Abstract
Apoptosis or programmed cell death is a physiological mechanism, characterized by specific morphological and biochemical changes such as cell shrinkage, chromatin condensation, protein cleavage, DNA breakdown and phagocytosis. Apoptosis is a significant contributor to the morphologic and functional development of multicellular organisms. It is also involved in the pathogenesis of several diseases including degenerative diseases of the central nervous system (CNS) like Alzheimers disease or Parkinsons disease, cancer and immune system dysfunction. There are many factors, mainly proteins, which are involved in the activation, regulation and execution of related events. A fairly detailed outline of apoptotic mechanisms has also started to emerge and to be verified. In this short, focused mini-review, we attempt to outline current evidence regarding the mechanisms and the regulation of apoptosis.
Keywords: protein, apoptosis
For the last 150 years, cell death is considered as the basis of embryogenesis and metamorphosis just as cell proliferation is associated with growth14,26. The maintenance of every human tissue results from a balance between cell production and cell death which keeps the overall numbers of cells within physiologically appropriate ranges. Cell death can occur in two ways: necrosis and apoptosis.
Necrosis is cell death following either mechanical damage or exposure to toxic chemicals, during which cells undergo a characteristic series of changes: (a) the cells and their organelles swell as the cell membrane looses its ability to control and balance the ionic currents and water flow in-and-out of the cell, and (b) the cell contents leak out, leading to inflammation of the surrounding tissues21. In contrast to necrosis, apoptosis follows a sequence of events which is triggered by specific signals that instruct the cell to undergo cell death. As such, apoptosis is a fundamental eukaryotic biological process whereby individual cells die by activating their own genetically programmed cell death mechanisms.
Apoptosis is of widespread biological significance. It is important for tissue homeostasis in multicellular organisms, because of its role in many physiological processes, including those that characterise the immune system, the nervous system, tissue development and cancer. In the CNS for example, apoptosis is involved in Alzheimers disease, in amyotrophic lateral sclerosis, in Parkinsons disease, in Huntingtons disease and in other debilitating diseases.
Apart from its importance in the development of multicellular organisms and in securing constancy of cell numbers for the different tissues, apoptosis is also involved in the deletion, of damaged and / or dangerous cells. In this way, many categories of cells are eliminated as inappropriate mitogenic signals that conflict with the environmental or cellular status of the cell result in cell cycle arrest and elimination. Moreover cells with severely damaged, non-repairable, DNA are removed while, in the immune system, auto-reactive cells are deleted. Finally, infected cells are eliminated. Therefore, it is not surprising that a dysfunction of the apoptotic mechanisms is implicated in many pathological conditions or that defects in apoptosis are suspected to be responsible for the development of cancer and autoimmune disease or to play a role in the spread of viral infections. On the other hand, current evidence suggests that neurodegenerative disorders, AIDS and ischemic diseases are either caused or enhanced by excessive apoptosis11.
Morphological and biochemical changes during apoptosis
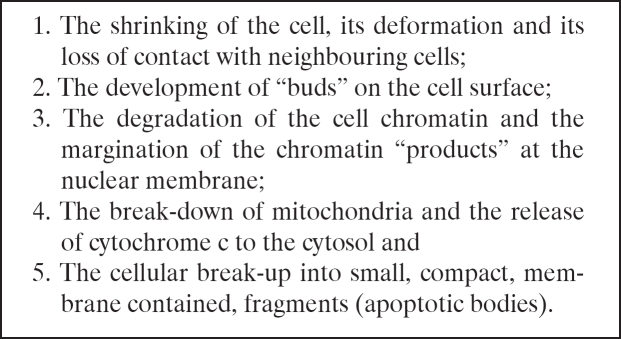
The process of apoptosis is characterized by many morphological and biochemical changes (Table 1) The morphological changes that occur during apoptosis are the result of the activation of proteolytic enzymes which (a) cleave DNA into oligonucleosomal fragments and (b) act upon the multitude of specific protein substrates which determine the integrity and shape of the cytoplasm and the cell organelles35.
Table 1. Changes taking place in the process of apoptosis in orderly sequence.
During these changes the phospholipid phosphatidyl- serine, which is normally hidden within the plasma membrane, is exposed on the surface of the apoptotic bodies. In this way phagocytic cells recognise and engulf the apoptotic bodies. At the same time, the phagocytic cells secrete cytokines (e.g., IL-10 and TGF-β) which inhibit inflammation of neighbouring tissues. In this way, apoptotic bodies are removed from the tissue without causing an inflammatory response.
The apoptosis proteins
There are many factors (mostly proteins) that have been identified to play a vital role in apoptosis. The most important are: the caspases, the amyloid-B peptide, the Bcl-2 family of proteins, the p53 gene and the heat shock proteins.
Caspases
The caspases are a family of aspartyl specific cysteine proteases. The term is an acronym for "cysteine-dependent aspartate-specific proteases". They exist within the cell as inactive pro-forms (pro-caspases) or zymogens. They contain one N-terminal "prodomain", a large subunit and a small subunit and give rise to active enzymes following the induction of apoptosis. The prodomain is usually removed during the process of activation.
Each active caspase is a tetramer composed of two identical big subunits and two identical small subunits. They can contain "death effector domains" (DED) or caspase recruitment domains (CARD). With the help of these domains, the active caspases can bind to other molecules whether inside or outside the cell. For example the death receptors [Fas/ CD95 (Cluster of differentiation), TNFR1 (Tumor necrosis factor receptor), DR3 (Death receptor) / TRAMP (TNFR-related apoptosis-mediated protein) DR4/ TRAIL (Tumor necrosis factor-related apoptosis-inducing ligand) -R1, DR5/TRAIL-R2) are a sub-group of the TNF superfamily that hold a death domain motif into the cytoplasm. Adapter molecules like e.g. FADD and TRADD bind to this death domain motif leading to the recruitment of caspases to the complex.
Caspases can be divided into 2 big subcategories8: (a) those that are involved and, consequently, are activated during apoptosis (caspase-2, -3,-6, -7, -8, -9 and -10) and (b) those which appear to be involved in the processing of pro-inflammatory cytokines during the immune response (caspase-1, -4, -5 and -11). In the case of death-receptor-activation induced apoptosis, the process starts with the activation of caspase 8 or caspase 9. Caspase-9, in turn, activates caspase-3 and caspase -7 almost simultaneously, which then activate other caspases, resulting in a cascade. Caspase-3, in particular, activates both caspase-2 and caspase-6, while caspase-6 activates caspases 8 and 1038 completing, in this way the recruitment of all relevant caspases in the death-receptor-activation induced apoptosis cascade.
Finally, regarding the caspases -1, -4, -5 and -11 and their possible role in the processing of pro-inflammatory cytokines during the immune response, it should be pointed out that mice that express a dominant negative mutant of caspase-1 and caspase-1 deficient mice are protected against ischemia induced brain damage. It is, therefore, postulated that, at least in some cases, suppression of caspase-1 may help prevent apoptosis.
Amyloid-B peptide (Abeta)
Since the early days of apoptosis studies, the fact that during brain development a significant amount of cellculling takes place in the CNS, has led to the hypothesis that apoptosis plays a significant role in the morphogenesis and development of the CNS. For example it is suggested5 that during neurogenesis and maturation one-half or more of the number of neurons born are eliminated by programmed cell death. Furthermore, studies with mutant mice deficient in the pro-apoptotic genes Casp3, Casp9 and Apaf1, all showed severe malformations of the CNS because of a reduction of developmental cell death21.
It is in the apoptotic mechanisms of the CNS in diseases such as Alzheimers disease and Parkinsons disease that apoptotic proteins have been identified and they appear to play an important role. Chief among them is amyloid-B peptide (Abeta), a protein derived from the amyloid-B precursor protein (APP). Abeta and Tau protein are major neuropathological hallmarks of Alzheimer's disease. Abeta plays a central role in the pathogenesis of Alzheimer's disease and induces neuronal apoptosis and its accumulation in the brain is considered the main cause of AD pathogenesis. In fact, results on primary mouse cortical cells29 indicate that Abeta induces apoptosis of neurons through a caspase-independent apoptosis pathway in neurosphere cultures.
Bcl-2 family
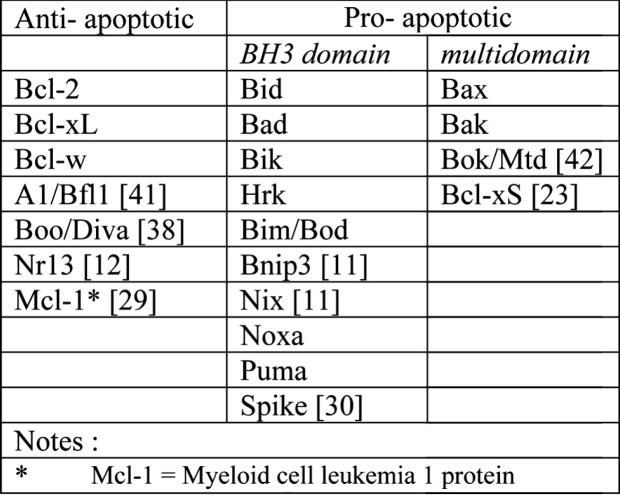
In addition to caspases the family of the Bcl-2 proteins plays a significant role in apoptosis. The Bcl-2 family (bcl-2 stands for B-cell lymphoma/leukemia-2 gene) consists of approximately 15 members some of which are anti-apoptotic while others are pro-apoptotic. The gene that codes for them was first identified because of its involvement in B-cell malignancies and it is located on chromosome segment 18q21.3.
Normally, the Bcl-2 family of proteins can be identified by the presence of some sequence motifs (Table 2) that are called Bcl-2 homology domains (BH1 to BH4). All Bcl-2 family members contain at least one of the Bcl-2 homology domains while those most similar toBcl- 2 itself have all four BH domains. Most anti-apoptotic members contain, at least, the BH1 and BH2 domains, while, in contrast to the pro-apoptotic Bcl-2 family members, they have four BH domains.
Table 2. The Bcl-2 Family of Proteins.
All pro-apoptotic family members, on the other hand, possess the BH3 domain, which is their central to their pro-apoptotic function, while they lack the BH4 domain. The pro-apoptotic members of the Bcl-2 family of proteins are divided into two subgroups: (a) the Baxsubfamily members which consist of Bax, Bak, and Bok and which contain combinations of the domains BH1, BH2, and BH3 and (b) the BH3-only proteins (Bid, Bim, Bik, Bad, Bmf, Hrk, Noxa, Puma, Blk, BNIP3, and Spike) which share homology only within the BH3 motif and are therefore called "BH3-only proteins"31.
There are many theories as to how the Bcl-2 family proteins are involved in apoptosis; all of them have to account not only for the presence or absence of Bcl-2 family members in the different events of the apoptotic process but, also, for the different roles each Bcl-2 family member may assume either in different organisms, different organs or, even, different stages of the apoptotic process. Targeted disruption of Bcl-2, for example, affects programmed cell death in specific neuronal subpopulations only during embryogenesis. Furthermore, after the period of naturally occurring cell death is over, neurons that lack both Bcl-XL and Bcl-2 are more susceptible to apoptosis (in vitro) than those neurons that lack either Bcl-XL or Bcl-2 only37.
One current view is that anti-apoptotic members of the Bcl-2 family inhibit the release of mitochondrial cytochrome c, whereas pro-apoptotic members induce its release2. The Bcl-2 family determines whether the multiprotein complex called apoptosome can assemble; specifically whether Bcl-2, or its nematode homologue, can prevent apoptosome formation1.
Another theory insists that Bcl-2 interacts with cell organelles such as mitochondria. Since the Bcl-2 family members apparently inhibit apoptosis by preventing the release of cytochrome c, which is essential for the formation of the apoptosome, according to this theory, Bcl-2 and Bcl-xL bind to the outer mitochondrial membrane and block cytochrome c release both in vitro and in vivo18,44 while pro-apoptotic proteins such as Bax and Bak facilitate the release of cytochrome c from the mitochondrial membrane. It appears that these proteins, through their BH3 parts, interact with the mitochondrial membrane and form channels through which cytochrome c is released3.
A third theory7 proposes that high concentrations of Bcl-2 or Bcl-xL, effectively prevent the caspase activation which is induced by the addition of cytochrome c in the cytosol.
Finally, as Bcl-xL is considered the key anti apoptotic protein, Bax has emerged as the crucial pro-apoptotic Bcl-2 family member during nervous system development. Bax along with the other main pro-apoptotic protein Bak induce cell death via mitochondrial membrane permeabilization (MMP) which results to the release of small pro-apoptotic molecules such as cytochrome c, Smac/DIABLO, Omi/HtrA2, AIF, and Endonuclease G17. Disruption and inhibition of bax decreases apoptosis in the nervous system resulting in the increase of the number of neurons in some neuronal populations19,9.
P53 gene
The tumor suppressor gene p53 is a gene with a key role in apoptosis. The protein it codes for belongs to a family of proteins that has three members: P53, P63 and P73. All of them have about 60-70% amino-acid identity of the DNA-binding region and all three can induce apoptosis38. A variety of stimuli such as DNA damage, ionizing radiation, UV irradiation, hypoxia, heat shock, oncogene activation and cytotoxic drugs activates p5341. P53 initiates responses that include cell cycle arrest, apoptosis, DNA repair and differentiation, through transcriptional activation of specific target genes that carry P53 DNA binding sites. P53 stimulates the expression of some Bcl-2 family genes including those for Bax and multiple BH3-only proteins, e.g. Bid, Noxa, and PUMA36. Also P53 can bind to one or more anti-apoptotic mitochondrial proteins, e.g. Bcl-XL, therefore inhibiting the Bax/ Bak mitochondrial pore formation and as a result cytochrome c release6, 27. Finally, P53 can also promote apoptosis activity through transcriptional repression of certain genes that lack consensus binding site motifs20,23.
Heat shock proteins
Heat-shock proteins, such as Hsp70, Hsp27 and Hsp90 are believed to be involved in the inhibition of apoptosis. In particular, Hsp70 protein prevents caspase activation directly by associating with Apaf-14. The heat shock proteins can inhibit the release of cytochrome c, which is essential for the formation of the apoptosome, from the mitochondria32.
As far as Hsp27 is concerned, it appears that32 there are two possible ways of interaction: Either Hsp27 interferes with the release of cytochrome c (directly or by interfering with an upstream signal) or it interacts with the cytochrome c itself, when it is released by the mitochondria. There are many newer research projects performed on the anti-apoptotic role of the heat-shock proteins. The heat shock protein Hsp70 blocks apoptosis mainly by the inhibition of Bax activation and as a result preventing the release of pro-apoptotic factors from mitochondria40.
Neuronal thread protein (AD7c-NTP)
According to10 AD7c-NTP protein was overexpressed in patients suffering with Alzheimers disease early in the course of disease. Increased levels of AD7c-NTP are observed in both CSF and urine of patients with early or moderately severe AD, therefore it might be used as a biochemical marker of the disease. AD7c-NTP is a 41kD membrane protein, which induces cell death through apoptosis and impaired mitochondrial function. Moreover levels of proteins involved in apoptosis, such as p53 and Fas/CD95 are increased with AD7c-NTP expression.
The activation of apoptosis
The current view of the apoptotic mechanism is that it consists of three parts: initiation, execution, and termination. It can be initiated by a number of factors such as oxidative stress, alkylating agents, ionizing radiation and chemotherapeutic agents or by external factors such as the tumor necrosis factor (TNF) superfamily of cytokines, the Fas ligand (FasL) and the TNF-related apoptosis inducing ligand (TRAIL).
Apoptosis can be triggered in two ways: (a) through an intrinsic pathway which is regulated by the protein Bcl-2 and is activated by internal signals. This pathway sub-serves cells that are subjected to stress such as DNA damage or growth factor deprivation. (b) by external signals such as death activators (e.g. FasL) that bind to receptors at the cell surface (e.g. Fas/CD95).
As far as the activation of apoptosis by internal signals is concerned, the nematode C. Elegans has provided us with a large number of data concerning the mechanism of apoptosis. The main cause of apoptosis in C. Elegans is the activation of the cysteine protease ced-3, which is mediated by its oligomerization at the activator protein ced-4. Programmed cell death in C. Elegans is quite similar to apoptotic cell death in mammals. During the development of an adult C. Elegans hermaphrodite, 131 out of the total 1090 cells undergo programmed cell death in a lineage-specific and, to a large extent, cellautonomous manner. The activity complex ced-3/ced-4 is, in turn, regulated by the apoptosis inhibitor ced-9 and the apoptosis inducer egl-1. The C. Elegans Bcl-2 protein family member ced -9 binds to the protein ced-4, and prevents it from activating the caspase ced-316. Several genes have been studied which have initiating or inhibiting effects in the process of apoptosis. For example, ced-3 and ced-4, promote apoptosis whereas ced-9, inhibits cell death45,15. In their case, it is postulated that the ced-3 and ced-9 genes are similar in structure and function to mammalian cell death genes. The mammalian homologs of ced-3 comprise a family of cysteinecontaining, aspartate-specific proteases called caspases18. The ced-4 homolog is identified as one of the apoptosis protease-activating factors [APAFs -44]. The mammalian homologs of ced-9 belong to the Bcl2 family of proteins, which share the Bcl2-homology (BH) domain and are either pro- or anti-apoptotic37. The cloning of egl-1 indicates that it is similar to the BH3-domain containing, pro-apoptotic subfamily of Bcl2 proteins.
Apart of C. Elegans, Drosophila has been used in the study of apoptosis34. In view of the fact that the number of the factors which are involved in the regulation of apoptosis depends on the complexity of the organism (e.g. there are 14 caspases in humans, 7 caspases in flies and 1 caspase in worms), Drosophila, by virtue of their evolutionary position between worms and mammals, have many advantages as an animal model of apoptosis, mainly because it shares many programmed cell death (PCD) components with the mammals. On the other hand, like in C. Elegans, Drosophila has a multi-protein complex, which initiates caspase activation in vitro and binds to the initiator caspases46. Based on the overlaps of the apoptosis mechanisms in different species, a global picture (Table 3) of the apoptotic process is emerging, which can be summarized as follows:
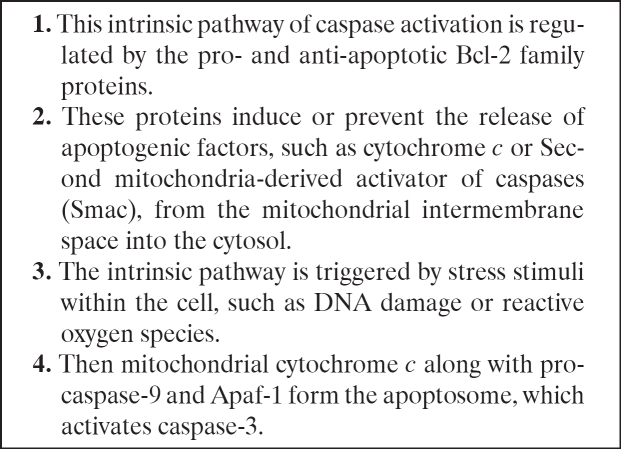
Table 3. Intrinsic pathway of caspase activation and formation of the apoptosome32.
In the healthy cell, the outer membranes of its mitochondria express the protein Bcl-2 on their surface.
Bcl-2 is bound to a molecule of the protein Apaf-1 (Apoptotic Protease Activating factor-1).
Internal damage to the cell causes Bcl-2 to release Apaf-1 which, together with the apoptosis related protein Bax, causes the activation of cytochrome c.
Cytochrome c is produced by the mitochondria and binds to the cytosolic protein Apaf-125.
The released cytochrome c and Apaf-1 bind to molecules of caspase 9.
This leads to the formation of a complex of high molecular weight that consists of cytochrome c, Apaf-1, caspase 9 and ATP, the apoptosome.
Apoptosomes aggregate in the cytosol.
Apaf-1 promotes caspase-9 activation in a way that is similar to the process by which death receptors achieve caspase aggregation while caspase 9 activates other caspases through cleavage.
In this way, through the sequential activation of one caspase by another, an expanding cascade of proteolytic activity is generated which leads to the digestion of structural proteins in the cytoplasm and the degradation of chromosomal DNA, leading to phagocytosis of the cell.
Still, not everybody agrees with this sequence of events. For example, the cell-death pathway controlled by Bcl-2 does not require caspase-9 or its activator Apaf- 1 in order to be functional27. Neither, in their view, is the apoptosome essential for the activation of apoptosis; its role, instead, is to amplify the whole procedure, rather than carry it out.
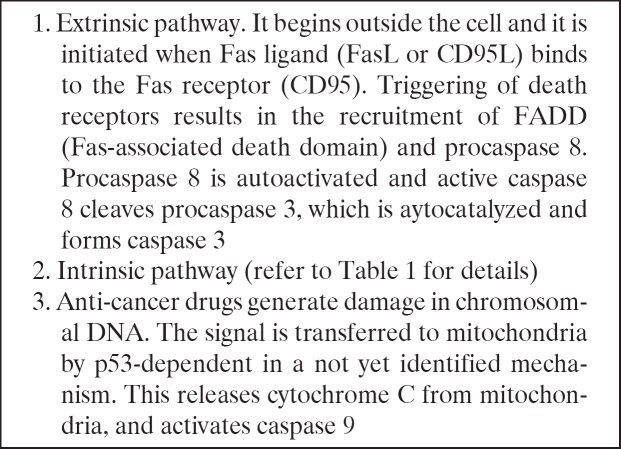
The activation of apoptosis by external signals and the way in which death receptors initiate the apoptosis pathway have been extensively studied and the death receptor pathway of Fas (APO-1 or CD95) is, currently, the most extensively studies and best characterized death receptor mediated apoptosis pathway. Fas/CD95 is a type 1 transmembrane protein and a member of the TNF receptor (TNFR) superfamily. This protein binds to a membrane protein ligand on the surface of an activated lympocyte called CD95 Ligand or CD95L or the Fas ligand. An adapter protein in the cell, FADD (Fasassociated death domain), binds to the aggregated cytoplasmic domain of CD95 and initiates the activation of caspase-8. The increased concentration of activated caspase- 8, in turn, leads to activation of the other caspases bringing about the phagocytosis of the cell (Table 4).
Table 4. Signal transduction of apoptosis.
Conclusion
The available evidence indicates that apoptosis or programmed cell death plays a crucial role in human development and also in some diseases. Over the past few years, our understanding of the biochemistry and the mechanism of apoptosis has grown rapidly. We presented above a selective review of the foundations of current research, which focuses on the mechanisms involved in apoptosis. These foundations include findings on the molecular mechanisms of apoptosis, the pro-apoptotic and anti-apoptotic factors and the way that apoptosis is triggered or inhibited. It is evident that many and, in many ways, important questions about apoptosis still remain unanswered. It is strongly believed that further improvement in our understanding of apoptosis will be of extraordinary importance and may lead to new therapies for major diseases including cancer, AIDS, neurodegenerative and ischemic diseases.
References
- 1.Adams JM, Cory S. Apoptosomes: engines for caspase activation. Curr Opin Cell Biol. 2002;14:715–720. doi: 10.1016/s0955-0674(02)00381-2. [DOI] [PubMed] [Google Scholar]
- 2.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 3.Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001;26:390–397. doi: 10.1016/s0968-0004(01)01844-8. [DOI] [PubMed] [Google Scholar]
- 4.Beere HM, Wolf BB, Cain K, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 5.Boonman Z, Isacson O. Apoptosis in neuronal development and transplantation: role of caspases and trophic factors. Exp Neurol. 1999;156:1–15. doi: 10.1006/exnr.1999.7056. [DOI] [PubMed] [Google Scholar]
- 6.Chipuk JE, Maurer U, Green DR, Schuler M. Pharmacologic activation of p53 elicits Bax-dependent apoptosis in the absence of transcription. Cancer Cell. 2003;4:371–381. doi: 10.1016/s1535-6108(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 7.Cosulich SC, Savory PJ, Clarke PR. Bcl-2 regulates amplification of caspase activation by cytochrome c. Curr. Biol. 1999;9:147–150. doi: 10.1016/s0960-9822(99)80068-2. [DOI] [PubMed] [Google Scholar]
- 8.Creagh EM, Martin SJ. Caspases: cellular demolition experts. Biochem Soc Trans. 2001;29(Pt 6):696–702. doi: 10.1042/0300-5127:0290696. [DOI] [PubMed] [Google Scholar]
- 9.Deckwerth TL, Elliott JL, Knudson CM, Johnson EM, Jr, Snider WD, Korsmeyer SJ. Bax is required for neuronal death after trophic factor deprivation and during development. Neuron. 1996;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 10.de la Monte SM, Wands JR. Alzheimer-associated neuronal thread protein-induced apoptosis and impaired mitochondrial function in human central nervous system-derived neuronal cells. J Neuropathol Exp Neurol. 2001;60:195–207. doi: 10.1093/jnen/60.2.195. [DOI] [PubMed] [Google Scholar]
- 11.Fadeel B, Gleiss B, Hogstrand K, et al. Phosphatidylserine exposure during apoptosis is a cell-type-specific event and does not correlate with plasma membrane phospholipid scramblase expression. Biochem Biophys Res Commun. 1999;266:504–511. doi: 10.1006/bbrc.1999.1820. [DOI] [PubMed] [Google Scholar]
- 12.Galvez AS, Brunskill EW, Marreez Y, et al. Distinct pathways regulate proapoptotic nix and BNip3 in cardiac stress. J Biol Chem. 2005 doi: 10.1074/jbc.M509056200. 2005 Nov 16; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Girard-Egrot A, Chauvet JP, Gillet G, Moradi-Ameli M. Specific interaction of the antiapoptotic protein Nr-13 with phospholipid monolayers is prevented by the BH3 domain of Bax. J Mol Biol. 2004;335:321–331. doi: 10.1016/j.jmb.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Gluecksmann A. Cell death in normal vertebrate ontogeny. Biological Reviews. 1951;26:59–86. doi: 10.1111/j.1469-185x.1951.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 15.Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992;356(6369):494–499. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- 16.Horvitz HR. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 1999;59(7 Suppl):1701–1706. [PubMed] [Google Scholar]
- 17.Kim R. Unknotting the roles of Bcl-2 and Bcl-xL in cell death. Biochem Biophys Res Commun. 2005;333:336–343. doi: 10.1016/j.bbrc.2005.04.161. [DOI] [PubMed] [Google Scholar]
- 18.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 19.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 20.Ko LJ, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 21.Kuan CY, Flavell RA, Rakic P. Programmed cell death in mouse brain development. Results Probl Cell Differ. 2000;30:145–162. doi: 10.1007/978-3-540-48002-0_6. [DOI] [PubMed] [Google Scholar]
- 22.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 23.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 24.Lindenboim L, Kringel S, Braun T, Borner C, Stein R. Bak but not Bax is essential for Bcl-xS-induced apoptosis. Cell Death Differ. 2005;12:713–723. doi: 10.1038/sj.cdd.4401638. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 26.Lockshin RA, Zakeri Z. Programmed cell death and apoptosis: origins of the theory. Nat Rev Mol Cell Biol. 2001;2:545–550. doi: 10.1038/35080097. [DOI] [PubMed] [Google Scholar]
- 27.Marsden VS, O'Connor L, O'Reilly LA, et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 2002;419(6907):634–637. doi: 10.1038/nature01101. [DOI] [PubMed] [Google Scholar]
- 28.Mihara M, Erster S, Zaika A, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 29.Millet P, Lages CS, Hark S, et al. Amyloid-B peptide triggers Fas-independent apoptosis and differentiation of neural progenitor cells. Neurobiology of Disease. 2005;19:57–65. doi: 10.1016/j.nbd.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Mori M, Burgess DL, Gefrides LA, et al. Expression of apoptosis inhibitor protein Mcl1 linked to neuroprotection in CNS neurons. Cell Death Differ. 2004;11:1223–1233. doi: 10.1038/sj.cdd.4401483. [DOI] [PubMed] [Google Scholar]
- 31.Mund T, Gewies A, Schoenfeld N, Bauer MK, Grimm S. Spike. A novel BH3-only protein, regulates apoptosis at the endoplasmic reticulum. . FASEB J. 2003;17:696–698. doi: 10.1096/fj.02-0657fje. [DOI] [PubMed] [Google Scholar]
- 32.Paul C, Manero F, Gonin S, et al. Hsp27 as a Negative Regulator of Cytochrome c Release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polster BMandFiskumG, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem. 2004;90:1281–1289. doi: 10.1111/j.1471-4159.2004.02572.x. [DOI] [PubMed] [Google Scholar]
- 34.Richardson H, Kumar S. Death to flies: Drosophila as a model system to study programmed cell death. J Immunol Methods. 2002;265:21–38. doi: 10.1016/s0022-1759(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 35.Saraste A, Pulkki K. Morphologic, biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;3:528–537. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 36.Sax JK, El Deiry WS. p53 downstream targets, chemosensitivity. Cell Death Differ. 2003;10:413–417. doi: 10.1038/sj.cdd.4401227. [DOI] [PubMed] [Google Scholar]
- 37.Shindler KS, Yunker AM, Cahn R, et al. Trophic support promotes survival of bcl-x-deficient telencephalic cells in vitro. Cell Death Diff. 1998;5:901–910. doi: 10.1038/sj.cdd.4400421. [DOI] [PubMed] [Google Scholar]
- 38.Slee EA, Harte MT, Kluck RM, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song Q, Kuang Y, Dixit VM, Vincenz C. Boo, a novel negative regulator of cell death, interacts with Apaf-1. EMBO J. 1999;18:167–178. doi: 10.1093/emboj/18.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stankiewicz AR, Lachapelle G, Foo CP, et al. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 41.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 42.Xiang Z, Ahmed AA, Mφller C, et al. Essential Role of the Prosurvival bcl-2 Homologue A1 in Mast Cell Survival After Allergic Activation. The Journal of Experimental Medicine. 2001;194:1561–1570. doi: 10.1084/jem.194.11.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yakovlev AG, Di Giovanni S, Wang G, et al. BOK and NOXA are essential mediators of p53-dependent apoptosis. J Biol Chem. 2004;279:28367–28374. doi: 10.1074/jbc.M313526200. [DOI] [PubMed] [Google Scholar]
- 44.Yang J, Liu X, Bhalla K, Kim CN, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 45.Yuan J, Horvitz HR. The Caenorhabditis elegans genes ced-3 and ced-4 act cell autonomously to cause programmed cell death. Dev Biol. 1991;38:33–41. doi: 10.1016/0012-1606(90)90174-h. [DOI] [PubMed] [Google Scholar]
- 46.Zhou L, Song Z, Tittel J, Steller H. HAC-1, a Drosophila homolog of APAF-1 and CED-4 functions in developmental and radiation-induced apoptosis. Mol Cell. 1999;4:745–755. doi: 10.1016/s1097-2765(00)80385-8. [DOI] [PubMed] [Google Scholar]