Abstract
Purpose
Temozolomide (TMZ), given concurrently with radiotherapy (RT) and as adjuvant monotherapy afterwards, has led to improved survival in glioblastoma multiforme (GBM). However, it is unclear whether its primary mechanism of action is through enhancement of radiation response or through independent cytotoxicity. We sought to determine the relative contribution of concomitant temozolomide in patients treated by concurrent and adjuvant TMZ versus adjuvant TMZ alone in the setting of newly-diagnosed GBM.
Patients and Methods
We identified patients diagnosed with GBM and treated with surgery, involved-field radiotherapy, and chemotherapy at MGH between January 1, 2002 and December 31, 2004. Eligible patients received either adjuvant temozolomide (group 1) alone or temozolomide concurrently with RT followed by adjuvant TMZ (group 2). The primary endpoint of this retrospective analysis is overall survival (OS), and the secondary endpoint is progression-free survival (PFS).
Results
Forty-three patients (group 1, n=21; group 2, n=22) were included in this study. The median follow-up is 33.7 months for surviving patients. There were no significant differences in baseline characteristics (including age, KPS, resection status, and RPA class) between these two groups. Thrombocytopenia was more pronounced in group 1 (p=0.0407), but there were no other significant differences in toxicity. On univariate analysis, patients who received concurrent and adjuvant temozolomide (Group 2) experienced a 2-year OS of 51% and a median survival of 25.5 months, compared with a 2-year OS of 36% and a median survival of 15.6 months for Group 1 patients. This difference was statistically significant (log-rank p=0.0495). On multivariable analysis, the hazard ratio (HR) favoring concurrent TMZ trended towards significance (HR = 0.508, p=0.0839) despite modest patient numbers. There was no statistically significant difference in PFS.
Conclusions
Concurrent and adjuvant TMZ is associated with improved survival compared to adjuvant TMZ alone. These results highlight the potentiation of radiation effect by temozolomide in the clinical setting.
Introduction
Glioblastoma (GBM), despite aggressive surgery and radiotherapy, had essentially been a uniformly fatal disease. Trials of systemic chemotherapy provided mixed results but overall were disappointing1, 2. However, the introduction of temozolomide (TMZ) in the setting of newly-diagnosed GBM has made a substantial impact in the treatment of this disease. The EORTC recently reported the results of a phase III randomized trial that showed significantly improved overall and progression-free survival in patients who received temozolomide3 compared to those who were managed with radiation alone. Patients in the TMZ arm of the trial received concurrent low-dose temozolomide during radiotherapy and then monthly adjuvant temozolomide for 6 months or until treatment failure, resulting in an absolute 16.1% increase in survival at 2 years3.
Despite these findings, it is unclear whether the primary benefit of temozolomide is mediated through its concurrent or adjuvant delivery. Pre-clinical evidence suggests that temozolomide acts as a radiation enhancing agent4-6. Therefore, it is possible that the observed survival benefit is a function of improved local control from radiation potentiation. Although temozolomide is well-tolerated, it is important to isolate the mechanism through which it exerts its effect. Thus far no clinical studies have compared the different modes of delivery of temozolomide in an attempt to distinguish this mechanism.
Glioblastoma patients at the Massachusetts General Hospital have been managed with temozolomide since 2001. Originally the drug was prescribed as adjuvant treatment, but after the results of phase III EORTC trial were reported, patients were treated with concurrent temozolomide as well. Since radiotherapy dose and technique were stable over this time, our experience is a natural comparison of the difference in survival between patients who receive concurrent and adjuvant TMZ (i.e. Stupp protocol) versus adjuvant drug alone. We report the survival outcomes in a cohort of patients who were treated with either the Stupp protocol or adjuvant temozolomide alone.
Materials and Methods
Study design
This study is a retrospective chart and radiology review. Data were abstracted from the electronic medical record and imaging computing system. This study was approved by the Massachusetts General Hospital (MGH) Investigational Review Board (IRB). The MGH Cancer Registry compiles a database of all patients diagnosed with a malignancy at its hospital, and the patients in this registry formed the basis of this study cohort.
Patient eligibility
Patient eligibility was strictly defined by the following characteristics. All patients had a diagnosis of glioblastoma multiforme (GBM) at MGH between 1/1/02 and 12/31/04. This time frame was selected to ensure adequate follow-up. All patients underwent surgery (and/or biopsy) at MGH, and the radiotherapy (RT) was delivered at MGH. Patients were excluded if they received less than 56 Gy of RT. Patients were required to have at least one documented post-RT follow-up visit at MGH in which the adjuvant therapy was specified.
Many patients were enrolled in a variety of clinical protocols over this time, many of which included temozolomide. Patients who received an experimental treatment in addition to TMZ were excluded, although patients who received concurrent celecoxib or rofecoxib were included. Patients were placed into the concurrent temozolomide arm (group 1) if they received daily, low-dose TMZ (75 mg/m2) and then adjuvant TMZ (typically 200 mg/m2 in 5 day cycles). Patients who underwent RT without concurrent TMZ were placed into the adjuvant TMZ-only arm if they received adjuvant TMZ alone after RT. Patients who clinically progressed during radiation where treatment had to be interrupted or was incomplete were not included in this study.
Radiation treatment
All patients were treated with involved-field radiotherapy (IFRT), either in 1.8 Gy or 2 Gy fractions using 3-dimensional conformal treatment planning, with a planned total dose of 60 Gy. Dose was in part determined by critical structure tolerance. Patients who received a stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT) boost were excluded. One patient received a total of 61.5 Gy, in which 54 Gy was delivered to the involved field, and 7.5 Gy was given using SRT in 2.5 Gy fractions in order to meet normal tissue constraints.
Temozolomide treatment
When taken during radiotherapy, temozolomide was initially prescribed at a dose of 75 mg/m2. The majority of patients received adjuvant TMZ at a dose of 150-200 mg/m2 in 5 day cycles every 4 weeks, but 10 patients in group 2 continued to receive daily low-dose TMZ as adjuvant therapy. Toxicity during treatment was defined by the NCI Common Toxicity Criteria version 3.0.
Radiology review
Each patient's serial MR images were reviewed by a neuroradiologist (JH) who was blinded to temozolomide status. The MRI scan closest to the start of radiation treatment was used as the baseline study. In some patients, the post-operative MRI was used as the baseline scan. Progression was defined using an adaptation of the RECIST criteria7. Progression was defined as an increase by 20% of the largest dimension of the tumor; measurements could be taken in the axial, sagittal and coronal planes. Nonmeasureable tumors were defined as masses whose largest cross-sectional dimension was less than 10 mm. The appearance of new lesions was also considered evidence of progression.
Statistical methods
The primary endpoint for this study was overall survival (OS), defined as the time between the date of resection and death from any cause. For patients who underwent biopsy only, the date of biopsy was used instead of the resection date. The secondary endpoint for this study was progression-free survival (PFS), defined as the time between date of surgery (or biopsy) and the MRI scan indicating progression.
Baseline and treatment characteristics between group 1 and group 2 were compared using Fisher's exact test. Differences in toxicity between the two groups were also assessed using Fisher's exact test. Continuous variables were tested for normality, and normally distributed characteristics were compared using a t-test. Non-normal variables were compared using a Wilcoxon rank sum test.
Univariate predictors of survival were tested using the log-rank statistic. The variable representing Karnofsky performance status (KPS) was dichotomized due to inadequate sample size in each strata.
A bivariable Cox regression model was created to evaluate OS and PFS after adjustment for RPA class, since that variable incorporates several clinical factors which are prognostic for survival. Only two covariates were included in this model due to the small number of events, and this parsimony was an attempt to avoid overfitting.
SAS software (Raleigh, NC) was used for all analyses.
Results
Patient selection and follow-up
Between January 1, 2002 and December 31, 2004, a total of 107 patients were treated with surgical resection and radiotherapy at MGH for GBM and had at least 1 follow-up visit which clearly specified the delivered adjuvant therapy (as well as any concurrent chemotherapy during RT). Nineteen patients received no concurrent or adjuvant treatment, 10 received concurrent TMZ only, 22 patients were given non-TMZ concurrent and/or adjuvant chemotherapy, and 12 patients received a stereotactic boost and were, hence, excluded. One patient who was prescribed concurrent and adjuvant TMZ received 54 Gy and was excluded.
Thus, a total of 43 patients form the final study cohort. Twenty-one patients received adjuvant TMZ only (group 1), and 22 patients were treated with concurrent and adjuvant temozolomide (group 2). Ten patients in group 2 received daily adjuvant temozolomide instead of higher-dose monthly TMZ. The median follow-up time for surviving patients is 33.7 months (interquartile range 28.4-36 months).
Clinical and treatment characteristics
Baseline clinical characteristics are listed in table 1. Although there was a trend for more favorable KPS and RPA class in group 2, these differences were not statistically significant.
Table 1.
Baseline clinical characteristics.
| Characteristic | Group 1 Adjuvant TMZ only N=21 | Group 2 Concurrent & adjuvant TMZ N=22 | P value |
|---|---|---|---|
| Age (mean, SE) | 57.8 (2.4) | 55.5 (2.6) | 0.65291 |
| Gender | |||
| Male | 11 (52%) | 15 (68%) | 0.35802 |
| Female | 10 (48%) | 7 (32%) | |
| KPS | |||
| <70 | 9 (43%) | 4 (18%) | 0.10402 |
| ≥70 | 12 (57%) | 18 (82%) | |
| Able to work | |||
| Yes | 17 (81%) | 15 (68%) | 0.48762 |
| No | 4 (19%) | 7 (32%) | |
| RPA class | |||
| 3 | 2 (9%) | 4 (18%) | |
| 4 | 8 (38%) | 9 (41%) | 0.61702 |
| 5 | 6 (29%) | 7 (32%) | |
| 6 | 5 (24%) | 2 (9%) | |
P value derived from t-test.
P value derived from Fisher's exact test.
Treatment characteristics are described in table 2. Treatment variables were well distributed between these two groups, with no significant differences between them. There was a trend towards more cycles of temozolomide in group 2, but this difference was not statistically significant. Among patients who received monthly adjuvant temozolomide, the dose (mg/m2) was not significantly different (group 1 median 176 mg/m2 vs. group 2 median 193 mg/m2, Wilcoxon p=0.1956).
Table 2.
Treatment characteristics.
| Characteristic | Group 1 Adjuvant TMZ only N=21 | Group 2 Concurrent & adjuvant TMZ N=22 | P value |
|---|---|---|---|
| Resection status | |||
| Biopsy | 6 (29%) | 5 (23%) | |
| STR | 8 (38%) | 12 (54%) | 0.58121 |
| GTR | 7 (33%) | 5 (23%) | |
| XRT dose | |||
| 56-58 Gy | 2 (10%) | 1 (5%) | 0.92011 |
| ≥59.4 Gy | 19 (90%) | 21 (95%) | |
| Cycles of TMZ | 6 [3,8] | 9.5 [4,16] | 0.22242 |
| (median, IQ range) | |||
| Salvage local tx | |||
| SRS | 2 (10%) | 1 (5%) | |
| SRT | 1 (5%) | 3 (14%) | 0.14731 |
| Brachytherapy | 1 (5%) | 1 (5%) | |
| Salvage surgery | 3 (14%) | 3 (14%) | |
P value derived from Fisher's exact test.
P value derived from Wilcoxon rank sum test.
Univariate survival analyses
Univariate survival analyses are reported in table 3. Treatment with concurrent temozolomide was associated with a significant survival benefit (MST 25.5 months vs 15.6 months, p=0.0495). Figure 1 displays a Kaplan-Meier graph comparing these two regimens. There was no difference in survival between patients who received daily or monthly adjuvant TMZ (log rank p =0.3701).
Table 3.
Univariate analysis of overall survival.
| Characteristic | N | Median survival | 2-year survival | P1 |
|---|---|---|---|---|
| Treatment regimen | ||||
| Group 1 (Adjuvant TMZ only) | 21 | 15.6 months | 36% | 0.0495 |
| Group 2 (Concurrenent & adjuvant TMZ) | 22 | 25.5 months | 51% | |
| Age | ||||
| Age ≤ 50 years | 12 | -- Not met -- | 80% | 0.0299 |
| Age > 50 years | 31 | 16.9 months | 32% | |
| Resection status | ||||
| Biopsy | 11 | 10.3 months | 0% | |
| STR | 20 | 28.7 months | 53% | 0.00022 |
| GTR | 12 | 28.3 months | 60% | 0.43953 |
| KPS | ||||
| <70 | 13 | 15.6 months | 35% | 0.1212 |
| ≥70 | 30 | 23 months | 48% | |
| RPA class | ||||
| 3 | 6 | -- Not met -- | 80% | |
| 4 | 17 | 28.7 months | 63% | 0.0139 |
| 5 | 13 | 17.6 months | 23% | |
| 6 | 7 | 10.3 months | 14% | |
All p values derived from log-rank test.
Survival differences between biopsy, STR and GTR.
Survival difference between STR and GTR.
Figure 1.
Kaplan-Meier graph of overall survival, stratified by protocol. Solid line = Adjuvant temozolomide only (group 1). Dashed line = concurrent and adjuvant treatment temozolomide (group 2). The difference between these curves is statistically significant (log-rank p=0.0495).
Younger age and non-biopsy resection status were also associated with improved survival. There was a trend for improved survival with higher KPS dichotomized at 70, but this relationship was not statistically significant.
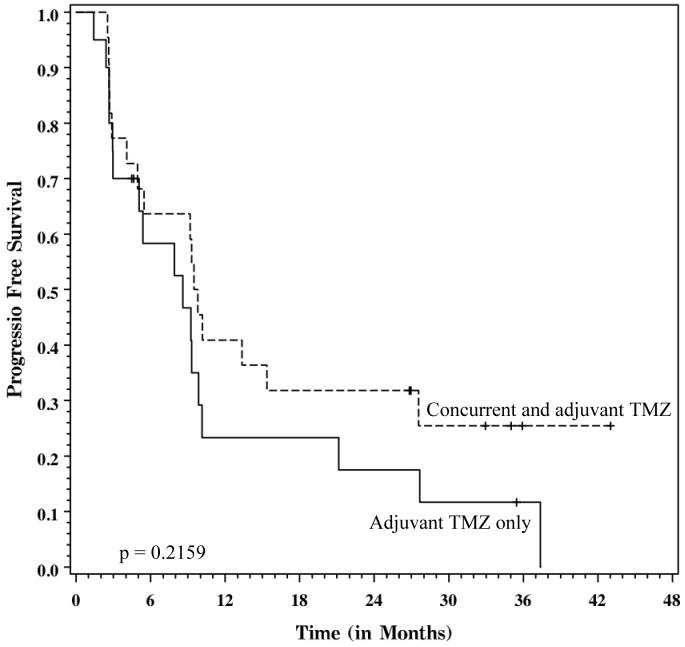
There was no significant difference in progression-free survival (group 1 MST 8.6 months vs. group 2 MST 9.7 months, p=0.2159), although there was a trend for improved progression-free survival at 2 years in the concurrent TMZ group (group 1 2-year PFS 18% vs. group 1 2-year PFS 32%).
Multivariate survival analyses
Due to the small number of events, a bivariable Cox proportional hazards model was designed to adjust for RPA class. The results are shown in table 3. Treatment with concurrent temozolomide was associated with a hazard ratio of 0.51 (95% CI 0.24-1.1) compared to adjuvant TMZ alone. There was a trend towards significance (p=0.0839). There was no significant relationship between radiographic progression and temozolomide status.
Toxicity
Temozolomide was generally well tolerated in both groups. There was significantly more thrombocytopenia in group 1, and there was a borderline significant increase in leukopenia in group 2. In total, there was no significant difference in the amount of grade 2 or higher toxicity (p=1.00, Fisher's exact test).
Discussion
In this highly selected cohort, we have shown that treatment with concurrent and adjuvant temozolomide is associated with a significant increase in overall survival compared to adjuvant temozolomide alone on univariate analysis. After adjustment for RPA class, this relationship maintained borderline significance. With additional follow-up, it is possible that concurrent treatment will again regain significance, as the survival curves are continuing to diverge.
These findings are consistent with pre-clinical and in vitro data which suggest that temozolomide acts as a radiation sensitizer. For example, Chakravarti et al. showed that glioma cell lines which do not express O(6)-methylguanine-DNA methyltransferase (MGMT) are significantly more susceptible to killing by radiation when the drug is given concurrent with the irradiation versus after irradiation4. The authors found that temozolomide enhances double-stranded DNA breaks and apoptosis after radiation, both of which could enhance radiosensitization.
Our data also bring up several other issues in the management of this disease. First, overall survival for GBM is improving. Patients who only received adjuvant temozolomide experienced a median survival of 15.5 months, which is on par with the median survival from the experimental arm of the EORTC trial3. The survival for patients who received TMZ according to the Stupp protocol fared extremely well, with a 2-year overall survival of 51%. In addition, traditional prognostic factors were confirmed in our results, as RPA class, age and non-biopsy resection status were all favorable characteristics.
One of the strengths of this study is the relative standardization of treatment. All patients underwent surgical treatment at MGH, and the radiotherapy was planned and delivered in one department. Surviving patients have been followed for over two years, so it is unlikely that these results are due to inadequate follow-up. Since the neurooncologists generally started using concurrent temozolomide after the preliminary results of the EORTC trial were released, the decision to use the drug was more a function of time and not patient characteristics. This natural experiment tends to reduce the influence of unmeasured confounders.
That said, this study has several limitations. First, it is a retrospective analysis and subject to the limitations of that study design. One critical issue is selection bias, as patients who were more fit were more likely to receive concurrent temozolomide; this confounding factor is seen in the bias towards slightly better RPA classes for group 2. Furthermore, selection bias likely enriched both groups for patients with more favorable disease, in part because patients were excluded if they progressed through radiation treatment and, hence, did not receive all of the planned treatment. In comparison, the EORTC randomized trial reported intention-to-treat results, including 22% of patients who never started adjuvant temozolomide. In addition, patients in both groups were eligible for many other experimental protocols. Thus patients who received adjuvant temozolomide alone were either not candidates for the other protocols or chose not to enroll, which could have selected that cohort for patients with worse prognoses. However, the same argument could be made for patients in group 2, so it is unclear the extent to which selection bias could account for these results. Finally, ten patients were treated with daily adjuvant TMZ, which is not standard treatment, although their survival outcomes were not improved.
The absence of a relationship between treatment regimen and progression-free survival is somewhat surprising. One potential explanation is the challenge of assessing radiographic progression. Necrosis and post-operative stroke can be confused with progressive disease, and the clinical picture may not be consistent with worsening disease8.
In summary, our results are consistent with the evolving paradigm of combined-modality therapy and fit in nicely with the experience from other disease sites9-12. It will be important to determine temozolomide's mechanism of action, since future approaches to GBM treatment should build upon the success of this agent. If the drug primarily acts through radiosensitization, then the use of additional non-TMZ adjuvant chemotherapy should be actively investigated.
Figure 2.
Kaplan-Meier graph of progression-free survival, stratified by protocol. Dashed line = Adjuvant temozolomide only (group 1). Solid line = concurrent and adjuvant treatment temozolomide (group 2). The difference between these curves is not statistically significant (log-rank p=0.2159).
Table 4.
Cox bivariable regression models for overall survival.
| Outcome and covariates | HR | 95% CI | P |
|---|---|---|---|
| Overall survival | |||
| Concurrent/adjuvant TMZ vs. adjuvant TMZ | 0.508 | 0.24-1.1 | 0.0839 |
| RPA class | 1.994 | 1.28-3.11 | 0.0024 |
| Progression-free survival | |||
| Concurrent/adjuvant TMZ vs. adjuvant TMZ | 0.679 | 0.34-1.4 | 0.2774 |
| RPA class | 1.788 | 1.23-2.59 | 0.0021 |
Table 5.
Toxicity during adjuvant temozolomide chemotherapy.
| Characteristic | Group 1 Adjuvant TMZ only N=21 | Group 2 Concurrent & adjuvant TMZ N=22 | P |
|---|---|---|---|
| Thrombocytopenia | |||
| Grade 1 | 3 (14%) | 3 (14%) | |
| Grade 2 | 0 | 0 | 0.0407 |
| Grade 3 | 2 (10%) | 0 | |
| Grade 4 | 4 (19%) | 0 | |
| Leukopenia | |||
| Grade 1 | 4 (19%) | 1 (5%) | |
| Grade 2 | 0 | 5 (23%) | 0.0685 |
| Grade 3 | 1 (5%) | 0 | |
| Grade 4 | 1 (5%) | 1 (5%) | |
| Anemia | |||
| Grade 1 | 1 (5%) | 0 | |
| Grade 2 | 1 (5%) | 1 (5%) | 0.7379 |
| Grade 3 | 0 | 1 (5%) | |
| Grade 4 | 0 | 2 (9%) | |
| Upper GI toxicity | |||
| Grade 1 | 5 (24%) | 5 (23%) | |
| Grade 2 | 1 (5%) | 3 (14%) | 0.5290 |
| Grade 3 | 2 (10%) | 0 | |
| Grade 4 | 0 | 1 (5%) | |
| Radiation dermatitis | |||
| Grade 1 | 8 (38%) | 4 (18%) | |
| Grade 2 | 1 (5%) | 3 (14%) | 0.2576 |
| Grade 3 | 0 | 0 | |
| Grade 4 | 0 | 0 | |
References
- 1.Fine HA, Dear KB, Loeffler JS, et al. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol. 2001;19:509–518. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Chakravarti A, Erkkinen MG, Nestler U, et al. Temozolomide-mediated radiation enhancement in glioblastoma: a report on underlying mechanisms. Clin Cancer Res. 2006;12:4738–4746. doi: 10.1158/1078-0432.CCR-06-0596. [DOI] [PubMed] [Google Scholar]
- 5.Wedge SR, Porteous JK, Glaser MG, et al. In vitro evaluation of temozolomide combined with X-irradiation. Anticancer Drugs. 1997;8:92–97. doi: 10.1097/00001813-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 6.van Rijn J, Heimans JJ, van den Berg J, et al. Survival of human glioma cells treated with various combination of temozolomide and X-rays. Int J Radiat Oncol Biol Phys. 2000;47:779–784. doi: 10.1016/s0360-3016(99)00539-8. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Ulmer S, Braga TA, Barker FG, 2nd, et al. Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology. 2006;67:1668–1670. doi: 10.1212/01.wnl.0000242894.21705.3c. [DOI] [PubMed] [Google Scholar]
- 9.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 10.Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990;323:940–945. doi: 10.1056/NEJM199010043231403. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 12.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]