Abstract
A cell culture immunofluorescence (CCIF) assay was optimized for detection of porcine pararotavirus (group C rotavirus) in intestinal contents. The greatest viral infectivity was observed when MA104 cells (5 days after subculturing) were rinsed and refed in serum-free medium before inoculation, pancreatin was added to the inocula, and the inocula were centrifuged onto the cells. Gentamicin treatment of pararotavirus samples to reduce bacterial contamination also reduced the viral infectivity of these samples for MA104 cells. An indirect CCIF assay was used to determine the prevalence of pararotavirus and rotavirus antibodies in pig sera. In pigs from four herds, pararotavirus antibodies were detected in 100% (68 of 68) of adults and 59% (24 of 41) of weanling pigs, while 86% (24 of 28) of nursing pigs from 12 herds had pararotavirus antibodies. The physicochemical properties of pararotavirus were examined and compared with those of group A rotaviruses by using the CCIF assay to quantitate in vitro changes in viral infectivity. Pararotavirus was inactivated (greater than or equal to 99% reduction in titer) by heating to 56 degrees C for 30 min, was slightly labile at pH 3 (16 to 34% reduction in titer), and was stable at pH 5 (0 to 17% reduction in titer) and in either (3 to 19% reduction in titer). One group A rotavirus (Gottfried strain) was stable at 56 degrees C (0% reduction in titer), whereas the OSU strain of group A rotavirus was inactivated at this temperature (99% reduction in titer).
Full text
PDF

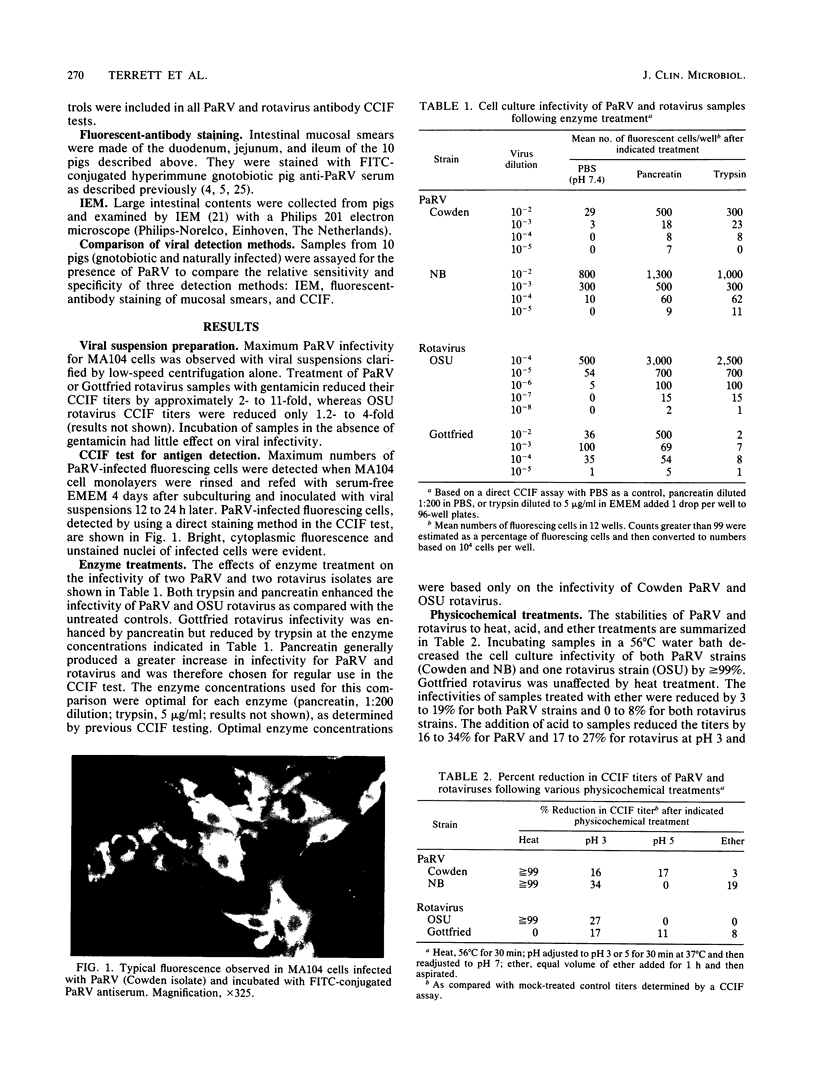


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Mohammed K. A. Trypsin and bovine rotavirus replication. Vet Rec. 1978 Jan 21;102(3):61–62. doi: 10.1136/vr.102.3.61. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Mohammed K., Spence L., Fauvel M., Petro R. Rotavirus isolation and cultivation in the presence of trypsin. J Clin Microbiol. 1977 Dec;6(6):610–617. doi: 10.1128/jcm.6.6.610-617.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banatvala J. E., Totterdell B., Chrystie I. L., Woode G. N. In-vitro detection of human rotaviruses. Lancet. 1975 Oct 25;2(7939):821–821. doi: 10.1016/s0140-6736(75)80057-2. [DOI] [PubMed] [Google Scholar]
- Bohl E. H., Kohler E. M., Saif L. J., Cross R. F., Agnes A. G., Theil K. W. Rotavirus as a cause of diarrhea in pigs. J Am Vet Med Assoc. 1978 Feb 15;172(4):458–463. [PubMed] [Google Scholar]
- Bohl E. H., Saif L. J., Theil K. W., Agnes A. G., Cross R. F. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982 Feb;15(2):312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohl E. H., Theil K. W., Saif L. J. Isolation and serotyping of porcine rotaviruses and antigenic comparison with other rotaviruses. J Clin Microbiol. 1984 Feb;19(2):105–111. doi: 10.1128/jcm.19.2.105-111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C., Brown J. F. Prevalence of antibody to typical and atypical rotaviruses in pigs. Vet Rec. 1985 Jan 12;116(2):50–50. doi: 10.1136/vr.116.2.50. [DOI] [PubMed] [Google Scholar]
- Bridger J. C. Detection by electron microscopy of caliciviruses, astroviruses and rotavirus-like particles in the faeces of piglets with diarrhoea. Vet Rec. 1980 Dec 6;107(23):532–533. [PubMed] [Google Scholar]
- Bridger J. C., Pedley S., McCrae M. A. Group C rotaviruses in humans. J Clin Microbiol. 1986 Apr;23(4):760–763. doi: 10.1128/jcm.23.4.760-763.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden A. S., Davies H. A., Thouless M. E., Flewitt T. H. Diagnosis of rotavirus infection by cell culture. J Med Microbiol. 1977 Feb;10(1):121–125. doi: 10.1099/00222615-10-1-121. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Inversion of the two segments of the herpes simplex virus genome in intertypic recombinants. J Gen Virol. 1983 Jan;64(Pt 1):1–18. doi: 10.1099/0022-1317-64-1-1. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. H., Estes M. K., Rangelova S. M., Shindarov L. M., Melnick J. L., Graham D. Y. Detection of antigenically distinct rotaviruses from infants. Infect Immun. 1983 Aug;41(2):523–526. doi: 10.1128/iai.41.2.523-526.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Puerto F., Soler C., González N. Characterization of a human pararotavirus. Infect Immun. 1984 Apr;44(1):112–116. doi: 10.1128/iai.44.1.112-116.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Smith E. M., Gerba C. P. Rotavirus stability and inactivation. J Gen Virol. 1979 May;43(2):403–409. doi: 10.1099/0022-1317-43-2-403. [DOI] [PubMed] [Google Scholar]
- MEYER R. C., BOHL E. H., KOHLER E. M. PROCUREMENT AND MAINTENANCE OF GERM-FREE SEINE FOR MICROBIOLOGICAL INVESTIGATIONS. Appl Microbiol. 1964 Jul;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas J. C., Cohen J., Fortier B., Lourenco M. H., Bricout F. Isolation of a human pararotavirus. Virology. 1983 Jan 15;124(1):181–184. doi: 10.1016/0042-6822(83)90302-1. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Bishop R. F., Holmes I. H. Detection of a rotavirus-like agent associated with diarrhea in an infant. J Clin Microbiol. 1982 Oct;16(4):724–726. doi: 10.1128/jcm.16.4.724-726.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Further biochemical characterization, including the detection of surface glycoproteins, of human, calf, and simian rotaviruses. J Virol. 1977 Oct;24(1):91–98. doi: 10.1128/jvi.24.1.91-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L. J., Bohl E. H., Kohler E. M., Hughes J. H. Immune electron microscopy of transmissible gastroenteritis virus and rotavirus (reovirus-like agent) of swine. Am J Vet Res. 1977 Jan;38(1):13–20. [PubMed] [Google Scholar]
- Saif L. J., Bohl E. H., Theil K. W., Cross R. F., House J. A. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J Clin Microbiol. 1980 Jul;12(1):105–111. doi: 10.1128/jcm.12.1.105-111.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil K. W., Bohl E. H., Agnes A. G. Cell culture propagation of porcine rotavirus (reovirus-like agent). Am J Vet Res. 1977 Nov;38(11):1765–1768. [PubMed] [Google Scholar]
- Theil K. W., Bohl E. H., Cross R. F., Kohler E. M., Agnes A. G. Pathogenesis of porcine rotaviral infection in experimentally inoculated gnotobiotic pigs. Am J Vet Res. 1978 Feb;39(2):213–220. [PubMed] [Google Scholar]
- Theil K. W., Bohl E. H. Porcine rotaviral infection of cell culture: effects of certain enzymes. Am J Vet Res. 1980 Jan;41(1):140–143. [PubMed] [Google Scholar]
- Theil K. W., Bohl E. H., Saif L. J. Techniques for rotaviral propagation. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):548–551. [PubMed] [Google Scholar]
- Theil K. W., Saif L. J. In vitro detection of porcine rotavirus-like virus (group B rotavirus) and its antibody. J Clin Microbiol. 1985 May;21(5):844–846. doi: 10.1128/jcm.21.5.844-846.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouless M. E., Bryden A. S., Flewett T. H., Woode G. N., Bridger J. C., Snodgrass D. R., Herring J. A. Serological relationships between rotaviruses from different species as studied by complement fixation and neutralization. Arch Virol. 1977;53(4):287–294. doi: 10.1007/BF01315627. [DOI] [PubMed] [Google Scholar]
- Vonderfecht S. L., Huber A. C., Eiden J., Mader L. C., Yolken R. H. Infectious diarrhea of infant rats produced by a rotavirus-like agent. J Virol. 1984 Oct;52(1):94–98. doi: 10.1128/jvi.52.1.94-98.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch A. B., Thompson T. L. Physiochemical characterization of a neonatal calf diarrhea virus. Can J Comp Med. 1973 Jul;37(3):295–301. [PMC free article] [PubMed] [Google Scholar]