Summary
Exonuclease (Exo1) mediates checkpoint induction in response to telomere dysfunction in yeast but it is unknown whether Exo1 has similar functions in mammalian cells. Here we show that deletion of the nuclease domain of Exo1 reduces accumulation of DNA damage and DNA damage signal induction in telomere dysfunctional mice. Exo1 deletion improved organ maintenance and lifespan of telomere dysfunctional mice but did not increase chromosomal instability or cancer formation. Deletion of Exo1 also ameliorated cell cycle arrest and DNA damage signal induction in response to irradiation and increased survival of cells in response to DNA damage induced by 6-thioguanine treatment. Exo1-deletion reduced ssDNA-formation and the recruitment of Replication Protein A (RPA) at laser induced DNA breaks and impaired activation of ATR in telomere dysfunctional mice. Together, these studies provide the first evidence that Exo1 contributes DNA damage signal induction in mammalian cells and deletion of Exo1 can prolong survival in the context of telomere dysfunction.
Introduction
Telomeres form the ends of eukaryotic chromosomes (Blackburn, 2001). The main function of telomeres is to cap chromosomal ends thus preventing induction of DNA damage pathways. Telomere capping function depends on telomere length and telomere structure (de Lange, 2005). Due to the end replication problem of DNA polymerase and the processing of telomeres during cell cycle progression, telomeres shorten with each round of cell division (Shay JW and Wright WE, 2000).
Telomere shortening limits the proliferative lifespan of primary human cells (Allsopp et al., 1992) and can reduces regenerative reserve and organ homeostasis during aging and chronic diseases (for review see Djojosubroto et al. 2003). In mammalian cells telomere shortening leads to telomere dysfunction inducing senescence or apoptosis (Lechel et al., 2005; Lee et al., 1998; Wright and Shay, 1992a). Critically short telomeres lose chromosome capping-function. The most upstream response to telomere uncapping is the formation of DNA-damage foci at dysfunctional telomeres (d’Adda di Fagagna et al., 2003; Takai et al., 2003). These DNA damage foci activate downstream DNA damage signals, which involve activation of the ATM and ATR kinases (d’Adda di Fagagna et al., 2003) inducing the p53-pathway (Chin et al. 1999; Vaziri and Benchimol, 1996). Cell cycle arrest and apoptosis in response to telomere dysfunction represent tumor suppressor mechanisms (Wright and Shay, 1992b). As a downside, these checkpoints may contribute to decreased regenerative reserve and impaired organ maintenance in response to telomere dysfunction and aging (Choudhury et al. 2007).
The generation of telomerase deficient mice, carrying a homozygous deletion of the telomerase RNA component (mTerc−/−), has delivered a unique tool to study consequences of telomere dysfunction in vivo (Blasco et al., 1997). Late generation (G3) mTerc−/− mice exhibit cellular and molecular phenotypes induced by telomere dysfunction, including activation of the p53-DNA-damage pathway, impaired proliferation, increased apoptosis, and chromosomal fusions ( Lee et al., 1998; Rudolph et al., 1999; Chin et al. 1999). Mice with dysfunctional telomeres show impaired maintenance of organs with high rates of cell turnover and premature ageing of these compartments (Herrera et al., 1999; Lee et al., 1998; Rudolph et al., 1999; Choudhury et al. 2007; Hao et al. 2005; Hemann et al. 2001).
Studies on telomere dysfunctional mice have shown that deletions of different components of the DNA damage pathway affect organ homeostasis, cancer, and lifespan of telomere dysfunctional mice. Deletion of ATM increased telomere dysfunction and accelerated premature aging of mTerc−/− mice (Qi et al., 2003; Wong et al., 2003). These findings were consistent with the known function of ATM in the maintenance of telomere length and function (Greenwell et al., 1995; Naito et al., 1998). Of note, ATM deletion did not rescue premature ageing of telomere dysfunctional mice (Wong et al 2003) indicating that ATM independent pathways can activate p53 in response to telomere dysfunction possibly involving ATR – a gene involved in activation of DNA damage signals in response to generation of single stranded DNA (Zou et al., 2003; d’Adda di Fagagna et al., 2003). The deletion of p53 rescued germ cell apoptosis and improved fertility of telomere dysfunctional mice (Chin et al., 1999). However, p53 deletion did not improve lifespan of telomere dysfunctional mice due to an increase in cancer formation (Artandi et al., 2000). Deletion of the Ink4a gene locus encoding for p16 and p19ARF did not rescue tissue atrophy in mTerc−/−mice, but telomere dysfunction suppressed tumor formation associated with Ink4a deletion (Greenberg et al., 1998). The deletion of p21, a downstream target of p53, improved stem cell function, organ maintenance and lifespan of telomere dysfunctional mice without accelerating cancer formation because apoptotic responses remained intact (Choudhury et al., 2007).
Studies in mTerc−/− mice have shown that telomere dysfunction has a dual role in cancer formation. On one hand telomere dysfunction inhibits tumor progression, on the other hand it induces chromosomal instability and increases tumor initiation, especially when p53 function is compromised (Artandi et al., 2000; Chin et al., 1999). The induction of chromosomal instability and tumor initiation in response to telomere dysfunction is linked to the evolution of chromosomal instability and the formation of non-reciprocal translocation (Artandi et al., 2000). This response appears to be initiated by DNA repair pathways inducing chromosomal fusions in response to telomere uncapping with the consequence of fusion-bridge-breakage cycles (Maser et al., 2007a; Smogorzewska et al. 2002).
Exo1 is a 5′–3′ exonuclease. A role of Exonuclease-1 (Exo1) for the induction of checkpoints in response to telomere dysfunction has been revealed in studies on yeast. Deletion of Exo1 rescued the induction of senescence in telomere dysfunctional yeast strains (Maringele and Lydall, 2002). In addition, Exo1 processing of dysfunctional telomere has been implicated in generating chromosomal translocation in response to telomere dysfunction (Hackett and Greider, 2003). Exo1 can also mediate survival of telomere dysfunctional yeast strains by increasing homologous recombination at dysfunctional telomeres (Maringele and Lydall, 2004). Knockout mice carrying a deletion mutation of the nuclease domain of Exo1 are viable, but show defects in DNA mismatch repair accompanied with slightly increased cancer susceptibility at advanced age (Wei et al., 2003).
It is unknown, whether Exo1 has a role in response to telomere dysfunction or extra-telomeric DNA breaks in mammalian cells. Here we show that Exo1 deletion rescues the accumulation of DNA damage in telomere dysfunctional mice as well as DNA damage signal induction in response to telomere dysfunction, γ-irradiation, and 6TG-induced DNA damage. Moreover, Exo1 can improve organ maintenance and prolong lifespan of telomere dysfunctional mice without accelerating chromosomal fusion or cancer formation.
Results
Exo1 deletion prolongs the lifespan of mice with dysfunctional telomeres without accelerating tumor formation
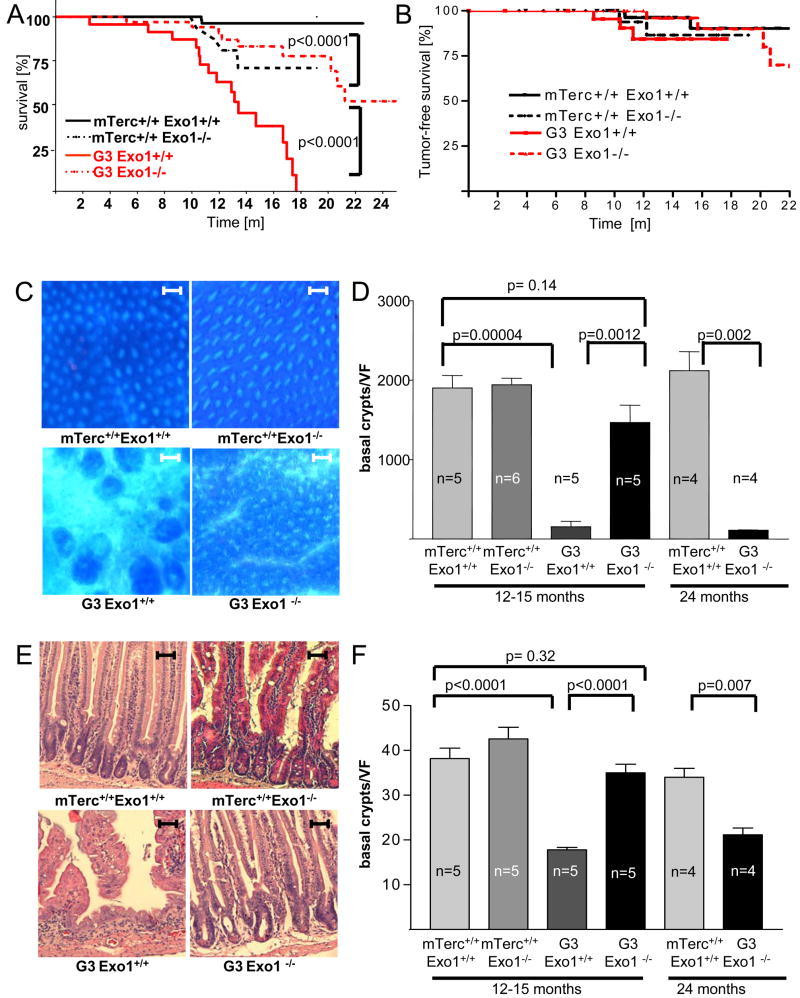
Mice carrying a deletion of the nulease domain (ΔExon 6) of Exo1 were crossed for 3 generations with mTerc−/− mice on a C57BL/6J background. As previously reported (Herrera et al., 1999) the lifespan of G3mTerc−/− mice (n=25) was shortened compared to mTerc+/+, Exo1+/+ mice (n=89, p<0.0001, Figure 1 A). Exo1 deletion shortened the lifespan of mTerc+/+ mice (p=0.009, Figure 1A, Wei et al. 2003). An increase in sponatneous cancer has been reported in mExo1−/− mice of a mixed genetic background (Wei et al. 2003). In agreement with the relative cancer resistance of the C57Bl/6J mouse strain, the overall cancer incidence was low in our mouse cohorts (Figure 1B, Suppl. Table 1) and Exo1 deletion did not result in a significant increase in cancer formation (Figure 1B) indicating that other factors may contribute to the lifespan reduction in mTerc+/+, Exo−/− mice.
Figure 1.
Exo1 deletion prolongs the lifespan and improves maintenance of intestinal epithelia in aging telomere dysfunctional mice. A) Survival curves for mTerc+/+, Exo1+/+ (n=89), mTerc+/+, Exo1−/− (n=27), G3, Exo1+/+ (n=25), and G3, Exo1−/− mice (n=34). Note that G3, Exo1−/− mice show a significantly prolonged lifespan compared to G3, Exo1+/+ (p<0.0001). B) Tumor free survival curves for the indicated mouse cohorts. There was a mild increase in tumor formation in G3mTerc−/−, Exo1+/+ mice compared to mTerc+/+, Exo1+/+ mice (p=0.03). Exo1 deletion did not accelerate tumor formation in G3mTerc−/−, Exo1−/− mice. C) Representative photographs of whole mount staining of the colon of 12–15 month old mice of the indicated genotypes (magnification bar: 500μm). D) Histogram on the number of crypts per low power vision field (35x) in the colon of 12–15 and 24 month old mice of the indicated genotypes. The number of crypts was counted on whole mount staining (n=4−6 mice per group). E) Representative photographs of H&E stained longitudinal sections of the small intestine of 12–15 month old mice of the indicated genotypes (magnification bar: 200μm). F) Histogram on the number of basal crypts per vision field (100x) in the small intestine of 12–15 and 24 month old mice of the indicated genotypes (n=4−5 mice per group). Note that Exo1 deletion rescues the depletion of colon crypts and basal crypts in small intestine of 12–15 month old G3 mTerc mice, but these phenotypes appear in 20–24 month old G3mTerc−/−, Exo1−/− mice.
In contrast to the effects on lifespan of mTerc+/+ mice, deletion of Exo1 significantly prolonged survival of G3mTerc−/−, Exo1−/− (n=34) compared to G3mTerc−/−, Exo1+/+ mice (p<0.0001, Figure 1A). This rescue in lifespan was associated with an improvement of overall fitness as reflected by an increase in body weight of aging G3mTerc−/−, Exo1−/− compared to G3mTerc−/−, Exo1+/+ mice (p=0.0002, Suppl. Figure 1A). Notably, Exo1 deletion did not accelerate tumor formation in G3 mTerc−/− mice (Figure 1B, p=0.3).
Exo1 deletion rescues organ maintenance in aging telomere dysfunctional mice
G3mTerc−/− mice are phenotypically normal at the age of 3 months, but show an atrophy of the intestinal epithelium and impaired hematolymphopoiesis at the age of 12–15 months (Herrera et al., 1999; Rudolph et al., 1999, Choudhury et al. 2007). The evolution of these phenotypic changes correlates with the progression of telomere dysfunction in aging mTerc−/−mice. A survey of our aging cohorts showed that Exo1 deletion rescued organ maintenance in 12–15 month old G3mTerc−/− mice (Figure 1C–F and 2A–F). Specifically, 12–15 month old G3mTerc−/− mice showed a severe atrophy of colonic crypts (Figure 1C,D) and a depletion of the progenitor compartment in the basal crypts of the intestine (Figure 1E,F) compared to age-matched mTerc+/+ mice. Exo1 deletion had no significant influence on crypt numbers in aging mTerc+/+ mice but improved maintenance of basal crypts and colonic crypt numbers in 12–15 month old G3mTerc−/−, Exo1−/− compared to G3mTerc−/−, Exo1+/+ mice (Figure 1C–F).
Figure 2. Exo1 deletion rescues lymophopoiesis in aging telomere dysfunctional mice.
A) Representative photographs of H&E stained longitudinal sections of the spleen of 12–15 month old mice of the indicated genotypes (magnification bar: 500μm). The lymphocyte containing white pulp is circled. B) Histogram on the frequency of B-lymphocytes in spleen of 12–15 and 24 month old mice of the indicated genotypes (n=4−6 mice/group). C) Histogram showing the total number of B-lymphocytes (B220+) and T-lymphocytes (CD8+ or CD4+) in total bone marrow (collected from 2 hind legs) of 12–15 month and 24 month old mice of the indicated genotypes (n=4–8 mice/group). D) FACS plot showing B-lymphocytes (B220+) in bone marrow of 12–15 month old mice of the indicated genotypes. E) Histogram showing the total number of thymocytes of 12–15 month old mice of the indicated genotypes (n=5). Note that the loss of thymocytes in G3, Exo1+/+ is rescued in G3, Exo1−/− mice. F) Representative FACS plots showing T- cell development in thymus of 12–15 month old mice of the indicated genotypes. Note that the percentage of CD8+, CD4+ double positive T-cells (immature T-cells) is reduced in G3, Exo1+/+ but rescued in G3, Exo1−/− mice.
In the hematopoietic system, 12–15 month old G3mTerc−/− mice showed a severe atrophy of the splenic white pulp (Figure 2A), a reduction in splenic B-lymphocytes (Figure 2B), and impaired B-lymphocyte development in bone marrow compared to mTerc+/+ mice (Figure 2C,D). In addition, an atrophy of thymus and reduction in thymic T-lymphocyte numbers was observed in 12–15 month old G3mTerc−/− mice compared to mTerc+/+ mice (Figure 2E,F). Exo1 deletion rescued these defects in lymphopoiesis in 12–15 month old G3mTerc−/− mice but had no apparent influence on lymphopoiesis in aging mTerc+/+ mice (Figure 2C–F).
In a cohort of 20–24 month old G3 mTerc−/−, Exo−/− mice (n=4) some of the phenotypes of impaired organ maintenance appeared but not in 20–24 month old mTerc+/+ mice (Figure 1D,F, Figure 2B,C) indicating that Exo1 deletion transiently rescued organ maintenance in G3 mTerc−/− mice but Exo1-independent mechanisms limited organ maintenance in very old G3mTerc−/−, Exo1−/− mice.
Deletion of Exo1 did not rescue testis atrophy in G3mTerc−/− mice because Exo1 deletion itself causes meiotic defects resulting in testis atrophy and sterility in both male and femal mTerc+/+ mice (Wei et al., 2003).
Exo1 deletion reduces cell cycle arrest and apoptosis in intestinal crypts of aging G3mTerc−/− mice
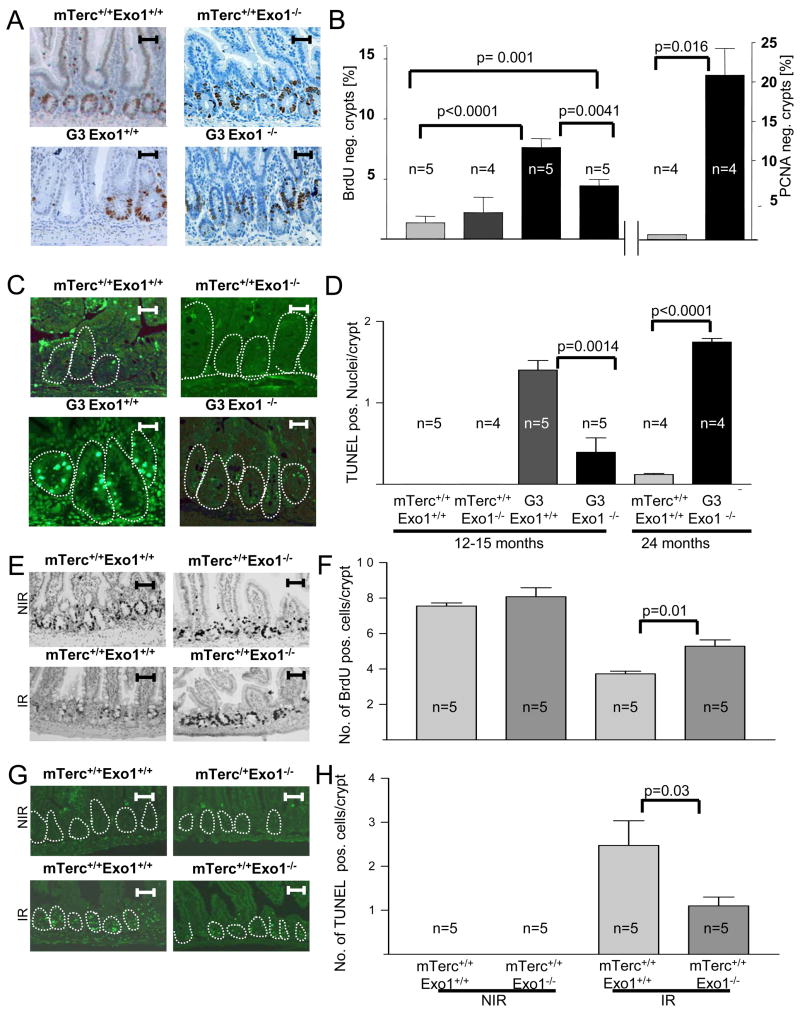
Impaired maintenance of high turnover organs in telomere dysfunctional mice has been linked to increased apoptosis and impaired cell proliferation (Lee et al., 1998). In agreement with these studies, the rate of BrdU incorporation was reduced in basal intestinal crypts of aged G3mTerc−/− mice compared to mTerc+/+ mice (Figure 3A,B). Exo1-deletion partially rescued proliferation rates of intestinal basal crypts in G3mTerc−/−, Exo1−/− double knockout mice (n=5 mice per group, p=0.0041,Figure 3A,B). TUNEL staining showed elevated rates of apoptosis in basal crypts of 12–15 month old G3mTerc−/− mice compared to age-matched mTerc+/+ mice (Figure 3C,D). Deletion of Exo1 reduced apoptosis in 12–15 month old G3mTerc−/−, Exo1−/− compared to G3mTerc−/−, Exo1+/+ mice (n= 5 mice per group, p= 0.001). An increase in apoptosis and cell cycle arrest appeared in intestinal crypts of 24 month old G3mTerc−/−, Exo1−/− mice (Figure 3B, D).
Figure 3. Exo1 deletion increases cell proliferation and prevents apoptosis in telomere dysfunctional mice. A,B) Mice were pulsed with BrdU for 4 hours before sacrificing.
A) Representative photographs of BrdU-stained crypts in the small intestine of 12–15 month old mice of the indicated genotypes. B) Histogram showing the percentage of BrdU and PCNA-negative crypts in the small intestine of 12–15 and 24 month old mice of the indicated genotypes (n=4–5 mice/group). C) Representative photographs of apoptotic cells (green nuclei) in basal crypts (circled) of the small intestine of 12–15 month old mice of the indicated genotypes. D) Histogram showing the number of TUNEL-positive nuclei per basal crypts in the small intestine of 12–15 and 24 month old mice of the indicated genotypes (n=4–5 mice/group).
Exo1 deletion does not rescue telomere dysfunction in aging G3mTerc−/− mice
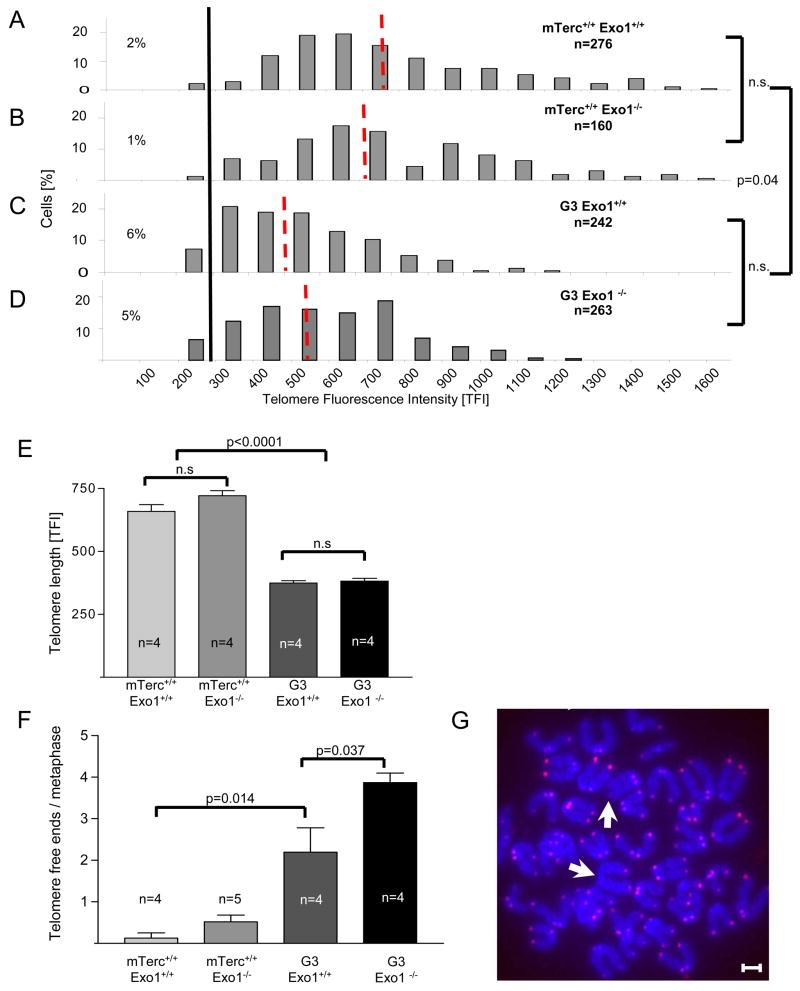
Studies in yeast have shown that defects in mismatch repair genes can promote telomerase-independent proliferation (Rizki and Lundblad, 2001). In mammalian cells, telomerase-independent immortalization of cells involves the activation of an alternative lengthening mechanism of telomeres (ALT), which is based on homologous recombination and characterized by an overall lengthening of telomeres and an increase in telomere length heterogeneity (Bryan et al., 1995; Chang et al., 2003). An analysis of telomere length in basal intestinal crypts (Figure 4A), splenic white pulp (data not shown), and bone marrow (Suppl. Fig. 1B) of 12–15 month old mice revealed a reduction of the average telomere length and an increase in the frequency of cells with critically short telomeres in G3mTerc−/− compared to mTerc+/+ mice. This reduction in telomere length in G3mTerc−/− mice was not rescued by Exo1 deletion (Figure 4A, Suppl Fig. 1B). Neither did we see an increase of telomere length heterogeneity in G3 mTerc−/−,Exo1−/−. Together, there is no evidence of systemic activation of ALT that rescued survival and organ maintenance in G3 mTerc−/−, Exo1−/− mice.
Figure 4. Exo1 deletion does not rescue telomere function in G3 mTerc−/− mice.
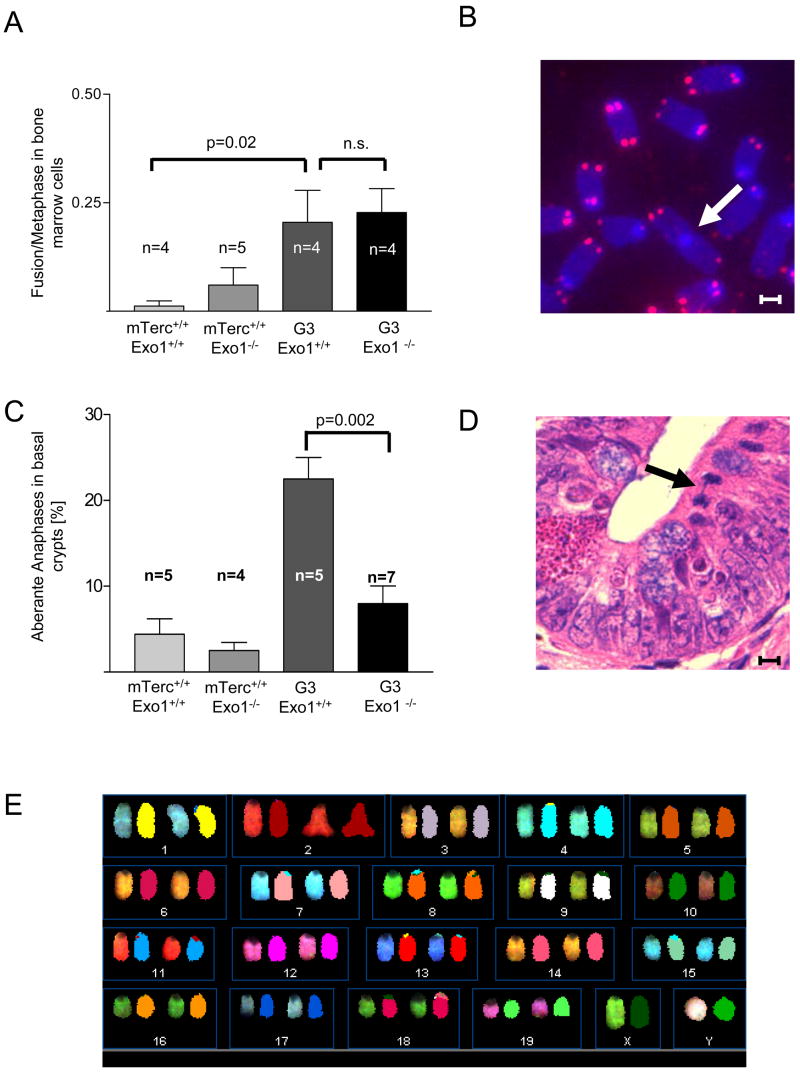
A) Telomere length was analyzed by qFISH in basal crypts of the small intestine of 12–15 month old mice (n=4/group) of the indicated genotypes. The histograms show the distribution of telomere fluorescence intensities (TFI) over all analyzed nuclei ofmTerc+/+, Exo1+/+ (n= 276 nuclei), mTerc+/+, Exo1−/− (n=160 nuclei), G3, Exo1+/+ (n=242 nuclei) and G3, Exo1−/− mice (n=263 nuclei). The red dotted lines indicate the mean value of each genotype. The black line marks the threshold of critically short telomeres (TFI <200 arbitrary units). B) Histogram on the rate of telomere-free ends per metaphase in bone marrow cells of 12–15 month old mice of the indicated genotypes (n=4–5). C) Representative photographs of a metaphase of a G3, Exo1−/− mouse, white arrows pointing on telomere-free ends (magnification bar: 4 pm). D) Histogram on the rate of anaphase bridges per total number of anaphases in basal crypts of small intestine of 12–15 month old mice of the indicated genotypes. E) Representative photograph showing an anaphase bridge in basal crypts of small intestine. F) Representative photograph showing SKY-analysis of metaphase spreads from bone marrow of 24 month old G3mTerc−/−, Exo1−/− mice. No aberrations were detected in 40 metaphases from G3, Exo1−/−mice (n=4).
To directly analyze the in vivo prevalence of telomere dysfunction, we quantified the number of telomere-free chromosomal ends on metaphase spreads derived from bone marrow of 12–15 month old mice. In agreement with previous reports, telomere-free ends were only seen in G3mTerc−/− mice but rarely in mTerc+/+ mice (Figure 4B,C). Exo1 deletion did not rescue the prevalence of telomere-free in G3mTerc−/− mice (Figure 4B,C). Instead there was a further increase of telomere free ends in G3 mTerc−/−, Exo1−/− mice likely due to the abrogation of checkpoint responses (Figure 3).
Exo1 deletion reduces chromosomal fusion and does not increase chromosomal translocations in telomerase knockout mice
Telomere dysfunction induces chromosomal fusion by activation of DNA repair pathways (Maser et al., 2007b, Smogorzewska et al. 2002). We analyzed the rate of anaphase bridges – a morphological correlate of chromosomal fusions (Kirk et al., 1997; Rudolph et al., 2001) in the intestine of aging mice. There was a significant increase in anaphase bridges in G3mTerc−/−, Exo1+/+ mice compared to age-matched wildtype mice, but Exo1 deletion rescued the formation of anaphase bridges in G3mTerc−/− mice (Figure 4D,E). SKY-analysis on metaphase spreads from bone marrow cells showed low rates of chromosomal fusions in this compartment, but did not reveal recurrent chromosomal translocations in 12–15 month old G3 mTerc−/−, Exo1+/+ mice (data not shown). Notably, deletion of Exo1 did not result in any detectable increase in chromsomal translocations in bone marrow cells from 24 month old G3mTerc−/−, Exo−/− mice (Figure 4F, n=4 mice). Together these results indicate that Exo1 deletion reduced the formation of chromosomal fusions and did not increase chromosomal instability in G3mTerc−/−, Exo1−/−mice with an extended lifespan. These data are consistent with observation in yeast showing that Exo1 promotes DNA recombination and chromosomal instability in response to telomere dysfunction ( Khazanehdari and Borts, 2000; Hackett and Greider, 2003).
Exo1 could also have a role in processing of normal, functional telomeres, which could be important for generation of the g-strand overhang at telomeres. However, the absence of telomere length abnormalities, telomere free ends, or chromosomal fusion in mTerc+/+, Exo1−/− mice indicated that Exo1 is not a major determinant for g-strand integrity and normal telomere function in mouse cells (Figure 4). In agreement with this assumption, we did not detect a significant effect of Exo1 deletion on telomeric g-strand integrity in mouse embryonic fibroblasts (MEFs) (Suppl. Figure 2A–D).
Exo1 deletion prevents DNA damage accumulation and reduces DNA damage signalling in telomere dysfunctional mice
The formation of DNA damage foci represents the very upstream molecular event associated with DNA damage signal induction in response to telomere dysfunction (d’Adda di Fagagna et al., 2003; Satyanarayana et al., 2004). DNA damage foci containing γH2AX and 53BP1 were detected in basal, intestinal crypts of 12–15 month old G3mTerc−/− mice (Figure 5A–D) but not in age-matched mTerc+/+ mice (Suppl Fig 3A,B). Exo1 deletion rescued the accumulation of DNA damage foci in G3mTerc−/−, Exo1−/− mice (Figure 5A–D). Co-staining of γH2AX with TUNEL-positive, apoptotic cells revealed that DNA damage foci were present in non-apoptotic cells of ageing G3mTerc−/− mice indicating that the DNA-damage foci staining did not reflect DNA breaks in apoptotic cells (Supple Fig. 4A). Co-staining of γH2AX or 53BP1 with telomeres (Immuno-FISH) revealed a co-localization of DNA damage foci with telomeric DNA in 14% of the total number of DNA damage foci (Suppl. Fig. 4B,C). These data indicated that Exo1 deletion impaired the accumualtion of DNA damage in telomere dysfunctional mice likely including extratelomeric DNA damage.
Figure 5. Exo1 deletion prevents accumulation of DNA-damage and reduces DNA damage signaling in telomere dysfunctional mice.
A) Representative photographs showing γ-H2AX positive cells (white arrows) in basal crypts of 12–15 month old mice of the indicated genotypes. Magnification bars 50μm. The inlet shows a nucleus containing γ-H2AX foci at high power magnification. B) Histogram showing the percentage of γ-H2AX positive basal crypts in 12–15 and 24 month old mice of the indicated genotypes (n= 4–5 mice/group). C) Representative photographs showing 53BP1 foci (white arrows) in basal crypts of 12–15 month old mice of the indicated genotypes. Magnification bars 50μm. The inlet shows a nucleus containing 53BP1 foci at high power magnification. D) Histogram showing the number of 53BP1 foci per basal crypts in 12–15 and 24 month old mice of the indicated genotypes (n= 4–5 mice/group). E) Representative photographs showing p53 positive cells (white arrows) in basal crypts of 12–15 month old mice of the indicated genotypes. Magnification bars 200 pm. F) Histogram showing the percentage of p53 positive basal crypts in 12–15 and 24 month old mice of the indicated genotypes (n= 4–5 mice/group). G) Representative photographs showing p21-positive nuclei (red arrows) in basal crypts of 12–15 month old mice of the indicated genotypes. Magnification bars 200 μm. H) Histogram showing the percentage of p21-positive intestinal basal crypts of 12–15 and 24 month old mice of the indicated genotypes (n=4–5 mice/group).
Activation of DNA damage signaling in response to dysfunctional telomeres and DNA damage involves p53 and p21 (Brown et al., 1997; Satyanarayana et al., 2003; Stein et al., 1999). We employed immunohistochemical analysis for p53 and p21 to assess whether this pathway was also perturbed in the G3mTerc−/−, Exo1−/− mice. In 12–15 month old G3 mTerc−/−, Exo1+/+ mice nuclear expression of p53 was detected in 47% and p21 in 9.5% of basal intestinal crypts (Figure 5E–H). p53 and p21 expression was not detected in mTerc+/+ mice (Figure 5F, H, Suppl. Fig. 3C,D). Both p53 and p21 up regulation was rescued in G3mTerc−/−Exo−/− mice compared to G3 mTerc−/−, Exo+/+ mice (p<0.001).
Exo1 deletion impairs checkpoint induction and DNA damage signaling in response to γ-irradiation and confers resistance to 6-thioguanine (6TG) induced DNA damage
To analyze whether the effects of Exo1 were specific for telomeric DNA damage or reflected a more general role of Exo1 in response to DNA damage, we analyzed cell cycle arrest and apoptosis in basal intestinal crypts of mTerc+/+, Exo1+/+ and mTerc+/+, Exo1−/− mice in response to 4Gy γ-irradiation (IR) (n= 5 mice per group, 24 hrs after IR). This analysis revealed that Exo1 deletion significantly rescued cell cycle arrest (p=0.01, Figure 6A,B) and apoptosis (p= 0.03, Figure 6A,C) in basal intestinal crypts in response to γ-IR. Next, we determined responses of cultured cells in response to γ–IR, hydroxyurea treatment and after exposure to 6TG – a purine analogue inducing DNA damage. 24 hours after IR (15 Gy), Exo1+/+ fibroblasts showed a strong cell cycle arrest (reduction of cells in S-phase, G1 accumulation), but Exo1−/− fibroblasts exhibited reduced checkpoint responses (Figure 6 D,E, Suppl. Fig. 5A). Exo1 deletion significantly increased the resistance of cells treated with 6-TG (Figure 6F) but had no significant effect on sensitivity to hydroxy urea treatment (Suppl. Fig. 5B). To further characterize the role of Exo1 in response to DNA damage, we analyzed DNA damage signal induction in response to γ-IR. The formation of γH2AX foci is an early response to double strand breaks, but was not significantly reduced in Exo1−/− vs. Exo1+/+ fibroblasts at early time points i.e., at 15, 30 minutes after 15Gy irradiation (data not shown). Similarly, Exo1 deletion did not significantly reduce γH2AX foci formation in the intestine of irradiated mice (Figure 6G). However, intestinal epithelial cells of irradiated Exo1−/− mice in comparison to irradiated Exo+/+ mice showed a significant reduction in 53BP1 foci formation (Figure 6H) and reduced induction of p53 and p21 (Suppl. Fig. 5C,D). In agreement with this in vivo data, DNA damage signaling (ATR, Chk2, p53) was ameliorated in irradiated Exo1−/−vs. Exo1+/+ fibroblasts (Figure 6I).
Figure 6. Exo1 deletion impairs DNA damage signal induction and confers resistance to DNA damaging agents.
A) Representative photographs of BrdU-positive cells or TUNNEL positive cells in the intestinal basal crypts of 4 month old mice of the indicated genotypes: 24 hours after 4Gy γ-irradiation (IR). Magnification bars: 200 μm. B,C) Histogram showing the number of BrdU-positive cells per crypt (B) or TUNNEL positive cells per crypt (C) in the small intestine of 4 month old irradiated (IR) mice of the indicated genotypes (n=5 mice/group). D) Histogram showing the relative reduction of BrdU positive cells in 15Gy irradiated (24 hours after IR) compared to non- irradiated cells of the indicated genotypes. E) Histogram showing the relative increase in cells in G1 stage of cell cycle in 15Gy irradiated (24 hours after IR) compared to non- irradiated cells of the indicated genotypes. F) Survival curve of mouse embryonic stem cells of the indicated genotypes at 3–4 days after 6TG treatment at indicated doses. Survival curves for Exo1−/− (complete gene knockout) and Exo1exonΔ−/− (knockout of the nuclease domain, which was used throughout this study) were generated in two separate experiments. The survival curves for Msh2−/− and WT are the averaged survival curves from two separate experiments. G) Histogram showing the number γH2AX foci per nuclei in the small intestine of 4 month old mice (n=5 per group) of the indicated genotypes: 24 hours after 4Gy γ-irradiation (IR). H) Histogram showing the number of 53BP1 foci per crypt in the small intestine of 4 month old mice (n=5 per group) of the indicated genotypes: 24 hours after 4Gy γ-irradiation (IR). I) Western blots showing expression of phosphorylated ATR (Ser345), phosphorylated Chk2 (T68), and phosphorylated p53 (Ser15) along with β-actin as a loading control in mouse ear fibroblasts of the indicated genotypes at 24hrs after 15Gy irradiation and in non-irradiated controls.
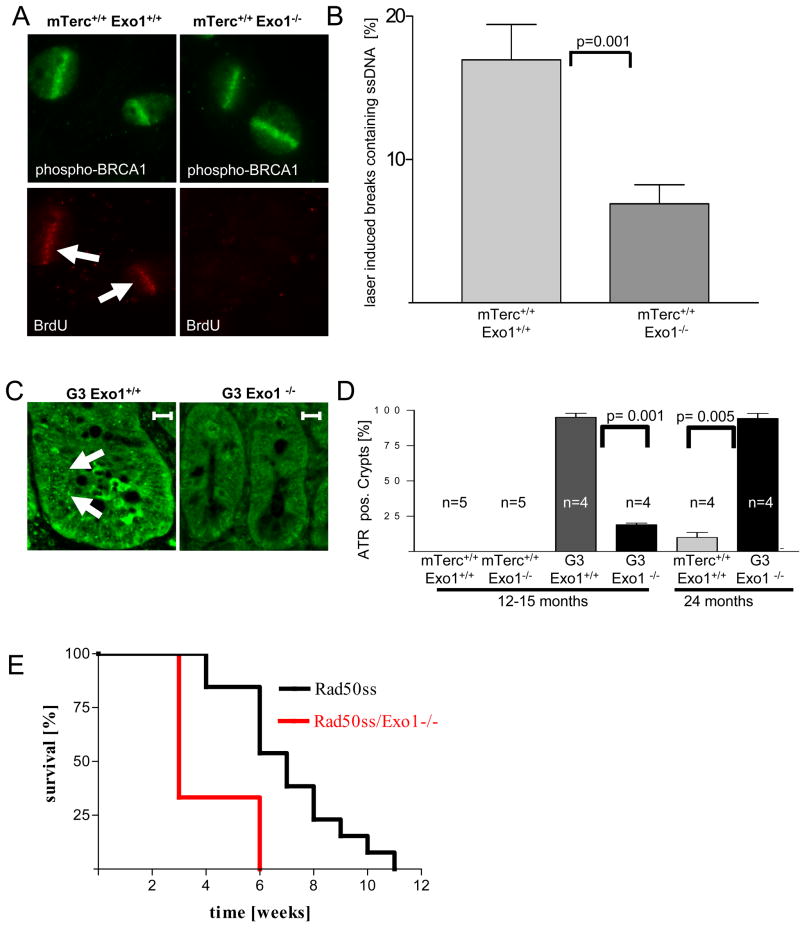
Exo1 deletion impairs the formation of single stranded DNA and RPA recruitment at DNA breaks and impairs ATR activation in telomere dysfunctional mice
Together, our results indicated that Exo1 deletion did not prevent DNA breakage and γH2AX foci formation in response to γ–IR, but impaired the induction of DNA damage signals at the breaks. Studies in yeast have shown that Exo1 generates single stranded DNA (ssDNA) at dysfunctional telomeres and the generation of ssDNA has been shown to induce DNA damge signals in yeast and mammallian cells (Garvik et al., 1995; Lee et al., 1998; Lydall and Weinert, 1995; Zou et al., 2003). To analyze whether Exo1 can generate ssDNA at DNA breaks in mammalian cells we used the experimental system of laser induced DNA breaks (Maser et al. 1997,Rogakou et al. 1999, Greenberg et al., 2006) on mouse embryonic fibroblast (MEF) cultures. Non-denaturing BrdU staining revealed a significant reduction in ssDNA formation in Exo1−/− vs. Exo1+/+ MEFs at laser-induced DNA breaks 15 minutes after damage induction (Figure 7A,B). Similar to the results on γ-IR, γH2AX staining at laser induced DNA breaks was not affected by Exo1 gene status (Figure 7C). In contrast, recruitment of Replication Protein A (RPA) to laser induced breaks was reduced in Exo1−/− vs. Exo1+/+ MEFs (Figure 7C,D). In agreement with previous reports (Bekker-Jensen et al., 2006) high resolution imaging revealed that RPA was recruited to the center of the laser-induced breaks overlapping the region of ssDNA formation, but it did not co-localized with γH2AX or MDC1 in the adjacent chromatin regions flanking the breaks (data not shown). An in vivo analysis of ssDNA by non-denaturing BrdU and RPA staining was not possible because these assays revealed background staining in proliferative organs due to ssDNA intermediates in replicating cells. However, the generation of ssDNA and RPA binding at ssDNA leads to an activation of ATR, which can thus serve as an indirect marker for ssDNA generation in response to DNA damage (Namiki and Zou, 2006). To analyze whether activation of ATR was detectable in organ system affected by telomere shortening immunhistochemistry staining was carried out on mouse intestine (Figure 7E,F). ATR foci were not present in mTerc+/+ mice (Suppl. Figure 5E). Notably, 12–15 month old G3 mTerc−/−, Exo1+/+ mice showed nuclear foci of ATR in intestinal basal crypts, but ATR foci formation was highly dependent on Exo1 gene status and it was not present in 12–15 month old G3 mTerc−/−, Exo1−/− mice (n=4 mice per group, p= 0.001, Figure 7F,G). In agreement with our above results on organ homeostasis, cellular checkpoints, and DNA damage signalling (Figures 1–3, and 5), ATR foci appeared in nuclei of intestinal basal crypts of 20–24 month old G3mTerc−/−, Exo1−/− mice (Figure 7F) indicating that Exo1-independent mechanisms can activate ATR in very old double knockout mice.
Figure 7. Exo1 deletion impairs formation of ssDNA at laser induced DNA breaks in MEFs and the formation of ATR-foci in telomere dysfunctional mice.
A) Representative photograph showing co-localization of ssDNA (stained by non-denaturing BrdU staining) and laser induced DNA breaks (stained by a phospho-BRCA1-antibody) in mTerc+/+, Exo1+/+ MEFs, but absence of such co-localization in mTerc+/+, Exo1−/− MEFs. B) Histogram showing the percentage of laser-induced DNA breaks (phospho-BRCA1) staining positive for ssDNA (non-denaturing BrdU) in MEFs of the indicated genotypes. C) Representative photograph showing γH2AX-formation and recruitment of RPA to laser induced DNA breaks in mTerc+/+, Exo1+/+ MEFs. Note that γH2AX-formation is independent of the Exo1-genotype but recruitment of RPA is reduced in mTerc+/+, Exo1−/− MEFs. D) Histogram showing the percentage of laser-induced DNA breaks staining positive for RPA in MEFs of the indicated genotypes. E) Representative photographs showing ATR-foci in nuclei of cells in basal crypts of 12–15 month old mice of the indicated genotypes (magnification bar 50 μm). F) Histogram showing the percentage of ATR positive basal crypts in 12–15 and 24 month old mice of the indicated genotypes (n= 4–5 mice/group).
Discussion
This study provides direct experimental evidence that Exo1 has a functional role for the induction of DNA damage responses in mammalian cells. Moreover, Exo1 deletion prevents the accumulation of DNA damage and prolongs lifespan of telomere dysfunctional mice. Different mechanisms appear to contribute to this rescue in lifespan.
Exo1 deletion impairs the induction of DNA damage signals at DNA breaks
The current study shows that Exo1 deletion impairs checkpoint responses and confers resistance of mammalian cells and organs to DNA damage. In response to IR and laser-induced damage, Exo1 deletion did not interfere with the formation of DNA breakage itself but reduced the induction of DNA damage signals. The study points to the formation of ssDNA as one possible mechanism by which Exo1 amplifies the generation of DNA damage signals at DNA breaks. Previous studies on yeast cells have shown that the generation of single stranded DNA is required for DNA damage signal induction (Garvik et al., 1995; Lee et al., 1998; Lydall and Weinert, 1995; Zou et al., 2003). Similar mechanisms of DNA damage signal induction exist in mammalian cells but have not been demonstrated to occur in vivo in response to telomere dysfunction or γIR (Zou et al., 2003). The molecular mechanisms leading to the formation of ssDNA in response to DNA damage in mammalian cells are unknown. In yeast, Exo1 has been shown to induce ssDNA in response to telomere dysfunction (Maringele and Lydall, 2002). Exo1 exhibits 5′–3′ exonuclease activity (Genschel et al., 2002), which, at dysfunctional telomeres in yeast, leads to the degradation of the sub-telomeric, lagging strand (the c-rich strand). Our study provides the first evidence that the nuclease domain of Exo1 participates in the formation of ssDNA and RPA recruitment at laser induced DNA breaks in mammalian cells. The deletion of the nuclease domain of Exo1 also impaired the formation of ATR foci in mTerc−/− mice. Given that ATR is a bona fide marker of damage signals induced by ssDNA (Zou et al., 2003), these data indicate that ssDNA formation contributes to DNA damage signal induction in telomere dysfunctional mice and thus aggravate phenotypes of telomere dysfunction and aging. Notably, Exo1 deletion did not rescue cell survival in response to HU treatment. A possible explanation is that HU induces ssDNA dependent signalling independent of Exo1 processing since HU induces single stranded DNA damage by itself.
Exo1 deletion impairs the accumulation of DNA damage in telomere dysfunctional mice
Although Exo1 does not interfere with DNA breakage and γH2AX foci formation in response to IR and laser treatment, Exo1 deletion markedly reduces the accumulation of DNA damage foci in vivo in mTerc−/− mice (Figure 5). These data indicate that dysfunctional telomeres induce an Exo1 dependent processes that leads to the accumulation of DNA damage and checkpoint induction. This process likely involves multiple mechanisms including the following:
The processing of DNA breaks and dysfunctional telomeres by Exo1 could increase the recruitment of some components of DNA damage foci (ATR, 53Bp1, RPA), which would amplify DNA damage signals at dysfunctional telomeres and extratelomeric DNA damage. Our data on laser induced DNA breaks support this concept (Figure 7).
Exo1 deletion may reduce the secondary formation of DNA damage in response to telomere dysfunction by inhibiting chromosomal fusions and fusion bridge-breakage cycles, which creates extratelomeric DNA damage in telomere dysfunctional mice. In agreement with this hypothesis Exo1 deletion reduces the number of anaphase bridges in telomere dysfunctional mice (Figure 4D,E). Cells having undergone replicative senescence due to telomere shortening display numerous γH2AX foci throughout the genome that do not colocalize with telomeric regions (Sedelnikova et al., 2004). Thus telomere shortening can induce DSBs and repair foci at non-telomeric regions of the genome.
Other less obvious mechanisms might be involved. It has been shown that telomere dysfunction increases intracellular ROS-levels in senescent cells (Passos et al., 2007). Although the exact mechanisms is not understood it is possible that deletion of Exo1 reduces the accumulation of ROS and thereby the accumulation of DNA damage.
Together, it is conceivable that Exo1 has different, additive effects on DNA damage accumulation and signal induction in telomere dysfunctional mice. Its role in DNA break processing could directly contribute to the induction of DNA damage signals at dysfunctional telomeres. In addition, Exo1 could increase the generation of extratelomeric DNA damage in the context of chronic telomere dysfunction. An accumulation of extratelomeric DNA damage has been associated with organismal aging (Sedelnikova et al., 2004). Based on our study it is intriguing to speculate that Exo1-dependent processes contribute to this phenomenon in the context of telomere dysfunction.
Exo1 deletion rescues organ maintenance and lifespan of telomere dysfunctional mice
This study reveals that Exo1 deletion improves the maintenance of high-turnover organs and prolongs survival of telomere dysfunctional mice. These findings indicate that Exo1-dependent mechanisms could represent therapeutic targets to improve regeneration and organ homeostasis in the context of telomere dysfunction. There is growing evidence that telomere dysfunction is one of the causes of human aging (Armanios et al., 2007; Cawthon et al., 2003; Tsakiri et al., 2007; Vulliamy et al., 2004) and limit regenerative reserve and organ function at the end stage of chronic diseases in humans (Ball et al., 1998; Wiemann et al., 2002). Since organ homeostasis is maintained by a balance of cell proliferation and apoptosis, it is likely that the inhibition of cell cycle arrest and apoptosis improved organ maintenance and lifespan of G3mTerc−/−, Exo1−/− mice compared to G3mTerc−/−, Exo+/+ mice. This interpretation of the data stands in agreement with our recent study having shown that deletion of p21 improves cell proliferation and organ maintenance in telomere dysfunctional mice (Choudhury et al., 2007). However, we can not exclude that telomere dysfunction also induces other, less obvious, Exo1-dependent mechanisms that limit organ function and survival, independent of cell proliferation and apoptosis, e.g. inflammation or environmental alterations.
The study shows that cell cycle arrest and apoptosis were not completely rescued in G3mTerc−/−, Exo−/− but checkpoint activation and phenotypes of impaired organ homeostasis appeared in very old double knockout mice but not in mTerc+/+ mice. These data suggest that mammalian cells possess Exo1-dependent and Exo1-independent mechanisms for checkpoint induction in response to telomere dysfunction. Consistent with this notion, studies in yeast provide evidence that other exonucleases participate in the processing of dysfunctional telomeres (Tomita et al., 2003). It is possible that such nucleases trigger checkpoint responses independent of Exo1 processing. Albeit this process seems to be less efficient since increased numbers of telomere-free ends in 12–15 month G3mTerc−/−, Exo−/− mice (Figure 4B) do not correlate with impaired organ homeostasis at this age.
Exo1 deletion does not increase the rate of cancer formation in aging telomere dysfunctional mice
The current study shows that Exo1 deletion did not increase cancer formation in telomere dysfunctional mice. Studies in telomerase deficient mice have shown that telomere dysfunction can increase tumor initiation by increasing chromosomal fusions, and the rate of chromosomal instability (Artandi et al., 2000). The current study shows that Exo1 deletion impaired the rate of chromosomal fusions in telomerase knockout mice with short telomere, and Exo1 deletion did not increase chromosomal instability in these mice. These data are in agreement with the model that the generation of chromosomal fusions and consequent induction of fusion-bridge-breakage cycles represent a mechanism inducing chromosomal instability (Maser and DePinho, 2002; Zhu et al., 2003; O’Hagan et al., 2002; Maser et al., 2007a). The current study suggests that Exo1 contributes to the formation of chromosomal fusions and to the evolution of chromosomal instability as a consequence of fusion-bridge-breakage cycles. In agreement with these data it has been shown that Exo1 mediates translocations at chromosome ends in response to telomere dysfunction in yeast (Hackett and Greider, 2003).
In conclusion, our studies show that Exo1 is a critical component for induction of DNA damage signals, cell cycle arrest, and apoptosis in telomere dysfunctional mice and in response to various kinds of DNA damage. Moreover, Exo1 deletion prevents the accumulation of DNA damage and can prolong organismal survival in the context of telomere dysfunction without accelerating chromosomal instability and cancer formation.
Material and Methods
Mouse crosses and survival
Exo1+/− mice were crossed with mTerc+/− mice to generate double heterozygous mice, which were then crossed through successive generations to produce G3 mTerc−/−, Exo1+/+ (n=25) and G3, Exo1−/− mice (n=34). Intercrosses between mTerc+/+, Exo1+/− generated mTerc+/+, Exo1+/+ (n=89) and mTerc+/+, Exo1−/− mice (n=27). All mice were on a C57BL/6J background.
Histopathology
Mice were humanely sacrificed at the age of 12–24 month. For some of the analysis on organ histology we included moribund animals. For FACS analysis we used animals that were killed at a fixed time point (12–15 month and 24 month) without being moribund. Whole mounts of colon and rectum were prepared as previously described (Rudolph et al., 2001).
In vivo BrdU incorporation assay
In vivo labeling of proliferating cells was performed by 4 hour of BrdU labelling [i.p. injection of BrdU 30 mg/kg body weight]. Immunostaining was performed on 5 μm thick paraffin sections using Cell Proliferation Kit (Amersham Biosciences) according to manufacturer’s instructions. The number of BrdU+ and BrdU− crypts was counted in 20 low power fields (200x) and the percentage of BrdU+ and BrdU-crypts was calculated. BrdU+ crypts showed >30% of the cells labelled with BrdU. BrdU–crypts did not show any BrdU+ cells.
In irradiated mice we evaluated the number of BrdU+ cells per crypt. This analysis was not suitable in telomere dysfunctional mice because G3mTerc−/−, Exo1+/+ mice showed enlarged and hyperplastic crypt containing higher cell numbers.
FACS analysis
Thymus, spleen and hind limb bones were dissected from young and old mice. Single cell suspensions (BM cells, thymocytes, splenocytes) were stained with antibody cocktails for 15 min on ice according to standard procedures. Acquisition was performed on BD FACS Calibur. All antibodies were purchased from BD Pharmingen.
Immunohistochemistry
Apoptosis
The rate of apoptosis was determined by TUNEL assay (In situ cell death detection kit, Roche, Mannheim, Germany) on 5 μm thick paraffin sections of small intestine. The number of apoptotic cells/crypt was counted in 20 low power fields (200x) per mouse (n=4–5 mice per group).
γH2AX, ATR, 53BP1, p53 and PCNA
Immunofluorescence was performed on 4-μm-thick paraffin sections of small intestine. Sections were deparaffinized and rehydrated in series of ethanol and permeabelised in 1mM sodium citrate buffer by heating at boiling temperature for 5 minutes and then heating at sub-boiling temperature for 10 min and then allowed to cool down at RT for 40 minutes. The slides were then washed in PBS twice and incubated with primary antibody: γH2ax (UpState, 1:750 dilution), ATR (cell signalling, 1:100), 53BP1 (Cell signalling. 1:100), p53 (R&D, 1:50) and PCNA (Calbiochem, 1:50 dilution) either over night at 4°C or for 2 hours in humid chamber at room temperature. The slides were washed twice with PBS before treated with secondary antibody: γH2ax and PCNA: Anti mouse (1:200 and 1:300 respectively) Cy3-Zymed, 53BP1: Anti rabbit (1:150) Fitc-Zymed, p53: Anti goat (1:100) Fitc-BD pharmingen, for 1 hour at RT and washed with PBS twice, air dried and mounted with Antifade + DAPI (1:1 ratio), covered with coverslip and observed under microscope.
The evaluation of γH2AX in the case of G3 and G3Exo−/− was made as the percent of positive crypts, to this end 20 low power fields (200x) per mouse was counted. Positive crypts showed positive nuclear staining in 2 or more nuclei per crypt and negative crypts did not show any nuclear staining. In the case of irradiated mice, γH2AX was evaluated as the number of foci per nuclei and was counted in 100 high power fields (1000x) per mouse.
In the case of 53BP1 the evaluation was made as the number of positive foci per crypt in all experiments. 53BP1 was counted in 100 high power fields (1000x) per mouse.
For ATR, p53 and PCNA staining, the evaluation was made as the percent of positive crypts and was counted in 20 low power fields (200x) per mouse. Positive crypts showed positive nuclear staining in 2 or more nuclei per crypt. Negative crypts did not show any nuclear staining.
p21
For p21 staining 5 μm thick paraffin sections of small intestine were blocked with M.O.M.™ blocking reagent (Vector Labs) for 1h and then incubated with mouse anti-mouse p21 (Santa Cruz) overnight at 4°C. The subsequent steps to detect p21 signal was performed using M.O.M.™ Immunodetection Kit (Vector Laboratories, USA) as per manufacturer’s recommendations. p21 staining was evaluated in 20 low power fields (200x) per mouse. Positive crypts showed positive nuclear staining in 2 or more nuclei per crypt. Negative crypts did not show any nuclear staining.
Quantitative Fluorescence in situ Hybridization (qFISH)
qFISH was performed as described (Satyanarayana et al., 2003; Wiemann et al., 2002) on 5 pm thick paraffin-sections of small intestine and methanol/acetic acid-fixed bone marrow cells respectively. Sections were boiled 10 min in 10 mM Citricacid-Buffer for antigen unmasking, and hybridized with telomere specific probe. Telomere length was analyzed from the nuclei of the basal crypts and bone marrow nuclei using the TFL-Telo Software from Peter Lansdorp.
Spectral karyotyping
Spectral karyotyping (SKY) was performed as described previously (Frank et al., 2004) and according to the manufacturer’s instructions (ASI; Applied Spectral Imaging, Ltd., Migdal HaEmek, Israel). Spectral images were acquired using an epifluorescence microscope equipped with an interferometer (SpectraCube™ ASI), a custom-designed optical filter and the SkyView™ software (ASI).
Western blot
Whole cell extracts of mouse ear fibroblasts were obtained in RIPA lysis buffer (50mM Tris-HCl (pH8.0) 150mM Nacl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 1mM sodium Meta venadate, 1mM DTT, 1mM PMSF and finally protease inhibitor). Protein was subjected to 10% SDS-PAGE and detected using antibody against phospho ATR (1:1000 dilution, cell signelling), Phospho Chk2-T68 (1:1000, Abcam), phospho-p53-Ser15 (1:1000 dilution; Cell Signalling and against β-Actin (1:1000 dilution, Santa Cruz).
Cytotoxicity assays
Cytotoxicity of 6TG and Hydroxy urea (HU) were determined by growth inhibition assays (Horton et al. 2000). Cells were seeded on 6-well plates and grown overnight. On the following day, the media was replaced with media containing a range of concentrations of either 6TG or HU. Cells were exposed to 6TG and HU for 24 hours. After drug exposure, the plates were washed and cells were grown in drug-free DMEM growth media for 3–4 days. Cells were counted in triplicates using a lysis procedure for 6TG experiment (Butler WB 1984.). For HU treated cells, after 48 hours of treatment cells were trypsinised and counted under microscope in Haemocytometer. Results are expressed in percent survival by comparing the number of surviving cells in drug treated wells to control cells in untreated wells.
Proliferation assay of cell cultures
Mouse ear fibroblasts were seeded in 10cm dishes and grown over night. Then cells were irradiated with 15 Gy irradiation and 24 hours later cells were trypsinised and fixed in 70% cold ethanol for analysis. BrdU (10mM) was added 3 hrs before harvesting the cells. BrdU staining was performed according to manufacturer’s instructions using anti-Brdu (BD pharmingen) and FACS was performed using FACS caliber Flow Cytometer (Becton Dickinson). Data were analysed using Flowjo.
Laser-generated DNA DSBs were generated using a P.A.L.M. MicroBeam laser microdissection system (Carl Zeiss MicroImaging, Inc.) as previously described (Greenberg et al., 2006; Lukas et al., 2003). Cells were grown on coverslips for 36–48 hours in media containing 10uM BrdU (Sigma-Aldrich) prior to laser treatment. Laser-stripes were performed on 70–100 cells per coverslip with the P.A.L.M Microbeam laser (337 nm) using the 40x objective at 52% power. Cells were returned to a cell culture incubator at 37°C for 15 minutes. Cells were pre-extracted with PBS/.5%Titon X-100/1mM PMSF for 5 minutes at 4°C then fixed in a solution containing 3% paraformaldehyde and 2% sucrose for 10 minutes at room temperature.
Bias was not introduced into this assay by knowledge of the genotypes since BrdU staining at laser induced DNA breaks was counted blindly with respect to genotype.
Immunofluorescence following laser stripes was performed as previously described (Greenberg et al., 2006). Following antibodies were used: anti BrdU (BD Bioscience, 1:120); Brca1 phospho-Ser1387 and Brca1 phospho-Ser1423 (Bethyl Labs 1:2500): secondary antibody: Rhodamine Red-X-conjugated AffiniPure Goat Anti-Mouse IgG (Jackson ImmunoResearch, 1:200) and Fluorescein (FITC)-conjugated AffiniPure Goat Anti-Rabbit IgG (Jackson ImmunoResearch, 1:200).
Co-staining of gH2AX and RPA at laser stripes
Immunofluorescence following laser stripes was performed as previously described. Cells were pre-permeabilized with PBS/0.5%Titon X-100 for 5 minutes at 4°C prior to fixation in 3% paraformaldehyde/2% sucrose solution in PBS for 10 minutes at room temperature. Fixed cells were washed twice in PBS and then permeabilized again in PBS/0.5% triton for 5 minutes on ice and then washed twice with PBST. Coverslips were placed on parafilm and cells incubated with primary antibody at 37°C for 20 minutes. Cells were washed three times with PBST prior to 20 minutes incubation at 37°C with secondary antibody. Cells were washed four times with PBST then mounted on slides with VectaShield Mounting media containing DAPI (Vector Labs). Microscopy was performed on a Nikon Eclipse 80i integrated with ImageProTM 6.2 imaging software (Phase 3 Imaging Systems) which was used to generate deconvolved images.
Statistics
Statistical analysis was done using Microsoft Excel and Graph Pad Prism software. Unpaired Students t-test was used to generate the ‘p’ values for the data sets. Survival curves were calculated using the method of Kaplan and Meier.
Supplementary Material
Acknowledgments
T.A.K and A.B.C. were supported by the Intramural Research Program of the NIH, NIEHS.
References
- Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- Ball SE, Gibson FM, Rizzo S, Tooze JA, Marsh JC, Gordon-Smith EC. Progressive telomere shortening in aplastic anemia. Blood. 1998;91:3582–3592. [PubMed] [Google Scholar]
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- Bekker-Jensen S, Lukas C, Kitagawa R, Melander F, Kastan MB, Bartek J, Lukas J. Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. J Cell Biol. 2006;173:195–206. doi: 10.1083/jcb.200510130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. Embo J. 1995;14:4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler WB. Preparing nuclei from cells in monolayer cultures suitable for counting and for following synchronized cells through the cell cycle. Anal Biochem. 1984 Aug 15;141:70–73. doi: 10.1016/0003-2697(84)90426-3. [DOI] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Chang S, Khoo CM, Naylor ML, Maser RS, DePinho RA. Telomere-based crisis: functional differences between telomerase activation and ALT in tumor progression. Genes Dev. 2003;17:88–100. doi: 10.1101/gad.1029903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Choudhury AR, Ju Z, Djojosubroto MW, Schienke A, Lechel A, Schaetzlein S, Jiang H, Stepczynska A, Wang C, Buer J, et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat Genet. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- Djojosubroto MW, Choi YS, Lee HW, Rudolph KL. Telomeres and telomerase in aging, regeneration and cancer. Mol Cells. 2003;15:164–175. [PubMed] [Google Scholar]
- Frank O, Rudolph C, Heberlein C, von Neuhoff N, Schrock E, Schambach A, Schlegelberger B, Fehse B, Ostertag W, Stocking C, Baum C. Tumor cells escape suicide gene therapy by genetic and epigenetic instability. Blood. 2004;104:3543–3549. doi: 10.1182/blood-2004-03-0852. [DOI] [PubMed] [Google Scholar]
- Garvik B, Carson M, Hartwell L. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol Cell Biol. 1995;15:6128–6138. doi: 10.1128/mcb.15.11.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genschel J, Bazemore LR, Modrich P. Human exonuclease I is required for 5′ and 3′ mismatch repair. J Biol Chem. 2002;277:13302–13311. doi: 10.1074/jbc.M111854200. [DOI] [PubMed] [Google Scholar]
- Greenberg RA, Allsopp RC, Chin L, Morin GB, DePinho RA. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene. 1998;16:1723–1730. doi: 10.1038/sj.onc.1201933. [DOI] [PubMed] [Google Scholar]
- Greenberg RA, Sobhian B, Pathania S, Cantor SB, Nakatani Y, Livingston DM. Multifactorial contributions to an acute DNA damage response by BRCA1/BARD1-containing complexes. Genes Dev. 2006;20:34–46. doi: 10.1101/gad.1381306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell PW, Kronmal SL, Porter SE, Gassenhuber J, Obermaier B, Petes TD. TEL1, a gene involved in controlling telomere length in S. cerevisiae, is homologous to the human ataxia telangiectasia gene. Cell. 1995;82:823–829. doi: 10.1016/0092-8674(95)90479-4. [DOI] [PubMed] [Google Scholar]
- Hackett JA, Greider CW. End resection initiates genomic instability in the absence of telomerase. Mol Cell Biol. 2003;23:8450–8461. doi: 10.1128/MCB.23.23.8450-8461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D, Greider CW. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Herrera E, Samper E, Martin-Caballero J, Flores JM, Lee HW, Blasco MA. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. Embo J. 1999;18:2950–2960. doi: 10.1093/emboj/18.11.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JK, Prasad R, Hou E, Wilson SH. Protection against methylation-induced cytotoxicity by DNA polymerase beta-dependent long patch base excision repair. J Biol Chem. 2000;275:2211–2218. doi: 10.1074/jbc.275.3.2211. [DOI] [PubMed] [Google Scholar]
- Khazanehdari KA, Borts RH. EXO1 and MSH4 differentially affect crossing-over and segregation. Chromosoma. 2000;109:94–102. doi: 10.1007/s004120050416. [DOI] [PubMed] [Google Scholar]
- Kirk KE, Harmon BP, Reichardt IK, Sedat JW, Blackburn EH. Block in anaphase chromosome separation caused by a telomerase template mutation. Science. 1997;275:1478–1481. doi: 10.1126/science.275.5305.1478. [DOI] [PubMed] [Google Scholar]
- Lechel A, Satyanarayana A, Ju Z, Plentz RR, Schaetzlein S, Rudolph C, Wilkens L, Wiemann SU, Saretzki G, Malek NP, et al. The cellular level of telomere dysfunction determines induction of senescence or apoptosis in vivo. EMBO Rep. 2005;6:275–281. doi: 10.1038/sj.embor.7400352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Blasco M, Gottlieb G, Horner Jn, Greider C, DePinho R. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- Lukas C, Falck J, Bartkova J, Bartek J, Lukas J. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat Cell Biol. 2003;5:255–260. doi: 10.1038/ncb945. [DOI] [PubMed] [Google Scholar]
- Lydall D, Weinert T. Yeast checkpoint genes in DNA damage processing: implications for repair and arrest. Science. 1995;270:1488–1491. doi: 10.1126/science.270.5241.1488. [DOI] [PubMed] [Google Scholar]
- Maringele L, Lydall D. EXO1-dependent single-stranded DNA at telomeres activates subsets of DNA damage and spindle checkpoint pathways in budding yeast yku70Delta mutants. Genes Dev. 2002;16:1919–1933. doi: 10.1101/gad.225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maringele L, Lydall D. EXO1 plays a role in generating type I and type II survivors in budding yeast. Genetics. 2004;166:1641–1649. doi: 10.1534/genetics.166.4.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RS, DePinho RA. Connecting chromosomes, crisis, and cancer. Science. 2002;297:565–569. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- Maser RS, Monsen KJ, Nelms BE, Petrini JH. hMre11 and hRad50 nuclear foci are induced during the normal cellular response to DNA double-strand breaks. Mol Cell Biol. 1997;17:6087–6096. doi: 10.1128/mcb.17.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RS, Choudhury B, Campbell PJ, Feng B, Wong KK, Protopopov A, O’Neil J, Gutierrez A, Ivanova E, Perna I, Lin E, Mani V, Jiang S, McNamara K, Zaghlul S, Edkins S, Stevens C, Brennan C, Martin ES, Wiedemeyer R, Kabbarah O, Nogueira C, Histen G, Aster J, Mansour M, Duke V, Foroni L, Fielding AK, Goldstone AH, Rowe JM, Wang YA, Look AT, Stratton MR, Chin L, Futreal PA, DePinho RA. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007a;447:966–971. doi: 10.1038/nature05886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RS, Wong KK, Sahin E, Xia H, Naylor M, Hedberg HM, Artandi SE, DePinho RA. DNA-dependent protein kinase catalytic subunit is not required for dysfunctional telomere fusion and checkpoint response in the telomerase-deficient mouse. Mol Cell Biol. 2007b;27:2253–2265. doi: 10.1128/MCB.01354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito T, Matsuura A, Ishikawa F. Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nat Genet. 1998;20:203–206. doi: 10.1038/2517. [DOI] [PubMed] [Google Scholar]
- Namiki Y, Zou L. ATRIP associates with replication protein A-coated ssDNA through multiple interactions. Proc Natl Acad Sci U S A. 2006;103:580–585. doi: 10.1073/pnas.0510223103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birket MJ, Harold G, Schaeuble K, Birch-Machin MA, Kirkwood TB, von Zglinicki T. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5:e110. doi: 10.1371/journal.pbio.0050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Strong MA, Karim BO, Armanios M, Huso DL, Greider CW. Short telomeres and ataxia-telangiectasia mutated deficiency cooperatively increase telomere dysfunction and suppress tumorigenesis. Cancer Res. 2003;63:8188–8196. [PubMed] [Google Scholar]
- Rizki A, Lundblad V. Defects in mismatch repair promote telomerase-independent proliferation. Nature. 2001;411:713–716. doi: 10.1038/35079641. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Millard M, Bosenberg MW, DePinho RA. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet. 2001;28:155–159. doi: 10.1038/88871. [DOI] [PubMed] [Google Scholar]
- Satyanarayana A, Greenberg RA, Schaetzlein S, Buer J, Masutomi K, Hahn WC, Zimmermann S, Martens U, Manns MP, Rudolph KL. Mitogen stimulation cooperates with telomere shortening to activate DNA damage responses and senescence signaling. Mol Cell Biol. 2004;24:5459–5474. doi: 10.1128/MCB.24.12.5459-5474.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana A, Wiemann SU, Buer J, Lauber J, Dittmar KE, Wustefeld T, Blasco MA, Manns MP, Rudolph KL. Telomere shortening impairs organ regeneration by inhibiting cell cycle re-entry of a subpopulation of cells. Embo J. 2003;22:4003–4013. doi: 10.1093/emboj/cdg367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1:72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, Karlseder J, Holtgreve-Grez H, Jauch A, de Lange T. DNA ligase IV-dependent NHEJ of deprotected mammalian telomeres in G1 and G2. Curr Biol. 2002;12:1635–1644. doi: 10.1016/s0960-9822(02)01179-x. [DOI] [PubMed] [Google Scholar]
- Stein GH, Drullinger LF, Soulard A, Dulic V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- Tomita K, Matsuura A, Caspari T, Carr AM, Akamatsu Y, Iwasaki H, Mizuno K, Ohta K, Uritani M, Ushimaru T, et al. Competition between the Rad50 complex and the Ku heterodimer reveals a role for Exo1 in processing double-strand breaks but not telomeres. Mol Cell Biol. 2003;23:5186–5197. doi: 10.1128/MCB.23.15.5186-5197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri H, Benchimol S. From telomere loss to p53 induction and activation of a DNA-damage pathway at senescence: the telomere loss/DNA damage model of cell aging. Exp Gerontol. 1996;31:295–301. doi: 10.1016/0531-5565(95)02025-x. [DOI] [PubMed] [Google Scholar]
- Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet. 2004 doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- Wei K, Clark AB, Wong E, Kane MF, Mazur DJ, Parris T, Kolas NK, Russell R, Hou H, Jr, Kneitz B, et al. Inactivation of Exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev. 2003;17:603–614. doi: 10.1101/gad.1060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, Flemming P, Franco S, Blasco MA, Manns MP, Rudolph KL. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. Faseb J. 2002;16:935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- Wong KK, Maser RS, Bachoo RM, Menon J, Carrasco DR, Gu Y, Alt FW, DePinho RA. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature. 2003;421:643–648. doi: 10.1038/nature01385. [DOI] [PubMed] [Google Scholar]
- Wright WE, Shay JW. Telomere positional effects and the regulation of cellular senescence. Trends Genet. 1992a;8:193–197. doi: 10.1016/0168-9525(92)90232-s. [DOI] [PubMed] [Google Scholar]
- Wright WE, Shay JW. The two-stage mechanism controlling cellular senescence and immortalization. Exp Gerontol. 1992b;27:383–389. doi: 10.1016/0531-5565(92)90069-c. [DOI] [PubMed] [Google Scholar]
- Zhu XD, Niedernhofer L, Kuster B, Mann M, Hoeijmakers JH, de Lange T. ERCC1/XPF removes the 3′ overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Mol Cell. 2003;12:1489–1498. doi: 10.1016/s1097-2765(03)00478-7. [DOI] [PubMed] [Google Scholar]
- Zou L, Liu D, Elledge SJ. Replication protein A-mediated recruitment and activation of Rad17 complexes. Proc Natl Acad Sci U S A. 2003;100:13827–13832. doi: 10.1073/pnas.2336100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.