Abstract
Infant rats cycle rapidly between periods of high muscle tone (indicative of wakefulness) and periods of atonia (indicative of sleep). Here, the influence of air temperature on sleep in 8-day-old rats was examined by testing pups at thermoneutrality (35°C) and during moderate (28°C) and extreme (20°C) cold challenge; also, pups were tested 1, 4, and 8 hours after infusion of milk to assess the effects of food deprivation on sleep. While moderate cooling slightly reduced sleep durations and altered the temporal patterning of myoclonic twitching, extreme cooling substantially decreased sleep durations and inhibited twitching. In contrast, food deprivation had little effect. Therefore, thermoregulatory mechanisms engaged during moderate cooling sustain sleep, whereas extreme cooling overwhelms these mechanisms, thereby promoting arousal.
Spontaneous activity, including myoclonic twitching, is a prominent feature of mammalian and avian behavior early in ontogeny (Corner, 1977). Twitches in infants resemble the movements exhibited by adult mammals during REM sleep and are readily distinguished from the high-amplitude coordinated movements that characterize awake behavior. Although the developmental relations between infant and adult sleep continue to be debated (Blumberg, Karlsson, Seelke, & Mohns, in press; Frank & Heller, 2003), it is clear that myoclonic twitching is a sleep-related behavior (Blumberg & Lucas, 1996; Gramsbergen, Schwartze, & Prechtl, 1970; Jouvet-Mounier, Astic, & Lacote, 1970).
In previous studies in which myoclonic twitching was used as the sole indicator of infant sleep, it was previously found that twitching is tightly linked to thermoregulatory competence during thermal challenges (Blumberg & Lucas, 1996; Sokoloff & Blumberg, 1998). For example, rat pups exhibited similar rates of twitching at thermoneutrality and during exposure to moderate air temperatures (defined as the range of air temperatures that stimulate endogenous heat production without exceeding thermogenic capacity), but that extreme air temperatures (defined as the range of air temperatures that exceed a pup’s thermogenic capacity) suppressed twitching.
Recent work has indicated that other measures of sleep can be used to supplement the information provided by myoclonic twitching. In particular, soon after birth in rats, a strong association already exists between myoclonic twitching and nuchal muscle atonia (Karlsson, Kreider, & Blumberg, 2004). Combining these behavioral and EMG measures, a typical sleep-wake cycle in a week-old rat evolves as follows: High-amplitude movements of the limbs and elevation of the head occurs during periods of high nuchal tone, after which muscle tone rapidly decreases and atonia begins; initially, atonia is accompanied by a brief period of behavioral quiescence, which has been designated as quiet sleep (QS) (Jouvet-Mounier et al., 1970); then, twitching of the limbs and tail begins while background levels of atonia are maintained, a state that has been designated as active sleep (AS) (Jouvet-Mounier et al., 1970); finally, there is an abrupt transition to high-amplitude waking movements and high muscle tone, thus completing the cycle. The effect of air temperature on this sleep-wake cycle – as defined using both behavioral and EMG criteria – has not been investigated.
In addition to temperature, food deprivation is also considered an important modulator of sleep. For example, Lorenz and colleagues found that active sleep in 11–13-day-old (P11–13) rats was suppressed after 9–12 h of separation from the dam, and that infusion of milk into the gut restored high levels of AS (Lorenz et al., 1998). In adults, food deprivation also suppresses sleep, but only after several days of fasting (Borbely, 1977; Danguir & Nicolaidis, 1979; Dewasmes, Duchamp, & Minaire, 1989; Jacobs & McGinty, 1971). It is not known, however, how infant sleep changes over the course of food deprivation; nor is it known how the effects of food deprivation on sleep interact with cold exposure.
In this experiment, myoclonic twitching and nuchal EMG were measured in P8 rats during exposure to three air temperature conditions: 35°C (thermoneutral), 28°C (moderate thermal challenge), and 20°C (extreme thermal challenge). Pups in the 35°C and 28°C conditions were tested across an 8-h period to assess the effects of food deprivation on sleep. Based on previous work, we hypothesized that moderate thermal challenge would have negligible effects on the expression of sleep and wakefulness and that the effects of extreme thermal challenge would be pronounced. Furthermore, we hypothesized that 8 h of food deprivation would result in increased wakefulness, especially during moderate thermal challenge because of the increased use of energy to support thermogenesis.
METHODS
All experiments were performed under National Institutes of Health guidelines for the care of animals in research and were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Subjects
A total of twenty-four 8-day-old (12 males and 12 females) rats from 24 litters were used. Body weights ranged from 18.0 to 22.6 g. All pups were born to Harlan Sprague-Dawley rats housed in the animal colony at the University of Iowa. The pups were raised in litters that were culled to 8 pups within 3 days of birth (day of birth = Day 0). Litters and mothers were raised in standard laboratory cages (48 cm long × 20 cm wide × 26 cm high), in which food and water were available ad libitum. All rats were maintained on a 12-hr light-dark schedule with lights on at 7 a.m.
Test Environment
Pups were tested inside an electrically shielded double-walled glass chamber (height = 17.0 cm; i.d. = 12.5 cm) with a Plexiglas lid. Air temperature inside the chamber was regulated using a temperature-controlled water circulator. Access holes in the side and lid of the chamber allowed for passage of air through the chamber (300 ml/min), as well as the passage of thermocouple wires and EMG electrodes. A round platform constructed of polyethylene mesh was fitted inside the chamber. The mesh allowed for the movement of air through the chamber. A perforated felt pad was placed on top of the mesh platform.
Procedure
On the day of testing, a pup with a visible milk band was removed from the litter and weighed. Under isoflurane anesthesia, copper-constantan thermocouples (Omega Engineering, Stamford, CT) were attached to the surface of the skin with collodion; one thermocouple was secured in the lumbar region (Tback) and a second was secured above the interscapular brown adipose tissue pad (Tis). To measure nuchal EMG, two bipolar stainless steel hook electrodes (California Fine Wire, Grover Beach, CA) were implanted bilaterally into the nuchal muscle while a ground wire was looped through the skin of the back. The pup was placed in an incubator maintained at 35°C and allowed to recover for 1 h, after which the pup was intubated with half-and-half (50% cream/50% milk; 3% of body weight in ml). Following infusion the pup was transferred to the testing chamber (maintained at 35°C, 28°C, or 20°C) and allowed to acclimate for 1 h.
Testing began 1 h post-infusion with the recording of 1 h of behavioral, EMG, and temperature data; in addition, for pups in the 28°C and 35°C conditions, testing was repeated at 4- and 8-h post-infusion. (Pilot studies indicated that sleep was dramatically altered at 20°C after only 1 h of food deprivation; therefore, pups in this condition were not subjected to longer deprivation times.) For all pups, the first recording period always began before 10 a.m. and the final recording period was completed by 7 p.m. Therefore, to control for the possible influence of circadian factors on sleep, 6 pups (3 males and 3 females) were tested at 35°C, 1 h after infusion with milk but late in the day at the same time as those pups that were tested after 8 h of deprivation.
EMG electrodes were connected to differential amplifiers (A-M Systems, Carlsborg, WA) and their signals were amplified (×10,000) and filtered (300–5000 Hz). Thermocouples were connected to a temperature meter (TC-1000; Sable Systems, Las Vegas, NV). EMG and thermocouple data were digitized at 1 kHz using a data acquisition system (BioPac Systems, Santa Barbara, CA) and simultaneously visualized by the experimenter during the test. A microcamera was placed above the chamber lid for monitoring and recording of behavior. EMG and video data were recorded to digital videotape with a data recorder (DV8; WinTron Technologies, Rebersberg, PA).
Data Analysis
The Tis and Tback values for each subject were derived by averaging the temperature data over the first and final 60 s of each recording period. Tis-Tback, a reliable measure of BAT thermogenesis in infant rats (Blumberg & Stolba, 1996), was then calculated.
Digitized EMG signals were summed (on two occasions, only one EMG signal was useable), integrated, and full-wave rectified. The EMG signal was then dichotomized into high-tone and atonia categories using the following method, as described previously (Karlsson et al., 2004): For each pup the amplitude of five 1-s segments of noise-free, uninterrupted atonia and high-tone periods were measured, averaged, and the midpoint between the two means was calculated. A period of muscle atonia (indicative of sleep) was defined as a period in which muscle tone was below the midpoint value for at least 1 s, while a period of high muscle tone (indicative of wakefulness) was defined as a period during which muscle tone was above the midpoint value. Sleep and awake durations were determined for all subjects over 60 min. Average sleep and awake durations were then calculated. These and all other analyses were performed using StatView 5.0 (SAS, Cary, NC).
Log-survivor analyses were also used to examine the distributions of atonia and high tone durations. As described previously (Karlsson et al., 2004), durations were collapsed across pups for these analyses. Regressions were performed to determine the fit of the data to an exponential distribution. Group differences in the distributions were tested using the Mantel-Cox X2 test.
To assess the relationship between nuchal EMG and myoclonic twitching, 15 min segments of data from each pup were digitized while a trained observer scored the pup’s behavior. As the pup’s behavior was observed on a monitor, an event recorder was activated when a myoclonic twitch or high-amplitude movement was detected. In this way, a synchronized digital record of the pup’s behavior and EMG activity was created. As described elsewhere (Blumberg & Lucas, 1994; Gramsbergen et al., 1970), twitching was defined as phasic, rapid, and independent movements of the limbs and tail. Because the animals in this experiment were freely moving (and thus movements of the limbs were not easily detected), twitches were scored from any visible limb (including the tail). High-amplitude movements, indicative of wakefulness, include locomotion, stretching, and yawning.
Twitches of the nuchal muscle can be detected as spikes in the EMG during periods that are dominated by atonia (Jouvet-Mounier et al., 1970). To determine whether these twitches are temporally related to twitches of the distal limbs and tail, a separate series of three analyses was performed. First, a nuchal muscle twitch was defined as a spike in the nuchal EMG, produced during a period of atonia, with an amplitude 3x the noise band. Second, a peri-event histogram was created relating the onset of nuchal muscle twitches and limb twitches. To do this, 10 atonia periods were selected from each animal in the 35°C, 1-h deprivation condition. The first limb twitch of the atonia period served as the triggering event. The number of nuchal twitches in the 10 1-s bins surrounding the triggering event was then counted and averaged for each pup. Paired t tests were performed across pups to compare the mean number of twitches in each bin to those in the preceding bin. Because of the multiple comparisons, a conservative alpha of .01 was used. Finally, the relationship between the overall quantity of nuchal and limb twitches was analyzed. Again, 10 atonia periods were selected from each of the 6 pups in the 35°C, 1-h deprivation condition. The number of nuchal muscle and limb twitches in each atonia period was counted and a regression analysis was performed.
Using the 15-min segments of data scored for twitching, 5 atonia periods from each animal in the 35°C and 28°C 1-h deprivation conditions were selected for analysis of the temporal distribution of twitching within atonia periods. First, each atonia period was segmented into sequential 2-s bins. For each bin, the presence or absence of nuchal muscle and limb twitches was determined and a X2 analysis was performed. Second, to evaluate the rate of twitching after the onset of atonia, the number of nuchal and limb twitches within each 2-s bin was summed across pups within each temperature condition. A log-survivor analysis was then performed and a Mantel-Cox X2 test was used to test for a difference between the distributions.
The record containing the EMG and scored behavioral data was used to establish the relationship between atonia periods and myoclonic twitching. First, the latency from the end of a high-tone period (as defined above) to the first twitch of an atonia period was determined (this latency to twitch, LT, defined the period of quiet sleep, QS), as was the latency from the last twitch of an atonia period to the beginning of the next high-tone period (latency to arousal, LA). The duration of twitching (DT, the period between the first and last twitch of an atonia period, defined as the period of active sleep, AS), as well as the number of twitches during that AS period (NT), were then determined. From the above measures, the total duration of the atonia period (LT + DT + LA), the percentage of each atonia period in AS (DT/(LT + DT + LA) × 100), and the number of twitches per second during AS (NT/DT) were calculated.
Because the pups in the 20°C condition were only tested at 1 h post-infusion, the following statistical approach was adopted. First, restricting the analysis to pups in the 35°C and 28°C conditions, a repeated-measures analysis of variance (ANOVA) was used to test for the effect of air temperature and deprivation time; planned between-group comparisons at 4 and 8 h of deprivation were made using unpaired t tests. Second, a single-factor ANOVA was used to test for the effect of air temperature in the 1-h deprivation condition; the post hoc test was Fisher’s PLSD.
Unless otherwise stated, alpha was set at .05. All means are presented with their standard errors.
RESULTS
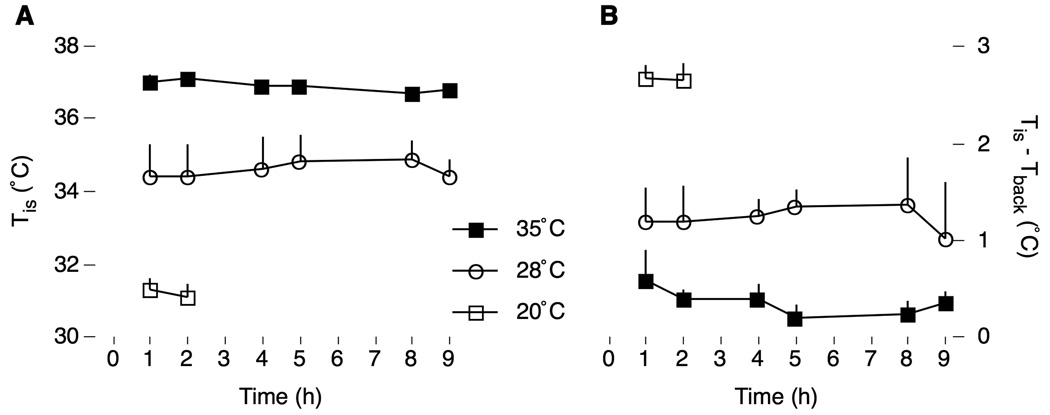
Figure 1 shows Tis and Tis-Tback over the duration of the experiment. In general, moderate and extreme cooling produced different levels of hypothermia, as well as different levels of BAT thermogenesis (as indicated by the increase in Tis-Tback), in relation to pups tested at thermoneutrality. Repeated-measures ANOVA restricted to the 35°C and 28°C conditions revealed significant effects of air temperature on Tis, F1,9 = 10.1, p < .05, and a trend for Tis-Tback, F1,7 = 5.2, p < .06, but no effects of deprivation time on either Tis, F2,18 = 0.2, or Tis-Tback, F2,12 = 0.2 (the interactions were also not significant). At the 1-h deprivation period, ANOVA revealed a significant effect of air temperature on Tis, F2,14 = 36.8, p < .0001, as well as on Tis-Tback, F2,13 = 19.6, p < .0001. Therefore, pups’ body temperatures stabilized within the 1-h acclimation period and remained stable throughout the entire testing period, even in moderately cooled pups exhibiting BAT thermogenesis.
Figure 1.
(A) Interscapular temperature (Tis) and (B) interscapular minus back-skin temperature (Tis-Tback) in P8 rats maintained at thermoneutrality (35°C) or exposed to moderate (28°C) or extreme (20°C) thermal challenge. Sleep behaviors were recorded at 1–2, 4–5, and 8–9 hours after infusion with milk (except for pups at 20°C for which the test was terminated after 2 h). Both thermal measures were stable across time. N = 6 pups per group.
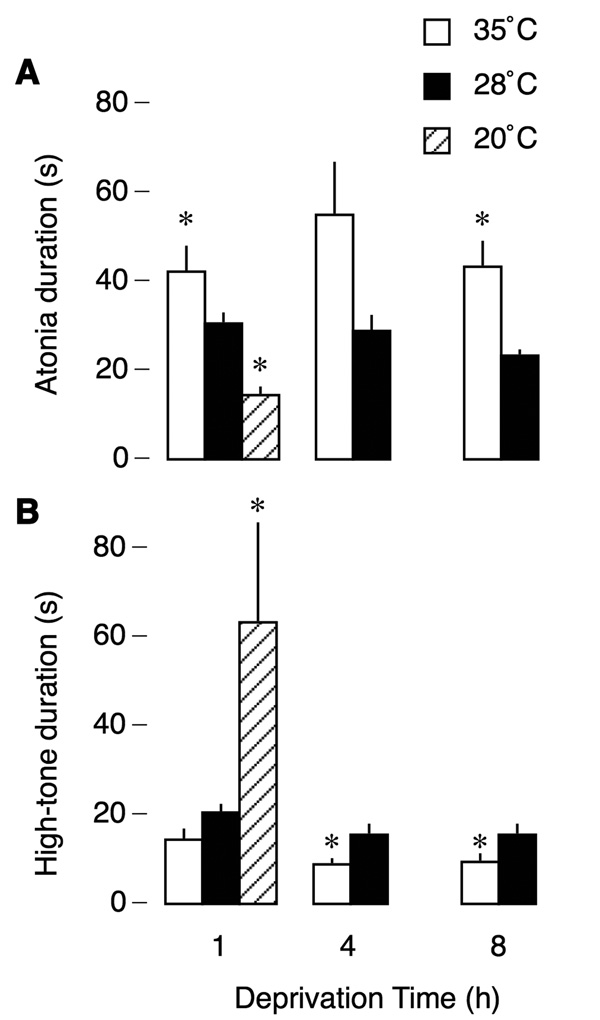
Figure 2 presents mean tone and atonia durations across air temperature and food-deprivation conditions. Mean atonia and high-tone durations remained relatively stable across 8 h of deprivation within air temperature conditions but decreased during cooling. Atonia occupied an average of 81% of total time at 35°C and 62% at 28°C, consistent with a previous study (Karlsson et al., 2004). In contrast, at 20°C, this value decreased to 19%. Thus, in comparison to moderate cooling, extreme cooling produced a disproportionate shift in the balance of high muscle tone and atonia durations. These observations were supported by statistical analyses: Repeated-measures ANOVA revealed a significant main effect of air temperature on mean atonia duration, F1,10 = 7.5, p < .05, but no effect of deprivation time, F2,20 = 2.4, and no interaction, F2,20 = 1.7. A repeated-measures ANOVA for high-tone duration revealed a significant main effect of air temperature, F1,10 = 9.2, p < .05, an effect of deprivation time, F2,20 = 7.2, p < .01, but no interaction, F2,20 = 0.1. Single-factor ANOVAs at 1-h of deprivation were significant for both atonia, F2,15 = 14.2, p < .0005, and high-tone, F2,15 = 4.1, p < .05, durations.
Figure 2.
Thermal and nutritional modulation of mean atonia (A) and high-tone (B) durations in P8 rats. Mean atonia durations decreased, and mean high-tone durations increased, with cooling. Food deprivation (1, 4, and 8 h) had a small but significant effect on high-tone durations. * significant difference in relation to the other temperature condition(s). N = 6 pups per group.
Because the 1-h deprived pups were always tested early in the morning and the 8-h deprived pups were always tested late in the afternoon, an additional group of 1-h deprived pups was tested late in the afternoon. These pups, tested at 35°C, did not differ from the 1-h deprived pups that were tested in the morning on any measure, including high-tone and atonia durations (high tone: t10 = 0.03, NS; atonia: t10 = 1.72, NS).
Consistent with the mean durations presented in Figure 2, the log-survivor distributions in Figure 3 indicate that air temperature significantly altered sleep-wake organization (in this figure, only the data for 1 h of deprivation are shown; the data at 4 and 8 h exhibited similar patterns). For the atonia data in Figure 3A, as well as the high-tone data at 35°C and 28°C in Figure 2B, the values of r2 are all greater than .98, indicating a good fit of the data to a negative exponential process in which the slope of each line is proportional to the rate at which events occurred (Fagen & Young, 1978). Thus, for pups exhibiting atonia, transitions to a state of high tone occurred at a similar rate at 35°C and 28°C but occurred more rapidly at 20°C. Similarly, for the high-tone data, transitions to a state of atonia occurred at a similar rate at 35°C and 28°C. In contrast, at 20°C, the pronounced inflection in the plot (indicated by the arrow in Figure 3B) signifies that the transition rate slowed considerably when high-tone durations exceeded 20 s. All together, these data indicate qualitatively different thermal sensitivities of the mechanisms that underlie transitions between high muscle tone and atonia.
Figure 3.
Log-survivor distributions of nuchal atonia (A) and high-tone durations (B) for P8 rats across temperature conditions in the 1-h deprived pups. Data are collapsed across subjects (390–413 points per plot). Values of r2 are indicated. Distributions on either side of an asterisk (*) are significantly different. The arrow indicates an inflection point for the 20°C distribution.
Close inspection of behavior during atonia periods indicates that they are typically composed of an initial period of quiescence followed by a longer period of phasic motor activity (i.e., myoclonic twitching of the limbs and tail). Examination of nuchal EMG records also reveals periods of phasic activity, which can be seen as spikes during periods that are dominated by atonia (Figure 4A). The temporal relationship between these nuchal muscle spikes and limb twitches was examined by constructing a peri-event histogram of the number of nuchal muscle twitches that occurred before and after the first limb twitch of an atonia period (Figure 4B). Typically, nuchal twitches began within 1 s of the first twitch of the atonia period, indicating that the AS phase of the atonia period is initiated concurrently in multiple muscle groups.
Figure 4.
Relationship between myoclonic twitching as measured by visual observation of the limbs and activity in nuchal EMG. (A) Representative cycle of high nuchal muscle tone and atonia in a P8 rat at 35°C. Nuchal muscle twitches against a background of atonia are indicated, as are instances of visually scored limb twitches. Based on our scoring criteria, this cycle has been divided into periods of wakefulness (W), quiet sleep (QS) and active sleep (AS). (B) Perievent histogram indicating increase in nuchal muscle twitching within 1 s of the first visually scored limb twitch of an atonia period. Data are from 10 atonia periods across 6 P8 rats tested at 35°C after 1 h of food deprivation. * significant difference from previous time bin, paired t test. There was no attempt to compensate for the lag produced by the observer’s reaction time in scoring limb twitches. (C) Regression relating the number of behaviorally scored limb twitches and the number of nuchal muscle twitches during periods of atonia. Data are from the same atonia periods as in (B). N = 60 data points. The best-fit line is shown. (D) Representative data from a single period of atonia in a P8 rat tested at 35°C showing the number of twitches measured during successive 2-s bins. Behaviorally scored limb twitches (filled circles) and nuchal muscle twitches (open circles) are shown separately and indicate synchronized bursts of phasic activity during the atonia period.
To further assess the association between nuchal muscle and limb twitching, the correlation between the number of nuchal muscle and limb twitches was determined. The individual values for 10 atonia periods from each of 6 pups at 35°C is shown in Figure 4C. The regression between the number of nuchal muscle twitches and the number of limb twitches was significant, F1,58 = 74.4, p < .0001. Moreover, all regressions performed individually on each pup were also significant.
Finally, Figure 4D shows the temporal relationship between limb and nuchal muscle twitches for successive 2-s bins within one representative atonia period. Atonia onset is followed by a period of quiescence, whereupon twitching of the nuchal muscle and limbs begins within the same time bin and continues until both forms of twitching briefly cease; twitching then commences, ceases, and commences again in close temporal succession until the pup arouses. Five such atonia periods were examined for 6 pups after 1 h of deprivation at an air temperature of 35°C; for each 2-s bin, the presence or absence of nuchal muscle and limb twitching was determined and the data were collapsed across subjects. A significant association between the two forms of twitching was found, X2 = 222.3, p < .0001. An identical analysis performed for pups in the 28°C condition was also significant, X2 = 103.9, p < .0001.
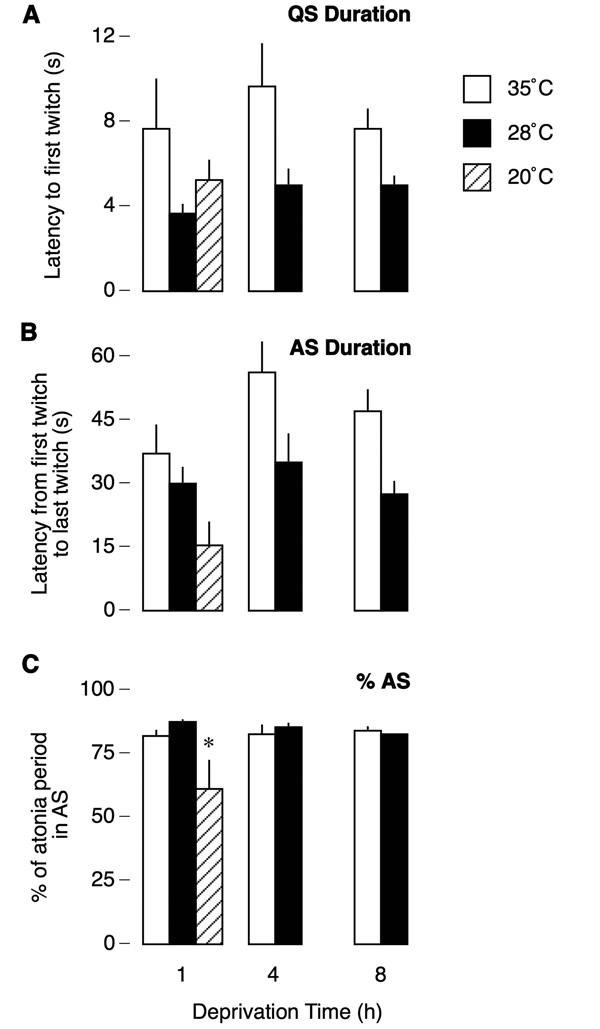
As previously discussed, the atonia period can be segregated into two states, QS and AS, based on the presence or absence of myoclonic twitching. Figure 5 depicts how these states vary under conditions of thermal challenge and food deprivation. In relation to the 35°C condition, QS durations were reduced by the same amount in the 28°C condition across all deprivation times (Figure 5A). Repeated-measures ANOVA revealed a significant main effect of temperature, F1,10 = 5.3, p < .05, but there was no effect of deprivation time, F2,20 = 2.0, NS, and no interaction, F2,20 = .8, NS. Single-factor ANOVA revealed no effect of temperature for the 1-h deprivation period, F2,14 = 1.6, NS.
Figure 5.
Measures of quiet sleep (QS; A), active sleep (AS, B), and %AS (C) in P8 rats tested at thermoneutrality (35°C) or exposed to moderate (28°C) or extreme (20°C) air temperatures over the course of food deprivation. Moderate cooling resulted in significant reductions in QS and AS that were similar across deprivation time; as a result, %AS did not differ between conditions. In contrast, extreme cooling significantly suppressed %AS. * significant difference in relation to other two temperature conditions. N = 5–6 pups per group.
AS duration followed a similar pattern as QS duration, with the highest values occurring in the 35°C condition (Figure 5B). A repeated-measures ANOVA revealed a significant effect of temperature, F1,10 = 6.0, p < .05, as well as deprivation time, F2,20 = 4.2, p < .05, but there was no significant interaction, F2,20 = 1.7. Single-factor ANOVA revealed a trend towards significance in the 1-h deprivation period, F2,14 = 3.6, p < .06.
The percent of each atonia period spent in AS (%AS) is presented in Figure 5C. Because QS and AS durations were proportionally affected by air temperature, %AS was similar in the 35°C and 28°C conditions and was stable across time. The value in the 20°C condition was much lower than the values in the other temperature conditions. Again, repeated-measures ANOVA revealed no significant effects of either air temperature, F1,10 = 1.3, or deprivation time, F2,20 = .6. A single-factor ANOVA revealed an effect of air temperature in the 1-h deprivation condition, F2,14 = 4.0, p < .05, with the value at 20°C differing significantly from both 28°C and 35°C.
The last twitch of an atonia period is not typically followed by a period of behavioral quiescence; rather, pups transition rapidly to wakefulness. Indeed, nearly 95% of the arousal latencies across all pups in this study were less than 3 s. Repeated-measures ANOVA revealed no significant differences, nor was the single-factor ANOVA at the 1-h deprivation period significant.
Quantitative aspects of limb twitching are shown in Figure 6A. There was no difference between the number of twitches between the 35°C and 28°C conditions, and the number of twitches remained stable across the deprivation period. However, there was a dramatic decrease in the number of twitches in the 20°C condition, consistent with previous reports (Blumberg & Stolba, 1996; Sokoloff & Blumberg, 1998). Repeated-measures ANOVA revealed no effect of temperature, F1,9 = 2.1, or deprivation time, F2,18 = 1.6, but a single-factor ANOVA revealed an effect of temperature at the 1-h deprivation time, F2,15 = 12.3, p < .001, with the 20°C condition differing significantly from both the 28°C and 35°C conditions. In contrast, the number of twitches per second of AS time increased with decreasing air temperature (Figure 6B). As determined by repeated-measures ANOVA, there was a significant effect of temperature, F1,10 = 10.0, p = .01, as well as a significant effect of deprivation time, F2,20 = 4.9, p < .05.
Figure 6.
Changes in myoclonic twitching in P8 rats tested at thermoneutrality (35°C) or exposed to moderate (28°C) or extreme (20°C) air temperatures over the course of food deprivation. (A) Number of behaviorally scored limb twitches regardless of behavioral state during the entire 15-min scoring period. † significant difference in relation to other two temperature conditions. (B) Rate of twitching during periods of active sleep. Data are from the same periods as in (A). * significant difference in relation to 28°C condition. N = 5–6 pups per group. (C) Log-survivor distributions of myoclonic twitching across atonia periods. Data are from 5 atonia periods collapsed across 5–6 P8 rats tested at 35°C or 28°C after 1 h of food deprivation. Each atonia period was divided into 2-s bins and the number of behaviorally scored limb twitches and nuchal muscle twitches was determined and summed for each bin. ** significant difference between the two distributions.
Figure 6C presents the log-survivor distributions of twitching during periods of atonia for pups in the 35°C and 28°C temperature conditions at 1 h of food deprivation (twitching in 20°C pups is not included in this analysis because twitching was rare). The two plots were significantly different, X2 = 144.3, p < .0001. The deviation of the two distributions soon after the onset of atonia is consistent with the shorter QS durations of pups at 28°C (see Figure 5A). Moreover, the steeper slope of the 28°C distribution signifies a higher rate of twitching over the atonia period, consistent with the higher rate of twitching in these pups (see Figure 6B). Thus, even though pups in the 28°C and 35°C conditions exhibited the same number of twitches during a fixed period of time (i.e., 15 min), the distribution of twitching differed at the two air temperatures such that moderately cooled pups began twitching sooner after the onset of atonia and twitched at a higher rate.
DISCUSSION
The aim of this experiment was to examine the effects of temperature and food deprivation on sleep duration and organization. Based on a previous report (Lorenz et al., 1998), we hypothesized that 8 h of starvation would result in increased behavioral arousal as well as decreased sleep duration, especially during moderate thermal challenge during which energy use is increased to support BAT thermogenesis. Contrary to expectation, food deprivation did not evoke pronounced levels of arousal, even during moderate cold exposure. In fact, the effects of food deprivation on both sleep and wakefulness were modest. Discrepancies between the present findings and those of Lorenz and colleagues (1998) may be due to the fact that the pups used in the earlier study were older (P11–13) and were deprived of food for 9–12 h before testing.
It has been found that 10 h of food deprivation inhibits BAT thermogenesis during cold exposure (Bignall, Heggeness, & Palmer, 1974; Blumberg, Deaver, & Kirby, 1999), and that pharmacological blockade of BAT thermogenesis inhibits sleep (Sokoloff & Blumberg, 1998). The deprivation times used here were insufficient to suppress BAT thermogenesis during cooling. It is possible, however, that longer deprivation times, especially if sufficient to inhibit BAT thermogenesis during moderate cooling, would have produced pronounced changes in the organization of sleep and wakefulness.
Based on previous reports in which twitching was used as the sole indicator of sleep (Blumberg & Stolba, 1996; Sokoloff & Blumberg, 1998), we hypothesized that moderate cold exposure would have negligible effects on atonia duration and that the effects of extreme thermal challenge would be pronounced. This hypothesis was only partly supported. Specifically, mean atonia durations in the 28°C condition were significantly shorter than those in the 35°C condition, while the opposite was true for mean high-tone durations. Thus, the added sensitivity provided by nuchal EMG revealed that sleep is significantly affected at moderate temperatures. In contrast, extreme cooling produced dramatic increases in high-tone duration accompanied by behavioral arousal, as expected from previous studies (Blumberg & Stolba, 1996; Sokoloff & Blumberg, 1998).
Although moderate cooling produced a significant decrease in atonia durations, the total number of twitches per 15 min of total observation time did not differ from pups tested thermoneutrality. It was this observation in earlier studies (Blumberg & Stolba, 1996; Sokoloff & Blumberg, 1998) that led to the conclusion that moderate cooling does not modulate sleep. It seems, however, that the amount of twitching during moderate cooling remained constant despite the reduction in atonia time. This apparent conservation of twitching during moderate cooling occurred because twitching occurred earlier in the atonia periods and at higher rates than in pups at thermoneutrality. This surprising finding provides additional support for the notion that twitching is selectively and independently regulated (Blumberg, Middlemis-Brown, & Johnson, in press).
Myoclonic twitching only occurs against a background of nuchal atonia at thermoneutrality and this relationship can be disrupted, for example, by lesions of the ventromedial medulla (Karlsson & Blumberg, in press; Karlsson et al., 2004). Importantly, in the present experiment, cold exposure only influenced the temporal organization of sleep and did not disrupt the cohesion among individual sleep components. Indeed, even during extreme cooling when periods of nuchal atonia were brief, the few instances of twitching always occurred during these periods. Thus, the entire sleep state, not just the individual sleep components, was modulated by temperature.
Numerous studies from our laboratory have documented the physiological and behavioral consequences of moderate and extreme thermal challenge. For example, exposure to moderate air temperatures (i.e., 25–35°C in P8 rats) is accompanied by increases in BAT thermogenesis, oxygen consumption, and respiratory rate, as well as maintenance of cardiac rate (Blumberg, 2001; Sokoloff & Blumberg, 1998). In contrast, during exposure to extreme air temperatures (i.e., all air temperatures below 25°C in P8 rats), BAT thermogenesis can no longer compensate for heat loss, resulting in hypothermia and bradycardia, as well as the emission of ultrasonic vocalizations. The present study further demonstrates the significance of the distinction between moderate and extreme cooling.
What is it, then, that accounts for the pronounced decrease in atonia duration during thermal challenge? First, as discussed above, a variety of physiological adjustments accompany exposure to both moderate and extreme air temperatures. For example, the dramatic increase in wakefulness during extreme cooling could result when bradycardia and the consequent challenges to cardiorespiratory homeostasis interfere with the maintenance of sleep. Second, cooling may modulate the activity of thermosensitive neurons in the preoptic/anterior hypothalamus that also appear to regulate sleep (Alam, McGinty, & Szymusiak, 1995a, 1995b). Finally, extreme temperatures can activate transient receptor potential ion channels (thermoTRPs) on peripheral or central neurons resulting in the transmission of nociceptive information (Patapoutian, Peier, Story, & Viswanath, 2003). These and other factors may contribute to the modulation of sleep and wakefulness by cooling.
By analyzing the timing and organization of limb twitches it was possible to categorize the atonia periods into two successive sleep states: QS followed by AS. Other researchers have used similar designations (Dugovic & Turek, 2001; Gramsbergen et al., 1970; Jouvet-Mounier et al., 1970). Nonetheless, the definitions of QS and AS used here may not accurately reflect the complexity of state organization at this age. For example, Figure 4D illustrates how a single period of AS may be better conceptualized as a series of AS periods – characterized by muscle atonia and twitching - with intervening QS periods – characterized by muscle atonia without twitching. In other words, just as infants cycle between sleep and wakefulness more rapidly than adults (Karlsson et al., 2004), infants may cycle between sleep states more rapidly than adults. Determining whether this perspective accurately captures infant sleep will require close attention to the fine temporal patterning of sleep; clearly, the use of conventional 10-s (or longer) epochs to categorize sleep states would mask such fine details.
One related issue that needs to be resolved is whether phasic activity during AS – as well as the intervening periods of behavioral quiescence – reflect the synchronized activity of all sleep-related phasic activity. The temporal association between twitches of the limbs and tail and spikes in the nuchal EMG provides strong evidence that AS does represent a global period of co-occurring phasic activity. Future experiments will need to examine the temporal patterning of other forms of phasic activity, including rapid eye movements (Van Someren et al., 1990) and PGO waves (Datta, Siwek, Patterson, & Cipolloni, 1998).
In summary, using nuchal EMG to provide a more sensitive measure of sleep, it was found that moderate cooling reduced total sleep time without decreasing the expression of twitching. In contrast, although the air temperature differentials between the three groups were similar (i.e., 7–8°C), extreme cooling had a disproportionate effect, suppressing both sleep and twitching. This modulatory effect of temperature was restricted to changes in the temporal patterning of sleep; at no time was the cohesion of individual sleep components disrupted. Surprisingly, pups deprived of food for as long as 8 h, even during moderate thermal challenge, did not exhibit the expected increase in wakefulness, perhaps because BAT thermogenesis was not inhibited. All together, these results reinforce the notion that sleep is protected during moderate cooling by the infant’s ability to activate BAT thermogenesis and thereby maintain critical physiological variables within tolerable limits (Blumberg, 2001).
ACKNOWLEDGMENTS
This research was supported by National Institute of Mental Health Grants MH50701 and MH66424. We thank Jessica Middlemis-Brown for technical assistance and Karl Karlsson and Ethan Mohns for helpful suggestions.
REFERENCES
- Alam MN, McGinty D, Szymusiak R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: Relation to NREM sleep. American Journal of Physiology. 1995a;269:R1240–R1249. doi: 10.1152/ajpregu.1995.269.5.R1240. [DOI] [PubMed] [Google Scholar]
- Alam MN, McGinty D, Szymusiak R. Preoptic/anterior hypothalamic neurons: Thermosensitivity in rapid eye movement sleep. American Journal of Physiology. 1995b;269:R1250–R1257. doi: 10.1152/ajpregu.1995.269.5.R1250. [DOI] [PubMed] [Google Scholar]
- Bignall KE, Heggeness FW, Palmer JE. Effects of acute starvation on cold-induced thermogenesis in the preweanling rat. American Journal of Physiology. 1974;227:1088–1093. doi: 10.1152/ajplegacy.1974.227.5.1088. [DOI] [PubMed] [Google Scholar]
- Blumberg MS. The developmental context of thermal homeostasis. In: Blass EM, editor. Handbook of Behavioral Neurobiology. Vol. 13. New York: Plenum Press; 2001. pp. 199–228. [Google Scholar]
- Blumberg MS, Deaver K, Kirby RF. Leptin disinhibits BAT thermogenesis in infant rats after maternal separation. American Journal of Physiology. 1999;276:R606–R610. doi: 10.1152/ajpregu.1999.276.2.R606. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Karlsson KÆ, Seelke AMH, Mohns EJ. The ontogeny of mammalian sleep: A response to Frank and Heller. Journal of Sleep Research. 2003 doi: 10.1111/j.1365-2869.2004.00430_1.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Lucas DE. Dual mechanisms of twitching during sleep in neonatal rats. Behavioral Neuroscience. 1994;108:1196–1202. doi: 10.1037//0735-7044.108.6.1196. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Lucas DE. A developmental and component analysis of active sleep. Developmental Psychobiology. 1996;29:1–22. doi: 10.1002/(SICI)1098-2302(199601)29:1<1::AID-DEV1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Middlemis-Brown JE, Johnson ED. Sleep homeostasis in infant rats. Behavioral Neuroscience. doi: 10.1037/0735-7044.118.6.1253. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Stolba MA. Thermogenesis, myoclonic twitching, and ultrasonic vocalization in neonatal rats during moderate and extreme cold exposure. Behavioral Neuroscience. 1996;110:305–314. doi: 10.1037//0735-7044.110.2.305. [DOI] [PubMed] [Google Scholar]
- Borbely AA. Sleep in the rat during food deprivation and subsequent restitution of food. Brain Res. 1977;124:457–471. doi: 10.1016/0006-8993(77)90947-7. [DOI] [PubMed] [Google Scholar]
- Corner MA. Sleep and the beginnings of behavior in the animal kingdom -- Studies of ultradian motility cycles in early life. Progress in Neurobiology. 1977;8:279–295. doi: 10.1016/0301-0082(77)90008-9. [DOI] [PubMed] [Google Scholar]
- Danguir J, Nicolaidis S. Dependence of sleep on nutrients' availability. Physiol Behav. 1979;22:735–740. doi: 10.1016/0031-9384(79)90240-3. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Patterson EH, Cipolloni PB. Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse. 1998;30:409–423. doi: 10.1002/(SICI)1098-2396(199812)30:4<409::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Dewasmes G, Duchamp C, Minaire Y. Sleep changes in fasting rats. Physiol Behav. 1989;46:179–184. doi: 10.1016/0031-9384(89)90252-7. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Turek FW. Similar genetic mechanisms may underlie sleep-wake states in neonatal and adult rats. Neuroreport. 2001;12(14):3085–3089. doi: 10.1097/00001756-200110080-00021. [DOI] [PubMed] [Google Scholar]
- Fagen RM, Young DY. Temporal patterns of behaviors: durations, intervals, latencies and sequences. In: Colgan PW, editor. Quantitative Ethology. New York: Wiley; 1978. pp. 79–114. [Google Scholar]
- Frank MG, Heller HC. The ontogeny of mammalian sleep: a reappraisal of alternative hypotheses. Journal of Sleep Research. 2003;12:25–34. doi: 10.1046/j.1365-2869.2003.00339.x. [DOI] [PubMed] [Google Scholar]
- Gramsbergen A, Schwartze P, Prechtl HFR. The postnatal development of behavioral states in the rat. Developmental Psychobiology. 1970;3(4):267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, McGinty DJ. Effects of food deprivation on sleep and wakefulness in the rat. Experimental Neurology. 1971;30:212–222. doi: 10.1016/s0014-4886(71)80002-x. [DOI] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Developmental Psychobiology. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. Active medullary control of atonia in week-old rats. Neuroscience. doi: 10.1016/j.neuroscience.2004.09.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Kreider JC, Blumberg MS. Hypothalamic contribution to sleep-wake cycle development. Neuroscience. 2004;123:575–582. doi: 10.1016/j.neuroscience.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Lorenz DN, Poppe CJ, Quail C, Seipel K, Stordeur SA, Johnson E. Filling the Gut Activates Paradoxical Sleep in Suckling Rats. Dev Psychobiol. 1998;32:1–12. doi: 10.1002/(sici)1098-2302(199801)32:1<1::aid-dev1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nature Reviews Neuroscience. 2003;4(7):529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- Sokoloff G, Blumberg MS. Active sleep in cold-exposed infant Norway rats and Syrian golden hamsters: The role of brown adipose tissue thermogenesis. Behavioral Neuroscience. 1998;112:695–706. doi: 10.1037//0735-7044.112.3.695. [DOI] [PubMed] [Google Scholar]
- Van Someren EJW, Mirmiran M, Bos NPA, Lamur A, Kumar A, Molenaar PCM. Quantitative analysis of eye movements during REM-sleep in developing rats. Developmental Psychobiology. 1990;23:55–61. doi: 10.1002/dev.420230106. [DOI] [PubMed] [Google Scholar]