Abstract
Dental enamel is comprised primarily of carbonated apatite, with less than 1% w/w organic matter and 4-5% w/w water. To determine the influence of each component on the microhardness and fracture toughness of rat incisor enamel, we mechanically tested specimens in which water and organic matrix were selectively removed. Tests were performed in mid-sagittal and transverse orientations to assess the effect of the structural organization on enamel micromechanical properties. While removal of organic matrix resulted in up to a 23% increase in microhardness, and as much as a 46% decrease in fracture toughness, water had a significantly lesser effect on these properties. Moreover, removal of organic matrix dramatically weakened the dentino-enamel junction (DEJ). Analysis of our data also showed that the structural organization of enamel affects its micromechanical properties. We anticipate that these findings will help guide the development of bio-inspired nanostructured materials for mineralized tissue repair and regeneration.
Keywords: dental enamel, fracture toughness, hardness, structure, hydroxyapatite
INTRODUCTION
Dental enamel is a highly mineralized tissue containing more than 95% w/w of carbonated apatite (dahlite), less then 1% w/w of organic matter, and 4-5% w/w of water (Robinson et al., 1971). Enamel is a uniquely organized, nanostructured material, comprised of long, thin crystals arranged in parallel arrays, 2-3 μm in diameter (enamel rods), forming an intricate 3D pattern (Warshawsky and Smith, 1971; Ten Cate, 1994). The organic content of mature enamel consists of short peptide fragments, rich in Pro, Glx, and Gly (Glimcher et al., 1964; Belcourt and Gillmeth, 1979), which are presumably breakdown products of the major enamel matrix protein, amelogenin (Yamakoshi et al., 2006). Enamel and dentin are coupled at the DEJ (Wang and Weiner, 1998; Fong et al., 2000; Marshall et al., 2001; Zaslansky et al., 2006). Cracks generated in enamel are stopped at the DEJ, preventing catastrophic failure of the tooth (Lin and Douglas, 1994; Xu et al., 1998) via a ligament-bridging reinforcement mechanism provided by collagen fibrils of Von Korff fibers (Imbeni et al., 2005).
It has been previously reported that human enamel is much tougher than geological apatites, suggesting that organic matrix and water have a significant toughening effect on the tissue (White et al., 2001). Significant differences in enamel hardness and toughness have also been observed between planes with different orientations of enamel rods (Staines et al., 1981; Xu et al., 1998; He et al., 2006), suggesting that structural organization also contributes to the mechanical properties of enamel.
The objective of the current study was to obtain new information on how the different components of enamel, specifically organic matrix and water, as well as its structural organization, affect its microhardness and fracture toughness. Special attention was paid to the effect of organic matrix on crack propagation at the DEJ.
MATERIALS & METHODS
Sample Preparation
For this study, we used 24 mandibular incisors from 8-week-old rats. The incisors were flash-frozen in liquid N2 and lyophilized at −55°C for 48 hrs. These teeth were generously provided by Charles Smith (Université de Montréal, Quebec, Canada). Murine incisors were chosen since, unlike other tooth types, they have a homogenous enamel pattern along their anterior-posterior axis (Fig. 1) (Warshawsky and Smith, 1971). Samples were mounted in Epofix (EMS, Hatfield, PA, USA) at room temperature. Half of the samples were cut in the mid-sagittal plane and half in the transverse plane (Fig. 1). All samples were ground (600-grit sandpaper) and polished with diamond suspensions (6, 1, and 0.25 μm particle size) in a Minimet 1000 polisher (Buehler, Lake Bluff, IL, USA). To ensure that the freeze-drying did not affect the mechanical properties of enamel, we performed microhardness studies of wet vs. freeze-dried and rehydrated human enamel and found no difference in hardness values (Appendix 1).
Figure 1.
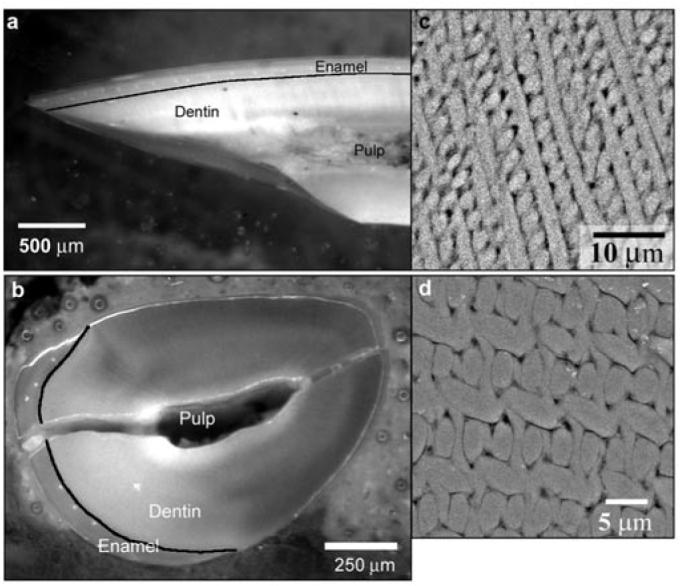
Optical micrographs of rat incisors cut in the mid-sagittal (a) and transverse (b) planes, which are normal to each other; black lines outline the dentino-enamel junction (DEJ). SEM-BSE micrographs of enamel polished in the mid-sagittal (c) and transverse (d) planes illustrate the difference in the enamel rod organization in these 2 planes. Note that the crack in (b) is a consequence of specimen preparation. Since the samples were not embedded, but rather were mounted in resin, the pulp cavity remained unfilled; this led to macrocracking of the sample during polishing. However, this crack did not affect the enamel integrity at the microscopic level.
For each orientation, 6 specimens were cold-ashed for 4 hrs with O2, in a PlasmaPrep II (SPI Supplies, West Chester, PA, USA) at 100 W and 0.5 torr, so that the maximal organic material would be removed. This method of organic removal was chosen since wet chemical techniques have been shown to alter the mineral content of bone, which, like dental tissues, contains carbonated apatite (Broz et al., 1997). The removal of proteins was confirmed by FTIR microspectroscopy of dentin (Appendix 2), since the organic content of mature enamel is at the detection limit of FTIR, and it was impossible to identify changes in the enamel organic content reliably by this approach. Furthermore, a SEM study of untreated and plasma-treated enamel etched with EDTA was carried out. SEM analysis of untreated samples revealed the presence of sheaths of organic material throughout the depth of enamel, whereas in the treated samples such organic material was not observed (Appendix 3). To verify that the plasma ashing had not affected the enamel mineral phase, we performed FTIR studies on untreated and treated samples, showing that there were no changes in the mineral phase (Appendix 4). Half of the plasma-treated and half of the untreated samples were re-hydrated in a humidity chamber at 37° and 100% relative humidity for 72 hrs before mechanical testing. Thus, for each orientation, 4 compositional groups of 3 samples each were obtained: wet untreated, dry untreated, dry treated, and wet treated.
Micromechanical Analyses
We performed mechanical tests in mid-sagittal and transverse planes to determine the influence of rod organization on the mechanical properties of the tissue (Fig. 1). For each sample (n = 3), 10-14 micro-indentations in the mid-sagittal plane and 5-9 in the transverse plane were performed in enamel with the tip corner oriented toward the DEJ at an average distance of 15 μm away. [It is important to mention here that since the micromechanical tests were performed close to the DEJ, the boundary effect could influence the results to some extent. Nevertheless, since the measurements were performed in exactly the same way in all sample groups, such an effect would not influence the trends observed in this study.] To minimize interactions, we made indentations at a distance that was at least twice the crack lengths from each other, from other artifacts, and from the samples' edges (Xu et al., 1998). Tests were carried out at room temperature with a load of 0.98 N and a dwell time of 5 sec, by means of a Leco M 400 H1 microhardness tester equipped with a Vickers diamond tip (St. Joseph, MI, USA). Vickers hardness and fracture toughness were computed according to previously published equations (Anstis et al., 1981; ASTM, 1991). Specifically, fracture toughness was calculated from optical images taken within 5 sec after the test. For each indentation, a circle enclosing all induced cracks was drawn, and its radius, developed from the center of the indentation, was taken as the crack length of the indentation (Anstis et al., 1981). (See Appendix 5 for more details.)
Statistical Analyses
Hardness and fracture toughness values were statistically compared among samples (n = 3) within each compositional group and in the same orientation (ANOVA; α = 0.05). Since no significant difference was found within each group (p ≥ 0.14), further comparisons were made for wet-untreated, dry-untreated, dry-treated, and wet-treated compositional groups. Comparisons among groups of the same orientation (ANOVA; α = 0.05) and between mid-sagittal and transverse groups with the same composition (t test; α = 0.05) were performed.
Scanning Electron Microscopy (SEM) Analysis
Samples were air-dried, sputter-coated with Pd/Au, and analyzed by scanning electron microscopy (JEOL 6400, Tokyo, Japan) in the back-scattered (BSE) and secondary (SE) electron modes at 15 KV and a working distance of 8 mm.
RESULTS
In the mid-sagittal plane, the lowest hardness values were observed in wet-untreated samples, followed by dry-untreated, dry-treated, and wet-treated specimens, which were found to be 23% harder than wet-untreated samples (Table, Fig. 2a). A similar trend was observed in the transverse orientation (Table, Fig. 2a). However, in this plane, the change was much smaller, and the hardness was only 7% higher in wet-treated vs. wet-untreated samples. The differences between treated and untreated specimens were significant in both mid-sagittal and transverse planes (Table). In the mid-sagittal plane, water had a considerable effect on the hardness of untreated enamel, which was 15% harder in dry vs. wet samples (Table; Fig. 2a). In the transverse plane, the effect of drying on hardness values in untreated samples was very small (2%), although statistically significant (Table; Fig. 2a). At the same time, water had no significant influence on the hardness of treated samples (Table; Fig. 2a).
Table.
Micromechanical Characteristics of Murine Enamel from 4 Compositional Groups* Used in the Study (Values represent x̄ ± SD) and Their Statistical Comparison (t test, α = 0.05)
| p values | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wet Untreated | Dry Untreated | Dry Treated | Wet Treated | Wet Untreated vs. Wet Treated |
Dry Untreated vs. Dry Treated |
Wet Untreated vs. Dry Untreated |
Wet Treated vs. Dry Treated |
||
| Hardness (HV0.981) | Mid-saggital plane | 301 ± 8 | 345 ± 4 | 364 ± 8 | 369 ± 12 | < 0.001 | < 0.001 | < 0.001 | 0.09 |
| Transverse plane | 319 ± 5 | 325 ± 11 | 336 ± 5 | 341 ± 11 | < 0.001 | < 0.001 | 0.02 | 0.06 | |
| Fracture toughness (MPa*m1/2) |
Mid-saggital plane | 1.57 ± 0.1 | 1.22 ± 0.1 | 1.01 ± 0.1 | 0.91 ± 0.13 | < 0.001 | < 0.001 | < 0.001 | 0.04 |
| Transverse plane | 0.57 ± 0.1 | 0.52 ± 0.1 | 0.33 ± 0.06 | 0.31 ± 0.06 | < 0.001 | < 0.001 | 0.13 | 0.23 | |
| Crack length (μm) | Mid-saggital plane | 13.8 ± 0.9 | 15.7 ± 0.9 | 17.4 ± 1.1 | 18.7 ± 1.9 | ||||
| Transverse plane | 27.1 ± 3.5 | 28.8 ± 4.4 | 38.4 ± 5.5 | 40.2 ± 5.1 | |||||
Three samples per group were tested; from 10 to 14 micro-indentations in the mid-sagittal plane and from 5 to 9 in the transverse plane were performed on each sample.
Figure 2.
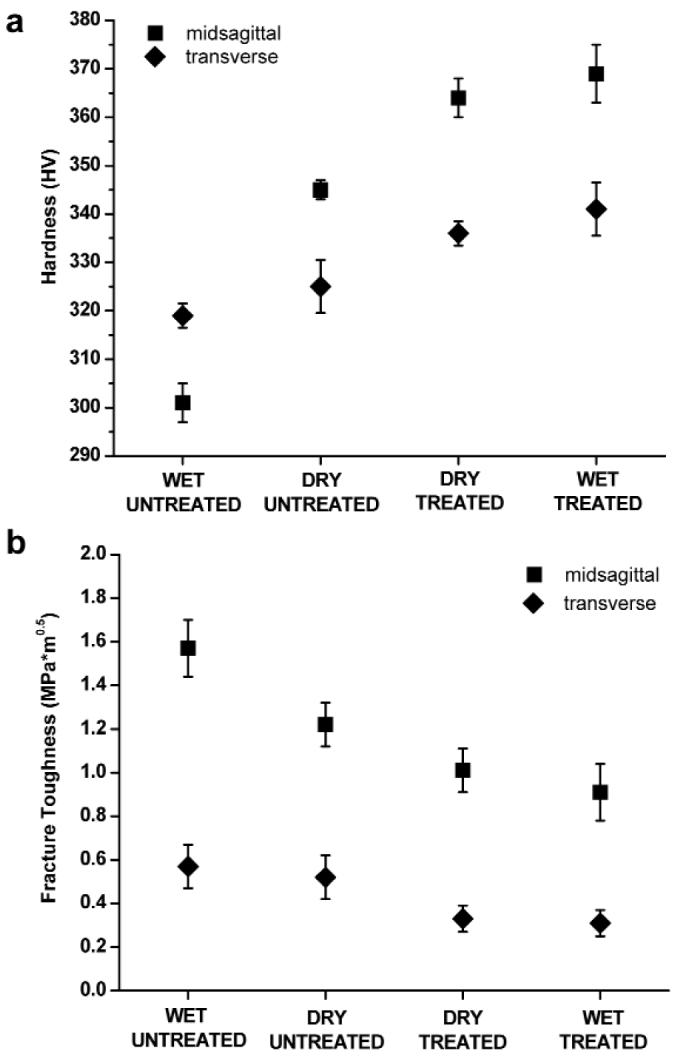
Plots of the hardness (a) and fracture toughness (b) of rat incisor enamel in 4 experimental groups, namely, wet untreated, dry untreated, dry treated, and wet treated, collected in the mid-sagittal (■) and transverse (◆) planes. Error bars represent standard deviation values based on data obtained from 3 incisors per compositional group.
The fracture toughness and hardness values were inversely correlated (Table, Fig. 2). Wet-untreated samples had the highest toughness, followed by dry-untreated, dry-treated, and wet-treated, in which the toughness values were 42% and 46% lower than those of wet-untreated samples in mid-sagittal and transverse planes, respectively (Table, Fig. 2). Removal of organic matter led to a substantial and statistically significant drop in fracture toughness in both planes (Fig. 2b, Table). In the mid-sagittal plane, water had a significant effect on toughness values in both treated and untreated samples (Table). They were 22% lower in dry vs. wet samples in untreated and 10% lower in wet vs. dry samples in treated preparations (Table, Fig. 2b). In the transverse plane, water had no significant effect on toughness values in both treated and untreated samples (Table).
Within each compositional group, hardness and toughness values were all significantly different between midsagittal and transverse orientations (p < 0.001) (Fig. 2). Hardness values were significantly higher in the mid-sagittal vs. the transverse plane for all groups except wet-untreated samples (p < 0.001) (Fig. 2). Remarkably, in the treated samples in the mid-sagittal orientation, toughness values remained relatively high, whereas in the transverse orientation they dropped to the level previously found for geological hydroxyapatite (0.37 ± 0.04 MPa*m1/2) (Fig. 3, Table) (White et al., 2001).
Figure 3.
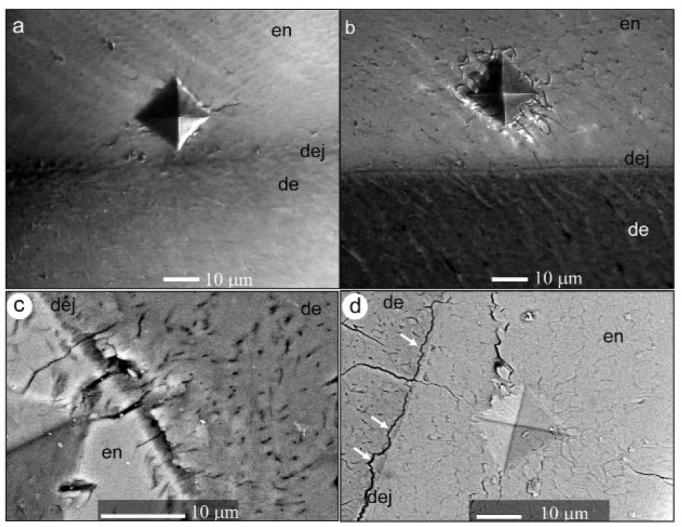
Optical micrographs representing characteristic damage produced by the microindenter in dry-untreated (a) and dry-treated (b) samples in the mid-sagittal plane. SEM-BSE micrographs demonstrating indentation damage in dry-treated samples in the mid-sagittal plane (c), and dry-treated samples in the transverse plane (arrows indicate areas of dentin attached to enamel) (d)
Optical microscopy and SEM analysis of the samples revealed that, in both orientations, removal of organic matrix led to the formation of longer and more numerous cracks and to buckling around the indentation (Fig. 3). In some cases, voids in the polished surface were observed around the indentation, suggesting that some of the material had been chipped away from the tissue (Fig. 3d). In the treated samples, delamination of dentin and enamel along the DEJ occurred, and cracks propagated through this junction (Figs. 3c, 3d). Interestingly, delamination occurred not exactly at the interface between dentin and enamel, but at the dentin side, suggesting a stronger physical bond between dentin and enamel crystals at the interface (Figs. 3c, 3d). Although the water content did not seem to have an influence on the appearance of the indented areas in SEM, we cannot safely conclude this, since all samples were dehydrated before SEM analysis.
DISCUSSION
The results of our study demonstrated that the organic content of enamel, although it comprises less than 1% of w/w, significantly influenced its mechanical properties, confirming previous hypotheses (Jameson et al., 1993; Spears, 1997; White et al., 2001; Zhou and Hsiung, 2006). However, those studies were performed on enamel with organic matrix, and their analyses were based on several assumptions concerning the mechanical properties of enamel components. To the best of our knowledge, our study is the first direct observation of the effect of organic matrix on the mechanical properties of enamel.
Several reports have suggested that the organic matrix of mature enamel is primarily composed of residual enamel protein (Glimcher et al., 1964; Belcourt and Gillmeth, 1979; Yamakoshi et al., 2006). Hence, the results of our study imply that the enamel matrix proteins are not only essential during amelogenesis, but also play a functional role in the mature tissue.
In general, water had a less prominent effect than organic matrix on the mechanical properties of enamel. Interestingly, the way in which water affected the mechanical properties of enamel depended on the presence of organic matrix. In the untreated samples, drying contributed to a decrease in toughness, in agreement with previous findings (Staines et al., 1981; Cuy et al., 2002). Hence, we can assume that dehydration leads to stiffening of enamel proteins that affects their ability to absorb and dissipate impact energy. In contrast, among plasma-treated samples, wet samples were less tough than dry samples. This phenomenon could be due to the differences in energy dissipation in aqueous medium vs. air and their interfaces with mineral. A model has been proposed in which the toughness of enamel can be influenced by interactions between an aqueous solution and the crystal surfaces in enamel pores, which could explain the differences observed between treated wet vs. dry samples (Fox, 1980).
In our experiments, the absolute fracture toughness values, as well as the magnitude of their variability among different compositional groups, were much higher in the mid-sagittal plane than in the transverse plane. Since murine incisor enamel is an anisotropic structure, with mineral rods arranged differently in the mid-sagittal vs. the transverse orientation (Warshawsky and Smith, 1971), analysis of these data suggests that the structural organization of enamel influences its mechanical properties. In the mid-sagittal plane, more rods are oriented at small angles to the plane, whereas they transect the transverse plane at much wider angles. As a result, the load applied normal to the transverse plane is more aligned with the direction of the enamel rods than when a load is applied normal to the mid-sagittal plane. Consistent with these findings, it has been previously shown that the structural organization of mineral rods in the plane of indentation significantly affects the mechanical properties of enamel (Staines et al., 1981; Xu et al., 1998; Habelitz et al., 2001; He et al., 2006). A model of micromechanical properties of human enamel, based on a finite element analysis, has been developed (Spears, 1997). This model suggests that when the applied forces are parallel to the general direction of the enamel rods, the mineral component has greater influence on the mechanical properties, making it harder and more brittle, whereas when rods are perpendicular to the direction of indentation, the organic matrix have more influence, making it softer and tougher. Our findings are consistent with this hypothesis. The difference in fracture toughness between mid-sagittal and transverse orientations in murine incisors may be functionally important. Murine incisors are constantly growing, self-sharpening teeth, with the proximal part ground down under a sharp angle to the transverse plane. It is possible, therefore, that the observed fracture toughness anisotropy is related to this self-sharpening mechanism and protects the enamel from cracking along the tooth axis during this essential process.
SEM analysis revealed significant differences between plasma-treated and untreated samples with and without organics. Specifically, in the plasma-treated samples, cracks generated in enamel crossed the DEJ. These observations are in agreement with a previous hypothesis suggesting that the organic content at the DEJ, specifically, collagen fibrils, is responsible for the prevention of crack propagation from enamel into dentin (Lin et al., 1993; Marshall et al., 2001; Imbeni et al., 2005). Delamination of enamel and dentin after plasma treatment suggests that the organic content is also important in maintaining the integrity of the interface between these two tissues, as has been previously suggested (Imbeni et al., 2005). At the same time, cracks occurring on the dentin side along, but not exactly at, the DEJ suggest strong physical interactions between dentin and enamel crystals.
In conclusion, our findings provide important information on the role of the residual enamel matrix, water, and microstructural organization of enamel mineral in its micromechanical properties. We have also provided new data regarding the role of organic matrix in maintaining the integrity of the interface between enamel and the underlying dentin. These results emphasize how even small differences in material composition can lead to significant changes in their mechanical properties. Analysis of these data contributes to a better understanding of the relationships among structural, compositional, and functional properties of dental tissues and should help guide the design of novel bio-inspired materials for hard-tissue repair and regeneration.
Supplementary Material
ACKNOWLEDGMENTS
We thank Charles Smith (Université de Montréal, Quebec, Canada) for providing rat mandibles. This work was supported by grants DE16703 (EB) and DE016376 (HCM) from the NIDCR. MB thanks Drs. Lorenzo Scalise and Enrico Primo Tomasini for their supervision.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.iadrjournals.org/cgi/content/full/87/7/645/DC1.
REFERENCES
- Anstis GR, Chantikul P, Lawn BR, Marshall DB. A critical evaluation of indentation techniques for measuring fracture toughness: I. Direct crack measurements. J Am Ceram Soc. 1981;64:533–538. [Google Scholar]
- ASTM . Standard test method for microhardness of materials. American Society for Testing and Materials; Philadelphia: 1991. ASTM designation E 384. [Google Scholar]
- Belcourt A, Gillmeth S. EDTA soluble-protein of human mature normal enamel. Calcif Tissue Int. 1979;28:227–231. doi: 10.1007/BF02441240. [DOI] [PubMed] [Google Scholar]
- Broz JJ, Simske SJ, Corley WD, Greenberg AR. Effects of deproteinization and ashing on site-specific properties of cortical bone. J Mater Sci: Mater Med. 1997;8:395–401. doi: 10.1023/a:1018545303184. [DOI] [PubMed] [Google Scholar]
- Cuy JL, Mann AB, Livi KJ, Teaford MF, Weihs TP. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch Oral Biol. 2002;47:281–291. doi: 10.1016/s0003-9969(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Fong H, Sarikaya M, White SN, Snead ML. Nano-mechanical properties profiles across dentin-enamel junction of human incisor teeth. Mater Sci Eng. 2000;C7:119–128. [Google Scholar]
- Fox PG. The toughness of tooth enamel, a natural fibrous composite. J Mater Sci. 1980;15:3113–3121. [Google Scholar]
- Glimcher MJ, Friberg UA, Levine PT. The isolation and amino acid composition of enamel proteins of erupted bovine teeth. Biochem J. 1964;93:202–210. doi: 10.1042/bj0930202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habelitz S, Marshall SJ, Marshall GW, Jr, Balooch M. Mechanical properties of human dental enamel on the nanometre scale. Arch Oral Biol. 2001;46:173–183. doi: 10.1016/s0003-9969(00)00089-3. [DOI] [PubMed] [Google Scholar]
- He LH, Fujisawa N, Swain MV. Elastic modulus and stress-strain response of human enamel by nano-indentation. Biomaterials. 2006;27:4388–4398. doi: 10.1016/j.biomaterials.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Imbeni V, Kruzic JJ, Marshall GW, Marshall SJ, Ritchie RO. The dentin-enamel junction and the fracture of human teeth. Nat Mater. 2005;4:229–232. doi: 10.1038/nmat1323. [DOI] [PubMed] [Google Scholar]
- Jameson MW, Hood JAA, Tidmarsh BG. The effects of dehydration and rehydration on some mechanical properties of human dentin. J Biomech. 1993;26:1055–1065. doi: 10.1016/s0021-9290(05)80005-3. [DOI] [PubMed] [Google Scholar]
- Lin CP, Douglas WH. Structure-property relations and crack resistance at the bovine dentin-enamel junction. J Dent Res. 1994;73:1072–1078. doi: 10.1177/00220345940730050901. [DOI] [PubMed] [Google Scholar]
- Lin CP, Douglas WH, Erlandsen SL. Scanning electron microscopy of type I collagen at the dentin-enamel junction of human teeth. J Histochem Cytochem. 1993;41:381–388. doi: 10.1177/41.3.8429200. [DOI] [PubMed] [Google Scholar]
- Marshall GW, Jr, Balooch M, Gallagher RR, Gansky SA, Marshall SJ. Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus, and fracture. J Biomed Mater Res. 2001;54:87–95. doi: 10.1002/1097-4636(200101)54:1<87::aid-jbm10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Robinson C, Weatherell JA, Hallsworth AS. Variation in composition of dental enamel within thin ground tooth sections. Caries Res. 1971;5:44–57. doi: 10.1159/000259731. [DOI] [PubMed] [Google Scholar]
- Spears IR. A three-dimensional finite element model of prismatic enamel: a re-appraisal of the data on the Young's modulus of enamel. J Dent Res. 1997;76:1690–1697. doi: 10.1177/00220345970760101101. [DOI] [PubMed] [Google Scholar]
- Staines M, Robinson WH, Hood JAA. Spherical indentation of tooth enamel. J Mater Sci. 1981;16:2551–2556. [Google Scholar]
- Ten Cate AR. Oral histology: development, structure, and function. 4th ed. Mosby; St. Louis: 1994. [Google Scholar]
- Wang RZ, Weiner S. Strain-structure relations in human teeth using Moiré fringes. J Biomech. 1998;31:135–141. doi: 10.1016/s0021-9290(97)00131-0. [DOI] [PubMed] [Google Scholar]
- Warshawsky H, Smith CE. A three-dimensional reconstruction of the rods in rat maxillary incisor enamel. Anat Rec. 1971;169:585–591. doi: 10.1002/ar.1091690308. [DOI] [PubMed] [Google Scholar]
- White SN, Luo W, Paine ML, Fong H, Sarikaya M, Snead ML. Biological organization of hydroxyapatite crystallites into a fibrous continuum toughens and controls anisotropy in human enamel. J Dent Res. 2001;80:321–326. doi: 10.1177/00220345010800010501. [DOI] [PubMed] [Google Scholar]
- Xu HH, Smith DT, Jahanmir S, Romberg E, Kelly JR, Thompson VP, et al. Indentation damage and mechanical properties of human enamel and dentin. J Dent Res. 1998;77:472–480. doi: 10.1177/00220345980770030601. [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y, Hu JCC, Zhang HM, Iwata T, Yamakoshi F, Simmer JP. Proteomic analysis of enamel matrix using a two-dimensional protein fractionation system. Eur J Oral Sci. 2006;114(Suppl 1):266–271. doi: 10.1111/j.1600-0722.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- Zaslansky P, Friesem AA, Weiner S. Structure and mechanical properties of the soft zone separating bulk dentin and enamel in crowns of human teeth: insight into tooth function. J Struct Biol. 2006;153:188–199. doi: 10.1016/j.jsb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Zhou JK, Hsiung LL. Biomolecular origin of the rate-dependent deformation of prismatic enamel. Appl Phys Lett. 2006:89–51904. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


