Abstract
Porphyrinogens serve as substrates for three heme biosynthetic enzymes. Porphyrinogens are highly unstable and must be generated as an integral part of enzyme assays. Methods commonly utilized to generate porphyrinogens include chemical reduction using sodium amalgam or sodium borohydride and enzymatic generation from porphobilinogen. Chemical reduction yields porphyrinogens in highly alkaline solutions with high ionic strength, while enzymatic generation requires purified enzymes, deproteination and complete buffer replacement. This report describes an improved method for reducing porphyrins to porphyrinogens using palladium on carbon as a catalyst under hydrogen at ambient temperature and pressure in the dark. The palladium catalyst is removed by filtration, the filtrate blown dry with an inert gas and the dried porphyrinogen can be dissolved in a buffer compatible with biological studies.
Keywords: porphyrinogen, porphyrin, porphyria, heme biosynthetic pathway, uroporphyrinogen decarboxylase, HPLC
INTRODUCTION
Porphyrinogens serve as substrates for three heme biosynthetic enzymes, uroporphyrinogen decarboxylase (UroD), coproporphyrinogen III oxidase (CoproOx) and protoporphyrinogen IX oxidase (ProtoOx). Porphyrinogens are highly unstable and readily auto-oxidize to porphyrins [1]. In the laboratory, these properties require that they be synthesized as an integral part of enzyme assays. Porphyrinogens have also been utilized in structural studies of enzyme-substrate complexes and, as with enzyme activity assays, the porphyrinogen of interest must be prepared as an integral part of the crystallization process [2]. Here we describe a unique method for preparing porphyrinogens by palladium catalyzed reductive hydrogenation of porphyrins. We concentrate on the preparation of uroporphyrinogen but the method is also applicable to the preparation of other porphyrinogens.
Two methods of preparing uroporphyrinogen for use in enzymatic and structural studies have been widely used [1; 3] One employs the coupled enzymatic synthesis of uroporphyrinogen I using porphobilinogen (PBG) and PBG deaminase (PBGD) [3]. Uroporphyrinogen III can be generated by adding uroporphyrinogen III synthase to the mixture [2]. The pH and ionic strength of the resulting porphyrinogen preparation are then adjusted to allow optimal UroD activity. The other employs chemical reduction of the porphyrin using either sodium amalgam or sodium borohydride under an inert gas [1]. The sodium amalgam method yields uroporphyrinogen, but with a high concentration of sodium hydroxide. Ionic strength and pH then have to be dramatically adjusted to render the preparation useful. Because porphyrinogens are unstable at acidic pH [1], titrations to the desired nearneutral pH must be very precise. Preparation of the amalgam, which has a shelf life of approximately two weeks, requires gram quantities of mercury to be dissolved with molten sodium metal, a step with explosive potential. Significant amounts of excess mercury and mercury-tainted laboratory ware also result and represent serious biohazards. Reduction with sodium borohydride has also been used to chemically reduce porphyrins [1], but again the resulting porphyrinogen solution has very high ionic strength and a very alkaline pH. Porphyrins and porphyrin esters in glacial acetic acid solutions have also been reduced by catalytic hydrogenation in the presence of hydrogen and either palladium on asbestos [4] or platinum [5]. All these chemical reduction methods require extra purification steps in order to generate highly concentrated and purified porphyrinogens.
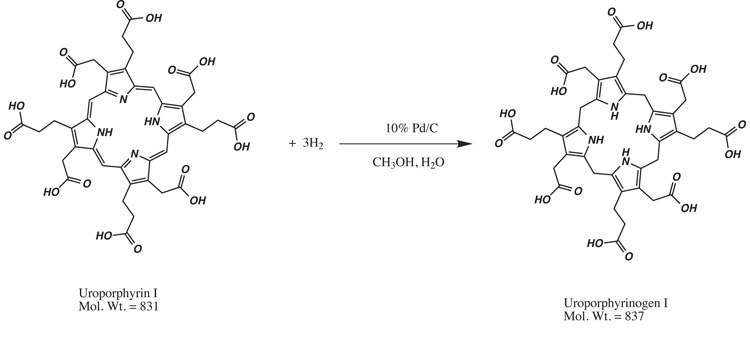
Palladium on carbon (Pd/C) is commercially available, is stable for years in a closed container and has been widely used to catalyze the hydrogenation of unsaturated organic compounds and porphyrins [6; 7] (Fig. 1). The Pd/C catalyst is easily removed by filtration, as the Pd/C particles are 28–34 µm in diameter [8] while syringe type glass fiber filters have a 1.6 µm pore size. The filtrate is then evaporated to obtain the dry porphyrinogen. Porphyrinogens prepared using this method can then be dissolved in a buffer suitable for biological studies. Although the toxicity of palladium relative to mercury is not clearly defined [9], the amount of waste Pd (in milligrams) and Pd-contaminated materials is greatly reduced compared to the gram amounts of mercury in the amalgam system.
Figure 1.
Reductive hydrogenation of uroporphyrin to uroporphyrinogen.
We determined the optimal conditions for the use of Pd/C and hydrogen in reducing uroporphyrin to uroporphyrinogen for use as a substrate for UroD. UroD catalyzes the decarboxylation of each of the four acetate groups of uroporphyrinogen to methyl groups, yielding coproporphyrinogen. UroD does not accept uroporphyrin or partially oxidized uroporphyrinogen as substrates [10]. The generation of partially and fully decarboxylated porphyrinogens by UroD can be used as an indicator of the concentration of uroporphyrinogen in a mixture of porphyrinogens and oxidized porphyrins. This measure of porphyrinogen •purity• can be used to determine the suitability of a preparation for biological and structural studies.
MATERIALS AND METHODS
Materials
Porphyrins were obtained from Frontier Scientific (Logan, Utah). Palladium, 10% by weight on activated carbon and the Atmosbag inflatable gas bag were purchased from Sigma-Aldrich (St. Louis, MO). Organic solvents and other chemical reagents were supplied by Fisher Scientific (Pittsburgh, PA). Recombinant human UroD (rhUroD) and recombinant PBGD were prepared as described [2].
Porphyrin reduction
Initial experiments were carried out as follows: A 13 × 100 mm borosilicate test tube containing 5.0 mg Pd/C and 2.0 mg (2.2 µmol) uroporphyrin I was wetted with 0.2 mL water to avoid potential explosion when vapor from the organic solvent came in contact with Pd/C powder. Then, 1.8 mL methanol was added and the mixture placed in a portable gas bag (Atmosbag) fully inflated with hydrogen. This was done under a dark room red light. The mixture was agitated using a magnetic flea and monitored with a UV light for 5 s, every 5 min. until all UV fluorescence disappeared, indicating maximal conversion of porphyrin to porphyrinogen (approximately 20–45 min). The Pd/C powder was removed by filtration through a wad of glass fiber or a syringe type glass fiber filter while still in the Atmosbag. The filtrate was removed and evaporated to dryness under blowing argon in a water bath at 60°. The dry porphyrinogen was then dissolved in deoxygenated 50 mM potassium phosphate, pH 6.80 with 10 mM dithiothreitol (DTT), a buffer optimal for the UroD reaction [3].
Uroporphyrinogen I was also prepared enzymatically using porphobilinogen and PBGD at pH 7.65 [3] for use as a control in optimizing the Pd/C method. Enzymatically generated porphyrinogen was kept under argon in the dark at pH 7.65 until just before use.
UroD assay
RhUroD was assayed with either enzymatically generated uroporphyrinogen I or with uroporphyrinogen from Pd/C catalyzed reduction of uroporphyrin. Enzymatically generated uroporphyrinogen I was prepared as described by Straka et al. [3], but using only 40% of the reagent quantities described. The assay with enzymatically generated substrate was carried out in two stages, A and B, at the optimal pH for the two enzymes used (PBGD and rhUroD). Incubation A generates uroporphyrinogen from PBGD and PBG at pH 7.65. The mixture is then adjusted to pH 6.80 and added to rhUroD to begin incubation B, the actual activity assay.
The dry Pd/C reduced porphyrinogen was dissolved in 1.0 mL of deoxygenated UroD assay buffer (50 mM potassium phosphate pH 6.80 and 10 mM DTT). Every 200-µL UroD assay mixture contained 120 µL of either Pd/C generated porphyrinogen or enzymatically generated substrate. To determine if oxidation of a portion of the porphyrinogen prepared by either method had occurred, an assay mixture containing 200 µg/mL UroD was used. Decarboxylation reaction mixtures always led to the removal of approximately 90% of the carboxyl moieties that could theoretically have been acted upon (data not shown). This measure of total UroD-reactive carboxylates (URC) present was used to calculate the proportion of a particular substrate sample that was the fully reduced porphyrinogen. All yields were presented as concentrations based on the original volume. All experiments were done in duplicate.
HPLC
The products of the rhUroD reaction were quantified using reverse phase HPLC as previously described [11].
RESULTS
Several mixtures of Pd/C, water and methanol were tested for their ability to reduce 2.0 mg uroporphyrin under hydrogen for different lengths of time (1–6 h). Water with no methanol led to very tight binding of the porphyrin to the Pd/C. It was predicted that reduction of the porphyrin to a more water-soluble porphyrinogen would result in release of the porphyrinogen into solution, whereas unreacted porphyrin would remain bound to the Pd/C solids. However, even after 6 hours of stirring under hydrogen, very little porphyrinogen was released. This was demonstrated by mixing the supernatant with an equal volume of 3 M HCl followed by exposure to UV light to oxidize any porphyrinogen present. Very little fluorescence was observed. Addition of methanol to the Pd/C sediment solubilized the adsorbed porphyrin from the Pd/C particles resulting in a highly fluorescent supernatant. Using 50 µL of water to initially wet the Pd/C and then adding 1.95 mL methanol kept the porphyrin in the liquid phase where it stayed unreduced and highly fluorescent even after 6 hours of stirring under hydrogen. Optimization of the aqueous volume showed that 10% v/v water in methanol produced the highest amount of porphyrinogen based on the URC content of the resulting product.
Water in the reduction mixture was then replaced with 0.1 M HCl or 0.1 M NH4OH to determine whether neutral or ionized porphyrin would yield more URC. Uroporphyrin is neutral in 0.1 M HCl, while all of its carboxylic acid moieties are ionized to carboxylate in 0.1 M NH4OH. The URC produced with 0.1 M NH4OH was 299±4 µM. Water or 0.1 M HCl yielded 36.8±0.1 and 21.8±1.2 µM URC, respectively. It was evident that only porphyrins in solution were reduced and that the activated charcoal prevented more of the neutral porphyrin molecules from dissolving. NH4OH ionized the uroporphyrin, rendering it readily soluble in the mixture and more easily reduced. The PBG+PBGD control system generated 129±2 µM URC, a value near the expected range. Our original UroD assay [3] was designed to measure enzyme activity in erythrocyte lysates or tissue homogenates, and a substrate concentration of approximately 30 µM proved to be saturating. Since each molecule of uroporphyrinogen contains four URC, the 30 µM substrate solution contained the equivalent of 120 µMURC.
Subsequent testing with 1, 3 and 5 mg of Pd/C was then performed to determine the optimal amount needed to convert 2 mg of uroporphyrin to the porphyrinogen. The 1 mg sample yielded only 90.6 µM URC, far less than that for the 3- and 5 mg Pd/C samples. The 5 mg sample produced the most URC (323 µM), followed by the 3-mg sample (278 µM).
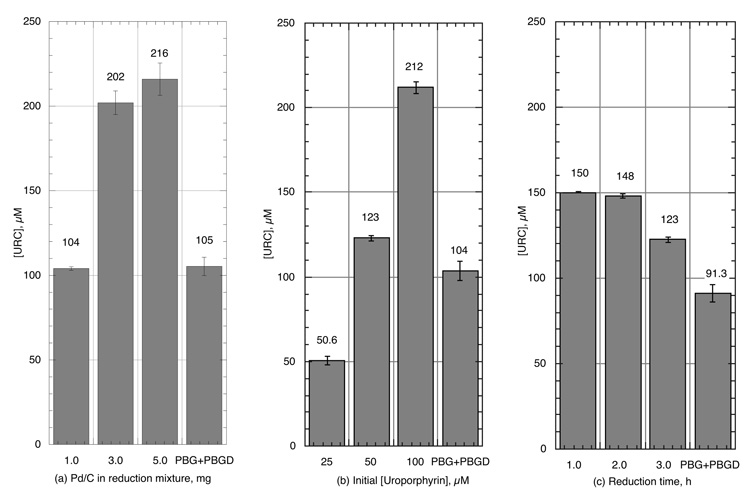
In optimizing the composition of the hydrogenation mixture, it was apparent that the adsorption of uroporphyrin onto the Pd/C caused significant losses. Zero loss to Pd/C adsorption plus total reduction is predicted to yield 5.3 mM URC in the UroD assay mixture, over 16 times more than the 323 µM yield for the 5 mg Pd/C sample obtained above. Hence 200 µL of 1 mM uroporphyrin in 0.1 M NH4OH (100 µM in the 2 mL Pd/C reduction mixture) was used in place of dry uroporphyrin as the starting material. This amount should supply a theoretical maximum of 480 µM URC to the UroD assay system, 11 times less than achievable from 2 mg of dry uroporphyrin. This system, using porphyrin in alkaline solution, was then optimized for maximal yield of uroporphyrinogen as determined by (a) amount of Pd/C catalyst, (b) initial porphyrin concentration and (c) time of reductive hydrogenation. The results are shown in Figure 2. A mixture of 3 to 5 mg Pd/C and 100 µM uroporphyrin reacted for 1 to 2 h proved optimal. The system that contained 3 mg Pd/C and 100 µM uroporphyrin reacted for 1 h was used in he succeeding experiments.
Figure 2.
Optimization of (a) the amount of Pd/C, (b) the initial porphyrin concentration and (c) the reaction time needed to produce the maximum amount of URC in the reductive hydrogenation reaction.
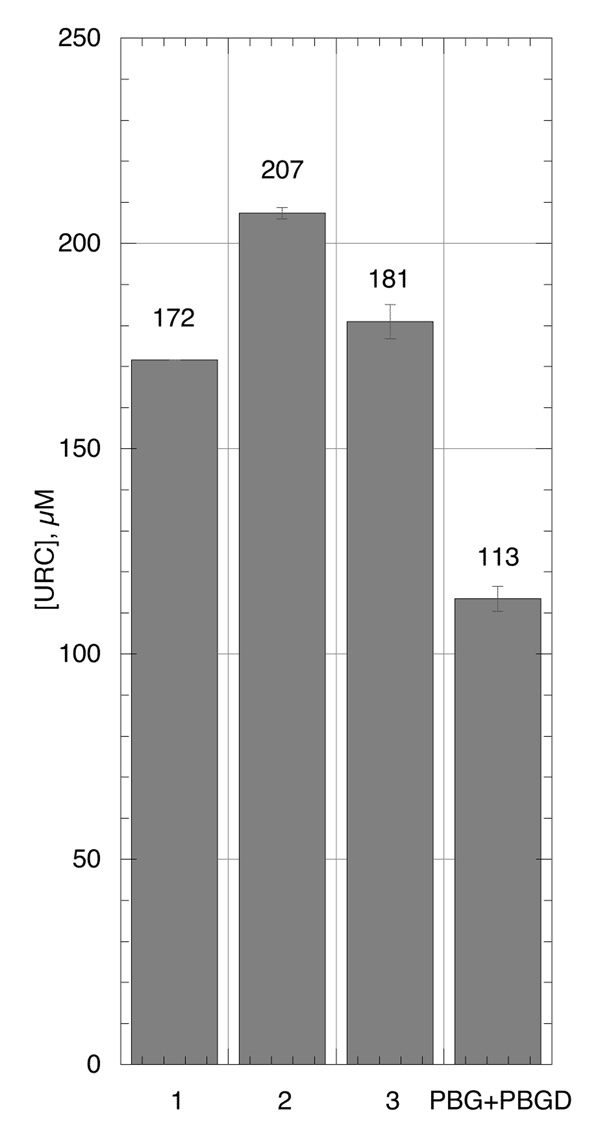
Three buffer systems used to dissolve the dried uroporphyrinogen prepared for use in the rhUroD activity assays were compared: (1) 50 mM potassium phosphate pH 6.80, (2) 50 mM potassium phosphate pH 6.80 plus 10 mM DTT and (3) 100 mM Tris pH 7.65 plus 3.75 mM DTT then neutralized with 1/5 volume of 150 mM KH2PO4 as in the PBG+PBGD system without the PBG and PBGD. The dry uroporphyrinogen was prepared via the 1 h hydrogenation of 100 µM uroporphyrin with 3 mg Pd/C in 200 µL 0.1 M NH4OH plus 1.8 mL methanol. All 3 buffers gave higher URC compared to the PBG+PBGD system but Buffer 2, with the highest amount of DTT, preserved the most URC (207 µM) (Fig. 3).
Figure 3.
The effect of different buffer solutions on the stability of uroporphyrinogen during UroD assay. Buffer 1: 50 mM potassium phosphate pH 6.80; Buffer 2: same as Buffer 1 plus 10 mM DTT; Buffer 3: 100 mM Tris, 25 mM KH2PO4, 3.75 mM DTT, the same buffer as in the PBG+PBGD substrate solution. Uroporphyrinogen was prepared optimized 100 µM uroporphyrin with 3 mg Pd/C in 200 µL 0.1 M NH4OH and 1.8 mL methanol.
In the PBG+PBGD sample, almost all of the tetrapyrrole synthesized by the system was uroporphyrinogen, as evidenced by minimal UV fluorescence and routine conversion of about 90% of the substrate to coproporphyrinogen. The enzymatic synthesis of uroporphyrinogen in the PBG+PBGD system (Incubation A) contained 3.75 mM DTT. After adjusting the pH to 6.80 for the UroD assay the DTT concentration is reduced to 2.25 mM, a concentration approximating that in Buffer 3.
In all experiments exclusion of oxygen was required to yield reproducible results. The Atmosbag was always thoroughly flushed with either hydrogen or argon as needed. Exposure to ambient laboratory atmosphere and light must be minimized.
DISCUSSION
The chemical reduction of porphyrin to porphyrinogen involves the addition of six hydrogen atoms to the porphyrin molecule, one to each of the four meso carbons and one to each of two pyrrole nitrogens. The four meso carbons are thus converted from methine to methylene carbons while the aromaticity of the individual pyrrole rings remain intact (Figure 1). Our results show that this hydrogenation reaction can be catalyzed by palladium on an inert support, activated charcoal. The general mechanism of heterogeneous catalysis requires the initial interaction of hydrogen and the double bonds with the surface of the metal catalyst [8]. A series of steps occur that include the addition of a hydrogen atom to the porphyrin molecule, bond/charge migrations, desorption from and adsorption onto the surface of the palladium catalyst. These steps give rise to the porphyrinogen as the major product under the optimal conditions described. The selectivity of the optimized conditions for converting porphyrin to porphyrinogen implies that further hydrogenation to disrupt the aromaticity of the pyrrole rings and to reduce the carbonyls requires more stringent conditions.
In the general mechanism for metal catalyzed hydrogenation of unsaturated compounds, the π bonds of the compound facilitate the binding to the metal atoms. After the reaction, the affinity of the now saturated carbon-carbon bond is much weaker and is released from the surface of the catalyst. The ease of hydrogenation is affected by the nature of the substituents on the two carbon atoms involved. In a porphyrin, the fully conjugated macrocyclic structure is stabilizing and hence may undergo hydrogenation more easily. The rigid macrocycle structure, however, may provide steric hindrance to effective binding with the palladium surface.
A problem we encountered relates to the tendency of the highly hydrophobic porphyrin molecule to remain adsorbed onto the carbon matrix, thus lowering the yield of the reaction. The optimal amount of Pd/C in the reduction mixture proved to be 3 mg. Less did not reduce porphyrins in the time allotted. More Pd/C did not lead to a significant increase in the amount of porphyrinogen produced due to losses via adsorption onto the Pd/C powder and other unwanted hydrogenation reactions.
A balance between solubility of the porphyrin and its various reduced counterparts and adsorption onto the catalytic Pd surface needed to be established in the reaction mixture to allow optimal conversion of porphyrin to porphyrinogen. This was resolved by the presence of NH4OH, which ionized all of the porphyrin carboxylate moieties and markedly reduced binding of the porphyrin to the carbon matrix. Sufficient hydrophobicity in the macrocycle prevented total eradication of this competitive binding, likely leading to losses and the inability to attain higher yields of URC.
Porphyrins are very slightly soluble in water but much more soluble in methanol, yet methanol alone as the solvent did not yield more complete reduction. The addition of NH4OH to ionize all the carboxylate moieties promoted solubility in the hydrogenation mixture and resulted in maximal reduction. A recent report described a homogeneous mode of catalysis wherein Pd nanoparticles are temporarily leached into solution as Pd(II) prior to activity [8]. It has been proposed that water and dissolved ions help stabilize the metal in solution. The Pd serves as a catalyst and then comes out of solution as Pd(0). Our observation that no significant hydrogenation occurs in pure methanol supports this mechanism.
This report describes a new method suitable for the selective hydrogenation of the meso carbons of a porphyrin using a member of the platinum group of metal catalysts, namely palladium. The method requires neither desalting nor deproteination steps in the preparation of the highly labile porphyrinogen for biochemical studies. We have utilized porphyrinogens prepared as described as substrates for crystallography/structural studies [12]. Preparation of porphyrinogens by this rapid, specific reduction method can also be used for assays of CoproOx and ProtoOx. Enzymatically generating coproporphyrinogen III or protoporphyrinogen IX through a coupled enzyme procedure is possible but complex. The rapid specific porphyrin reduction procedure described here circumvents these problems and is ideally suited for diagnostic enzymatic assays useful for the detection of porphyria cutanea tarda (UroD), hepatoerythropoietic porphyria (UroD), hereditary coproporphyria (CoproOx) and porphyria variegata (ProtoOx).
ACKNOWLEDGEMENTS
We wish to acknowledge the valuable information on the properties and uses of Pd/C in pyrrole chemistry provided by Dr. Jerry Bommer of Frontier Scientific, Inc., Logan, Utah. This study was supported by the National Institute of Health grants RO1 DK020503, P30 DK072437.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Smith KM. Porphyrins and metalloporphyrins. Amsterdam: Elsevier North Holland Medical Press; 1976. [Google Scholar]
- 2.Phillips JD, Whitby FG, Kushner JP, Hill CP. Structural basis for tetrapyrrole coordination by uroporphyrinogen decarboxylase. EMBO J. 2003;22:6225–6233. doi: 10.1093/emboj/cdg606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straka JG, Kushner JP, Pryor MA. Uroporphyrinogen decarboxylase: A method for measuring enzymatic activity. Enzyme. 1982;28:170–185. [PubMed] [Google Scholar]
- 4.Conant JB, Hyde JF. Studies in the chlorophyll series. II. Reduction and catalytic hydrogenation. J Am Chem Soc. 1930;52:1233–1239. [Google Scholar]
- 5.Fischer H, Zerweck W. The natural porphyrins. VIII. Uroporphyrinogen heptamethyl ester and a new conversion of uro- into coproporphyrin. Z. Physiol. Chem. 1924;137:242–264. [Google Scholar]
- 6.Blanchard DE. Quantitative Microscale Hydrogenation of Vegetable Oils. Journal of Chemical Education. 2003;80:544–546. [Google Scholar]
- 7.Sessler JL, Johnson MR, Lynch V. Synthesis and crystal structure of a novel tripyrrane-containing porphyrinogen-like macrocycle. J. Org. Chem. 1987;52:4394–4397. [Google Scholar]
- 8.Chen J-S, A.N V, A.P P, J.G K. Pd-leaching and Pd-removal in Pd/C-catalyzed Suzuki couplings. Applied Catalysis A. 2007;325:76–86. [Google Scholar]
- 9.Kielhorn J, Melber C, Keller D, Mangelsdorf I. Palladium--a review of exposure and effects to human health. Int J Hyg Environ Health. 2002;205:417–432. doi: 10.1078/1438-4639-00180. [DOI] [PubMed] [Google Scholar]
- 10.Phillips JD, Bergonia HA, Reilly CA, Franklin MR, Kushner JP. A porphomethene inhibitor of uroporphyrinogen decarboxylase causes porphyria cutanea tarda. Proc Natl Acad Sci U S A. 2007;104:5079–5084. doi: 10.1073/pnas.0700547104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips JD, Kushner JP. Measurement of Uroporphyrinogen Decarboxylase Activity. In: Maines MD, Costa LG, Reed DJ, Sassa S, Sipes IG, editors. Current Protocols in Toxicology. New York: John Wiley & Sons, Inc.; 1999. pp. 8.4.1–8.4.13. [DOI] [PubMed] [Google Scholar]
- 12.Mathews MA, Schubert HL, Whitby FG, Alexander KJ, Schadick K, Bergonia HA, Phillips JD, Hill CP. Crystal structure of human uroporphyrinogen III synthase. Embo J. 2001;20:5832–5839. doi: 10.1093/emboj/20.21.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]