figure 5.
Analysis of Kleibsiella pneumonia with two antibodies. The over night culture of Kleibsiella pneumonia was used for the extraction of outer membrane protein (OMP) and whole membrane proteins. OMPs were digested in the presence or absence of proteinase K (concentration 40 µg/ml for 20 minutes) and the membrane was probed with 3F4-HRP or SAF61-HRP. Samples ID: O4 = Total extract of bacteria − PK, O3 = Total extract of bacteria + PK, O2 = Total membrane proteins + PK, O1 = All membrane proteins − PK, p = Recombinant PrP, M = Marker. 4A: Membrane was probed with SAF61-HRP followed with ECL Plus addition. Membrane documentation was with Kodak Imager with 20 minutes exposure time. 4C: Membrane was probed with 3F4-HRP. Membrane documentation was with scanner, after it was incubated for 20 minutes in Opti-4CN (Bio-Rad) solution and rinsed subsequently in H2O. 4B: Gel staining of the samples with Ez-Blue dye. Samples ID: 4 = Total extract of bacteria − PK, 3 = Total extract of bacteria + PK, 2 = Total membrane proteins + PK, 1 = Whole membrane proteins − PK, p = Recombinant PrP, M = Marker.
Discussion
In the present study we have tried to address the question of whether the urine of prion disease affected individuals contains PK resistant PrP. We examined enriched urines from CJD patients, one vCJD patient under PPS-treatment, disease control patients and healthy individuals for the existence of PK resistant PrP. To overcome the obstacle of the interaction of aggregated immunoglobulins with the secondary antibodies, as described elsewhere,47 anti-PrP-antibodies were labeled directly with a HRP-conjugate. Additionally we combined an immunobloting system with a selective concentration method.
We found PK-resistant proteins were frequently detected in the urine of patients affected with prion disease and other neurodegenerative diseases. The PK resistant bands were detected in western blots using monoclonal anti-PrP-HRP and anti-IgG-HRP antibodies. Probing with SAF61-HRP antibody showed several high MW bands (Fig. 2A), which co-localized with PK resistant bands on membranes analyzed with anti-IgG-HRP, with additional bands detected only with SAF61-HRP antibody.
The range of bands varied from sample to sample, and the molecular weights were different from those reported by Furukawa et al.5 The 35–37 kD bands appeared in the majority of samples, which we believe to represent nonspecific interaction of the probing antibody with PK resistant protein. In addition, some samples showed 22–28 kD bands and further bands between 10–98 kD. Membranes analyzed with another anti-C-terminal-PrP antibody, 3F4-HRP showed PK resistant bands of 55–60 kD.
Increasing the PK concentration and incubation time affected the number of samples showing PK resistant bands i.e., for majority of them the high MW bands disappeared when probed with SAF61-HRP. It appears that increasing the PK concentration and incubation time leads to stronger proteolytic digestion of high MW proteins in the urine samples. The 37 kD band appearing in the majority of urines including healthy controls, could be interpreted as non-specific interaction of antibody with PK as mentioned before. Yuan and colleagues reported that silent PK resistant PrP was found in the brain homogenate of healthy individuals,55 with a MW of 30 kD and lower. Although the 37 kD bands appearing in several samples including the healthy controls, are larger than the PK resistant bands reported by Yuan et al. there is a possibility that the observed 37 kD band is related to silent PrP, as it was detected in healthy controls.
Western blot analyses results with OMPs were consistent with those of Furukawa et al.5 and we confirm that OMPs from K. pneumoniaea are resistant to PK, resulting in a 37 kD band by SDS-PAGE. Western blot analysis of OMPs using monoclonal antibodies 3F4-HRP and SAF61-HRP did not show any reactive bands.
As the interaction of the anti-PrP antibodies with OMP was negative, it is unlikely that these antibodies detected PK resistant OMP on membranes containing urine samples i.e., the non-specific interaction of these antibodies with PK resistant OMPs can be excluded. In addition, PK resistant OMP has a molecular weight of 37 kD, which does not correspond to the detected high MW in the western blots.
Tsukui and colleagues reported high MW bands, reactive with anti-PrP antibodies, in the PK digested blood of scrapie infected hamster which disappear after PNGase F and acetic saline treatment. They also mention the involvement of carbohydrate side chains in PrPres-plasma proteins aggregation and the formation of multiple MW PrPres-like proteins with other plasma proteins.54
In agreement with Tsukui and colleagues, we observed the disappearance of higher MW bands in four of tested enriched urines after elimination of carbohydrate side chains following their protocol54 (except for one high MW band in one tested sample, which we cannot explain). The fact that the MW of the reactive bands were found in a variable range, different from previously reported 27–30 MW for PrP, can be explained by the linkage of PrP to other proteins.
In addition, western blot analysis of PK treated enriched urines with anti-IgG and anti-C-terminal-PrP antibodies showed a co-migration of reactive bands on the membranes, but failed to show them after treatment with PNGase F, PK and acetic saline treatment which eliminated carbohydrate chains. A likely explanation for this result is that carbohydrate side chains linked the PrPres proteins to immunoglobulin proteins in urine.
The fact that the distinct 18 kD bands reported by Tsukui et al. were not observed in this study could be explained by experimental differences. Tsukui and colleagues initially performed PK digestion, followed with PNGase F treatment. Thus, they digested the PK accessible peptides first, followed with elimination of carbohydrate side chains, releasing the non-digested peptides that were then detected by the anti-PrP antibody. In our study, in contrast samples were treated initially with PNGase F to eliminate the carbohydrate side chains, followed with PK digestion and acetic SDS treatment.
The comparison of blots analyzed with anti-IgG-HRP and anti-C-terminal-PrP-HRP revealed equivalent PK resistant bands, with additional bands detected only with anti-PrP antibodies (Fig. 4B). The question of whether immunoglobulin proteins interact to PrP, resulting in co-localization of bands on western blots analyzed with anti-PrP and anti-IgG antibodies, was addressed.
The attempt to isolate immunoglobulin proteins which we suggest to interact with PrP, by Protein-A column chromatography resulted in the detection of PK resistant bands from eluates of samples F and G (Sporadic and variant CJD samples) which partially co-localize with bands on membranes probed with 3F4-HRP and anti-IgG-HRP (Fig. 7A and B), although the bands appear fainter on the 3F4-HRP probed membrane. This supports our suggestion of interaction between PrP and immunoglobulin proteins, but finding co-localized PK resistant bands in the flow-throughs (Fig. 7A and B, samples F* and G*), was unexpected. This could be interpreted as the unbound immunoglobulin proteins exceeding the binding capacity of the Protein-A column and passing through the column. As creatinine concentration measurement was used to adjust the volumes of used urines, this might have not represented the concentration of proteins which were detected by western blots.
The possibility however of PrP linked to other not immunoglobulin related proteins remains and this would not support an interaction between PrP and immunoglobulin proteins. If these proteins are not immunoglobulin protein, we could not then explain why an interaction with anti-IgG-HRP occurs, unless immunoglobulin proteins are one of the compounds linked to PrP?
The 3F4 preparation which was used was purified IgG of mouse monoclonal antibody. Although majority of the product is specific anti-PrP monoclonal antibody, a minor percentage of other immunoglobulin proteins produced by hybridoma cells which were purified alongside 3F4 would be in the sample. During labeling process these would be labeled with HRP, alongside 3F4 antibody. As a result they might generate to a minor extent, nonspecific binding to proteins other than PrP during immunobloting of the urine samples, which could be the reason for the detection of PK resistant band in eluates and flow throughs.
Our initial goal was to explore PK resistant PrP in the urine of prion disease patients; however this study raised other questions with the scope for further investigation. What is the role of Immunoglobulin proteins' interaction with PrP? Is there a link between these two types of proteins in the urine samples.
In conclusion, our results were mostly consistent with previous reports for detection of PK resistant PrP in urine. Hence PK resistant bands were not detected in all CJD samples; at this stage we can not confirm the suitability of urine for ante-mortem diagnostic tests. We found PK resistant bands in several sCJD samples and in urine from patients with other neurodegenerative disorders. We suggest a possible interaction between PrP and immunoglobulin proteins in the urine forming a PK resistant complex, but a mechanism for this cannot be obtained from this study. Although the typical 27–30 kD PK resistant PrP does not correspond to the high molecular weight bands detected with anti-PrP antibodies in our study, similar high molecular weight bands have been reported else where, when probing the blood of Scrapie infected hamsters with anti-PrP antibodies.54 In addition the fact that two anti-C-terminal-PrP antibodies targeting different epitopes of C-terminus-PrP detected PK resistant protein in urine and the anti-PrP-N-terminal antibody did not, minimizes the possibility of nonspecific binding. We conclude that further investigation of immunoglobulin interaction with PrP in prion disease affected individuals is necessary.
Material and Methods
Urine samples.
After agreement with the German TSE-platform and the Department of Neurology, University of Goettingen, Germany, urine samples were collected under informed consent from patients with prion disease (Table 1). A clinical diagnosis of prion disease was made by neurologists following the diagnostic criteria proposed by the WHO.48,49 The individuals were diagnosed with probable sCJD, possible sCJD, genetic CJD or other neurodegenerative disorders. Samples were dispatched on dry ice and stored at −80°C prior to use. Additional CJD urine samples were obtained from the University of Verona, Italy. Control urines were collected from healthy UK resident donors from both genders (age range 24–56). None of the donors suffered from kidney or bladder disease. Urine was collected from a male vCJD patient, being treated with pentosanepolysulphate (PPS) after informed consent was obtained from the next of kin.
Reagents.
Monoclonal antibodies SAF32 (octapeptide repeat region at the N-terminal part of PrP)50 and SAF61 (142–160 of PrP)51 were purchased from Spi-Bio (Massy, France). 3F4 (109–112 of PrP)52 was from Dako (Cambridgshire, UK). Recombinant human 23–231 PrP was from Abcam (Cambridge, UK). Revalationbio (Bournemouth, UK) conjugated SAF32, 3F4 and SAF61 monoclonal antibodies with horse radish peroxidase (HRP).
Proteinase K (PK), 12% Bis-Tris Nu-precast gels, electrophoresis instruments, running buffer, Iblot western blot instrument with accessories, and protein markers were from Invitrogen (Paisley, UK). Amicon ultra-4 centrifugal filter tubes were from Millipore (Watford, UK). Pefa-block was from Roche (Brighton, UK), Starting Block, stripping buffer, immobilized Protein-A column, elution buffer and binding buffer were from Pierce (Cramlington, UK). ECL Plus was from Amersham (Amersham, UK), Opti-4CN colorimetric detection kit, Laemmli buffer, PBS, western blot instrument and Criterion 12% Bis-Tris gels were from Bio-Rad (Hemel Hempstead, UK). Anti-IgG-conjugated with HRP, phosphate buffered saline (PBS), Tris buffered saline (TBS), EZ-Blue, Tween-20, SDS, NP40, EDTA were from Sigma (Poole, UK).
Preparation of urine samples, PK digestion and western blot analysis.
Human urine specimens were enriched for proteins bigger than 10 kD in a pre-cooled Eppendorf bench centrifuge (Sunderland, UK) for 20 minutes (min) at 4°C and 4,000 rpm, using Amicon tubes with filtration cut-off for 10 kD proteins. Urinary creatinine concentrations were determined using a creatinine assay kit (IDS, Boldon, UK).
Enriched urine samples were digested with PK (concentrations 40 or 60 µg/ml) at 37°C for 20 or 30 min and the reaction was stopped by pefa-block addition. Subsequently Laemmli buffer was added and samples were heated for 10 min at 100°C prior to loading on 12% Bis-Tris gels for electrophoresis. Proteins were transferred to nitrocellulose membrane using the Iblot (Invitrogen) or Bio-Rad instruments. Western blot analysis was performed after blocking of membranes with PBST (PBS + 0.1% Tween-20) containing 5% BSA for 1 hour at RT followed with Starting Block for 1 hour at RT and subsequently incubated for one hour with the HRP-conjugated 3F4 (1:5,000) or SAF61 (1:1,500) or SAF32 (1:2,000) or anti-IgG (1:2,000) diluted in PBST. Membranes were washed vigorously in PBST before developing for 5 min at RT with ECL Plus. Subsequently membranes were documented using a chemiluminescence imager instrument Kodak 4000R from Carestreamhealth (London, UK).
Measuring creatinine in urine.
The creatinine content of all enriched urines was measured using a creatinine assay kit from IDS (Newcastle Upon Tyne, UK). For subsequent PK digestion, the final volumes of the urines were adjusted by dilution with H2O for the concentration of the measured creatinine.
Stripping of western blot membranes.
Some of the membranes were stripped with 40 ml of stripping buffer (‘Restore western Blot Stripping buffer’ from Pierce) for 30 min at 37°C in a dark chamber. Membranes were subsequently supplemented with ECL Plus (see above) to verify if the detecting antibody has been removed fully and were afterwards rinsed thoroughly with 50 ml of PBST, before exposing to another antibody. If membrane was not used immediately, it was stored in PBS at 4°C for further use.
Preparation of OMPs.
OMPs were extracted and isolated from Klebsiella pneumoniae, a gift of Dr. Doumith. The protocol used has been described previously.53
Total bacterial extract was isolated from an over night culture of Klebsiella pneumoniae by centrifugation of the culture at 3,000 rpm, followed with 3 PBS washes and cell breakage via water addition and heating at 100°C for 5 min. Subsequently, Laemmli buffer containing reducing agents was added and heated at 100°C for 10 min prior to gel electrophoresis. Proteolytic digestion was with 40 µg/ml of PK for 20 min at 37°C. The reaction was stopped by addition of pefa-block and Laemmli buffer, heated for 10 min at 100°C, and subsequently treated as described above for western blot analysis.
Affinity measurement of europium labelled antibodies.
Calibration measurements were performed for all three detecting antibodies using recombinant human PrP. Microtitre plates were precoated overnight with FH11 capture antibody at 4°C. Subsequently, the coated plates were washed with DELFIA wash buffer and blocked prior to use with PBS containing 2% BSA with shaking at 900 rpm, for one hour at 22°C. They were then re-washed. After loading PrP, plates were sealed with plastic film using a plate-sealer (Scientific Laboratory Supplies, Nottingham, UK) and incubated on a plate-shaker (Eppendorf, Sunderland, UK), run at 900 rpm and 19°C, and then rewashed. Europium-labelled detector antibodies (3F4 or SAF32 or SAF61) were diluted in assay buffer (0.07 µg/ml final concentration) mixed and filtered through a 0.2 µM filter (Scientific Laboratory Supplies) before adding to the plate. The plate was further incubated on the shaker at 900 rpm and 19°C, and subsequently washed. To remove excess reagent the plates were inverted and tapped on absorbent paper after each wash cycle. To dissociate the Eu3+ from detector antibody, 200 µL enhancement solution (Perkin-Elmer) was added, and the plate incubated for an additional 5 min at 900 rpm, 19°C, and then read with a Victor fluorometer.
Enzyme treatment with PNGase F and acidic SDS precipitation.
Enriched urine samples were treated overnight with PNGase F to remove the carbohydrate side chains on the proteins, followed with 20 min PK digestion (40 µg/ml). The reaction was stopped with PMSF addition and mixed with equal volume of cold acetic saline as described by Tsukui and colleagues54 and centrifuged subsequently for 10 minutes at 13,000 rpm. The pellet was resuspended in TBS containing 5 mM EDTA. The addition of acetic saline step was repeated and rinsed subsequently in ice-cold methanol. The resulting pellet was resuspended in Laemmli buffer.
Protein-A column chromatography of urine.
Enriched CJD and healthy control urines were applied to an immobilized protein-A column. 500 µl of enriched urine was mixed with 500 µl of binding buffer and loaded on the protein-A column. The flow through was collected and the column was washed with 15 ml of binding buffer. This was collected and pooled with the flow through. Bound protein was recovered from the column with 5 ml elution buffer.
All collected fluids were subsequently enriched, concentrating the solution to approximately 3% of original volumes. The creatinine concentration was measured, adjusting the concentration of urine equally before digesting the samples with PK (60 µg/ml for 30 minutes).
Acknowledgements
We would like to express our gratitude to German TSE platform, for providing urine samples, special thanks to the vCJD patient's relatives for kind support and Dr. Michel Doumith for the help with all experiments involving Klebsiella pneumoniae.
Abbreviations
- PrPC
cellular prion protein
- PrPTES/res
abnormal form of prion protein
- CJD
Creutzfeldt-Jakob disease
- PK
proteinase K
- HRP
horse radish peroxidase
- OMP
outer membrane protein
- MW
molecular weight
Footnotes
Previously published online as a Prion E-publication: http://www.landesbioscience.com/journals/prion/article/8068
References
- 1.Prusiner SB. The prion diseases. Brain Pathol. 1998;8:499–513. doi: 10.1111/j.1750-3639.1998.tb00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collinge J. Molecular neurology of prion disease. J Neurol Neurosurg Psychiatry. 2005;76:906–919. doi: 10.1136/jnnp.2004.048660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishida C, Okino S, Kitamoto T, Yamada M. Involvement of the peripheral nervous system in human prion diseases including dural graft associated Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 2005;76:325–329. doi: 10.1136/jnnp.2003.035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregori L, Kovacs GG, Alexeeva I, Budka H, Rohwer RG. Excretion of transmissible spongiform encephalopathy infectivity in urine. Emerg Infect Dis. 2008;14:1406–1412. doi: 10.3201/eid1409.080259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narang HK, Dagdanova A, Xie Z, Yang Q, Chen SG. Sensitive detection of prion protein in human urine. Exp Biol Med (Maywood) 2005;230:343–349. doi: 10.1177/153537020523000508. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Romero D, Barria MA, Leon P, Morales R, Soto C. Detection of infectious prions in urine. FEBS Lett. 2008;582:3161–3166. doi: 10.1016/j.febslet.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrievskaia O, Algire J, Balachandran A, Nielsen K. Prion protein in sheep urine. J Vet Diagn Invest. 2008;20:141–146. doi: 10.1177/104063870802000201. [DOI] [PubMed] [Google Scholar]
- 8.Tatzelt J, Winklhofer KF. Folding and misfolding of the prion protein in the secretory pathway. Amyloid. 2004;11:162–172. doi: 10.1080/1350-6120400000723. [DOI] [PubMed] [Google Scholar]
- 9.Halliday S, Houston F, Hunter N. Expression of PrPC on cellular components of sheep blood. J Gen Virol. 2005;86:1571–1579. doi: 10.1099/vir.0.80561-0. [DOI] [PubMed] [Google Scholar]
- 10.Brandel JP, Preece M, Brown P, Croes E, Laplanche JL, Agid Y, et al. Distribution of codon 129 genotype in human growth hormone-treated CJD patients in France and the UK. Lancet. 2003;362:128–130. doi: 10.1016/S0140-6736(03)13867-6. [DOI] [PubMed] [Google Scholar]
- 11.Wientjens DP, Rikken B, Wit JM, Hofman A, Stricker BH. A nationwide cohort study on Creutzfeldt-Jakob disease among human growth hormone recipients. Neuroepidemiology. 2000;19:201–205. doi: 10.1159/000026256. [DOI] [PubMed] [Google Scholar]
- 12.Lemmer K, Mielke M, Pauli G, Beekes M. Decontamination of surgical instruments from prion proteins: in vitro studies on the detachment, destabilization and degradation of PrPSc bound to steel surfaces. J Gen Virol. 2004;85:3805–3816. doi: 10.1099/vir.0.80346-0. [DOI] [PubMed] [Google Scholar]
- 13.Blattler T. Implications of prion diseases for neurosurgery. Neurosurg Rev. 2002;25:195–203. doi: 10.1007/s101430100170. [DOI] [PubMed] [Google Scholar]
- 14.Peden AH, Ritchie DL, Ironside JW. Risks of transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Folia Neuropathol. 2005;43:271–278. [PubMed] [Google Scholar]
- 15.Sneath PH. Estimation of the size of the vCJD epidemic. Antonie Van Leeuwenhoek. 2004;86:93–103. doi: 10.1023/B:ANTO.0000036145.53052.3b. [DOI] [PubMed] [Google Scholar]
- 16.Glatzel M, Ott PM, Linder T, Gebbers JO, Gmur A, Wust W, et al. Human prion diseases: epidemiology and integrated risk assessment. Lancet Neurol. 2003;2:757–763. doi: 10.1016/s1474-4422(03)00588-x. [DOI] [PubMed] [Google Scholar]
- 17.Spinney L. vCJD epidemic could be first of many, experts warn. Nat Med. 2003;9:1096. doi: 10.1038/nm0903-1096a. [DOI] [PubMed] [Google Scholar]
- 18.Trevitt CR, Singh PN. Variant Creutzfeldt-Jakob disease: pathology, epidemiology and public health implications. Am J Clin Nutr. 2003;78:651–656. doi: 10.1093/ajcn/78.3.651S. [DOI] [PubMed] [Google Scholar]
- 19.Ramasamy I, Law M, Collins S, Brooke F. Variant Creutzfeldt-Jakob disease and the potential for its accidental transmission following surgery with contaminated instruments: the risk of transmission in Australia. Folia Neuropathol. 2003;41:1–10. [PubMed] [Google Scholar]
- 20.Pedersen NS, Smith E. Prion diseases: epidemiology in man. Apmis. 2002;110:14–22. doi: 10.1034/j.1600-0463.2002.100103.x. [DOI] [PubMed] [Google Scholar]
- 21.Barnard G, Helmick B, Madden S, Gilbourne C, Patel R. The measurement of prion protein in bovine brain tissue using differential extraction and DELFIA as a diagnostic test for BSE. Luminescence. 2000;15:357–362. doi: 10.1002/1522-7243(200011/12)15:6<357::AID-BIO621>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 22.Bellon A, Seyfert-Brandt W, Lang W, Baron H, Groner A, Vey M. Improved conformation-dependent immunoassay: suitability for human prion detection with enhanced sensitivity. Journal of General Virology. 2003;84:1921–1925. doi: 10.1099/vir.0.18996-0. [DOI] [PubMed] [Google Scholar]
- 23.Bieschke J, Giese A, Schulz-Schaeffer W, Zerr I, Poser S, Eigen M, Kretzschmar H. Ultrasensitive detection of pathological prion protein aggregates by dual-color scanning for intensely fluorescent targets. Proc Natl Acad Sci USA. 2000;97:5468–5473. doi: 10.1073/pnas.97.10.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biffiger K, Zwald D, Kaufmann L, Briner A, Nayki I, Purro M, et al. Validation of a luminescence immunoassay for the detection of PrP(Sc) in brain homogenate. J Virol Methods. 2002;101:79–84. doi: 10.1016/s0166-0934(01)00421-9. [DOI] [PubMed] [Google Scholar]
- 25.Jackman R, Schmerr MJ. Analysis of the performance of antibody capture methods using fluorescent peptides with capillary zone electrophoresis with laser-induced fluorescence. Electrophoresis. 2003;24:892–896. doi: 10.1002/elps.200390112. [DOI] [PubMed] [Google Scholar]
- 26.Kenney K, Brechtel C, Takahashi H, Kurohara K, Anderson P, Gibbs CJ., Jr An enzyme-linked immunosorbent assay to quantify 14-3-3 proteins in the cerebrospinal fluid of suspected Creutzfeldt-Jakob disease patients. Ann Neurol. 2000;48:395–398. [PubMed] [Google Scholar]
- 27.Klohn PC, Stoltze L, Flechsig E, Enari M, Weissmann C. A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc Natl Acad Sci USA. 2003;100:11666–11671. doi: 10.1073/pnas.1834432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacGregor I, Hope J, Barnard G, Kirby L, Drummond O, Pepper D, et al. Application of a time-resolved fluoroimmunoassay for the analysis of normal prion protein in human blood and its components. Vox Sanguinis. 1999;77:88–96. doi: 10.1159/000031082. [DOI] [PubMed] [Google Scholar]
- 29.Oesch B, Doherr M, Heim D, Fischer K, Egli S, Bolliger S, et al. Application of Prionics western blotting procedure to screen for BSE in cattle regularly slaughtered at Swiss abattoirs. Arch Virol Suppl. 2000:189–195. doi: 10.1007/978-3-7091-6308-5_18. [DOI] [PubMed] [Google Scholar]
- 30.Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 31.Safar JG, Scott M, Monaghan J, Deering C, Didorenko S, Vergara J, et al. Measuring prions causing bovine spongiform encephalopathy or chronic wasting disease by immunoassays and transgenic mice. Nat Biotechnol. 2002;20:1147–1150. doi: 10.1038/nbt748. [DOI] [PubMed] [Google Scholar]
- 32.Sayers R. Chemiluminescent ELISA of prion proteins. In: Nunally B, Krull IS, editors. Prions and Mad Cow Disease. 2003. [Google Scholar]
- 33.Schaller O, Fatzer R, Stack M, Clark J, Cooley W, Biffiger K, et al. Validation of a western immunoblotting procedure for bovine PrP(Sc) detection and its use as a rapid surveillance method for the diagnosis of bovine spongiform encephalopathy (BSE) Acta Neuropathol (Berl) 1999;98:437–443. doi: 10.1007/s004010051106. [DOI] [PubMed] [Google Scholar]
- 34.Schmerr MJ, Jenny AL, Bulgin MS, Miller JM, Hamir AN, Cutlip RC, Goodwin KR. Use of capillary electrophoresis and fluorescent labeled peptides to detect the abnormal prion protein in the blood of animals that are infected with a transmissible spongiform encephalopathy. Journal of Chromatography A. 1999;853:207–214. doi: 10.1016/s0021-9673(99)00514-2. [DOI] [PubMed] [Google Scholar]
- 35.Zerr I, Bodemer M, Otto M, Poser S, Windl O, Kretzschmar HA, et al. Diagnosis of Creutzfeldt-Jakob disease by two-dimensional gel electrophoresis of cerebrospinal fluid. Lancet. 1996;348:846–849. doi: 10.1016/S0140-6736(96)08077-4. [DOI] [PubMed] [Google Scholar]
- 36.Barnard G, Sy M. The diagnosis of transmissible spongiform encephalopathies using differential extraction and DELFIA. In: Krull I, editor. Prions and Mad Cow Disease. New York: Marcel Dekker; 2003. pp. 277–315. [Google Scholar]
- 37.Grassi J. Pre-clinical diagnosis of transmissible spongiform encephalopathies using rapid tests. Transfus Clin Biol. 2003;10:19–22. doi: 10.1016/s1246-7820(02)00279-3. [DOI] [PubMed] [Google Scholar]
- 38.Meyer RK, Oesch B, Fatzer R, Zurbriggen A, Vandevelde M. Detection of bovine spongiform encephalopathy-specific PrP(Sc) by treatment with heat and guanidine thiocyanate. J Virol. 1999;73:9386–9392. doi: 10.1128/jvi.73.11.9386-9392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Safar JG, Geschwind MD, Deering C, Didorenko S, Sattavat M, Sanchez H, et al. Diagnosis of human prion disease. Proc Natl Acad Sci USA. 2005;102:3501–3506. doi: 10.1073/pnas.0409651102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JI, Wang C, Kuizon S, Xu J, Barengolts D, Gray PC, Rubenstein R. Simple and specific detection of abnormal prion protein by a magnetic bead-based immunoassay coupled with laser-induced fluorescence spectrofluorometry. J Neuroimmunol. 2005;158:112–119. doi: 10.1016/j.jneuroim.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Frosh A, Smith LC, Jackson CJ, Linehan JM, Brandner S, Wadsworth JD, Collinge J. Analysis of 2000 consecutive UK tonsillectomy specimens for disease-related prion protein. Lancet. 2004;364:1260–1262. doi: 10.1016/S0140-6736(04)17143-2. [DOI] [PubMed] [Google Scholar]
- 42.Glatzel M. Testing for prions: a novel protocol for vCJD prevalence studies. Lancet. 2004;364:1196–1197. doi: 10.1016/S0140-6736(04)17155-9. [DOI] [PubMed] [Google Scholar]
- 43.Hilton DA, Ghani AC, Conyers L, Edwards P, McCardle L, Ritchie D, et al. Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J Pathol. 2004;203:733–739. doi: 10.1002/path.1580. [DOI] [PubMed] [Google Scholar]
- 44.Furukawa H, Doh-ura K, Okuwaki R, Shirabe S, Yamamoto K, Udono H, et al. A pitfall in diagnosis of human prion diseases using detection of protease-resistant prion protein in urine. Contamination with bacterial outer membrane proteins. J Biol Chem. 2004;279:23661–23667. doi: 10.1074/jbc.M400187200. [DOI] [PubMed] [Google Scholar]
- 45.Shiga Y, Miyazawa K, Takeda A, Arai H, Doh-ura K, Itoyama Y. Laboratory and imaging studies for the diagnosis of prion disease. Rinsho Shinkeigaku. 2003;43:810–812. [PubMed] [Google Scholar]
- 46.Kariv-Inbal Z, Halimi M, Dayan Y, Engelstein R, Gabizon R. Characterization of light chain immunoglobulin in urine from animals and humans infected with prion diseases. J Neuroimmunol. 2005;162:12–18. doi: 10.1016/j.jneuroim.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Serban A, Legname G, Hansen K, Kovaleva N, Prusiner SB. Immunoglobulins in urine of hamsters with scrapie. J Biol Chem. 2004;279:48817–48820. doi: 10.1074/jbc.M409107200. [DOI] [PubMed] [Google Scholar]
- 48.Will RG, Zeidler M, Stewart GE, Macleod MA, Ironside JW, Cousens SN, et al. Diagnosis of new variant Creutzfeldt-Jakob disease. Ann Neurol. 2000;47:575–582. [PubMed] [Google Scholar]
- 49.Ingrosso L, Vetrugno V, Cardone F, Pocchiari M. Molecular diagnostics of transmissible spongiform encephalopathies. Trends Mol Med. 2002;8:273–280. doi: 10.1016/s1471-4914(02)02358-4. [DOI] [PubMed] [Google Scholar]
- 50.Vincent B, Paitel E, Frobert Y, Lehmann S, Grassi J, Checler F. Phorbol ester-regulated cleavage of normal prion protein in HEK293 human cells and murine neurons. J Biol Chem. 2000;275:35612–35616. doi: 10.1074/jbc.M004628200. [DOI] [PubMed] [Google Scholar]
- 51.Mouillet-Richard S, Ermonval M, Chebassier C, Laplanche JL, Lehmann S, Launay JM, Kellermann O. Signal transduction through prion protein. Science. 2000;289:1925–1928. doi: 10.1126/science.289.5486.1925. [DOI] [PubMed] [Google Scholar]
- 52.Kascsak RJ, Rubenstein R, Merz PA, Tonna-DeMasi M, Fersko R, Carp RI, et al. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987;61:3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.George M, Carlone MLT, Rumschlag Hella S, Sottnek Frances O. Rapid Microprocedure for Isolating Detergent-Insoluble Outer Membrane Proteins from Haemophilus Species. Journal of Clinical Microbiology. 1986;24:330–332. doi: 10.1128/jcm.24.3.330-332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsukui K, Takata M, Tadokoro K. A potential blood test for transmissible spongiform encephalopathies by detecting carbohydrate-dependent aggregates of PrPres-like proteins in scrapie-Infected hamster plasma. Microbiol Immunol. 2007;51:1221–1231. doi: 10.1111/j.1348-0421.2007.tb04009.x. [DOI] [PubMed] [Google Scholar]
- 55.Yuan J, Xiao X, McGeehan J, Dong Z, Cali I, Fujioka H, et al. Insoluble aggregates and protease-resistant conformers of prion protein in uninfected human brains. J Biol Chem. 2006;281:34848–34858. doi: 10.1074/jbc.M602238200. [DOI] [PubMed] [Google Scholar]