Abstract
Rhabdomyolysis constitutes a common cause of acute renal failure and presents paramount interest. A large variety of causes with different pathogenetic mechanisms can involve skeletal muscles resulting in rhabdomyolysis with or without acute renal failure. Crush syndrome, one of the most common causes of rhabdomyolysis presents increased clinical interest, particularly in areas often involved by earthquakes, such as Greece and Turkey. Drug abusers are another sensitive group of young patients prone to rhabdomyolysis, which attracts the clinical interest of a variety of medical specialties.
We herein review the evidence extracted from updated literature concerning the data related to pathogenetic mechanisms and pathophysiology as well as the management of this interesting syndrome.
Keywords: Rhabdomyolysis, acute renal failure, myoglobin, crush syndrome
The first case of the crush syndrome, which constitutes one of the main causes of rhabdomyolysis, was reported in Sicily in 1908, after an earthquake1,2. In 1930, in the Baltic area, an epidemic of myoglobinuria was observed due to consumption of contaminated fish. Interest in rhabdomyolysis and crash syndrome was stimulated during the World War II particularly after the bombing in London, where the victims developed acute renal failure and myoglobinuria1.
Rhabdomyolysis is a rupture (lysis) of skeletal muscles due to drugs, toxins, inherited disorders, infections, trauma and compression3. Lysis of muscle cells releases toxic intracellular components in the systemic circulation which leads to electrolyte disturbances, hypovolemia, metabolic acidocis, coagulation defects and acute renal failure due to myoglobin4.
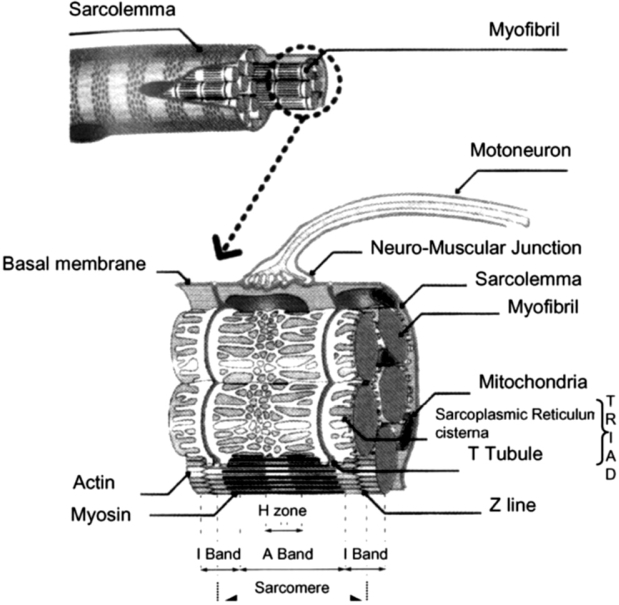
The skeletal muscle consists of cylindrical myofibrils, which contain variant structural and contraction proteins. Actin and myosin, arranged in thin and thick filaments respectively, form the repeated functional units of contraction, the sarcomeres5. The sarcoplasmic reticulum constitutes an important cellular calcium storage. It is structurally connected to the t-tubules, that are formed by invaginations of the muscle cell plasma membrane, the sarcelemma, around every fibril (Figure 1). After the sarcelemma depolarization, the stimulation arrives, through the t-tubules junctions, at the sarcoplasmic reticulum, inducing the calcium ions release and triggering muscle contraction6.
Figure 1. The skeletal muscle fibre (from ref. 3).
Causes of rhabdomyolysis and pathogenetic mechanisms
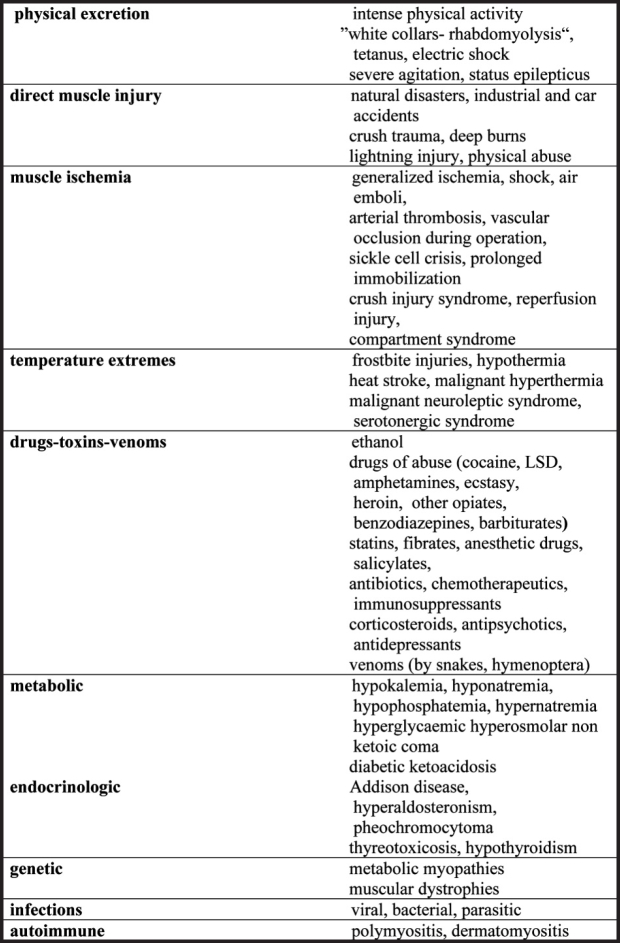
Rhabdomyolysis can be induced by many different causes, but it is usually the result of multiple contributing factors (Table 1). Although it had been initially associated almost exclusively with traumatic conditions, now the non-traumatic causes appear to be at least 5 times more frequent7. Alcohol and drug abuse, the crush syndrome, seizures and some metabolic derangements are considered to be the commonest factors that lead to rhabdomyolysis1.
Table 1. Causes of rhabdomyolysis.
Excessive physical exertion
Excessive physical exertion of any kind provokes rhabdomyolysis, especially in previously untrained individuals ("white-collars rhabdomyolysis"), while the high temperature, the humidity, the cocaine or alcohol use and the dehydration may act as additional risk factors8. There have been many reported cases of rhabdomyolysis in marathon runners, weight lifters and also in conditions of severe agitation, such as the protracted tonicclonic seizures in status epilepticus or due to the use of psychostimulant drugs or in psychiatric diseases, such as the delirium tremens.
It seems that the intense physical exertion leads to the exhaustion of cellular ATP supplies and to pumb dysfunction in muscle cell membranes, which results in their disruption, producing rhabdomyolysis4.
Direct muscle injury
Another widely recognized mechanism that triggers rhabdomyolysis is the direct muscle trauma, often as a result of natural or human-made disasters. This is described by the term "crush injury syndrome", usually referring to people entrapped under collapsed buildings. There were more than 1000 cases of rhabdomyolysis reported during the Armenian earthquake of 19884 , while after the more recent 1999 earthquake at Turkey 462 people required dialysis, due to rhabdomyolysis-induced acute renal failure9. Furthermore, incidents of rhabdomyolysis have occurred in industrial and car accidents, in victims with deep burns, after beating and severe physical abuse as well as after lightning injuries4,10.
Pathophysiologically, it seems that the direct disruption of the muscle membrane leads to the influx of calcium into the cytoplasm, which eventually results in rhabdomyolysis4.
Muscle ischemia
Conditions of generalized ischemia and hypoxemia, such as shock, CO poisoning and status asthmaticus are considered as possible causes of rhabdomyolysis, as they are associated with insufficient ATP production and sarcelemma dysfunction4. Arterial thrombosis, prolonged vascular occlusion during surgical operations, air emboli and severe sickle cell crisis are also causes of muscle cell lysis1,4,9.
The compartment syndrome can be considered as both a cause and a complication of rhabdomyolysis4. As the intracompartmental pressure rises, due to bleeding or tissue-swelling, initially the venular and then the arterial blood flow are blocked, leading in muscle cell lysis11,12. The compartment syndrome can also be complicated by irreversible peripheral nerves damage, due to compression13.
Moreover, rhabdomyolysis can be the result of muscle ischemia, due to tissue compression in cases of prolonged immobilization, such as comatose situations after illicit drug overtake4, elderly immobilization after a hip fracture14, immobilization during prolonged operations, especially at the lithotomy position15, physical restraint of psychiatric patients16 and certainly crush injury syndrome4.
In conditions of ischemia and hypoxemia, the cellular energy supplies are gradually reduced and more than 4 hours ischemia can provoke irreversible damage to skeletal muscle. However, rhabdomyolysis occurs mainly after muscle reperfusion and therefore the reperfusion injury is considered to be more deleterious than extended ischemia. In the reperfused area, a reactive hyperemia occurs, trying to repay the oxygen demand of the previously ischemic muscles. The increased capillary permeability results in fluid exit from the vasculature, which leads to intravascular hypovolemia and localized tissue edema subsequently. Polymorphonuclear neutrophils, leukotrienes and other inflammatory mediators accumulate in the postischemic tissues. After the onset of reperfusion, the oxygen availability and the large intracellular concentration of calcium ions induce the oxygen-derived free radical release from the neutrophils, leading to the peroxidation of the lipid membranes and consequently to impairment of their ion permeability. The later causes influx of fluid in the myocytes and finally their lysis17. Furthermore, the release of mitochondrial respiratory chain components triggers the myocyte apoptotic mechanisms, resulting in programmed cell death1.
Temperature extremes
The exposure to severely low temperature is known to cause vasoconstriction, which can lead to cell damage, when it is prolonged. This mechanism explains the occurrence of rhabdomyolysis in cases of frostbite injuries and generalized hypothermia.
On the other hand, hyperthermia is considered as a hypermetabolic condition that triggers rhabdomyolysis, when the energy supplies are not adequate for the cell needs. The pathophysiological mechanism is common in various clinical hyperthermic syndromes4.
Heat stroke
he heat stroke is a serious and often life-threatening disorder, characterized by core body temperature > 40℃. Multiple contributing factors, such as aging, dehydration, poor physical condition and certain genetic polymorphisms are associated with low cellular expression of heat shock proteins, contributing to inability of dissipating heat and eventually to heat stroke18. Moreover, the exertional heat stroke occurs at physically active individuals, after excessive physical exertion. Its clinical manifestations include high fever, muscle weakness, neurological dysfunction that can potentially be complicated by electrolyte disturbances, acid-base disorders, acute renal failure, rhabdomyolysis and disseminated intravascular coagulation19.
Malignant hyperthermia
As malignant hyperthermia is defined the hypermetabolic syndrome that occurs more frequently at genetically predisposed individuals bearing certain mutations at the ryanodine receptor of the sarcoplasmic reticulum gene. Clinically, is manifestated with fever, tachycardia, hyperventilation, generalized skeletal muscle contraction and rigidity, metabolic acidosis and rhabdomyolysis, due to massive calcium release from the sarcoplasmic reticulum1, 20, 21.
Malignant neuroleptic syndrome
In patients with malignant neuroleptic syndrome the dopaminergic block in hypothalamus, the sudden interruption of dopaminergic drugs or the striatal dopamine block trigger the syndrome clinical manifestations that involve fever, generalized muscular contraction, rigidity and potentially rhabdomyolysis. The central anticholinergic syndrome represents a mild variant of this syndrome1.
Serotonergic syndrome
This syndrome is caused by an excessive activation of 5-HT1A and 5-HT2A serotonergic receptors, resulting in the cerebral neuronal dopaminergic block. It emerges with fever, altered mental state, autonomal dysfunction and neuromuscular excitability, leading to rhabdomyolysis1, 22.
Drugs, toxins and venoms
A variety of drugs, toxins and venoms play a role in approximately 80% of cases with rhabdomyolysis. Ethanol, abuse drugs and statins are the drugs mostly implicated1.
Ethanol
Acute or chronic alcohol abuse induces the hepatic cytocrome p450 incorporating toxic metabolites. Beside the direct toxic effects on myocytes, the alcohol intoxication can lead to electrolyte and acid-base disorders, like metabolic acidosis, hypokalemia, hypomagnesemia, hypocalcemia and hypophosphatemia, that distort the function of sarcelemma and contribute to cell death23. Furthermore, ethanol may lead to rhabdomyolysis, because of the initial agitation it provokes9.
Finally, its central depressant effect after acute inebriation may result in comatose situation, prolonged immobility, muscle compression and rhabdomyolysis24,25.
Abuse drugs
t is widely recognized that cocaine, heroin, other opiates, amphetamines, other club drugs, like "ecstasy" and benzodiazepines cause rhabdomyolysis4.
Cocaine blocks the presynaptic re-uptake of norepinephrine and dopamine, inducing sympathetic stimulation, which leads to increased muscular activity and can be complicated by seizures26. Moreover, the cocaine intoxication can be established through hyperthermia, as well as through direct myotoxicity. Besides the aforementioned mechanisms, cocaine can cause rhabdomyolysis through vasoconstriction, which results in muscle ischemia27,28.
Heroin is also considered to have possible direct myotoxicity29, while amphetamins are etiologically connected to the serotonergic syndrome22. However, the most common mechanism through which all these drugs induce rhabdomyolysis is the muscle compression and ischemia, due to prolonged immobilization on a rigid ground, after an acute intoxication and subsequent unconsciousness or coma4.
Statins
Rhabdomyolysis as a side effect of statins administration is an issue of intense interest. According to several studies, elevation of CPK occurs in the 3-5 % of statin administration, while significant rhabdomyolysis occurs only in 0.04-0.2% of them3.
The precise mechanism has not yet been clarified, but several theories have been suggested. One of them suggests that the sarcelemma cholesterol decrease is involved in membrane's dysfunction and statins' myotoxicity30.
According to another theory, this myotoxicity is attributed to the depletion of mevalonic acid and ubiquinone, which results in reduced cellular ATP production and membrane's distortion. Moreover, the blockade of chloride channels of sarcelemma is another possible pathogenetic mechanism, contributing to prolonged muscle contraction31.
All statins can potentially lead to rhabdomyolysis, even as a monotherapy. Statins' myotoxicity seems to be dose-dependent32. Furthermore, the additional administration of drugs, such as fibrates, cyclosporine, macrolide antibiotics, digoxine, coumarin anticoagulants that are metabolized by cytP-4503A4 or that inhibit this enzyme result in increase of serum statins concentration. Finally, the coadministration of myotoxic medications, as well as the coexistence of other risk factors may contribute to rhabdomyolysis31.
Other drugs-toxins-venoms
Various drugs, such as corticosteroids, immunosuppressants, salicylates, fibrates, antibiotics, chemotherapeutic agents, antidepressants, antipsychotics and anesthetics have been associated with rhabdomyolysis, not only in toxic, but also in therapeutics doses. Rhabdomyolysis occurs through multiple mechanisms, including direct myotoxicity, metabolic and electrolyte derangements, muscle compression and ischemia due to prolonged immobility, agitation and physical exertion. Moreover, the anesthetic halothane and some depolarizing muscle relaxants can potentiate malignant hyperthermia and rhabdomyolysis, while lithium, phenothiazines, neuroleptics and antiparkinsonism agents abrupt withdrawal may lead to malignant neuroleptic syndrome. Furthermore, the administration of selective serotonin reuptake inhibitors, other antidepressants, pethidine and amphetamine-like substances may induce the serotonergic syndrome and trigger rhabdomyolysis .
On the other hand, certain types of mushrooms and snake venoms are causes of rhabdomyolysis1,4.
Metabolic endocrine disorders
lectrolyte disorders, such as hypokalemia, hypophosphatemia, hyponatremia, but also hypernatremia can result in rhabdomyolysis, distorting the functions and the permeability of sarcelemma4,9.
Hypokalemia is the most common electrolytic cause of rhabdomyolysis. It depolarizes the muscle membrane and inhibits the production and storage of glycogen at myocytes1. Additionally, potassium depletion is associated with inadequate muscle blood flow during exercise, ischemia and rhabdomyolysis33.
Hyponatremia potentiates rhabdomyolysis, probably through extracellular hypoosmolarity and subsequent cell swelling. This disorder often acts complementary to the coexisting hypokalemia34.
Hypophosphatemia constitutes a notable cause of rhabdomyolysis, particularly in cases of weak, exhausted individuals, since phosphate is involved in biochemical pathways of ATP production and in the affinity of oxygen for haemoglobin in erythrocytes3,35. It is impressive that in case of hypokalemic and hypophosphatemic rhabdomyolysis, potassium and phosphate serum levels may be maintained normal or even raised, not always reflecting their real deficiency, as these intracellular ions are released from the destroyed myocytes4.
On the other hand, some endocrinologic disorders, such as pheochromocytoma and thyreotoxicosis, potentiate rhabdomyolysis, due to hypermetabolism. Diabetic ketoacidosis and hyperaldosteronism cause rhabdomyolysis through hypokalemia, whereas Addison disease through hyponatremia4.
Genetic disorders
Metabolic myopathies
Metabolic myopathies are considered as an uncommon cause of rhabdomyolysis. The cause is a genetically determined abnormality of an enzyme implicated in a biochemical pathway of ATP production which results in inadequate muscular energy supply. The disorder may concern glycolysis, glycogonolysis, as in McArdle disease, fatty acid oxidation, as in CPT1 and CPT2 deficiencies, oxidative phosphorylation, lipolysis, purine metabolism, or the function of the mitochondrial respiratory chain (mitochondrial cytopathies). A metabolic myopathy should be suspected, when the episodes of rhabdomyolysis start in childhood, are recurrent and occur after physical activity or in combination with fasting or infection and finally when there is a family history of exercise intolerance1,3.
Muscular dystrophies
Muscular dystrophies and particularly Duchenne and Becker dystrophies may be complicated by rhabdomyolysis3.
Infections:
Some infections can lead to rhabdomyolysis through various pathophysiological mechanisms. Streptococcus, staphylococcus and salmonella infections can cause direct myotoxicity. In other cases, toxins by clostridia may indirectly contribute to muscle damage. Moreover, fever and inflammatory mediators, as well as drugs, such as zidovudine which is used against HIV, induce the muscle cell lysis3.
Autoimmune disorders:
Polymyositis and dermatomyositis are autoimmune myopathies that result in progressive muscle weakness and rarely in rhabdomyolysis1,36.
Pathophysiology
The cell membrane can be injured by mechanical pressure, burn, chemicals, toxins and poisons. The lysis of cell membrane releases organic and inorganic intracellular components, such as myoglobin, potassium, lactic acid, purines, and phosphate which entering the circulation, after the restoration of blood flow, can be toxic and life threatening. When reperfusion starts, leukocytes migrate into the damaged area, cytokines and prostaglandins increase whereas free radicals are produced in the presence of oxygen2.
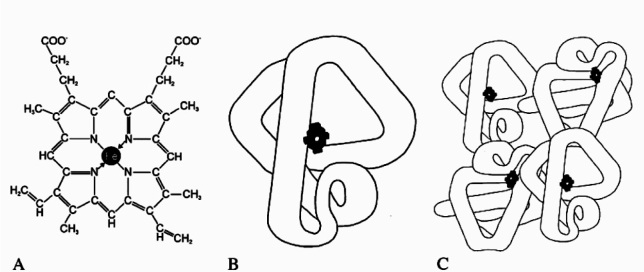
Myoglobin is a 17 kDa small chromoprotein like hemoglobin (Figure 2). They are both filtered through the glomeruli and reabsorbed in the proximal tubules by endocytosis. In acidic environment (pH<5.6) the globin chain dissociates from the iron-containing ferrihemate portion of the molecule. This normally happens in lysosomes, where free iron is rapidly converted to ferritin. However, in rhabdomyolysis the amount of myoglobin delivered to the proximal tubule cells overwhelms their ability to convert iron to ferritin, resulting in intracellular ferrihemate accumulation. Iron as a metal has the ability to donate and accept electron as well as the capability to generate oxygen free radicals. This leads to oxidative stress and injury of the renal cell. The decreased acidic pH of the urine because of the metabolic acidosis (damaged muscle cells release acids) has an important role in iron release37.
Figure 2. (A) the iron-containing porphyrin ring, (B) myoglobin, (C) hemoglobin (From ref. 17).
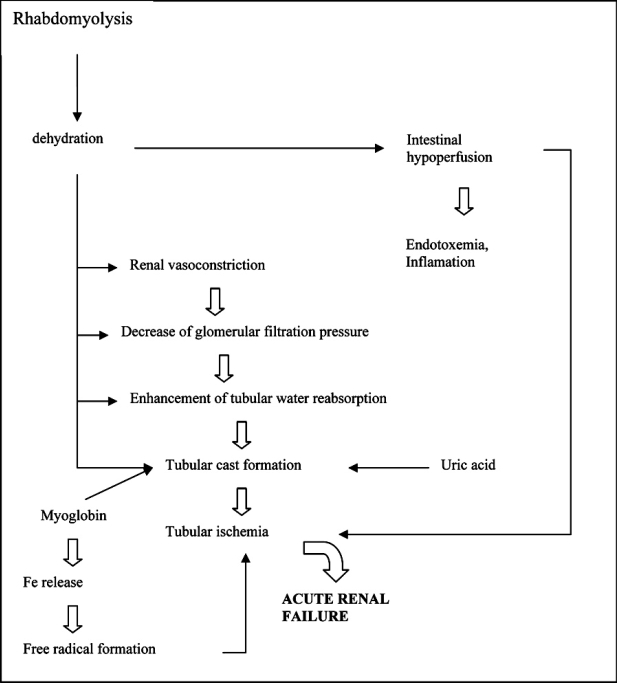
Myoglobin can not be reabsorbed when in excessive amounts in the tubules. Systemic vasoconstriction and hypovolemia result in water reabsorbion in renal tubules which in turn increases further myoglobin concentration in urine38. The later causes formation of casts that obstruct renal tubules. Apoptosis of epithelial cells contributes in casts formation. Besides iron toxic effect, the heme center of myoglobin initiates lipid peroxidation and renal injury39.
Therefore, the obstruction of renal tubules by the myoglobin casts, the formation of free radicals from iron, the vasoconstriction and hypoxia due to hypovolemia are the main causes of acute renal failure in rhabdomyolysis (Figure 3).
Figure 3. Pathophysiology of ARF in rhabdomyolysis (From ref. 2).
The integral myoglobin molecule is not toxic but becomes detrimental in acidic medium, as it has already been mentioned. Thus, if the amount of the urine is high and the pH alkaline, myoglobin is stabilized and its toxic effects are prevented4.
Lactic acid which is the main organic acid released which causes metabolic acidosis. Acidosis has an effect on numerous metabolic functions and enhances hyperkalemia. The low pH in urine facilitates intratubular precision of myoglobin and uric acid.
Released nucleotides are metabolized in the liver to purines such as xanthine and hypoxanthine resulting in uric acid overproduction, which contribute to casts formation and to tubular obstruction.
Dehydration causes hyperalbuminemia at an early stage. At a later stage, inflammation, malnutrition, capillary leak and fluid overload cause hypoalbuminemia. Under this condition, total calcium levels must be counted according to the levels of albumine.
Massive lysis of muscle cells substantially increases potassium in circulation. Renal insufficiency and acidosis increase the potassium levels further on. Hyperkalemia is a life threatening condition because of potassium cardiotoxicity and it requires immediate treatment. In the presence of hyperkalemia, hypocalcaemia may lead to severe cardiac arrhythmias.
Phosphorus is released by damaged muscle resulting in hyperphosphatemia. Renal insufficiency is also a cause of hyperphosphatemia which causes deposition of calcium-phosphate complexes in tissues and suppresses 1a-hydroxylase, the enzyme responsible for the production of the active vitamin D, contributing further to hypocalcemia.
Phosphocreatine is abundant in the muscle cells and it plays a role in energy delivery. When released it is transformed into creatinine and is removed through kidneys. Although, it raises in rhabdomyolysis, its levels are not as high as expected in proportion to muscle damage and renal insufficiency. Released thromboplastine and tissue plasminogen could cause intravascular coagulation. Creatine kinase (CK) extremely increases in the serum but it has no toxic effects2.
Clinical Features
The severity of metabolic disturbances due to rabdomyolysis depends on the mass of the muscle damaged. Hypovolemia, hypotension, body temperature, sypsis, hypokalemia, intake of toxic drugs, physical status, hormone environment and genetic susceptibility contribute in the severity of the clinical features (Table 2)1.
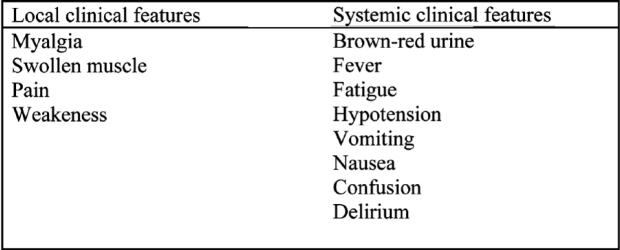
Table 2. local and systemic clinical features of rhabdomyolysis. (From ref. 40 modified).
Acute muscle necrosis causes tenderness and local oedema. The patients have fever, weakness, fatigue, cramps and pain. The majority of patients suffer from back pain and some from limb pain37. Hypotension and metabolic acidosis are common in these patients. Hyperkalemia can cause life threatening arrhythmias3.
Apart from myalgia, the initial clinical sigh of rabdomyolysis is the appearance of discolored urine. The presence of myoglobin gives urine a brown-red color (Figure 4).
Figure 4. Dark urine due to the presence of myoglobin (From ref. 17).
A good history, focusing on the patient's activity, is essential for establishing the diagnosis. The physical examination and the laboratory findings confirm the diagnosis.
Laboratory findings
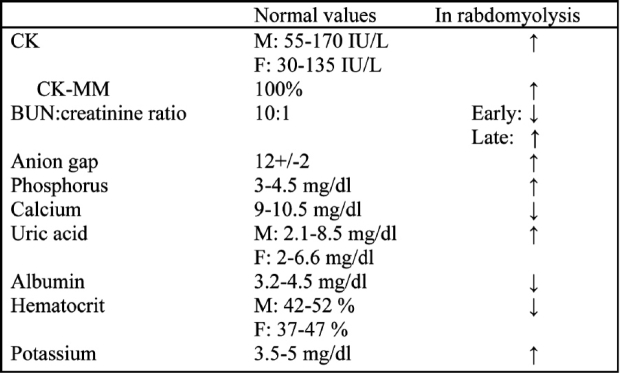
CK levels are the most sensitive indicators of myocyte injury. Under normal condition, CK levels are 45- 260 U/L. After rhabdomyolysis, the levels of CK can be raised to 10.000-200.000 U/L or even to 3.000.000.000 U/L. No other condition except rabdomyolysis can cause such extreme CK elevation4, 37.
CK is consisted of three isoenzyme which are: CK-MM mostly found in muscles, CK-MB mostly found in heart and CK-BB mostly found in the brain and kidneys. When a differential diagnostic problem occurs, all the three isoenzyme should be counted (Table 3).
Table 3. Serum lab values in adults (M: male, F: female) in rhabdomyolysis. (From ref. 37).
Carbonic anydrase III elevation is the most specific marker for muscle injury but the method is expensive and difficult, and therefore is not measured. Aldolase, lactic dehydrogenase and transaminases are also frequently found elevated.
Plasma myoglobin is elevated early but this finding is not a reliable index because myoglobin is quickly removed through kidneys.
The increase of creatinine is disproportional to the increase of urea, so the BUN:creatinine ratio, which under normal condition is 10:1, becomes 6:1. This is attributed to the increased formation of creatinine from creatine and to acute renal failure. Later on, catabolism of muscle proteins leads to an increase in urea production and the ratio rise to higher than normal levels37.
The levels of uric acid can be raised to 40 mg/dl. Hypoalbuminamia, anaemia, leukocytocis, thrombopenia and coagulation disturbances can be observed.
Phosphorus and potassium are elevated while calcium is reduced. The serum anion gap {Na (Cl + HCO2)} is significantly higher than normal levels (12±2).
Hypoxia and metabolic acidosis can be detected in blood gas analysis41.
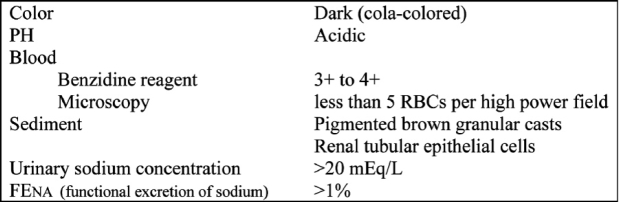
Albumin, uric acid crystals, brown casts and myoglobin are found in urinalysis (Table 4). Myoglobin can be detected with the same urostick as hemoglobin. If myoglobin is more than 25 µg/ml, urine gets a dark brown red color. Normally myoglobin is less than 5 ng/ml. Myoglobin is more than 25 µg/ml when more than 100 gr of the muscle is damaged. In urine there are epithelial cells and erythrocytes42.
Table 4. Urinalysis findings in rhabdomyolysis. (From ref. 37).
Urine electrolytes, especially Na, are very useful indicators of the functional integrity of the renal tubules in patients with acute renal failure. The functional excretion of sodium (FENa) is calculated with the following formula: 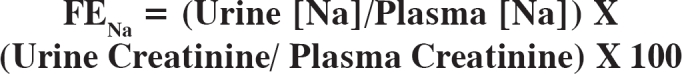
In patients with prerenal azothemia the FENa is <1% while in acute tubular necrosis is >1%. In rabdomyolysis is greater than 1%43.
In case of suspected intoxication the levels of toxic agents should be measured for diagnostic purposes and treatment.
Management
Early diagnosis and intervention is the key for treating rhabdomyolysis. It is essential to recognize the possible cause and limit it, while coping with the pathophysiological complications of rhabdomyolysis4.
Preventing further muscle damage
It is important to extricate immediately the entrapped patients, suffering from crush injuries and to interrupt the immobilization of any kind4.
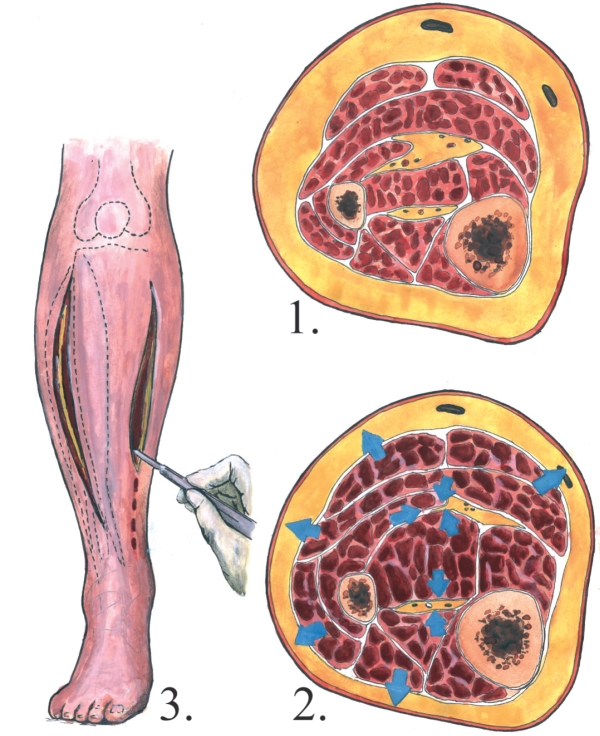
In case of compartment syndrome, it is widely accepted that a fasciotomy should be performed, when the intracompartmental pressure rises above 40 mmHg, as it is associated with clinically significant muscle ischemia (Figure 5)17, 44.
Figure 5. Compartment syndrome and fasciotomy incisions. 1. Normal anatomy of the right calf 2. Compartment syndrome 3. Fasciotomy incisions.
On the other hand, it is also important to rapidly administrate antidotes or antibiotics, in cases of rhabdomyolysis due to venoms or infections respectively4.
Prevention and treatment of acute renal failure
When rhadomyolysis occurs, the need of continuous monitoring of vital signs, diuresis (colour, pH, volume, specific gravity) arterial pH, electrolytes and CPK values, compartment pressures, cardiac status, as well as avoidance of nephrotoxic agents is necessary4.
The most essential intervention for the prevention of acute renal failure must be the early and vigorous fluid administration, which at the crush injury syndrome should start before victims' removement45. The fluid infusion expands the intravascular volume, inducing diuresis and clearance of toxins, resulting in the reduction in cast formation and obstruction of proximal tubules. The volume of fluid administrated to adults should be 10 or more litres per day, so that they achieve a urine output of 150 - 300 ml per hour4. It is essential to carefully monitor the urine output, as the massive fluid infusion can lead to congestive heart failure and pulmonary edema, particularly at the elderly and those with cardiologic history44.
The ideal fluid regimen is quite controversial. According to some authors, the initial administration of 1L isotonic saline and 1L dextrose 5% to which 100 mmol sodium bicarbonate has been added is the ideal combination. As far as the patient maintains a urine output of 20 ml/hour, 10 ml/hour of mannitol 15% can be also added9,13.
The administration of bicarbonate aims at the urine alkalinisation and appears to be very beneficial at the correction of metabolic acidosis, the enhancement of myoglobin washout and the reduction in cast formation, which is induced by acidic conditions. The alkalinisation of urine also prevents renal vasoconstriction and the lipid peroxidation, caused by myoglobin44.
On the other hand, mannitol enhances the renal blood flow and the glomerular pressure. It is an osmotic diuretic that decompresses the interstitial and muscular compartments, while expanding the intravascular volume, promoting diuresis, inducing the clearance of toxins and preventing the cast formation at proximal tubules. It is also believed to have some antioxidant properties9. However, the administration of mannitol should be done under close monitoring and must stop, if a patient develops oliguria or anuria13.
If the administration of fluids and mannitol fails to preserve an adequate urine output, loop diuretics may be used, although they potentially acidify the urine4.
Treating rhabdomyolysis also requires to cope with subsequent electrolyte disorders. Particularly, hyperkalemia is a really dangerous complication, which can lead to life-threatening arrhythmias. If the intravascular volume expansion, the bicarbonate and the insulin with glucose solution administration do not prove effective, dialysis is required44. On the contrary, hypocalcemia does not need to be corrected, unless it causes clinical complications, as its correction results in intramuscular calcification9.
In case of nonresponding to treatment oliguria and azotemia, severe metabolic acidosis or hyperkalemia and iatrogenic fluid overload, emergency dialysis is required. Conventional haemodialysis, continuous dialysis strategies or even peritoneal dialysis can be beneficial and have certain indications4,9.
Furthermore, many agents may have a role in treatment of rhabdomyolysis. Dandrolene and bromocriptine are considered to stabilize the membrane calcium channels of sarcoplasmic reticulum in myocytes and thus to be useful at malignant hyperthermia and malignant neuroleptic syndrome44. Dopamine is believed to increase renal blood flow and enhance natriuresis, whereas the 21-aminosteroids are considered to inhibit the lipid peroxidation of membranes. Many other agents have antioxidant properties and act as free radical scavengers. Iron chelation therapy diminishes the renal injury by preventing the hydroxyl radical connected iron toxicity. Pentoxifylline reduces the neutrophil accumulation and the cytokine release. Moreover, allopurinol, mannitol and vitamins E and C, as well as a few minerals and glutathione appear to have some antioxidant activity9,17.
Prognosis
The prognosis depends on the extent and clinical severity of rhabdomyolysis, as well as on the early and prompt medical intervention. Some patients develop a mild renal impaiment and recover quickly, whereas others require dialysis for more than 3 weeks. In most of the cases the renal function is totally restored and the general survival rate after a rhabdomyolysis-induced acute renal failure is 78,6 %37. The patients should be investigated after their recovery for predisposing factors, such as a metabolic myopathy, while individuals who use potentially myotoxic drugs, such as statins, must regularly monitor their CK levels1,32.
References
- 1.Warren JD, Blumbergs PC, Thompson PD. Rhabdomyolysis: a review. Muscle Nerve. 2002;25:332–347. doi: 10.1002/mus.10053. [DOI] [PubMed] [Google Scholar]
- 2.Singh D, Chander V, Chopra K. Rhabdomyolysis. Methods Find Exp Clin Pharmacol. 2005;27:39–48. doi: 10.1358/mf.2005.27.1.875435. [DOI] [PubMed] [Google Scholar]
- 3.Guis S, Mattei J-P, Cozzone PJ, Bendahan D. Pathophysiology and clinical presentations of rhabdomyolysis. Joint Bone Spine. 2005;72:382–391. doi: 10.1016/j.jbspin.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Criddle L. Rhabdomyoysis. Pathophysiology, recognision, and management. Critical Care Nurse. 2003;23:14–32. [PubMed] [Google Scholar]
- 5.Au Y. The muscle ultrastructure: a structural perspective of the sarcomere. Cell Mol Life Sci. 2004;61:3016–3033. doi: 10.1007/s00018-004-4282-x. [DOI] [PubMed] [Google Scholar]
- 6.Serysheva I. Structural insights into excitation-contraction coupling by electron cryomicroscopy. Biochemistry (Mosc) 2004;69:1226–1232. doi: 10.1007/s10541-005-0068-5. [DOI] [PubMed] [Google Scholar]
- 7.Visweswaran P, Guntupalli J. Rhabdomyolysis. Crit Care Clin. 1999;15:415–428. doi: 10.1016/s0749-0704(05)70061-0. [DOI] [PubMed] [Google Scholar]
- 8.Line R, Rust G. Acute exertional rhabdomyolysis. American Family Physician. 1995;52:502–506. [PubMed] [Google Scholar]
- 9.Vanholder R, Sever M, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553–1561. doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- 10.Okafor U. Lightning injuries and acute renal failure: A Review. Renal Failure. 2005;27:129–134. [PubMed] [Google Scholar]
- 11.Blaisdell F. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovascular Surgery. 2002;10:620–630. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 12.Kumar P. Compartment syndrome: pathophysiology. Burns. 2005;31:120. doi: 10.1016/S0305-4179(03)00136-0. [DOI] [PubMed] [Google Scholar]
- 13.Vanholder R, Sever M, Erek E, Lameire N. Acute renal failure related to the crush syndrome: towards an era of seismo-nephrology? Nephrol Dial Transplant. 2000;15:1517–1521. doi: 10.1093/ndt/15.10.1517. [DOI] [PubMed] [Google Scholar]
- 14.Lachiewicz PF, Latimer HA. Rhabdomyolysis following total hip arthroplasty. J Bone Joint Surg Br. 1991;73:576–579. doi: 10.1302/0301-620X.73B4.2071638. [DOI] [PubMed] [Google Scholar]
- 15.Biswas S, Gnanasekaran I, Ivatury RR, et al. Exaggerated lithotomy position- related rhabdomyolysis. Am Surg. 1997;63:361–364. [PubMed] [Google Scholar]
- 16.Mohr W, Petti T, Mohr B. Adverse effects associated with physical restraint. Can J Psyciatry. 2003;48:330–337. doi: 10.1177/070674370304800509. [DOI] [PubMed] [Google Scholar]
- 17.Slater MS, Mullins RJ. Rhabdomyolysis and myoglobinuric renal failure in trauma and surgical patients: A review. J Am Coll Surg. 1998;186:693–716. doi: 10.1016/s1072-7515(98)00089-1. [DOI] [PubMed] [Google Scholar]
- 18.Bouchama A, Knochel JP. Heatstroke. N Engl J Med. 2002;346:1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 19.Yeo TP. Heat Stroke: a comprehensive review. AACN Clin Issues. 2004;15:280–293. doi: 10.1097/00044067-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Ali SZ, Taguchi A, Rosenberg H. Malignant hyperthermia. Best Pract Res Clin Anaesthesiol. 2003;17:519–533. doi: 10.1016/j.bpa.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Pedrozzi NE, Ramelli GP, Tomasetti R, Nobile-Buetti L, Bianchetti MG. Rhabdomyolysis and anesthesia : A report of two cases and review of the literature. Pediatr Neurol. 1996;15:254–257. doi: 10.1016/s0887-8994(96)00171-3. [DOI] [PubMed] [Google Scholar]
- 22.Jones D, Story DA. Serotonin syndrome and the anaesthetist. Anaesth Intensive Care. 2005;33:181–187. doi: 10.1177/0310057X0503300205. [DOI] [PubMed] [Google Scholar]
- 23.Sulowicz W, Walatek B, Sydor A, et al. Acute renal failure in patients with rhabdomyolsis. Med Sci Monit. 2002;8:24–27. [PubMed] [Google Scholar]
- 24.Sakamoto K, Ohata M, Hashimoto K, et al. Two cases of alcoholics associated with rhabdomyolysis and acute renal failure. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2002;37:505–512. [PubMed] [Google Scholar]
- 25.Suzuki S, Nonaka A, Kumazawa T. Compartment syndrome in a young male caused by acute alcoholic rhabdomyolysis. Masui. 2002;51:1127–1128. [PubMed] [Google Scholar]
- 26.Doctora J, Williams C, Bennett C, Howlett B. Rhabdomyolysis in the acutely cocaine-intoxicated patient sustaining maxillofacial trauma: report of a case and review of the literature. J Oral Maxilofac Surg. 2003;61:964–967. doi: 10.1016/s0278-2391(03)00241-6. [DOI] [PubMed] [Google Scholar]
- 27.Fokko J. Cocaine use and kidney damage. Nephrol Dial Transplant. 2000;15:299–301. doi: 10.1093/ndt/15.3.299. [DOI] [PubMed] [Google Scholar]
- 28.Pogue V, Nurse H. Cocaine-associated acute myoglobinuric renal failure. The American Journal of Medicine. 1989;86:183–186. doi: 10.1016/0002-9343(89)90266-0. [DOI] [PubMed] [Google Scholar]
- 29.Gomez M, Castaneda M, Araujo AM, et al. Consequences of heroin consumption: compartmental syndrome and rhabdomyolysis. An Sist Sanit Navar. 2006;29:131–135. doi: 10.4321/s1137-66272006000100013. [DOI] [PubMed] [Google Scholar]
- 30.del C. Myopathy and rhabdomyolysis with lipid-lowering drugs. Toxicology Letters. 2002;128:159–168. doi: 10.1016/s0378-4274(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 31.Jamal S, Eisenberg M, Christopoulos S. Rhabdomyolysis associated with hydroxymethylglutaryl-coenzyme A reductase inhibitors. American Heart Journal. 2004;147:956–965. doi: 10.1016/j.ahj.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 32.Martin J, Krum H. Cytochrome P450 drug interactions within the HMG-CoA reductase inhibitor class. Are they clinically relevant? Drug Safety. 2003;26:13–21. doi: 10.2165/00002018-200326010-00002. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen C, Mazzone P. Muscle pain after exercise. Lancet. 1999;353:1062. doi: 10.1016/S0140-6736(98)11354-5. [DOI] [PubMed] [Google Scholar]
- 34.Trimarchi H, Gonzalez J, Olivero J. Hyponatremia-associated rhabdomyolysis. Nephron. 1999;82:274–277. doi: 10.1159/000045413. [DOI] [PubMed] [Google Scholar]
- 35.Shiber J, Mattu A. Serum phosphate abnormalities in the emergency department. The Journal of Emergency Medicine. 2002;23:395–400. doi: 10.1016/s0736-4679(02)00578-4. [DOI] [PubMed] [Google Scholar]
- 36.Thakur V, DeSalvo J, McGrath H, Jr, Weed S, Garcia C. Case report: polymyositis-induced myoglobinuric ARF. Am J Med Sci. 1996;312:85–87. doi: 10.1097/00000441-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Russell T. Acute renal failure related to rhabdomyolysis: pathophysiology, diagnosis, and collaborative management. Nephr Nurs J. 2005;32:409–417. [PubMed] [Google Scholar]
- 38.Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996;49:314–326. doi: 10.1038/ki.1996.48. [DOI] [PubMed] [Google Scholar]
- 39.Holt S, Moore K. Pathogenesis of renal failure in rhabdomyolysis: The role of myoglobin. Exp Nephrol. 2000;8:72–76. doi: 10.1159/000020651. [DOI] [PubMed] [Google Scholar]
- 40.Sauret J, Marinides G. Rhabdomyolysis. American Family Physician. 2002;65:907–912. [PubMed] [Google Scholar]
- 41.Haskins N. Rhabdomyolysis and acute renal failure in intensive care. Nurs Crit Care. 1998;3:283–288. [PubMed] [Google Scholar]
- 42.Bonventre J, Shah S, Walker P, Humphreys M. Rhabdomyolysis-induced acute renal failure. In: Jacobson, Striker, Klahr, editors. The principles and practice of nephrology. 2nd ed. St Louis: Mospy; 1995. pp. 569–573. [Google Scholar]
- 43.David WS. Myoglobinuria. Neurol Clin. 2000;18:215–243. doi: 10.1016/s0733-8619(05)70187-0. [DOI] [PubMed] [Google Scholar]
- 44.Holt SG, Moore KP. Pathogenesis and treatment of renal dysfunction in rhabdomyolysis. Intensive Care Med. 2001;27:803–811. doi: 10.1007/s001340100878. [DOI] [PubMed] [Google Scholar]
- 45.Gunal A, Celiker H, Dogukan A, et al. Early and vigorous fluid resuscitation prevents acute renal failure in the crush victims of catastrophic earthquakes. J Am Soc Nephrol. 2004;15:1862–1867. doi: 10.1097/01.asn.0000129336.09976.73. [DOI] [PubMed] [Google Scholar]