Abstract
This study describes the use of a novel magnetic resonance imaging (MRI) compatible system capable of measuring isometric ankle, knee and hip joint torques in real-time during functional MRI (fMRI) testing in healthy volunteers. The motor representations of three isometric torques - ankle dorsiflexion, ankle plantarflexion and knee extension – were studied at two time points. The reliability of motor performance and fMRI-derived measures of brain activity across sessions was examined. Reproducible motor performance was observed for each of the tasks; torques of the requested amplitude, assisted by visual feedback, were generated at the relevant joint with good accuracy, both within and across the two sessions. Significant blood oxygen level dependent (BOLD) signal increases were observed in left primary sensorimotor cortex (SM1) in the paracentral lobule and in secondary motor areas for all tasks. Within these areas there was substantial overlap of the motor representations though differential activation was observed in SM1, with greater activation of inferior paracentral lobule during knee extension than for either ankle task. Also, BOLD signal decreases were observed bilaterally within SM1 in the hand knob region for all tasks. No major session related effects were identified at the group level. High intraclass correlation coefficients were observed for t-values of voxels in cortical motor areas for each contraction type for individuals, suggesting that fMRI-derived activity across time points was reliable. These findings support the use of this apparatus in serial studies of lower limb function.
Introduction
To assess cortical adaptations for the control of lower limb movements over the course of rehabilitation interventions for walking, investigators have developed experimental designs to assess the motor representations of unilateral movements of the ankle or knee with fMRI.(Dobkin, 2005) As with all neuroimaging studies of motor control, there is a need to reduce task-correlated head movement and to isolate movement to the joint of interest without contraction of additional musculature. This has been addressed by use of restraints or supports of other parts of the lower limb or pelvis to facilitate isolated movement at the joint of interest (Debaere et al., 2001; Luft et al., 2002; Carey et al., 2004; Sahyoun et al., 2004; Luft et al., 2005; Ciccarelli et al., 2005; Kapreli et al., 2006; Ciccarelli et al., 2006; Kapreli et al., 2007). In order to control the direction of the movements some investigators employed restraints that constrained the angle through which the joint of interest could be moved (Debaere et al., 2001; Dobkin et al., 2004; Sahyoun et al., 2004; Ciccarelli et al., 2005; Kapreli et al., 2006; Christensen et al., 2006; Ciccarelli et al., 2006; Kapreli et al., 2007).
In addition to limiting associated movements, measurement and feedback of motor output is likely to reduce trial-wise variation in subject performance and standardize behavior between scans in longitudinal studies. Use of electrogoniometers has proved successful for monitoring and providing feedback of movements of the ankle (Debaere et al., 2001; Macintosh et al., 2004; Christensen et al., 2006) and knee (Carey et al., 2004). However, in previous studies, measurement of lower limb motor output has been limited to the joint of interest. Associated contractions of other stabilizing joint muscle groups and compensatory torques across joints may not have been appreciated during fMRI scanning. In addition, aspects of locomotor control are likely to be reflected by differential flexor or extensor movements at the hip, knee, and ankle. Thus, prior scan-compatible devices had inherent limitations on evaluating changes in motor control of the lower extremities.
Here we describe the use of a novel MR compatible system capable of measuring torques1 generated at the ankle, knee and hip joints simultaneously, as well as providing visual feedback of the torque at the relevant joint to the subject. The apparatus is based on technology used previously to measure isometric forces generated by the hand and joint moments along wrist flexion–extension and wrist ulnar–radial deviation axes during wrist extension in an MR environment (Hidler et al., 2006).
The aim of the present study was to investigate the motor representations of three different types of isometric contractions - ankle dorsiflexion, ankle plantarflexion and knee extension – and to assess the reliability of motor performance and fMRI-derived measures of brain activity across two time points in each participant. We hypothesize that use of this novel apparatus will result in reliable motor output and motor representations and that this will facilitate future longitudinal studies of lower limb motor control.
Materials and Methods
Subjects
Nine right-handed healthy volunteers with no history of neurological abnormality were studied (4 male, 5 female; age range = 21–58 years, median = 39 years). Written consent was obtained from all participants in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board at the University of California Los Angeles. Volunteers participated in the full protocol (task training and scanning) on two sessions with a mean interval of 15 days (range: 3–30 days).
Instrumentation
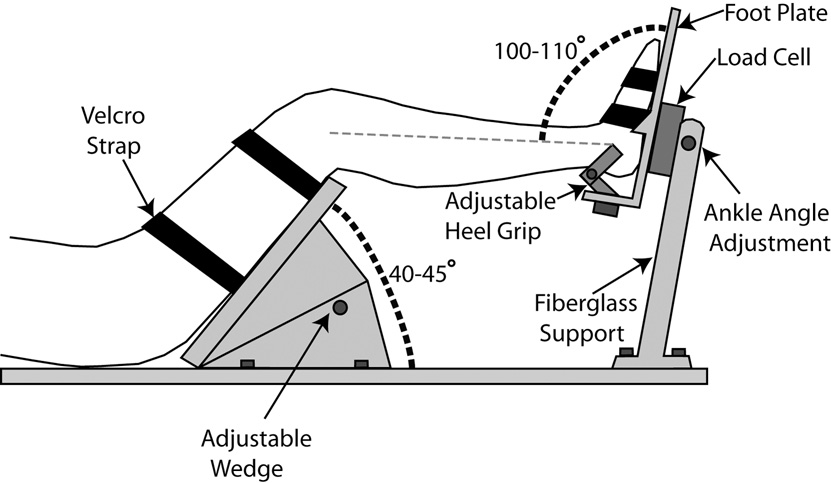
The novel test apparatus used in this study was custom built, allowing for the simultaneous measurement of ankle and knee torques generated by subjects during fMRI testing (Figure 1). A 6-axis non-magnetic load cell built by JR3 Inc. (model 45E15A; Woodland, CA) was mounted to a fiberglass support structure, providing side-to-side adjustability so that either leg can be tested, and also allowing for the ankle angle to be adjusted. The subject’s foot was firmly coupled to the load cell through a custom footplate made of a combination of aluminum and brass. The entire fiberglass assembly was mounted to a long sheet of polyethylene, on which the subject laid during testing. In this capacity, the subject’s body-weight acted to anchor the system down preventing movement.
Figure 1.
Cross-sectional diagram showing subject positioning and restraint within the torque-measuring apparatus.
In order to minimize head movement resulting from forces exerted at the foot, two different strategies were employed. First, the subject’s hip and knee joints were flexed to approximately 45° each. With the leg in this posture, exertions at the foot have a tendency to rotate the leg rather than propagate directly to the head. To capture this effect, the thigh was firmly strapped to a polyethylene wedge mounted to the base (Figure 1).
A 30’ cable extended from the load cell and connected to an optical isolation panel with a non-magnetic connector, where the load cell signals and sensor power were transferred between the control room and scanner room. All analog signals were read into a PC through a 12-bit data acquisition card (DAS1200, Measurement Computing, Middleboro, MA) using custom software (Matlab, Mathworks, Natick MA). Signals were low-pass filtered at 50 Hz before being sampled at 500 Hz.
Forces and torques measured at the load cell were used to compute the subject’s ankle and knee torques in real-time along all three planes (e.g. flexion-extension, inversion-eversion, and internal-external rotation) using an inverse statics analysis (Craig, 1989; Hidler et al., 2007). This involves transformation of the force and torques measured at the load cell back to the different joints using skew and rotation matrices formed from anatomical measurements made for each subject (shank and thigh lengths, knee and shank angles). (Craig, 1989) The details of the inverse static analysis for a slight variant of the current apparatus have been described previously.(Neckel et al., 2006) These transformations have been shown to result in errors in joint torque predictions of less than 3% (Hidler et al., 2007). Hip torque was also estimated using the same technique. However, the resulting measurements will not have the same accuracy as those for the ankle and knee torques due to the presence of the wedge beneath the subject’s thigh. The estimated values of hip torque will be underestimated as a consequence of the opposing forces occurring at the wedge. Removal of the wedge would eliminate this issue; however, we have found that the wedge is very effective at reducing head movement as a consequence of lower limb torque generation. Though the current study focuses on the control of torque generation at the ankle and knee joints, we chose to retain and report the incomplete information provided about hip torque as the influence of the wedge is likely identical for each subject across the two sessions. The torques generated by subjects throughout each trial were saved to file for later analysis.
Tasks
Three isolated lower limb isometric contractions were studied using the novel MRI compatible device. With the subject supine, the right foot was secured to the custom footplate with the ankle joint fixed at an angle of between 100–110° for each subject. The right thigh was secured to the thigh wedge, adjusted to hold the knee at an angle of between 135–140°. The ankle angle was measured as the angle formed between the shank and the surface of the footplate while the knee was measured as the angle formed between the thigh and shank.
Volunteers were trained to generate three isolated isometric torques prior to each scan session: ankle dorsiflexion, ankle plantarflexion and knee extension. The target torque amplitude for each contraction was set at 5 Nm, which is a modest percentage of maximal voluntary force, to help prevent associated joint stabilization forces that may arise when subjects attempt to exert larger forces. In addition, this torque is compatible with efforts that can be made by subjects with hemiparesis or paraparesis. The torque generated at the joint of interest was displayed in real-time on video goggles, represented as a horizontal line whose vertical position varied linearly with the joint torque (Hidler et al., 2007). Each contraction was cued by the appearance of a rectangular target, at a location equivalent to a target range of 4 Nm to 6 Nm.
Volunteers were instructed to make a brief, smooth contraction, moving the line representing the generated torque into the target range, at which point the contraction could be released. The target range was shown for 1 s, with a 2 s delay before the next contraction was cued.
Though the volunteers received visual feedback of only the ankle or knee torque, depending on which joint was being tested, the torques at all three joints (ankle, knee and hip) were displayed simultaneously on a monitor to allow constant observation by the investigators. During training, participants were provided with verbal feedback and guidance concerning the relative isolation of the each contraction when required.
MRI Data Acquisition
All scans were performed on a 1.5 T Siemens Sonata MRI System (Siemens, Erlangen, Germany) using a standard head coil. In each session, MRI data were acquired in separate runs in which volunteers performed each of the three contraction types. The order in which the contraction types were tested within each session was randomized and counterbalanced.
Immediately prior to each fMRI run, volunteers briefly practiced the requested contraction. Each functional acquisition run consisted of four blocks of cued contractions (each 32 s in length), interleaved with five rest periods of 28 s duration. Each active block began with a warning signal (display turns yellow, duration 2 s), followed by a series of 10 pairs of ‘go’ cues (display turns green, when the target torque range was displayed, duration 1 s) and ‘relax’ cues (target line returns to baseline level).
The functional data acquired during task performance consisted of T2*-weighted MRI transverse echo-planar images (TE = 60 ms) with blood oxygenation level dependent (BOLD) contrast. Each echo-planar image comprised 25 contiguous axial slices (3 mm thick, 3 mm in-plane resolution) positioned to cover the whole cerebral cortex with an effective repetition time (TR) of 2.5s per volume. Each session consisted of 110 volumes acquired continuously. The first two volumes were discarded to allow for T1 equilibration effects. In addition, a T1-weighted structural image was acquired with an isotropic spatial resolution of 1 mm.
Image Analysis
All fMRI analyses were performed in SPM5 (http://www.fil.ion.ucl.ac.uk/spm/). For each session, the EPI time series were co-registered to the first volume of the session, unwarped to correct for additional movement-by-susceptibility interactions and adjusted for slice timing effects. The T1-weighted structural image was then co-registered with the first volume of the EPI time series. All volumes were transformed into standard MNI space using parameters determined by combined segmentation and normalization of the co-registered T1-weighted image (Ashburner and Friston, 2005). All normalized volumes were resampled to 3 mm isotropic voxels and smoothed with an isotropic 8 mm full-width half-maximum Gaussian kernel. The time series in each voxel were high pass filtered at 1/128 Hz to remove low frequency confounds and an AR(1) process was used to model temporal autocorrelation across scans.
Statistical analysis was performed in two stages. In the first stage, a single-subject fixed effects model was used. Lower limb contractions were modeled as delta functions for each of the six experiment runs. The onset of each contraction was defined as the time when 10 % of the maximum torque was reached at the joint of interest. The peak torques generated at each joint (relative to resting baseline) for each contraction event formed parametric modulators of the original delta function. This set of three regressors was included to reduce error variance associated with variation in torque amplitude between contractions events in each experiment run and were not included in the subsequent group-level analysis. All covariates were convolved with a canonical synthetic hemodynamic response function (HRF) and used in a general linear model together with a single covariate representing the mean (constant) term over scans. Parameter estimates for each covariate were calculated using maximum likelihood estimation. Statistical parametric maps of the t statistic (SPM{t}) were generated for the linear contrasts of covariates representing the main effect of each contraction type across both sessions. In addition, for the purpose of assessing test-restest reliability, SPM{t}s were generated for contrasts corresponding to the individual covariates for the main effect of each contraction type in each session. Also, differential t contrasts were created to compare the effects of session (Session 1 vs Session 2) for each contraction type as well as the effect of contraction type (Ankle Dorsiflexion vs Ankle Plantarflexion, Ankle Dorsiflexion vs Knee Extension, Ankle Plantarflexion vs Knee Extension).
In the second stage of analysis, the contrasts from each individual subject were taken to the second level, and random-effects group statistics were computed. Contrast images for each subject were entered into a one sample t-test and the resulting SPM{t}s were thresholded at p < 0.05, corrected for multiple comparisons across whole brain. Additional searches at p < 0.001 and p < 0.005 (uncorrected) were performed within bilateral SM1, Area 6 and the cerebellum to reveal areas of the motor system showing trends for these contrasts.
Assessment of Test-Retest Reliability
To estimate the reliability of the activity measured in each session for each contraction type, we calculated the intraclass correlation (ICC) of t-values for pairs of activation maps collected at each time point extracted from each voxel contained within contralateral SM1 and bilateral premotor areas for each individual. ICC values were calculated using a two–way random model ICC for absolute agreement between the measurements (Shrout and Feliss, 1979; Raemaekers et al., 2007); the values are high when the between-session variance is small and the between-voxel variance is large.
The two anatomical regions of interest (ROIs) were created using cytoarchitectonic maps with the SPM Anatomy toolbox (Eickhoff et al., 2005; Eickhoff et al., 2006). The left SM1 ROI consisted of Brodmann areas 4a, 4p, 1, 2, 3a and 3b, whereas the bilateral premotor ROI comprised Brodmann Area 6 in both hemispheres. All voxels within these ROIs were included in the ICC calculation regardless of T-value.
Behavioral Analysis
Repeated measures ANOVAs were performed on the torques generated at each joint for each movement to identify differences between the two sessions.
Results
Behavior
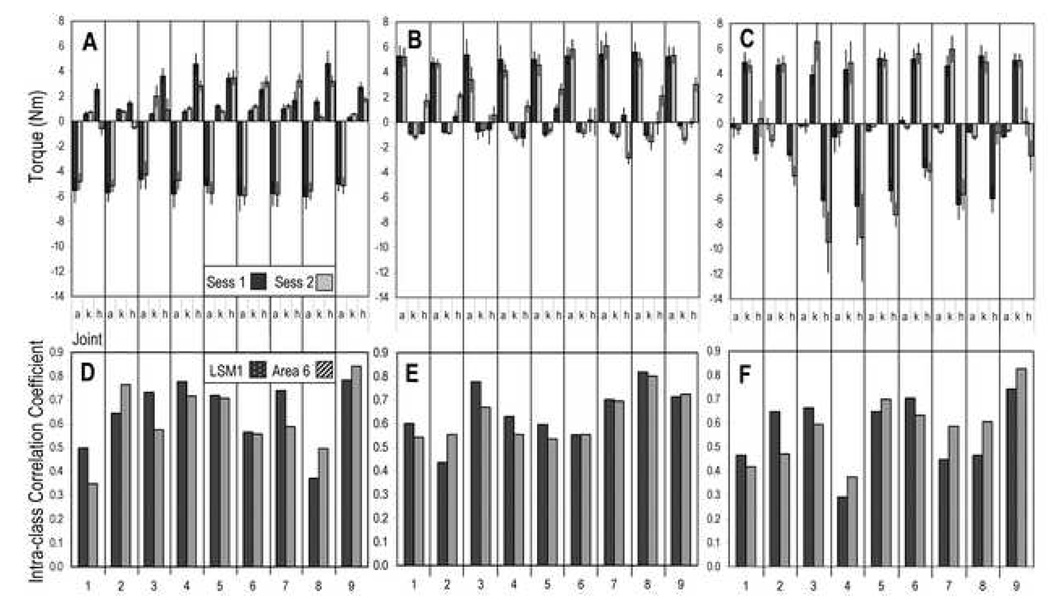
During fMRI scanning, all subjects were able to make controlled exertions of the lower limb to match the target torque at the requested joint, as shown in Figure 2. Positive torque values indicate extension of the joint (plantarflexion for the ankle joint) whereas negative values indicate joint flexion (dorsiflexion for the ankle). For the ankle dorsiflexion task, the mean amplitude of the torque at the ankle joint was −5.39 Nm (0.15) (mean of means ± standard error mean, for all subjects, data combined across both sessions). The mean amplitude of the torques at the knee and hip joints during the ankle dorsiflexion task were 0.91 Nm (0.08) and 2.47 Nm (0.39), respectively.
Figure 2.
Reliability of behavioral performance and brain activation across sessions presented for individual subjects.
(A–C) Mean torques generated at the ankle (a), knee (k) and hip (h) for ankle dorsiflexion (A), ankle plantarflexion (B) and knee extension (C) tasks for each session. Error bars signify standard deviation. Positive torque values indicate extension of the joint (termed plantarflexion for the ankle joint) whereas negative values indicate joint flexion (dorsiflexion for the ankle).
(D–F) ICC values calculated from the t-values for each task in the first and second session for voxels within left SM1 and bilateral Area 6 for ankle dorsiflexion (D), ankle plantarflexion (E) and knee extension (F) tasks.
The mean amplitude of the torque at the ankle during ankle plantarflexion was 5.06 Nm (0.16), whereas the mean torques at the knee and hip joints were −0.93 Nm (0.06) and 0.57 Nm (0.31), respectively.
For knee extension, the mean amplitude of the torque at the knee joint was 5.02 Nm (0.09). The mean amplitude of the torques at the ankle and hip joints were −0.53 Nm (0.10) and −4.51 Nm (0.87), respectively.
Repeated measures ANOVAs performed on the torques generated at each joint for each movement did not identify significant session-related differences, as reported in Table 1. For the ankle tasks, between-session differences in secondary, associated torques were approaching significance (p<0.1, at hip for ankle dorsiflexion, at knee and hip for ankle plantarflexion).
Table 1.
Mean torque amplitudes measured at each joint for each contraction type for each session.
| Mean Torque (SEM) Nm | |||||
|---|---|---|---|---|---|
| Movement | Joint | Session 1 | Session 2 | F(1,8) | P-value |
| Ankle Dorsiflexion | Ankle | −5.51 (0.16) | −5.26 (0.19) | 2.15 | 0.181 |
| Knee | 0.86 (0.13) | 0.96 (0.16) | 0.19 | 0.674 | |
| Hip | 3.00 (0.38) | 1.93 (0.55) | 4.28 | 0.072 | |
| Ankle Plantar Flexion | Ankle | 5.22 (0.08) | 4.91 (0.28) | 1.33 | 0.282 |
| Knee | −0.78 (0.08) | −1.07 (0.11) | 3.98 | 0.081 | |
| Hip | −0.06 (0.25) | 1.19 (0.60) | 3.52 | 0.097 | |
| Knee Extension | Ankle | −0.43 (0.15) | −0.63 (0.13) | 1.01 | 0.344 |
| Knee | 4.79 (0.16) | 5.26 (0.21) | 1.98 | 0.197 | |
| Hip | −4.30 (0.79) | −4.71 (1.16) | 0.18 | 0.685 | |
Brain Activation Patterns
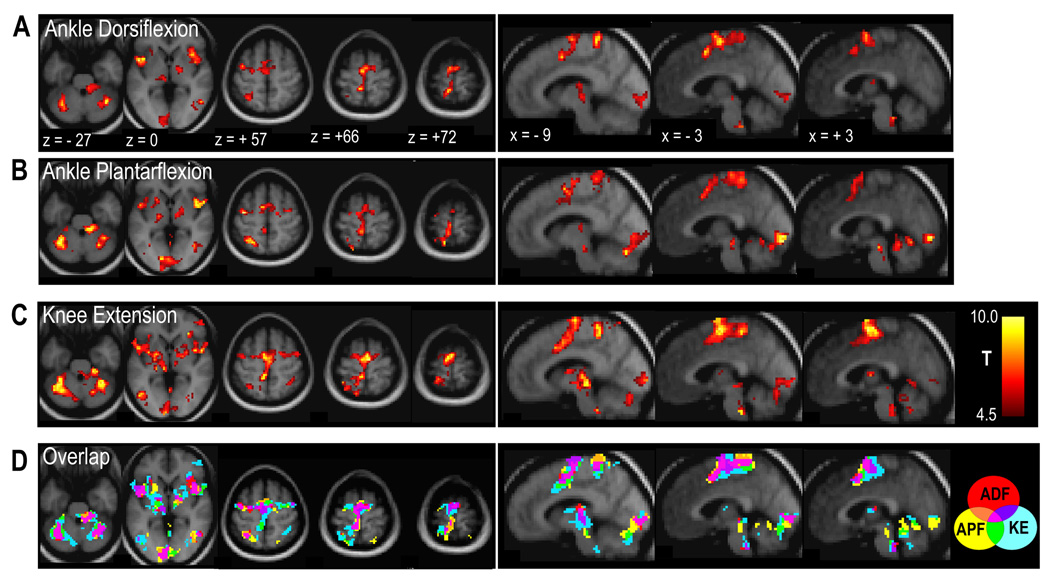
All three lower limb tasks resulted in significant BOLD signal increases in the left paracentral lobule, corresponding to SM1, as well as in left dorsolateral premotor cortex (PMd), and bilaterally in supplementary motor area (SMA) and secondary somatosensory cortex (Sii). Significant subcortical activation was observed bilaterally in the putamen, thalamus and cerebellum. (See Table 2 and Figure 3)
Table 2.
Regions showing significant task-related BOLD signal increases
| Co-ordinates in MNI Space | Peak Z-score | Volume (cm3) | |||
|---|---|---|---|---|---|
| Region | x | y | z | ||
| ANKLE DORSIFLEXION | |||||
| R Rolandic operculum | 51 | 6 | 9 | 4.61 | 13.53 |
| R anterior insula | 36 | 15 | 3 | 4.30 | |
| R putamen | 21 | 6 | 9 | 3.87 | |
| L SMA | −6 | −12 | 63 | 4.60 | 15.28 |
| L preSMA | −6 | 3 | 48 | 4.27 | |
| L paracentral lobule, M1 | −9 | −39 | 69 | 4.21 | |
| L precuneus/cingulate sulcus | −12 | −45 | 75 | 4.04 | |
| L PMd | −39 | −3 | 57 | 4.00 | |
| R preSMA | 6 | 9 | 51 | 3.76 | |
| R SMA | 12 | −6 | 66 | 3.53 | |
| L anterior paracentral lobule | −3 | −27 | 69 | 3.50 | |
| L Rolandic operculum | −48 | 6 | 3 | 4.57 | 6.83 |
| L inferior frontal gyrus | −60 | 9 | 18 | 3.96 | |
| L anterior insula | −30 | 24 | 3 | 3.71 | |
| L inferior parietal lobule | −54 | −36 | 42 | 4.57 | 6.13 |
| L cerebellum | −39 | −54 | −33 | 4.52 | 5.27 |
| R cerebellum | 27 | −63 | −24 | 4.39 | 10.34 |
| R anterior cerebellum | 9 | −36 | −24 | 4.10 | |
| R supramarginal gyrus (Sii) | 63 | −24 | 30 | 4.25 | 4.48 |
| R posterior caudate nucleus | 24 | −33 | 12 | 4.13 | 2.97 |
| R thamalus | 18 | −9 | 0 | 3.76 | |
| L thalamus | −18 | −24 | 6 | 4.03 | 4.56 |
| L putamen | −27 | −18 | 3 | 3.51 | |
| L superior parietal lobule | −30 | −54 | 57 | 3.81 | 2.16 |
| R supramarginal gyrus | 51 | −39 | 33 | 3.71 | 3.40 |
| L calcarine sulcus | −6 | −96 | −3 | 3.71 | 2.43 |
| ANKLE PLANTAR FLEXION | |||||
| R cerebellum | 27 | −60 | −21 | 5.73 | 42.85 |
| R anterior cerebellum | 9 | −36 | −24 | 4.81 | |
| L calcarine fissure | −3 | −93 | −6 | 4.68 | |
| L cerebellum | −33 | −51 | −27 | 4.51 | |
| L calcarine fissure | 6 | −90 | −3 | 4.44 | |
| R anterior cerebellum | 18 | −33 | −30 | 4.22 | |
| R putamen | 30 | 3 | 12 | 4.79 | 11.83 |
| R Rolandic operculum | 51 | 6 | 0 | 4.71 | |
| R insula | 39 | 3 | 6 | 4.45 | |
| R thalamus | 9 | −12 | −3 | 4.00 | |
| R posterior caudate nucleus | 24 | −33 | 12 | 3.95 | |
| R putamen | 24 | 9 | 6 | 3.71 | |
| R globus pallidus | 18 | −3 | 0 | 3.65 | |
| L superior parietal lobule | −27 | −54 | 57 | 4.79 | 20.33 |
| L precuneus | −12 | −54 | 72 | 4.34 | |
| L rostral SMA/preSMA | −9 | 0 | 57 | 4.11 | |
| R PMd, superior precentral gyrus | 30 | −6 | 54 | 4.06 | |
| R middle cingulate cortex | 12 | 15 | 42 | 4.03 | |
| L paracentral lobule, M1 | −6 | −39 | 69 | 3.99 | |
| L posterior/inferior paracentral lobule | −6 | −42 | 54 | 3.93 | |
| L anterior paracentral lobule | −3 | −27 | 72 | 3.93 | |
| L middle cingulate cortex | −12 | −3 | 42 | 3.83 | |
| L SMA | −12 | −9 | 63 | 3.81 | |
| R SMA | 12 | −3 | 54 | 3.65 | |
| L inferior parietal lobule | −54 | −33 | 36 | 4.78 | 6.05 |
| L parietal operculum | −60 | −24 | 27 | 4.65 | |
| L rolandic operculum | −48 | 0 | 6 | 4.69 | 5.27 |
| L anterior insula | −36 | 6 | 6 | 4.34 | |
| L putamen | −21 | 12 | 3 | 4.11 | |
| L PMd, superior seg precentral gyrus | −36 | −9 | 54 | 4.63 | 2.30 |
| R supramarginal gyrus/parietal operculum | 60 | −27 | 33 | 4.14 | 3.92 |
| L thalamus | −21 | −27 | 6 | 4.05 | 3.54 |
| KNEE EXTENSION | |||||
| R anterior cerebellum | 24 | −33 | −30 | 5.18 | 100.20 |
| L SMA superior | −3 | −6 | 75 | 5.01 | |
| L Rolandic operculum | −48 | 0 | 6 | 4.98 | |
| R Rolandic operculum | 54 | 6 | 9 | 4.90 | |
| L SMA | −3 | −9 | 60 | 4.69 | |
| L paracentral lobule/ cingulate sulcus, SM1 | −9 | −39 | 54 | 4.65 | |
| L putamen | −30 | 0 | 6 | 4.64 | |
| R PMv, inferior precentral sulcus | 60 | 9 | 30 | 4.62 | |
| R cerebellum | 33 | −51 | −27 | 4.46 | |
| R SMA | 6 | −12 | 66 | 4.44 | |
| R putamen | −24 | −3 | 6 | 4.39 | |
| R thalamus | 9 | −15 | 3 | 4.37 | |
| R insula | 42 | 6 | 9 | 4.36 | |
| L thalamus | −12 | −15 | −3 | 4.36 | |
| L middle cingulate cortex | −9 | 12 | 39 | 4.04 | |
| R middle cingulate cortex | 9 | 3 | 45 | 3.94 | |
| L PMd, precentral gyrus | −39 | −9 | 54 | 3.84 | |
| R PMd, precentral gyrus | 30 | −3 | 54 | 3.60 | |
| L cerebellum | −39 | −60 | −27 | 4.96 | 25.11 |
| L middle occipital gyrus | −21 | −93 | 15 | 4.24 | |
| L parietal operculum | −66 | −27 | 24 | 4.70 | 6.86 |
| L inferior parietal lobule | −54 | −36 | 39 | 3.96 | |
| R middle occipital gyrus | 36 | −81 | 15 | 4.66 | 2.08 |
| R inferior parietal lobule | 33 | −54 | 51 | 4.65 | 9.59 |
| R parietal operculum | 63 | −27 | 27 | 4.11 | |
Figure 3.
Brain regions showing significant positive BOLD signal changes for the main effects of ankle dorsiflexion (A), ankle plantarflexion (B) and knee extension (C) tasks across both sessions. Contrast maps show voxels at p < 0.001, uncorrected. Lowest panel (D) shows overlap of thresholded contrast maps for each task (p < 0.001, uncorrected), according to color key where ADF = ankle dorsiflexion, APF = ankle plantarflexion and KE = knee extension. Activation maps overlaid on the average of the normalized T1-weighted structural images; images displayed in neurological convention (left = left).
Within the sensorimotor network, there was substantial overlap of activation associated with each of the contraction types. However, some somatotopic organization was observed within the paracentral lobule. For example, activation extended more inferiorly during knee extension than during either of the ankle movements, as shown in Figure 3D.
As presented in Table 3 and Figure 4, significant BOLD signal decreases were observed for all lower limb tasks bilaterally within SM1 in the region of the hand knob, though for the knee extension task this effect was only seen without correction for multiple comparisons (p<0.001 uncorrected voxel threshold only). In addition, both ankle tasks resulted in significant BOLD signal decreases in anterior cingulate cortex, precuneus and right angular gyrus.
Table 3.
Regions showing significant task-related BOLD signal decreases
| Co-ordinates in MNI Space | Peak Z-score | Volume (cm3) | |||
|---|---|---|---|---|---|
| Region | x | y | z | ||
| ANKLE DORSIFLEXION | |||||
| Anterior cingulate cortex | 0 | 39 | 15 | 4.72 | 14.77 |
| L superior frontal gyrus | −18 | 57 | 12 | 4.23 | |
| R angular gyrus | 60 | −57 | 27 | 4.52 | 2.21 |
| R posterior wall of central suclus, S1 | 45 | −24 | 66 | 4.35 | 3.27 |
| R anterior wall of central sulcus, M1 | 39 | −21 | 57 | 4.16 | |
| L angular gyrus | −60 | −57 | 33 | 4.33 | 5.89 |
| L superior frontal gyrus | −21 | −18 | 54 | 4.26 | 2.08 |
| Precuneus | 0 | −66 | 33 | 3.83 | 3.19 |
| L base of central sulcus, Area 4p/S1 | −36 | −18 | 36 | 3.49 | 0.92 |
| L posterior wall of central sulcus, S1 | −42 | −18 | 54 | 3.48 | |
| ANKLE PLANTAR FLEXION | |||||
| R superior frontal gyrus | 21 | 48 | 9 | 5.45 | 15.85 |
| Anterior cingulate cortex | 0 | 33 | −12 | 3.79 | |
| L middle occipital gyrus | −39 | −78 | 36 | 5.2 | 2.57 |
| L superior frontal sulcus | −21 | 18 | 54 | 5.14 | 2.38 |
| R parieto-occipital sulcus | 18 | −57 | 18 | 4.93 | 8.91 |
| L parieto-occipital sulcus | −12 | −63 | 18 | 4.74 | |
| R postcentral gyrus | 48 | −21 | 63 | 4.79 | 6.91 |
| R rolandic operculum | 45 | −12 | 21 | 4.49 | |
| L hippocampus | −30 | −36 | −12 | 4.53 | 1.05 |
| R angular gyrus | 63 | −51 | 24 | 4.44 | 2.05 |
| Precuneus | 0 | −48 | 45 | 4.41 | 2.11 |
| R superior temporal gyrus | 60 | −9 | −3 | 4.27 | 2.32 |
| L posterior wall of central sulcus, S1 | −42 | −24 | 66 | 4.26 | 3.67 |
| L parietal operculum | −39 | −15 | 24 | 4.22 | 1.13 |
| R hippocampus | 30 | −27 | −15 | 4.16 | 0.76 |
| R superior frontal sulcus | 27 | 30 | 48 | 3.71 | 1.00 |
| KNEE EXTENSION | |||||
| L posterior wall of central sulcus, S1 | −39 | −24 | 60 | 3.83 | 1.03 |
| R anterior wall of central sulcus, M1 # | 36 | −21 | 57 | 3.71 | 0.46 |
Observed at p<0.001 uncorrected voxel threshold.
Figure 4.
Brain regions showing significant negative BOLD signal changes for the main effect of ankle dorsiflexion (A), ankle plantarflexion (B) and knee extension (C) tasks across both sessions. Contrast maps show voxels at p < 0.001 and p<0.005, uncorrected. Lowest panel (D) shows overlap of thresholded contrast maps for each task (p < 0.001, uncorrected), according to the color key in Figure 3.
Differential Activity between Movement Types
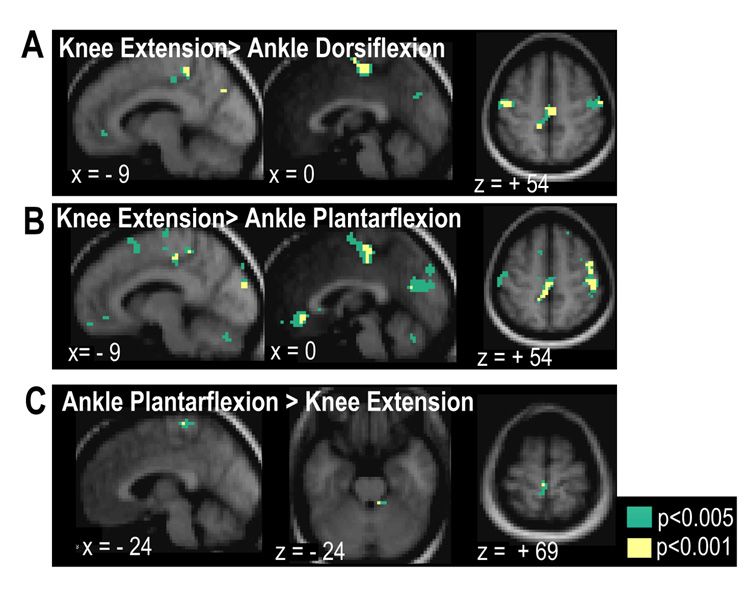
Significant differential activity was observed between the contraction types, as reported in Table 4 and Figure 5. The most striking differences were observed when comparing the knee extension task to either of the ankle tasks. When compared with ankle dorsiflexion, knee extension resulted in significantly greater activation of SMA, right PMv and contralateral M1 adjacent to the hand knob. (voxels significant at p<0.001 (uncorrected) and clusters significant at p<0.05 (corrected)) Further clusters of voxels within sensorimotor areas showed greater activity for knee extension than ankle dorsiflexion, but did not survive correction for multiple comparsions. These included portions of left SM1 at inferior levels within the paracentral lobule, as well as right M1, adjacent to the hand knob. (voxels significant at p<0.001 (uncorrected), see Figure 5A)
Table 4.
Regions showing significant differences in activation between tasks
| Co-ordinates in MNI Space | Peak Z-score | Volume (cm3) | ||||
|---|---|---|---|---|---|---|
| Contrast | Region | x | y | z | ||
| Ankle: dorsiflexion >plantar flexion | R cerebellum * | 18 | −33 | −39 | 3.84 | 0.66 |
| L central sulcus, SM1* | −39 | −24 | 60 | 3.17 | 0.22 | |
| Ankle: plantar flexion> dorsiflexion | n/a | - | - | - | - | - |
| Ankle Dorsiflexion > Knee Extension | R cerebellum * | 15 | −33 | −24 | 2.91 | 0.19 |
| Knee Extension > Ankle Dorsiflexion | SMA | 0 | −12 | 66 | 4.65 | 0.95 |
| 3 | −18 | 60 | 4.59 | |||
| L paracentral lobule, SM1 # | −9 | −36 | 54 | 4.15 | 0.24 | |
| R PMD, precentral gyrus # | 30 | −15 | 69 | 4.11 | 0.30 | |
| R precentral gyrus, PMv | 57 | 3 | 42 | 3.77 | 1.11 | |
| R central sulcus, SM1 # | 24 | −30 | 69 | 3.75 | 0.41 | |
| L anterior wall of central sulcus, M1 | −48 | −12 | 54 | 3.75 | 0.57 | |
| R anterior wall of central sulcus, M1 # | 42 | −15 | 51 | 3.37 | 0.16 | |
| Ankle plantarflexion > Knee Extension | R anterior cerebellum * | 9 | −39 | −24 | 3.17 | 0.16 |
| L paracentral lobule, SM1 * | −3 | −30 | 69 | 3.10 | 0.32 | |
| Knee Extension > Ankle Plantarflexion | L postcentral gyrus, S1 | −27 | −45 | 69 | 4.65 | 0.51 |
| R anterior wall of central sulcus, M1 | 48 | −12 | 48 | 4.63 | 0.97 | |
| R precentral gyrus, PMd | 45 | −3 | 57 | 3.81 | ||
| R postcentral gyrus, S1 | 36 | −24 | 63 | 4.41 | 1.27 | |
| R superior frontal sulcus | 21 | 36 | 48 | 4.41 | 0.81 | |
| R parieto-occipital sulcus | 6 | −63 | 21 | 4.16 | 0.59 | |
| L superior wall of cingulate sulcus | −9 | −27 | 48 | 3.88 | 1.22 | |
| L paracentral lobule, M1 | −6 | −36 | 54 | 3.48 | ||
| L postcentral gyrus, S1 # | −42 | −30 | 63 | 3.57 | 0.27 | |
Observed at p<0.005 uncorrected voxel threshold.
Observed at p<0.001 uncorrected voxel threshold.
Figure 5.
Brain regions showing significant differential activation between movements: Regions showing greater response during knee extension compared with ankle dorsiflexion (A) and ankle planatarflexion (B). Regions showing greater activity during ankle plantarflexion than during knee extension (C). Contrast maps show voxels at p<0.001 and p<0.005, uncorrected.
Comparison of knee extension with ankle plantarflexion also identified significantly greater knee-related BOLD signal changes in sensorimotor areas including clusters in left M1 within the paracentral lobule and extending into SMA, as well as in bilateral S1, right M1 at the hand knob, right PMd, right superior frontal sulcus and right parieto-occipital sulcus. (Figure 5B, voxels significant at p<0.001 (uncorrected), clusters significant at p<0.05 (corrected))
The region of the left paracentral lobule showing greater activation during the knee task compared with either of the ankle tasks corresponds to the inferior portion of SM1 that shows specific activation to knee extension only in the maps of overlap of the main effect of task type. (Figure 3D)
When contrasted with the knee extension task, the ankle tasks resulted in marginally greater activation in right anterior cerebellum (for both ankle tasks) and left SM1 within the paracentral lobule (z=69, ankle plantar flexion only) as shown in Figures 5C and 5D. However, these clusters only survived voxel-level thresholding of p<0.005 uncorrected.
Similarly, as shown in Figure 5D, only small differences were observed when contrasting ankle dorsiflexion with ankle plantarflexion. Dorsiflexion resulted in marginally greater activation of right cerebellum and left SM1 at the level of the hand knob when compared with plantarflexion (voxel-level threshold of p<0.005 uncorrected) There were no regions showing significantly greater activation for ankle plantarflexion when compared with dorsiflexion.
Reliability of fMRI measurements
Contrast of the activation between different sessions for each task did not reveal widespread differences in sensorimotor areas. Comparison of the two sessions for the ankle dorsiflexion task showed greater activation in right superior temporal sulcus, right supramarginal gyrus, right cuneus and right superior parietal lobule for the first session compared with the second session. No regions showed greater activity of the second session compared with the first. No significant differences were detected between sessions for the ankle plantarflexion task, even with searches at the uncorrected threshold of p<0.005. Contrast of the sessions for the knee extension task revealed greater activation in left hippocampus, right S1 within the paracentral lobule and right PMd for the second session compared with the first session, though the latter was only observed at an uncorrected voxel threshold of p<0.005.
The lack of major session related effects at the group level was supported by high intraclass correlation coefficients for t-values of voxels in contralateral SM1 and bilateral Area 6 for each of the contraction types, as shown in Figure 2D–F.
For individual subjects, the ICC of t-values between the sessions for voxels within contralateral SM1 ranged from 0.37 to 0.78 for ankle dorsiflexion, from 0.44 to 0.82 for ankle planarflexion and from 0.29 to 0.74 for knee extension. For voxels within bilateral Area 6, ICC values for individual subjects ranged from 0.35 to 0.84 for ankle dorsiflexion, from 0.54 to 0.80 for ankle planarflexion and from 0.38 to 0.83 for knee extension.
In Figure 6, t-values of voxels in each anatomical ROI for the ankle dorsiflexion task in the first session are plotted against those of the second session for the individuals at each extreme of the reported ICC range.
Figure 6.
Scatterplots of the t-values for ankle dorsiflexion in the first session plotted against the t-values of the second session for the two subjects with the highest (A–B) and lowest (C–D) ICC values for voxels within SM1 (A,C) and bilateral Area 6 (B–D).
Discussion
Use of a novel apparatus for estimating torque in the lower limb
The lack of major performance differences between sessions on each of the three tasks demonstrates the utility of the isometric torque monitoring apparatus for longitudinal studies of lower limb motor function. Volunteers were able to generate torques of the requested amplitude at the relevant joint with good accuracy, both within and across the two sessions. At the group level, no major differences in activation were found within the motor system, even when conducting searches with voxel-level uncorrected thresholds of p<0.001 and P<0.005. The reliability of the functional activity associated with the motor tasks across sessions was good; for individual subjects, assessment of intraclass correlation of t-values from the two sessions within contralateral SM1 and bilateral Area 6 revealed good test-retest reliability. These high values of test-retest reliability, calculated for each subject, support future use of this apparatus in longitudinal studies of patients, which may be reported as individual cases.
As shown in Figure 2, generation of the target torque at the joint of interest resulted in associated torques at the other two joints. For example, ankle dorsiflexion was accompanied by extensor torques at the knee and hip, whereas ankle plantarflexion occurred with slight knee flexion in all volunteers. During cued knee joint torque exertion there was accompanying hip flexion torque.
When interpreting the impact of these associated torques, it is important to remember that total isolation of isometric torque generation to a single joint is impossible due to the involvement of bi-articular muscles in the related contractions as well as the need for stabilization at multiple joints. For example, the rectus femoris muscle is one of the primary extensors of the knee, but as it crosses both the knee and the hip joints its contraction can generate torques at both joints, resulting in hip flexion. Further muscle activity may occur due to attempts to stabilize the pelvis in this scenario.
Though volunteers did not receive feedback of any changes in torque generated at the other two joints during fMRI acquisition, there were no significant differences in associated torque amplitudes between the two sessions. However, for the ankle tasks, between-session differences in the secondary torques did approach significance at the hip for ankle dorsiflexion and at knee and hip for ankle plantarflexion, suggesting that there were differences in performance between sessions for at least some of the subjects. Inspection of the mean torques per session for the group (Table 1) and for each subject (Figures 2A–B), reveal that these secondary torques are relatively small compared with the torques generated at the joint of interest for these tasks. However, these subtle differences may result in differences in task-related activity in the fMRI data between sessions.
Continuous collection of data from the three joints of the lower limb allows informed interpretation of changes in functional activation patterns associated with torque generation: measures of inter-session, as well as intra-session, variability in performance can be included in analytical models. This level of monitoring adds much insight when conducting longitudinal studies of patients with difficulties in walking and lower limb movement control in whom impairment and performance may vary over time. (Holly et al., 2007)
Robust performance of torque generation is likely to depend on standardization of subject positioning between sessions and the pre-scanning training. Verbal feedback and guidance was provided during the training phase and immediately prior to the acquisition of the functional data; subjects were trained until the requested torque was reached with minimal associated torques at other joints at each time point. Volunteers were encouraged to develop mental strategies, mostly involving visualization of the requested torque in a real-world scenario, which maximized the isolation of the torque. Feedback and instruction has a large influence on performance; Nozaki and colleagues, using a similar force cell apparatus, have shown that it is possible to reverse the associated torque at the hip joint during knee torque generation by providing verbal feedback about how to alter their strategy for generating knee extension (Nozaki et al., 2005).
The apparatus used in the current study is advantageous for limiting head movement associated with movements of the lower limb. Use of isometric torque generation and restraint of the foot, ankle and thigh limited displacement of the trunk and hence head movements. In addition, the sensitivity of the load cell enabled use of relatively small torques in the experiment: the target torque was set to an amplitude of 5Nm in all tasks, which is a low percentage of the maximum possible torque measured with this apparatus in healthy individuals – average maximum torques of 35 Nm for ankle dorsiflexion, 45 Nm for ankle plantarflexion and 80Nm for knee extension were measured in the individuals tested during pilot work and the current study.
Utilizing isometric exertions during fMRI studies has a distinct advantage over paced movements in individuals with motor impairments (e.g. stroke). The targets presented to the subject are scaled to their maximum ability so even subjects with trace motor function can execute the protocol. Additionally, because the limb maintains a constant posture, issues such as spasticity and limitations in joint range of motion are eliminated. These benefits allow for highly impaired subjects to participate in fMRI studies looking at motor recovery.
Topographic Organization of Lower Limb Motor Representations
We observed significant overlap of the motor representations for each of the torque-generation tasks; portions of contralateral SM1, ipsilateral cerebellum and bilateral SMA were common to the activation patterns of all three contraction types. Using differential contrasts between the tasks, we also observed separation of the movement representations and trends of greater activation of these regions during generation of particular torques. We found that knee extension was associated with greater activity at more inferior levels of the paracentral lobule compared with either ankle contraction (z=54), whereas ankle plantarflexion resulted in greater activity at superior levels (z=69) when compared with knee extension, albeit with an uncorrected threshold of p<0.001 and p<0.005, respectively. This distinction between representations of movement at these two joints suggested by these trends has also been observed in a center-of-mass analysis of motor representations of active extension-flexion cycles of these joints; the knee related maxima were located more lateral and inferior within SM1 than those of the ankle (Kapreli et al., 2006; Kapreli et al., 2007). This inferior location of the knee representation is supported by other studies that investigated the motor representation of the knee, though without concurrent testing of the ankle (Fink et al., 1997; Rotte et al., 2002; Luft et al., 2005).
In the current study no significant differences between activations associated with ankle dorsiflexion and plantarflexion were found within SM1. In addition, the maximal peaks within SM1 for the main effects of the two ankle contractions were identical. Similarly, Johansen and colleagues did not find significant differences in a voxel-wise analysis of activation related to tonic isometric dorsiflexion and plantarflexion contractions using PET, though they identified varying vector differences between the locations of the group-level peak activation depending on whether subjects performed tonic or dynamic isometric contractions (Johannsen et al., 2001).
However, due to the relatively small sample size employed in the current study, we may not have sufficient power to discern subtle differences in activation patterns between these different lower limb contractions, particularly using random effects analysis. Further studies with larger numbers of volunteers will be required to gain definitive information on this issue.
The considerable overlap of the representation for each contraction task may be due to the lack of complete isolation of torque generation to the instructed joint and the need for stabilization of these other joints, even during generation of small isometric torques as used in the current study. However, the distributed organization of motor representations for these lower limb contractions is in keeping with the general principles of somatotopic organization of the upper limb within M1: convergence of output from broad territories of M1 to individual joints or muscles, as well as divergence of output from a single neuron in M1 to multiple spinal motoneuron pools (Schieber, 2001). These principles appear especially relevant to efficient control of the musculature of the lower limb as locomotion is predicated on the coordination of torques at multiple joints in a timely manner throughout the gait cycle.
Influence of Lower Limb Contractions on Hand Representations in SM1
The observation of decreased BOLD signal in the vicinity of hand knob of SM1 in the current study suggests some form of interaction between lower limb motor output and upper limb representations at this level of the motor system. Modulation of wrist extensor and flexor excitability by ipsilateral foot movements has been demonstrated previously in neurophysiology studies and it possible that this modulation is related to the BOLD signal decreases we observe. For example, when the resting forearm was pronated, excitability of the H-reflex in wrist flexor increased during plantarflexion and decreased during dorsiflexion of the ankle, whereas the opposite modulatory coupling was observed for excitability of wrist extension (Baldissera et al., 2002; Cerri et al., 2003; Borroni et al., 2004). In addition, motor evoked potentials resulting from transcranial magnetic stimulation (TMS) of the cortical hotspot for inducing wrist movement were larger for the wrist extensor during ankle dorsiflexion than during plantarflexion, while the converse was true for responses measured in the wrist flexor (Byblow et al., 2007).
Paired-pulse TMS revealed that short interval cortical inhibition (SICI) of the wrist extensor representation was reduced during active dorsiflexion of the ankle compared with rest or active ankle plantarflexion, though this reduction in intracortical inhibition in the hand muscle representation was not seen for passive movements of the ankle (Byblow et al., 2007). These findings support the view that active contractions of the lower limb can alter excitability of upper limb corticospinal neurons at the level of the hand representation of SM1 and that this is not driven primarily by somatosensory feedback.
These previous electrophysiological investigations did not test responses in SM1 ipsilateral to the moved foot. (Byblow et al., 2007) If the bilateral BOLD signal decreases we observe in bilateral SM1 are related to these modulatory phenomena in SM1 contralateral to the moved foot, one may expect similar findings if the TMS experiments were repeated for the other hemisphere. A mechanism for bilateral mechanism is suggested by Byblow and colleagues, who also used paired-pulse TMS to demonstrate that PMd conditioning facilitated MEPs of the wrist extensor during dorsiflexion, whereas SMA conditioning produced facilitatory effects during both dorsiflexion and plantarflexion (Byblow et al., 2007). Both areas are known to have interhemispheric connections (McGuire et al., 1991; Rouiller et al., 1994; Marconi et al., 2003) as well as ipsilateral connections within the cortical motor system (Picard and Strick, 1996; Dum and Strick, 2005), so bilateral modulation of hand representations by unilateral foot movement would seem plausible.
In the current study, knee extension resulted in significantly less BOLD signal decrease in hand representation of SM1 in both hemispheres when compared with either ankle task, suggesting that this effect may be greatest for distal effectors. In addition, ankle dorsiflexion was associated with less BOLD signal decrease in left SM1 hand area than ankle plantarflexion. Naturally, the results of the present study cannot determine if these signal changes we observe in the hand area of SM1 are the result of modulation of excitability in this location, whether by inhibitory or excitatory means. As modulation of the excitability of upper limb neurons by lower limb movement is relevant to walking performance, both for arm swing (to assist locomotion) and for carrying tasks, it should be the subject of further investigation.
Summary
The simultaneous measurement of torques at the hip, knee and ankle with this novel apparatus along with real-time feedback enabled reliable investigation of selective lower limb motor control with fMRI. The reproducibility of torques monitored by visual feedback, as well as the BOLD signals they evoke in the representations for lower extremity movements, may make this apparatus and experimental design valuable for longitudinal studies of lower limb motor control.
Table 5.
Regions showing significant differences in activation between sessions for each task.
| Co-ordinates in MNI Space | Peak Z-score | Volume (cm3) | ||||
|---|---|---|---|---|---|---|
| Contrast | Region | x | y | z | ||
| Ankle dorsiflexion: | ||||||
| Session 1>2 | R superior temporal sulcus | 54 | −24 | −9 | 4.02 | 0.78 |
| R supramarginal gyrus | 51 | −42 | 39 | 3.95 | 0.57 | |
| R cuneus | 21 | −84 | 39 | 3.76 | 0.76 | |
| R superior parietal lobule | 33 | −57 | 54 | 3.72 | 0.76 | |
| Session 2>1 | n/a | |||||
| Ankle plantarflexion: no differences between sessions | ||||||
| Knee Extension: | ||||||
| Session 1>2 | n/a | |||||
| Session 2>1 | L hippocampus | −15 | −36 | 0 | 4.40 | 1.32 |
| R posterior wall of precentral suclus, PMd * | 18 | −12 | 66 | 2.84 | 0.14 | |
| R paracentral lobule, S1 | 15 | −45 | 66 | 2.81 | 0.22 | |
Observed at p<0.005 uncorrected voxel threshold.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In physiological terms, torque generated at a joint results from the sum of all muscle forces acting on both sides of the joint that tend to rotate the limb.
Reference List
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Borroni P, Cavallari P, Cerri G. Excitability changes in human corticospinal projections to forearm muscles during voluntary movement of ipsilateral foot. J Physiol. 2002;539:903–911. doi: 10.1113/jphysiol.2001.013282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni P, Cerri G, Baldissera F. Excitability changes in resting forearm muscles during voluntary foot movements depend on hand position: a neural substrate for hand-foot isodirectional coupling. Brain Res. 2004;1022:117–125. doi: 10.1016/j.brainres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Byblow WD, Coxon JP, Stinear CM, Fleming MK, Williams G, Muller JF, Ziemann U. Functional connectivity between secondary and primary motor areas underlying hand-foot coordination. J Neurophysiol. 2007;98:414–422. doi: 10.1152/jn.00325.2007. [DOI] [PubMed] [Google Scholar]
- Carey JR, Anderson KM, Kimberley TJ, Lewis SM, Auerbach EJ, Ugurbil K. fMRI analysis of ankle movement tracking training in subject with stroke. Exp Brain Res. 2004;154:281–290. doi: 10.1007/s00221-003-1662-7. [DOI] [PubMed] [Google Scholar]
- Cerri G, Borroni P, Baldissera F. Cyclic h-reflex modulation in resting forearm related to contractions of foot movers, not to foot movement. J Neurophysiol. 2003;90:81–88. doi: 10.1152/jn.00030.2003. [DOI] [PubMed] [Google Scholar]
- Christensen MS, Lundbye-Jensen J, Petersen N, Geertsen SS, Paulson OB, Nielsen JB. Watching Your Foot Move--An fMRI Study of Visuomotor Interactions during Foot Movement. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl101. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Toosy AT, Marsden JF, Wheeler-Kingshott CM, Miller DH, Matthews PM, Thompson AJ. Functional response to active and passive ankle movements with clinical correlations in patients with primary progressive multiple sclerosis. J Neurol. 2006;253:882–891. doi: 10.1007/s00415-006-0125-z. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Toosy AT, Marsden JF, Wheeler-Kingshott CM, Sahyoun C, Matthews PM, Miller DH, Thompson AJ. Identifying brain regions for integrative sensorimotor processing with ankle movements. Exp Brain Res. 2005;166:31–42. doi: 10.1007/s00221-005-2335-5. [DOI] [PubMed] [Google Scholar]
- Craig JJ. An introduction to robotics: mechanics and control. Reading, MA: Addison–Wesley Publishing; 1989. [Google Scholar]
- Debaere F, Swinnen SP, Beatse E, Sunaert S, Van Hecke P, Duysens J. Brain areas involved in interlimb coordination: a distributed network. Neuroimage. 2001;14:947–958. doi: 10.1006/nimg.2001.0892. [DOI] [PubMed] [Google Scholar]
- Dobkin BH. Rehabilitation and functional neuroimaging dose-response trajectories for clinical trials. Neurorehabil Neural Repair. 2005;19:276–282. doi: 10.1177/1545968305281892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin BH, Firestine A, West M, Saremi K, Woods R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage. 2004;23:370–381. doi: 10.1016/j.neuroimage.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci. 2005;25:1375–1386. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32:570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 1997;77:2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- Hidler J, Hodics T, Xu B, Dobkin B, Cohen LG. MR compatible force sensing system for real-time monitoring of wrist moments during fMRI testing. J Neurosci Methods. 2006;155:300–307. doi: 10.1016/j.jneumeth.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidler JM, Carroll M, Federovich EH. Strength and coordination in the paretic leg of individuals following acute stroke. IEEE Trans Neural Syst Rehabil Eng. 2007;15:526–534. doi: 10.1109/TNSRE.2007.907689. [DOI] [PubMed] [Google Scholar]
- Holly LT, Dong Y, Albistegui-DuBois R, Marehbian J, Dobkin B. Cortical reorganization in patients with cervical spondylotic myelopathy. J Neurosurg Spine. 2007;6:544–551. doi: 10.3171/spi.2007.6.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen P, Christensen LO, Sinkjaer T, Nielsen JB. Cerebral functional anatomy of voluntary contractions of ankle muscles in man. J Physiol. 2001;535:397–406. doi: 10.1111/j.1469-7793.2001.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapreli E, Athanasopoulos S, Papathanasiou M, Van Hecke P, Keleki D, Peeters R, Strimpakos N, Sunaert S. Lower limb sensorimotor network: issues of somatotopy and overlap. Cortex. 2007;43:219–232. doi: 10.1016/s0010-9452(08)70477-5. [DOI] [PubMed] [Google Scholar]
- Kapreli E, Athanasopoulos S, Papathanasiou M, Van Hecke P, Strimpakos N, Gouliamos A, Peeters R, Sunaert S. Lateralization of brain activity during lower limb joints movement. An fMRI study. Neuroimage. 2006;32:1709–1721. doi: 10.1016/j.neuroimage.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Luft AR, Forrester L, Macko RF, McCombe-Waller S, Whitall J, Villagra F, Hanley DF. Brain activation of lower extremity movement in chronically impaired stroke survivors. Neuroimage. 2005;26:184–194. doi: 10.1016/j.neuroimage.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Luft AR, Smith GV, Forrester L, Whitall J, Macko RF, Hauser TK, Goldberg AP, Hanley DF. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Hum Brain Mapp. 2002;17:131–140. doi: 10.1002/hbm.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintosh BJ, Mraz R, Baker N, Tam F, Staines WR, Graham SJ. Optimizing the experimental design for ankle dorsiflexion fMRI. Neuroimage. 2004;22:1619–1627. doi: 10.1016/j.neuroimage.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Giannetti S, Molinari M, Caminiti R. Callosal connections of dorso-lateral premotor cortex. Eur J Neurosci. 2003;18:775–788. doi: 10.1046/j.1460-9568.2003.02807.x. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Bates JF, Goldman-Rakic PS. Interhemispheric integration: I. Symmetry and convergence of the corticocortical connections of the left and the right principal sulcus (PS) and the left and the right supplementary motor area (SMA) in the rhesus monkey. Cereb Cortex. 1991;1:390–407. doi: 10.1093/cercor/1.5.390. [DOI] [PubMed] [Google Scholar]
- Neckel N, Pelliccio M, Nichols D, Hidler J. Quantification of functional weakness and abnormal synergy patterns in the lower limb of individuals with chronic stroke. J Neuroeng Rehabil. 2006;3:17. doi: 10.1186/1743-0003-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki D, Nakazawa K, Akai M. Uncertainty of knee joint muscle activity during knee joint torque exertion: the significance of controlling adjacent joint torque. J Appl Physiol. 2005;99:1093–1103. doi: 10.1152/japplphysiol.00365.2005. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Vink M, Zandbelt B, van Wezel RJ, Kahn RS, Ramsey NF. Test-retest reliability of fMRI activation during prosaccades and antisaccades. Neuroimage. 2007;36:532–542. doi: 10.1016/j.neuroimage.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Rotte M, Kanowski M, Heinze HJ. Functional magnetic resonance imaging for the evaluation of the motor system: primary and secondary brain areas in different motor tasks. Stereotact Funct Neurosurg. 2002;78:3–16. doi: 10.1159/000063834. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res. 1994;102:227–243. doi: 10.1007/BF00227511. [DOI] [PubMed] [Google Scholar]
- Sahyoun C, Floyer-Lea A, Johansen-Berg H, Matthews PM. Towards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot movements. Neuroimage. 2004;21:568–575. doi: 10.1016/j.neuroimage.2003.09.065. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Feliss JL. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychological Bulletin. 1979;2:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]